Abstract
Conservation biologists worry that fragmenting a bloc of natural habitat might reduce its species diversity. However, they also recognize the difficulty and importance of isolating the effect of fragmentation from that of simple loss of area. Using two different methods (species–area curve and Fisher's α index of diversity) to analyse the species diversities of plants, tenebrionid beetles and carabid beetles in a highly fragmented Mediterranean scrub landscape, we decoupled the effect of degree of fragmentation from that of area loss. In this system, fragmentation by itself seems not to have influenced the number of species. Our results, obtained at the scale of hectares, agree with similar results at island and continent scales.
Keywords: island biogeography, habitat loss, species area curves, Fisher's alpha, Carabidae, Tenebrionidae
1. Introduction
Ecologists believe that species diversity in a landscape declines as that landscape becomes more fragmented (Haila 2002). But this hypothesis may depend on ill-conditioned datasets in which area correlates with diversity, and diversity correlates inversely with the degree of fragmentation. Therefore, the suspected fragmentation effect may be an artefact (Harrison & Bruna 1999; Fahrig 2003). Using two complementary methods, we decoupled area and degree of fragmentation in an extraordinary set of natural patches and found that fragmentation may not have severe effects on species diversity after all.
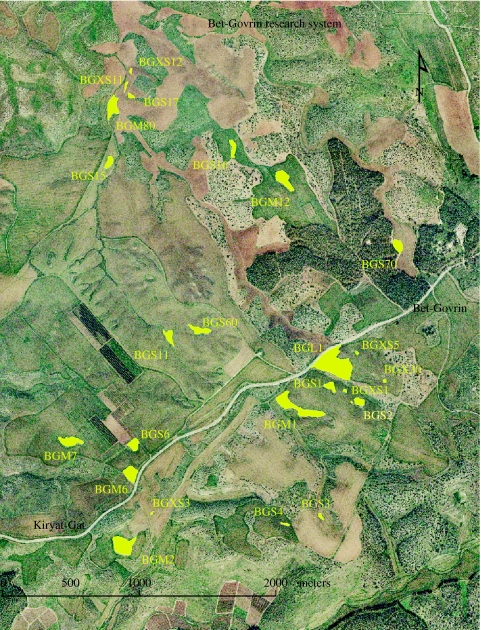
We worked in the agricultural plains of the southern Judea Lowland in Israel near Bet-Govrin (figure 1). This landscape is highly fragmented (less than 13% of its original habitat area), studded with variable-size, small patches of native Mediterranean scrub vegetation growing on thin rocky soils that make them uneconomical to farm. We chose 25 such patches within an area of approximately 13 km2. By coincidence, the 15 largest of these 25 patches form a logarithmic series that decouples fragmentation from area. The very largest (3.8 ha) is approximately twice the size of each of the next two largest; these two are each approximately twice the size of the next four largest; and each of these four is approximately twice the size of the next eight largest (see table 1). This coincidence effectively decouples area from fragment size and makes the set of 15 patches unusual if not unique (the remaining 10 patches were all much smaller than those in the set of 15).
Figure 1.
The highly fragmented landscape of the southern Judea Lowland with the studied patches marked in yellow. The third letter of each patch name indicates its size. XS, extra small; S, small; M, medium; L, large.
Table 1.
The special patch set and its beetles.
| groups (total area) | largest patch (3.8 ha) | next two patches (3.5 ha) | next four patches (3.5 ha) | next eight patches (3.4 ha) |
|---|---|---|---|---|
| Carabidae | ||||
| N | 619 | 612 | 1431 | 2631 |
| S | 22 | 19 | 20 | 25 |
| Fisher's α | 4.45 | 3.71 | 3.29 | 3.82 |
| Tenebrionidae | ||||
| N | 820 | 694 | 1523 | 1404 |
| S | 13 | 12 | 18 | 17 |
| Fisher's α | 2.19 | 2.06 | 2.86 | 2.72 |
| perennial plants | ||||
| S | 18 | 19 | 18 | 18 |
2. Material and methods
We measured the diversities of three groups of native species on the 25 patches: perennial flowering plants (31 species), darkling beetles (Tenebrionidae; 22 species, 4807 individuals) and predatory ground beetles (Carabidae; 32 species, 6316 individuals). We used randomly assigned repeated line transects to survey plants. To avoid trapping bias, we collected beetles with wet pit-fall traps for a total of 70 days. The sampling effort per unit area in all patches was relatively constant (see detailed information in Yaacobi et al. 2007). None of the beetle species can live in a habitat composed entirely of the surrounding agricultural matrix. Both Tenebrionidae and Carabidae are flightless. Carabidae wander broadly, whereas Tenebrionidae tend to stay very much within a confined home range. We found no significant change in beetle species turnover (β) diversity with distance among the studied patches across our landscape.
We analysed the set of 25 patches with two methods. Method one, the species–area relationship (SPAR) method based on Rosenzweig (1995, 2004), uses all 25 patches and all three groups. We applied the species–area curve (SPAR) method as follows. We determined the SPAR for each group. Then we extrapolated one group's SPAR to a point above the total area (14.2 ha) of the set of patches. The value of diversity at that point represents the predicted value of that group's diversity for an imaginary patch of that size, providing fragmentation has no effect on diversity. If the actual number of species in the set of 25 patches is less than this predicted value, then fragmentation will have depressed diversity. If it is greater, then fragmentation will have increased diversity. We repeated this procedure with the other two groups.
Method two uses only the 15 patches in the special set. For each of its four levels of fragmentation, we counted the number of species of both beetle taxa as well as their abundances. Then we calculated Fisher's α index of diversity (Fisher et al. 1943) for each level. If fragmentation does tend to decrease the species diversity of these groups, the single large patch should harbour the highest species diversities, the patch pair should have fewer, the set of four fewer still and the set of eight the least. We could not use this method on the plant data because one needs abundances to calculate Fisher's α. To get abundances, we would have either to uproot all the plants or to perform DNA fingerprinting for all of them. It is not clear what analogue of Fisher's α one might calculate in order to reduce the bias introduced by unequal sample sizes. However, we do report the observed number of plant species to permit simple comparisons with the beetle results.
3. Results
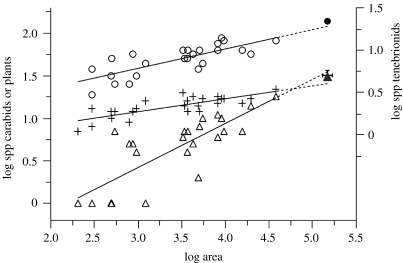
The SPAR method showed that the set of fragments had more species of both Tenebrionidae and Carabidae than would have been present in an unfragmented area equal in size to that of the set of 25 patches (Carabidae, 32>25.54; Tenebrionidae, 22>19.14; figure 2). Meanwhile, the set of fragments had fewer plant species than would have been present in an unfragmented area (31<35.22; figure 2).
Figure 2.
SPAR of the plant, carabid and tenebrionid beetles (open triangle, open circle and +, respectively) in the different patches and their total number of species (filled triangle, filled circle and †, respectively). The lines show the expected diversity if fragmentation has no effect.
None of these differences was severe. The deviations of all three from expectation lay well within the bounds of the empirical deviations of individual patches from their species–area curve, indicating that no ensemble deviation was significant.
The Fisher's α values revealed no significant trend of species diversity in either beetle family with increase of fragmentation level (table 1). Note that the Carabidae had their second highest diversity in the set of eight, and the Tenebrionidae had their highest and their second highest diversities in the set of four and the set of eight, respectively. In addition, the Fisher's α method independently provided the same conclusion as the SPAR method. Thus, although the data yielded only four points per taxon, our result is unlikely to reflect type II error. Fragmentation, independent of area loss, does not have a negative effect on the diversity of our studied groups.
4. Discussion
The negative effect of fragmentation is a cornerstone of the policy recommendations, research agenda and fiscal priorities of conservation biology (Lubchenco et al. 1991). In our work, we separated the effects of fragmentation from that of area loss. The results suggest that fragmentation has no consistent or significant effect on species diversity. Fahrig (2003) reports that most studies claiming that fragmentation leads to loss of diversity do not separate fragmentation from area. However, Fahrig lists 17 studies that do strive to achieve this separation. She concludes that they do not support the hypothesis that fragmentation reduces diversity. This is our conclusion too. Nevertheless, we looked at each of these 17 studies. Many deal only with a single species. Some deal with abundances; many deal with flying species not well isolated by the fragments; and one uses an analytical technique now known to be faulty. So we do not claim that they parallel and support our own. Yet we do endorse Fahrig's conclusion and have followed her recommendation to separate the two variables from each other.
The results of Tscharntke et al. (2002) support our conclusion that fragmentation does not have a negative effect on species diversity. In the latter study, small grassland fragments showed higher species diversities—of butterflies, legume-feeding herbivores and of rape-pollen beetles and their parasitoids—than did those same groups in larger grassland fragments (and see references within Tscharntke et al. 2002). However, this latter study did not explicitly analyse the effect of habitat loss relative to that of fragmentation per se processes on total species diversity, which is the purpose of our study.
The question of fragmentation was introduced to help design nature reserves using the theory of island biogeography (Wilson & Willis 1975). The scale of our study is smaller, involving not islands but patches of mainland. Yet, just as fragmentation seems not to matter at the latter scale, it appears not to matter at the island scale (Lomolino 1994; Rosenzweig 2004). Theoretically, it also does not matter at the scale of whole continents, i.e. a world divided into n separate biogeographical provinces should have about the same steady-state species diversity as an undivided one (Rosenzweig 2001). So the hypothesis that fragmentation depresses the number of species would appear to be one of those reified doctrines that Slobodkin (2001) warns against.
However, fragmentation will retain considerable importance for the conservation of individual species that require unbroken tracts for territories. These species may well be among the most charismatic, exerting an influence on decision making well beyond their contribution to species numbers. Even supposing that we are correct in our conclusion, even if we are right to expect that other species from the available pool can be expected to take the place of the charismatic ones that may disappear owing to fragmentation, society will not be satisfied. Nor, in our opinion, should it be. The simple number of species is hardly the sole matter of conservation importance. Nonetheless, fragmentation studies would seem best focused on those charismatic species likely to be endangered by fragmentation, and more general claims of fragmentation's severe consequences viewed with great caution.
Acknowledgments
We thank Nils Stenseth and two anonymous reviewers for improving the current version of our paper. This study has been supported by funds provided to the International Arid Lands Consortium (IALC) by the USDA Forest Service and by the USDA Cooperative State Research, Education, and Extension Service.
References
- Fahrig L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003;34:487–515. doi:10.1146/annurev.ecolsys.34.011802.132419 [Google Scholar]
- Fisher R.A, Corbet A.S, Williams C.B. The relation between the number of species and the number of individuals in a random sample from an animal population. J. Anim. Ecol. 1943;12:42–58. doi:10.2307/1411 [Google Scholar]
- Haila Y. A conceptual genealogy of fragmentation research: from island biogeography to landscape ecology. Ecol. Appl. 2002;12:321–334. [Google Scholar]
- Harrison S, Bruna E. Habitat fragmentation and large-scale conservation: what do we know for sure? Ecography. 1999;22:225–232. doi:10.1111/j.1600-0587.1999.tb00496.x [Google Scholar]
- Lomolino M.V. An evaluation of alternative strategies for building networks of nature-reserves. Biol. Conserv. 1994;69:243–249. doi:10.1016/0006-3207(94)90423-5 [Google Scholar]
- Lubchenco J, et al. The sustainable biosphere initiative: an ecological research agenda. Ecology. 1991;72:371–412. doi:10.2307/2937183 [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Rosenzweig M.L. The four questions: what does the introduction of exotic species do to diversity? Evol. Ecol. Res. 2001;3:361–367. [Google Scholar]
- Rosenzweig M.L. Applying species–area relationships to the conservation of species diversity. In: Lomolino M.V, Lawrence R.H, editors. Frontiers of biogeography: new directions in the geography of nature. Sinauer Associates; Sunderland, MA: 2004. pp. 325–344. [Google Scholar]
- Slobodkin L.B. The good, the bad and the reified. Evol. Ecol. Res. 2001;3:1–13. [Google Scholar]
- Tscharntke T, Steffan-Dewenter I, Kruess A, Theis C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 2002;12:354–363. [Google Scholar]
- Wilson E.O, Willis E.O. Applied biogeography. In: Cody M.L, Diamond J.M, editors. Ecology and evolution of communities. Harvard University Press; Cambridge, UK: 1975. pp. 522–534. [Google Scholar]
- Yaacobi G, Ziv Y, Rosenzweig M.L. Effects of interactive scale-dependent variables on beetle diversity patterns in a semiarid ecosystem. Landscape Ecol. 2007;22:687–703. doi:10.1007/s10980-006-9061-7 [Google Scholar]