Abstract
The social brain hypothesis argues that large brains have arisen over evolutionary time as a response to the social and ecological conflicts inherent in group living. We test predictions arising from the hypothesis using comparative data from birds and four mammalian orders (Carnivora, Artiodactyla, Chiroptera and Primates) and show that, across all non-primate taxa, relative brain size is principally related to pairbonding, but with enduring stable relationships in primates. We argue that this reflects the cognitive demands of the behavioural coordination and synchrony that is necessary to maintain stable pairbonded relationships. However, primates differ from the other taxa in that they also exhibit a strong effect of group size on brain size. We use data from two behavioural indices of social intensity (enduring bonds between group members and time devoted to social activities) to show that primate relationships differ significantly from those of other taxa. We suggest that, among vertebrates in general, pairbonding represents a qualitative shift from loose aggregations of individuals to complex negotiated relationships, and that these bonded relationships have been generalized to all social partners in only a few taxa (such as anthropoid primates).
Keywords: brain size, pairbonding, mammals, birds, primates
1. Introduction
Several decades ago, the cognitive demands imposed by social complexity was proposed as an explanation for why primates have unusually large brains for their body size (Jolly 1966; Byrne & Whiten 1988). This proposal since has become crystallized as the social brain hypothesis (SBH; Dunbar 1992, 1998). The essence of the SBH is that the need to solve (ecological) problems in a social context, rather than in a demographic vacuum, imposes significant cognitive demands. Within a social environment, individual decisions must be responsive to the decisions made by other group members and the constraints these impose. For example, in order for a foraging group to maintain coherence and not fission, individuals must make choices that not only allow their energetic needs to be met but also permit other group members to meet theirs.
One widely cited prediction resulting from the SBH is that social group size should correlate with brain size because the number of potential dyadic relationships (interpreted as one index of social complexity) is proportional to group size. Although strong support for this prediction has been found in primates (Dunbar 1992, 1998; Barton 1996), several recent analyses suggest that this prediction may be too simplistic: group size does not consistently correlate with brain size in some taxonomic groups (Beauchamp & Fernandez-Juricic 2004; Shultz & Dunbar 2006), implying that the relationship between brain size and sociality may be more complex than previously supposed. Thus, the nature and stability of relationships may be more important than the shear number of aggregating individuals. Some groups, such as flocks of wading birds, are little more than aggregations and exhibit a fluid structure: individuals join and leave the group as their needs, or the environment, dictate. However, in other cases such as primates, groups appear to be more structured, with membership relatively stable over time. Thus, a ‘complex’ social environment may be more the result of an individual's role within the group and its relationships with other group members rather than the total number of individuals with whom it associates. Indeed, in primates, the SBH has received considerable support from analyses that focus more explicitly on aspects of behavioural complexity: alternative indices of social complexity such as deception rates (Byrne & Whiten 1988), mating strategies (Pawlowski et al. 1998), grooming clique size (Kudo & Dunbar 2001) and coalition rates (Dunbar & Shultz 2007) all correlate with relative brain size.
Relative brain size in mammals (including primates) has also been shown to correlate with a number of non-social life history and ecological variables (Clutton-Brock & Harvey 1980; Armstrong 1985; Harvey & Krebs 1990). It is important to appreciate that many of these are constraints on the evolution of large brain size rather than selection pressures (they generally do not provide an adaptive explanation for an evolutionary increase in relative brain size). We have elsewhere shown, using path analysis, that for both birds (Shultz & Dunbar submitted) and primates (Dunbar & Shultz 2007), life history and diet act as constraints on brain evolution, and do so quite independently of effects due to sociality. However, to understand how brain size and behaviour are associated, it is necessary to consider and appreciate that constraints potentially exist.
Here, we evaluate predictions generated by the SBH across different vertebrate taxa. To do this, we compiled a database for 86 species of carnivores, 69 species of artiodactyls and 45 species of primates for whom anatomical data on actual brain volumes are available (see electronic supplementary material). In addition, we compare these taxa with relationships documented elsewhere between social system and brain size for 135 species of birds (S. Shultz and R. Dunbar 2007, unpublished analyses) and 54 species of bats (from Pitnick et al. 2006). First, we ask whether the group size effect that has been documented in primates applies more widely. Second, we evaluate whether different social systems (as opposed to group size) are associated with encephalization in any consistent way. Third, we identify models incorporating both behaviour and ecology which best predict relative brain size across groups. Finally, we evaluate whether we can identify commonalities across these taxonomic groups, both in terms of factors associated with relative brain size and in terms of the actual nature of sociality itself. In the latter respect, we identify two specific indices of social intensity whose frequency we can estimate and compare relatively easily for a number of species (social ‘bondedness’ and time devoted to social interaction: we define these below).
2. Material and methods
(a) Data sources
Information on the species included in the analyses and the phylogenetic trees constructed for each taxon can be found in the electronic supplementary material. To analyse the relationship between social system and brain size, each species was assigned to one of four social categories: solitary (individuals spend most of the year alone or only with their most recent offspring), pairbonded (breeding pairs establish bonds that last beyond the immediate mating season, including cooperative breeding species based on a single breeding pair), harem/single sex (groups composed of more than one adult female and one (seasonal) adult male) and multimale groups (several adult males and females in association, typically with polygynous breeding; see electronic supplementary material). We deliberately use the term pairbonded here rather than monogamous because the term ‘monogamy’ has been rather loosely used to cover a variety of social, mating and parenting relationships that do not always covary (Fuentes 1998). Our concern is explicitly with social monogamy, irrespective of whether this involves monogamous mating or biparental care. This four-way categorization of social systems is standard in the primate literature (Smuts et al. 1987), but it can be applied perfectly well to any order. More importantly, it has two advantages in the present context. First, it is based primarily on female bonding patterns (see below) and, second, it has an implicit quantitative continuum in terms of group size running through it.
As various ecological factors have been shown to be associated with brain size (Harvey et al. 1980; Reader & Laland 2002), we identified potentially relevant ecological factors (diet, habitat use, strata use and activity pattern; see table 1 for categories and electronic supplementary material for detailed criteria and data sources) associated with brain size.
Table 1.
Univariate results for all factors (for definitions, see §2) using phylogenetic brain size residuals.
| taxon | factor | categories | brain | ||
|---|---|---|---|---|---|
| d.f. | F | p | |||
| primates | social system | solitary, pair, harem, multimale | 3,38 | 12.47 | <0.001 |
| diet | folivore, folivore–frugivore, frugivore, omnivore | 3,38 | 2.36 | 0.09 | |
| strata | arboreal, terrestrial | 2,39 | 3.74 | 0.03 | |
| habitat | open, closed, mixed | 2,39 | 1.50 | 0.24 | |
| activity | diurnal, nocturnal | 1,40 | 40.76 | <0.001 | |
| carnivores | social system | solitary, pair, harem, multimale | 3,82 | 3.10 | 0.03 |
| diet | vegetarian, mixed-invertebrates, mixed-small vertebrates, large vertebrates | 3,82 | 3.87 | 0.006 | |
| strata | arboreal, terrestrial | 1,84 | 9.95 | 0.002 | |
| habitat | open, closed, mixed | 2,83 | 1.15 | 0.34 | |
| activity | diurnal, nocturnal | 2,83 | 0.77 | 0.47 | |
| artiodactyls | social system | solitary, pair, harem, multimale | 3,65 | 2.86 | 0.04 |
| diet | grazer, grazer/browser, browser, browser/frugivore, omnivore | 4,64 | 2.81 | 0.03 | |
| habitat | open, closed, mixed | 2, 66 | 0.01 | 0.99 | |
(b) Relative brain size
For the three focal taxa (primates, carnivores and artiodactyl ungulates), we employed phylogenetic generalized least squares (PGLS) analysis (Grafen 1989) to estimate residual brain size controlling for body size and phylogeny (as a fixed factor) because related species are likely to share traits by common decent (Harvey & Pagel 1991; Ihaka & Gentleman 1996). To incorporate phylogeny into the estimate of relative brain size, we regressed log brain size against log body size with an attached covariance matrix of relatedness as an error term. This covariance matrix can be modified to accommodate the degree to which trait evolution deviates from Brownian motion, using a measure of phylogenetic correlation (λ) derived by Pagel (1999; see also Freckleton et al. 2002). A Brownian motion model of evolution assumes that λ=1 (traits are directly proportional to relatedness), while in models that assume phylogenetic independence λ=0 (no correlation between traits and phylogenetic relatedness). The PGLS approach was executed in R (Ihaka & Gentleman 1996) using the APE (Analysis of Phylogenetics and Evolution) package (Paradis et al. 2005) with code provided by R. P. Duncan.
We used the PGLS approach again to evaluate the association between social and ecological and relative brain size for each focal taxon. As a second step to check whether ecological factors were confounds with our social measures, we constructed minimum adequate models (MAMs; table 2) using forward and backward stepwise selection, identifying models where all other factors remaining are significant.
Table 2.
Minimum adequate models identified for each taxon.
| taxon | factors | d.f. | F | p | r2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| primates | activity | 1,32 | 12.74 | 0.001 | 0.83 | ||||
| diet | 3,32 | 15.91 | <0.001 | ||||||
| social | 3,32 | 7.65 | 0.001 | ||||||
| strata | 2,32 | 4.60 | 0.02 | ||||||
| carnivores | social | 3,81 | 5.29 | 0.002 | 0.25 | ||||
| strata | 1,81 | 16.30 | <0.001 | ||||||
| ungulates | social | 3,61 | 4.51 | 0.006 | 0.30 | ||||
| diet | 4,61 | 4.11 | 0.005 |
| taxon | model | factors | categories | lambda | AIC | d.f. | F | p |
|---|---|---|---|---|---|---|---|---|
| primates | 1 | social | 0.78 | −80.61 | 3,35 | 7.64 | 0.0005 | |
| diet | 3,35 | 3.65 | 0.02 | |||||
| 2 | activity | diurnal, cathemeral, nocturnal | 0.82 | −61.67 | 2,39 | 4.197 | 0.02 | |
| 3 | strata | arboreal, terrestrial | 0.92 | −54.34 | 2,39 | 0.31 | 0.74 | |
| 4 | habitat | open, closed, mixed | 0.92 | −53.90 | 2,39 | 0.15 | 0.86 | |
| 5 | diet | folivore, folivore–frugivore, frugivore, omnivore | 0.95 | −67.10 | 3,38 | 5.25 | 0.004 | |
| 6 | social | solitary, pair, harem, multimale | 0.89 | −74.14 | 3,38 | 8.164 | 0.0003 | |
| carnivores | 1 | social | 0.43 | −127.43 | 3,81 | 3.54 | 0.018 | |
| strata | 1,81 | 17.27 | 0.0001 | |||||
| 2 | activity | diurnal, cathemeral, nocturnal | 0.09 | −108.55 | 2,83 | 0.43 | 0.65 | |
| 3 | strata | semi-arboreal, terrestrial | 0.20 | −124.83 | 1,84 | 16.10 | 0.0001 | |
| 4 | habitat | open, closed, mixed | 0.26 | −109.76 | 3,82 | 1.45 | 0.234 | |
| 5 | diet | vegetarian, mixed-vegetarian, mixed-invertebrates, mixed-small vertebrates, large vertebrates | 0.07 | −117.60 | 4,81 | 3.39 | 0.013 | |
| 6 | social | solitary, pair, harem, multimale | 0.0 | −112.71 | 3,82 | 2.90 | 0.04 | |
| ungulates | 1 | social | 0.76 | −122.83 | 3,61 | 3.00 | 0.04 | |
| diet | 4,61 | 2.46 | 0.05 | |||||
| 2 | habitat | open, closed, mixed | 0.69 | −119.06 | 2,66 | 3.87 | 0.03 | |
| 3 | diet | grazer, grazer/browser, browser, browser/frugivore, omnivore | 0.67 | −118.86 | 4,64 | 2.74 | 0.04 | |
| 4 | social | solitary, pair, harem, multimale | 0.71 | −119.43 | 3,65 | 3.42 | 0.02 |
(c) Social relationships
We obtained data on two measures of social intensity: the proportion of species in each group that exhibit a bonded social structure and, for a subset of species, the total daytime devoted to social activities. Each of us independently classified all primate, carnivore and ungulate (artiodactyl) genera as a function of the kinds of bonds exhibited by females (female–female bonded, pairbonded or non-bonded), based on species descriptions in Nowak (1999). We did not include the behaviour of males since (i) male-bonded social systems are rare in any order and (ii) the key factor in primate sociality has long been thought to be female-bondedness (Wrangham 1980). The criteria for female-bonded was a statement that a genus is characterized by stable groups composed of related females or that females remain in their natal group and do not disperse (and not merely that their adult ranges partially overlap those of their mothers). Pairbonded species were defined as those species that are behaviourally monogamous (males and females live together and coordinate their activities). All other social systems (i.e. those that have unstable group composition over time or those whose females disperse at maturity at least as far as males do) were defined as unbonded. We calculated Cohen's kappa (Cohen 1960) as a measure of inter-observer agreement: our overall agreement for 205 genera was 93% (κ=0.87, τ=14.59, p<0.001). We used primary sources to clarify the 7% of genera where our initial classifications disagreed.
A key problem faced by animals that live in bonded groups is the need to maintain relationships with their social partners. With the exception of primates (where social grooming is a widely accepted index), there has been little attempt to define behavioural measures of bondedness in animals. This makes it extremely difficult to make direct comparisons across taxa. However, as a preliminary attempt to explore this issue in more detail, we here use the amount of time invested in social activity as a measure of investment in social relationships. Activity budget data for primate and artiodactyl populations were obtained from the literature (see the electronic supplementary material for sources): social activity was defined broadly as all non-maintenance activities, including social grooming and other affiliative behaviour, social play, agonistic behaviour, territorial behaviour, etc.
3. Results
(a) Social correlates of brain size
Our analyses confirm previous findings for primates (Sawaguchi & Kudo 1990; Dunbar 1992; Barton 1996): there is a positive correlation between group size and relative brain size (λ=0.80, F1,40=8.14, p<0.01). In contrast, for the two non-primate taxa with reliable estimates of species average group sizes, the relationship between relative brain size and group size was not significant (artiodactyls: λ=0.63, F1,67=0.30, p=0.58; carnivores: λ=0.11, F1,84=0.07, p=0.80).
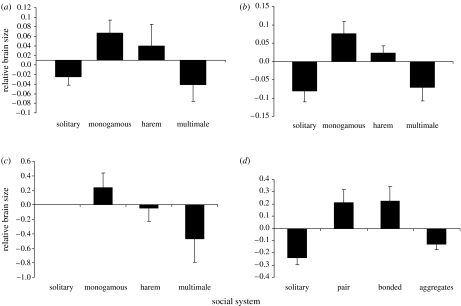
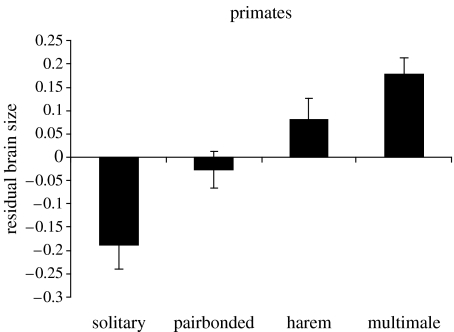
In contrast, social system was significantly associated with relative brain size for all three taxa (table 1). Figure 1 reveals that artiodactyl and carnivore species with pairbonded mating systems consistently had the largest brain sizes, and this pattern is closely paralleled in both birds and bats. In addition to a clear association between relative brain size and group size in primates, brain size is also associated with social system, but in their case brain size increases progressively from solitary to multimale groups. A comparison of pairbonded versus all other social systems combined confirms that pairbonded species have significantly larger relative brains than non-pairbonded species in all taxa except bats and primates (one-tailed tests with a directional hypothesis; carnivores: t=−2.86, p=0.0025; ungulates: t=−2.53, p=0.0007 birds, t=−5.67, p<0.001; bats: t=−1.34, p=0.095; primates: t=−0.13, t=0.445). All the five taxa exhibit a consistent trend in the same direction (Fisher's log-likelihood test: excluding primates, Χ82=46.42, p<0.001; including primates, Χ102=48.04, p≪0.001), although primates appear to be a clear outlier (figure 2).
Figure 1.
Mean relative brain size (±s.e.m.) as a function of social system in (a) carnivores and (b) ungulates. For comparison, the relationship between relative brain size and social system are presented for two additional vertebrate taxa: (c) bats and (d) birds, based on data provided by S. Shultz & R. Dunbar 2007 (unpublished data) and Pitnick et al. (2006).
Figure 2.
Mean relative brain size (±s.e.m.) as a function of social system in primates.
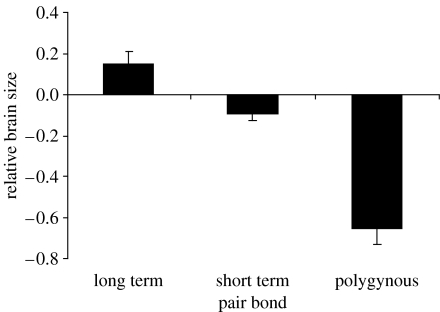
Thus, in all four non-primate taxa, pairbonded species have much larger brains than would be predicted for group size. This is given added weight by the fact that bird species with long-term pairbonds have significantly larger relative brain sizes than those with annual pairbonds (figure 3). It is particularly surprising that pairbonded species should so consistently have larger brains than species that mate polygamously since it has been widely assumed that managing polygamous mating relationships is cognitively more taxing (e.g. Gittleman 1986).
Figure 3.
Relationship between pairbond duration and type and relative brain size across bird species (mean±s.e.m.; data from Shultz & Dunbar, unpublished data).
To check whether there were potential confounds between ecological and behavioural factors, we identified MAMs for the three focal taxa (carnivores, artiodactyls and primates). These analyses confirm that social system is consistently included in all the best models as an independent predictor of brain volume (table 2).
(b) Indices of social intensity
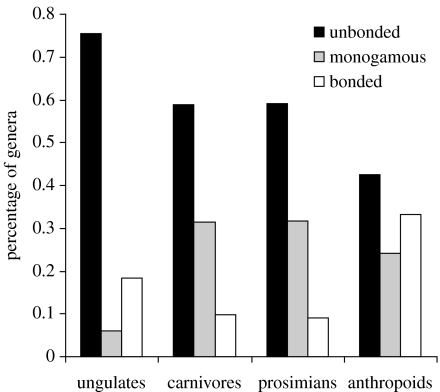
The contrast in sociality between anthropoid primates and other mammalian taxa (including strepsirhine primates) is emphasized by the extent to which bonded relationships (defined as having either female–female bonds or pairbonds; see §2), which are rare in mammals as a whole, are unusually common in anthropoid primates (figure 4). The four taxa differ significantly (Χ62=19.74, p=0.003). Partitioning Χ2 shows that artiodactyls and carnivores differ significantly (Χ22=10.99, p=0.01), but strepsirhine primates do not differ from ungulates and carnivores combined (Χ22=1.89, p=0.39), while anthropoids differ from all the three combined (Χ22=8.49, p=0.01). Note that strepsirhine primates (who have smaller brains for body size than anthropoids) seem to resemble carnivores more than they do anthropoid primates.
Figure 4.
Differences in percentage of genera that are bonded in individual ungulate (artiodactyl), carnivore, prosimian and anthropoid genera (for details and definitions, see §2). Bondedness in ungulates is heavily influenced by the suids (5 out of 9 suid genera are female bonded). Sample sizes are: 49 ungulate species, 54 carnivores, 23 prosimians and 38 anthropoid primates (data from Nowak 1999).
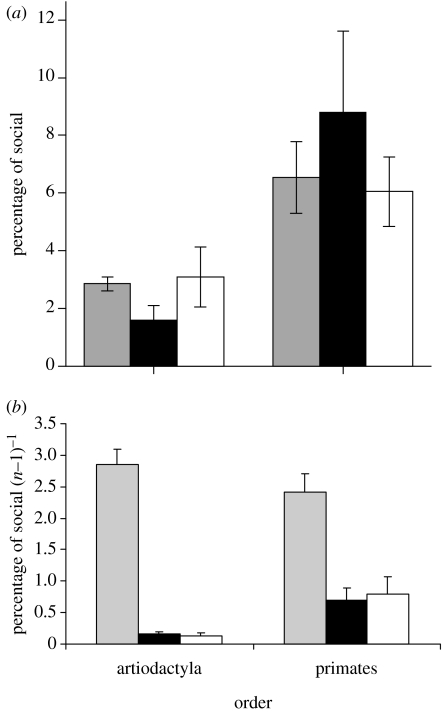
Overall, anthropoid primates devote significantly more time to social activity than ungulates (ANOVA arcsin proportion of social time: F1,39=9.59, p=0.004, figure 5a). As this may simply reflect the fact that primates are typically found in larger social groups, we also evaluated the amount of social time adjusted for group size. When social time is corrected for the number of other individuals in the group (N−1), pairbonded species devote significantly more time to social activities than do harem based or multimale grouping species (figure 5b; ungulates: LSD pairbonded (P) versus harem (H), p<0.001, and P versus multimale (M), p<0.001; primates: LSD P versus H, p=0.01 and P versus M, p=0.005). There is no difference in social time between pairbonded primates and pairbonded ungulates (ANOVA with arcsin proportion of time: F1,12=0.10, p=0.76), but harem and multimale primates devote more time per individual group member than ungulates (F1,25=6.05, p=0.02) and, in general, primates devote more time to social activity than ungulates do (F1,39=9.59, p=0.004). In sum, although non-pairbonded primates devote more time to other individuals than non-pairbonded ungulates, pairbonded primates and pairbonded ungulates do not differ significantly from each other and both devote significantly more time to social activities than do non-pairbonded species of either taxon.
Figure 5.
(a) Mean±SE total social time, and (b) corrected for number of other individuals in the group for monogamous (grey bars), harem (black bars) and multimale (white bars) ungulates and primates. Sample sizes are: 38 primate species and 6 ungulates (for sources: see §2).
4. Discussion
We have shown that, across a wide range of non-primate taxa, pairbonded species of vertebrates have significantly larger brains for body size than species with all other mating systems, which implies that they are cognitively more demanding. There are several reasons why pairbonding might be cognitively demanding. Individuals in lifelong monogamous mating relationships forego opportunities to mate or search for other partners (Komers & Brotherton 1997), making it imperative that the risk of cuckoldry is minimized and that individuals optimize mate choice. In addition, maintaining a lasting bond entails inevitable conflict between partners over resources, parental investment decisions and time budgets, which in turn necessitates an ability to resolve conflicts and coordinate scheduling (Dunbar & Dunbar 1980) in order to maximize reproductive success.
Conversely, the relatively small brains of carnivores, ungulates, bats and birds in multimale groups can be explained by a lack of cognitive demand placed on individuals in relatively unstable groupings, even when these are large. Unlike those in bonded groups, individuals in transitory groups may not need to invest in cognitive resources for cataloguing previous experiences (or identifying cheats), judging relative resource holding potential, manipulating the behaviour of other individuals or recognizing relatedness. Making decisions about these issues might be done more effectively, for example, on an encounter-by-encounter basis. This suggestion is given some support by the relatively large brains of species that live in stable mixed groups (e.g. canids) as compared to those social carnivores that forage solitarily (e.g. badgers: see the electronic supplementary material for species data).
There are at least three possible alternative explanations for these results. First, it could be that, since a number of other ecological and life-history variables are known to correlate with brain size in mammals, the relationship with social system is simply an artefact of the fact that mating system correlates with one of these ecological variables. However, the MAMs analysis confirms that, irrespective of any ecological and life-history covariates, social system independently influences brain size in all taxa.
Second, it has been suggested that, in the case of bats, there is a trade-off between two different kinds of expensive tissue, namely brain and testes (Pitnick et al. 2006); if this is applied more generally, it would offer an alternative explanation for our findings. In fact, a more likely scenario is that large testis size is a response to sperm competition and the relationship between testis size and brain size is an artefact of both covarying with mating system, as has been demonstrated in other vertebrate taxa (Harcourt et al. 1981; Møller & Briskie 1995). This suggestion is supported by the fact that Pitnick et al. (2006) themselves show that both brain and testis sizes are strongly associated with mating system, while the relationship between relative brain and testis sizes is much weaker. A more plausible alternative explanation for their bat results is thus that, rather than polygamous species trading brain tissue for larger testes, monogamous species require more brain volume.
Third, the large brains of pairbonded species may be related in some way to the demands of biparental care rather than the demands of pairbonding (i.e. relationship maintenance). Conventional views assume that, in both non-human primates (Kleiman 1977; Wittenberger & Tilson 1980) and early hominids (Lovejoy 1981; Key & Aiello 2000), monogamy evolved to facilitate dual parental investment in large-brained offspring. However, there is little if any paternal care in monogamous ungulates (Brotherton & Rhodes 1996), despite the fact that they also show a strong effect of pairbonding on brain size. More importantly, biparental care does not correlate with brain size in carnivores (Gittleman 1986) and is relatively rare in primates (Dunbar 1988), while in birds brain size correlates with pairbonding independently of biparental care (Shultz & Dunbar submitted). That pairbonded species have large brains across taxonomic groups, independent of patterns of parental care, suggests that pairbonding (or at least the mechanisms of bonding that underpin these mating systems) arose for some other reason such as mate guarding, reproductive coordination or predator avoidance (Dunbar & Dunbar 1980; van Schaik & van Hooff 1983; van Schaik & Dunbar 1990; Komers & Brotherton 1997).
One further possible source of confound might be the particular phylogenies we have used. This is a perennial problem given that phylogenies are always subject to change with time as new data become available. We have used those that are the most recent and widely accepted; indeed, that for primates (Purvis 1995) is the standard phylogeny used in all comparative analyses, and is not in serious dispute. For those orders where there is disagreement, this usually concerns the insertion points of major taxonomic groups, rather than the detailed arrangement of species within families; since such disagreements are likely to affect only a handful of high-level nodes in the phylogeny, they will have rather limited statistical impact on the results. Nonetheless, as a check on this, we ran the analyses both with and without phylogenetic correction for the mating system analysis for the three focal mammalian orders and the results remain the same. We can be confident that more minor disagreements are unlikely to significantly alter the conclusions.
Our analyses reveal a marked contrast between primates and all the other taxa (including both birds and a wide range of non-primate mammals) in that primates exhibit a strong signature for a quantitative group size effect on brain size, but all the other taxa do not. In primates, the relationship between brain and group sizes is much stronger than the relationship between brain size and any other factor (including social system or pairbonding). Why should primates apparently be so different from all other birds and mammals? One primary difference between primates and other mammalian taxa is that pairbonded social systems are more common among primates (Kleiman 1977) and, more generally, that bonded social groups are especially characteristic of primates (many anthropoid primate species, in particular, have large social groups with a stable structure of related individuals, often based on matrilineal or patrilineal kinship, as well as coalition and grooming relationships between genetically unrelated individuals: Smuts et al. 1987; Dunbar 1988).
Additionally, there is a qualitative difference (at least in terms of cognitive demands) between bonded and non-bonded social systems, suggesting that there may be two separate evolutionary routes between asociality, aggregations and bonded social systems. One possibility is that reproductive pairbonds arise for the reasons noted and then larger social groups result from an extension of the pairbond to non-reproductive group members; in other words, additional individuals are bolted onto an essentially pairbonded relationship. Possible examples of this type of sociality are wolves (and other group-living canids), callitrichids and many lemurid prosimians (van Schaik & Kappeler 1993), all of whose social groups are based around breeding pairs. A second possible evolutionary pathway to bonded social systems is by extending a female–offspring dependency into adulthood (Broad et al. 2006). Old World monkeys (especially cercopithecines) are a prime example of social groups based around stable matrilines. Although female-bonded social systems appear to be relatively common in primates, they seem to have happened only in isolated and rather exceptional cases among non-primates (e.g. elephants, equids). This might explain why primates exhibit a much stronger quantitative signature for group size than other taxa. It would also explain why primate sociality seems to be so different in quality from that found in other social vertebrates (see also Harcourt 1992).
These results undermine conventional notions of what constitutes social complexity. Indeed, we doubt that many would have seriously suggested that pairbonded social systems are more complex (and by implication, more cognitively demanding) than large polygamous or promiscuously mating groups. More detailed studies of the cognitive role in maintaining social and reproductive relationships are clearly needed. In addition, analyses looking at the differences in brain size between the sexes may help elucidate both the adaptive role of brain size evolution and the selection pressures acting on it (Lindenfors 2005; Lindenfors et al. 2007). These findings also suggest that, if we are really to understand the processes of social evolution, we will need to explore the behavioural mechanisms of sociality in different mating/social systems in rather greater detail than has previously been the case. By the same token, if we are to understand the cognitive processes that underpin these behavioural differences, we will need to undertake comparative analyses of cognitive processes in primates and other vertebrates that are a great deal more sophisticated than those done hitherto.
Acknowledgments
R.I.M.D. is supported by a British Academy Research Professorship. S.S. is supported by the British Academy Centenary Research Project.
Supplementary Material
Data, methods and analyses
References
- Armstrong E. Relative brain size in monkeys and prosimians. Am. J. Phys. Anthropol. 1985;66:263–273. doi: 10.1002/ajpa.1330660303. doi:10.1002/ajpa.1330660303 [DOI] [PubMed] [Google Scholar]
- Barton R.A. Neocortex size and behavioural ecology in primates. Proc. R. Soc. B. 1996;263:173–177. doi: 10.1098/rspb.1996.0028. doi:10.1098/rspb.1996.0028 [DOI] [PubMed] [Google Scholar]
- Beauchamp G, Fernandez-Juricic E. Is there a relationship between forebrain size and group size in birds? Evol. Ecol. Res. 2004;6:833–842. [Google Scholar]
- Brotherton P.N.M, Rhodes A. Monogamy without biparental care in a dwarf antelope. Proc. R. Soc. B. 1996;263:23–29. doi: 10.1098/rspb.1996.0005. doi:10.1098/rspb.1996.0005 [DOI] [PubMed] [Google Scholar]
- Broad K.D, Curley J.P, Keverne E.B. Mother–infant bonding and the evolution of mammalian social relationships. Phil. Trans. R. Soc. B. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. doi:10.1098/rstb.2006.1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W, Whiten A, editors. Machiavellian intelligence. Oxford University Press; Oxford, UK: 1988. [Google Scholar]
- Clutton-Brock T.H, Harvey P.H. Primates, brains and ecology. J. Zool. Lond. 1980;190:309–323. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Measure. 1960;20:37–46. doi:10.1177/001316446002000104 [Google Scholar]
- Dunbar R.I.M, editor. Primate social systems. Chapman & Hall; London, UK: 1988. [Google Scholar]
- Dunbar R.I.M. Neocortex size as a constraint on group-size in primates. J. Human Evol. 1992;22:469–493. doi:10.1016/0047-2484(92)90081-J [Google Scholar]
- Dunbar R.I.M. The social brain hypothesis. Evol. Anthropol. 1998;6:178–190. doi:10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8 [Google Scholar]
- Dunbar R.I.M, Dunbar E.P. Pairbond in klipspringer. Anim. Behav. 1980;28:219–229. doi:10.1016/S0003-3472(80)80026-1 [Google Scholar]
- Dunbar R.I.M, Shultz S. Understanding primate brain evolution. Phil. Trans. R. Soc. B. 2007;362:649–658. doi: 10.1098/rstb.2006.2001. doi:10.1098/rstb.2006.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. doi:10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. Am. Anthropol. 1998;100:890–897. doi:10.1525/aa.1998.100.4.890 [Google Scholar]
- Gittleman J.L. Carnivore brain size, behavioral ecology, and phylogeny. J. Mammal. 1986;67:23–36. doi:10.2307/1380998 [Google Scholar]
- Grafen A. The phylogenetic regression. Phil. Trans. R. Soc. B. 1989;326:119–157. doi: 10.1098/rstb.1989.0106. doi:10.1098/rstb.1989.0106 [DOI] [PubMed] [Google Scholar]
- Harcourt A.H. Coalitions and alliances: are primates more complex than non-primates? In: Harcourt A.H, de Waal F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford University Press; Oxford, UK: 1992. pp. 445–472. [Google Scholar]
- Harcourt A.H, Harvey P.H, Larson S.G, Short R.V. Testis weight, body-weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. doi:10.1038/293055a0 [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Krebs J.R. Comparing brains. Science. 1990;249:150–156. doi: 10.1126/science.2196673. doi:10.1126/science.2196673 [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Harvey P.H, Clutton-Brock T.H, Mace G.M. Brain size and ecology in small mammals and primates. Proc. Natl Acad. Sci. USA. 1980;77:4387–4389. doi: 10.1073/pnas.77.7.4387. doi:10.1073/pnas.77.7.4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R.R. A language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. doi:10.2307/1390807 [Google Scholar]
- Jolly A. Lemur social behavior and primate intelligence—step from prosimian to monkey intelligence probably took place in a social context. Science. 1966;153:501–508. doi: 10.1126/science.153.3735.501. doi:10.1126/science.153.3735.501 [DOI] [PubMed] [Google Scholar]
- Key C, Aiello L.C. A prisoner's dilemma model of the evolution of paternal care. Folia Primatol. 2000;71:77–92. doi: 10.1159/000021732. doi:10.1159/000021732 [DOI] [PubMed] [Google Scholar]
- Kleiman D.G. Monogamy in mammals. Q. Rev. Biol. 1977;52:39–69. doi: 10.1086/409721. doi:10.1086/409721 [DOI] [PubMed] [Google Scholar]
- Komers P.E, Brotherton P.N.M. Female space use is the best predictor of monogamy in mammals. Proc. R. Soc. B. 1997;264:1261–1270. doi: 10.1098/rspb.1997.0174. doi:10.1098/rspb.1997.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo H, Dunbar R.I.M. Neocortex size and social network size in primates. Anim. Behav. 2001;62:711–722. doi:10.1006/anbe.2001.1808 [Google Scholar]
- Lindenfors P. Neocortex evolution in primates: the ‘social brain’ is for females. Biol. Lett. 2005;1:407–410. doi: 10.1098/rsbl.2005.0362. doi:10.1098/rsbl.2005.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenfors P, Nunn C.L, Barton R.A. Primate brain architecture and selection in relation to sex. BMC Biol. 2007;5:20. doi: 10.1186/1741-7007-5-20. doi:10.1186/1741-7007-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy C.O. The origin of man. Science. 1981;211:341–350. doi: 10.1126/science.211.4480.341. doi:10.1126/science.211.4480.341 [DOI] [PubMed] [Google Scholar]
- Møller A.P, Briskie J.V. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 1995;36:357–365. [Google Scholar]
- Nowak R.M. Johns Hopkins University Press; Baltimore, MD: 1999. Walker's mammals of the world. [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999;48:612–622. doi:10.1080/106351599260184 [Google Scholar]
- Paradis E, Claude J, Strimmer K. Ape: analyses of phylogenetics and evolution in R language. Bioinformatics. 2005;20:289–290. doi: 10.1093/bioinformatics/btg412. doi:10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Pawlowski B, Lowen C.B, Dunbar R.I.M. Neocortex size, social skills and mating success in primates. Behaviour. 1998;135:357–368. [Google Scholar]
- Pitnick S, Jones K.E, Wilkinson G.S. Mating system and brain size in bats. Proc. R. Soc. B. 2006;273:719–724. doi: 10.1098/rspb.2005.3367. doi:10.1098/rspb.2005.3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. A composite estimate of primate phylogeny. Phil. Trans. R. Soc. B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. doi:10.1098/rstb.1995.0078 [DOI] [PubMed] [Google Scholar]
- Reader S.M, Laland K.N. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. doi:10.1073/pnas.062041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Kudo H. Neocortical development and social-structure in primates. Primates. 1990;31:283–289. doi:10.1007/BF02380949 [Google Scholar]
- van Schaik C.P, Dunbar R.I.M. The evolution of monogamy in large primates—a new hypothesis and some crucial tests. Behaviour. 1990;115:30–62. [Google Scholar]
- van Schaik C.P, Kappeler P. Life history, activity period and lemur social systems. In: Kappeler P.M, Ganzhörn J.U, editors. Lemur social systems and their ecological basis. Plenum Press; New York, NY: 1993. pp. 241–260. [Google Scholar]
- van Schaik C.P, van Hooff J. On the ultimate causes of primate social-systems. Behaviour. 1983;85:91–117. [Google Scholar]
- Shultz S, Dunbar R.I.M. Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B. 2006;273:207–215. doi: 10.1098/rspb.2005.3283. doi:10.1098/rspb.2005.3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz, S. & Dunbar R.I.M. submitted. Life history, social bonding and adaptive peak shifts in avian brain evolution.
- Smuts B.B, Cheney D.L, Seyfarth R.M, Wrangham R.W, Struhsaker T.T, editors. Primate societies. University of Chicago Press; Chicago, IL: 1987. [Google Scholar]
- Wittenberger J.F, Tilson R.L. The evolution of monogamy—hypotheses and evidence. Annu. Rev. Ecol. Syst. 1980;11:197–232. doi:10.1146/annurev.es.11.110180.001213 [Google Scholar]
- Wrangham R.W. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data, methods and analyses