Abstract
Large-scale patterns of isotope ratios are detectable in the tissues of organisms, but the variability in these patterns often obscures detection of environmental trends. We show that plants and animals at lower trophic levels are relatively poor indicators of the temporal trend in atmospheric carbon isotope ratios (δ13C) when compared with animals at higher trophic levels. First, we tested how differences in atmospheric δ13C values were transferred across three trophic levels. Second, we compared contemporary δ13C trends (1961–2004) in atmospheric CO2 to δ13C patterns in a tree species (jack pine, Pinus banksiana), large herbivore (moose, Alces alces) and large carnivore (grey wolf, Canis lupus) from North America. Third, we compared palaeontological (approx. 30 000 to 12 000 14C years before present) atmospheric CO2 trends to δ13C patterns in a tree species (Pinus flexilis, Juniperus sp.), a megaherbivore (bison, Bison antiquus) and a large carnivore (dire wolf, Canis dirus) from the La Brea tar pits (southern California, USA) and Great Basin (western USA). Contrary to previous expectations, we found that the environmental isotope pattern is better represented with increasing trophic level. Our results indicate that museum specimens of large carnivores would best reflect large-scale spatial and temporal patterns of carbon isotopes in the palaeontological record because top predators can act as ecological integrators of environmental change.
Keywords: stable isotopes, CO2, δ13C, ecological integrator, palaeoenvironment, wolf
1. Introduction
There is considerable interest in inferring environmental attributes and climate histories based on stable isotope ratio data derived from animals (Koch 1998; Kohn & Cerling 2002; Hedges et al. 2004; Leng 2004; Hoppe et al. 2006; West et al. 2006). Such inferences are based on the logic that since producer isotopic values reflect, in part, their growing conditions (Dawson et al. 2002), then consumer isotopic values in turn reveal information about the producer's environment. However, few experimental isotopic studies have examined the validity of such inferences and/or established general patterns in the transfer of environmental information across trophic levels.
A useful approach to understand the fidelity of environmental records at the producer level inferred from consumer-derived isotope data is to conduct complementary observational and experimental analyses. Although such an approach is frequently used to infer dietary histories from stable isotope records from animal tissues (Macko et al. 1982; Hobson & Schwarcz 1986; Hilderbrand et al. 1996; Ostrom et al. 1997), similarly derived climate proxies often lack corresponding experimental studies. The call for more comparative experiments to properly reconstruct dietary, trophic level and body condition based on stable isotope analysis (Gannes et al. 1997) is equally pertinent to reconstructing climate histories and habitat use.
In contemporary and palaeontological climate studies, the stable isotope values of carbon (δ13C) have proved particularly useful. For example, ecosystem changes such as carbon starvation in trees growing under conditions of reduced atmospheric CO2 concentration have been studied using δ13C analysis of fossil plant tissues (Ward et al. 2005). Also, in the context of current climate change issues (Karl & Trenberth 2003; Kennedy 2004) and research on global carbon cycling (Falkowski et al. 2000), atmospheric δ13C chronologies are of interest to researchers modelling past and future atmospheric CO2 scenarios (Ciais et al. 1995, 2005; Rayner et al. 1999; Nakicenovic & Swart 2000).
Since plants assimilate atmospheric CO2 directly, it has been argued that shifts in atmospheric δ13C values would be most accurately recorded in plant tissues (Dawson et al. 2002; Long et al. 2005). Consumer tissues are thought to more poorly reflect isotopic patterns in atmospheric CO2 owing to variations in diet, assimilation efficiencies of dietary components, isotope fractionation (a change in isotope ratios between source substrate and products), tissue turnover and differential allocation of nutrients among tissues (Gannes et al. 1997; Long et al. 2005). However, ecological theory suggests that organisms at higher trophic levels act as integrators, linking lower pathways in space and time (de Ruiter et al. 2005). Primary producer tissue reflects an integration of the environmental conditions over the time of tissue growth. Herbivores feed upon numerous primary producers, and carnivores ingest tissues from multiple herbivores. Hence, stable isotope values derived from organisms at higher trophic positions may effectively increase sample size, thereby reducing variation (i.e. a carnivore represents multiple herbivores, which in turn represent many primary producers).
Here, we present the results of one experiment and two observational studies designed to independently investigate patterns of δ13C records across trophic levels. In the experimental test, we expected the variability in δ13C values to decrease with increasing trophic level based on the untested idea that effective sample size is greater at higher trophic levels, and thereby should statistically provide less variable reflections of environmental factors. The observational studies explored the ability of contemporary species to act as ecological integrators of trends in atmospheric δ13C values and concentrations over historical and palaeontological time scales. In each study, we focus on the dominant global atmospheric variable (δ13C value and CO2 concentration) responsible for temporal variability in plant and animal δ13C values over a given time period. The dietary sources of carbon for insect and mammalian consumers are predominately plant-derived carbohydrates (for herbivores) and protein-rich body tissues (for carnivores; Hedges 2003). Both insect and mammalian carnivores ingest a range of prey body tissues; but, as whole-body consumers, we might expect insect carnivores to use a wider range of dietary carbon sources. Thus, we do not directly compare the experimental insect and observational mammalian consumer datasets, but instead report similar trends in isotopic variance with relation to trophic level.
2. Material and methods
First, we describe our experimental study in which we tested how differences in atmospheric δ13C values were transferred across a three-trophic level food chain (cabbage plant–caterpillar–insectivore). We manipulated the atmospheric δ13C values in semi-contained model systems and then measured the δ13C values for the growth chamber air and the organismal food chain. This experiment tested the theoretical prediction that the variability in δ13C values would decrease with increasing trophic level because consumers act as ecological integrators of environmental δ13C conditions. Second, we describe our two analyses of δ13C chronologies from organisms at three trophic levels (producer–herbivore–carnivore). In the first analysis, we relate historic to modern organismal chronologies to corresponding changes in atmospheric δ13C values. In the second analysis, we compare fossil plant and animal δ13C chronologies with late Pleistocene changes in the CO2 concentration of the atmosphere.
(a) Experimental study
Producers were grown in open top chambers (OTCs) in which atmospheric δ13C values were manipulated. Insect herbivores fed in situ on producers within the OTCs and were subsequently fed to an insectivore. δ13C values were measured for the OTC environment and for organisms at all three trophic levels, thereby permitting the assessment of how precisely atmospheric δ13C values are reflected at each trophic position.
Cabbage (Brassica oleracea var. capitata) was chosen for this study owing to its rapid growth rate, small size, ease of cultivation, seed availability and palatability to common insect herbivores. Approximately two to three weeks after greenhouse germination twelve 15 cm pots, each containing one plant, were placed into each of ten 0.5 m3 OTCs. The OTC construction followed that of Drake et al. (1989). OTCs were arranged into five blocks with each block containing one of two randomly assigned treatments: one relatively 13C-enriched (hereafter ‘heavy’) OTC and one 13C-depleted (hereafter ‘light’) OTC. Heavy and light 13C treatments were obtained by elevating CO2 above ambient levels in all OTCs using two isotopically different CO2 sources mixed with atmospheric air: heavy treatment OTCs (n=5) received additional CO2 with a δ13C value of −11‰ and light treatment OTCs (n=5) received additional CO2 with a δ13C value of −35.5‰. The mean (±1 s.d.) CO2 concentration within each OTC (528±10 ppm) was monitored automatically via continuous sampling of chamber air and an infrared CO2 gas analyser for 3 min at least every 5 hours (Drake et al. 1989). CO2 flow to each OTC was adjusted via manual rotometer. Cabbages were watered daily and fertilized once per week (100 ml containing 25 mg of Miracle Grow 15-30-15 fertilizer).
Egg-laying female cabbage butterflies (Pieris rapae) were net captured locally and held (approx. two weeks) in a net enclosure (2×2×2 m) with a sufficient food source (Lantana camara). Eight days after plants were placed in OTCs, 1–2 butterflies were transferred to each OTC and contained by temporarily screening the chamber top. Egg laying on multiple plants in each chamber was confirmed visually; if egg laying did not readily occur (i.e. within approx. 1 hour), butterflies were released and replaced with one to two new individuals until egg laying was observed. Caterpillars were allowed to feed in situ for the duration of the experiment (approx. eight weeks).
Eggs of spined soldier bugs (Podisus maculiventris) were obtained from Rincon-Vitova Insectaries, Inc. (Ventura, CA). Soldier bugs were reared until the fifth instar on the experimentally raised P. rapae caterpillars at 25 and 20°C, coinciding with a 16 : 8 hours light : dark cycle in an environmental chamber. Larvae were housed and fed daily in 100×15 mm plastic Petri dishes containing moistened filter paper, which was replaced daily. Two Petri dishes, each containing 2–5 soldier bug larvae, corresponded to each OTC to ensure adequate survival and archive tissue. Soldier bug larvae were fed caterpillars (randomly selected from plants) of the same instar daily.
Air samples for isotopic analysis were collected from each OTC four times during the experiment: once when plants were first placed into each chamber and then one, four and eight weeks subsequently. Air from each chamber was collected between 22.00 and 24.00 hours in septum-capped 10 ml vials, previously evacuated with argon gas.
Cabbage leaf tissue, caterpillars and soldier bug larvae corresponding to each growth chamber were randomly selected for elemental and isotopic analysis once soldier bug larvae reached the fifth instar. Two caterpillars of the most recent instar fed to the soldier bugs (i.e. fourth) were randomly selected from chambers; one for elemental analysis and the other for isotope analysis. The entire cabbage leaf on which the caterpillars were found was collected for analysis. Caterpillars tended to feed on newer leaves, hence leaf samples were of a similar age. Caterpillars were isolated for at least 24 hours before analysis to evacuate gut contents. All samples, plant and insect, were double rinsed with distilled water, air dried and then lyophilized. Leaf tissues were ground to a fine powder in a mill (model 8000M, SPEC CertiPrep, Inc., Metuchen, NJ).
Lipids were removed from insect samples because during biosynthesis lipids can become depleted in 13C (DeNiro & Epstein 1977) resulting in lower δ13C values for lipids versus proteins and carbohydrates; hence, whole-body δ13C can vary appreciably with lipid levels even if diet is constant. To extract lipids prior to isotope analysis, insect samples (i.e. caterpillars and soldier bugs) were soaked in 1 : 1 chloroform : methanol solution three times, sonicated for 10 min each time and then rinsed with distilled water and air-dried (Focken & Becker 1998; Sotiropoulos et al. 2004). Once dry, insect samples were ground to a fine powder using a mortar and pestle.
Isotopic analysis was conducted at the University of Michigan Biological Station's Analytical Laboratory on a ThermoFinnigan Deltaplus Continuous-Flow Stable Isotope Ratio Mass Spectrometer interfaced with a GasBench II and a Costech 4010 Elemental Analyzer. Gas sample injection volume was 100 μl to generate output voltage peaks of approximately 400 mV at m/e=44. Five injections were done per gas sample. Stable isotope values are reported in standard δ notation and are referenced to Vienna PeeDee Belemnite. Precision based on repeated measures of internal standards was ±0.25‰ for δ13C.
Separate elemental analysis was done to assess gross nutritional quality of producers and herbivores. Ground cabbage leaf tissues and caterpillar tissues without lipid extraction were combusted in a Fisons NA1500 Elemental Analyzer at Michigan Technological University's Ecosystem Science Centre to determine total carbon and nitrogen.
Homogeneity of variance tests and t-tests were used to test for differences in equality of variance across trophic levels, in isotopic fractionation between atmosphere and plant, with each trophic step, and in nutritional quality between heavy and light treatment producers and herbivores. These comparisons tested the a priori hypotheses that δ13C variability would decrease with increasing trophic level, hence differences where p<0.05 were considered significant.
(b) Observational study—Isle Royale
We compared trends (1961–2004) in the δ13C values of atmospheric CO2 to δ13C values in a species of tree (jack pine, Pinus banksiana), large herbivore (moose, Alces alces) and large carnivore (grey wolf, Canis lupus) from North America. Annual δ13C values of atmospheric CO2 for 1962–1975 (n=8) were obtained from high-precision ice core records (Francey et al. 1999) and for 1978–2002 (n=25) from direct atmospheric measurements (Keeling et al. 2005). Jack pine δ13C data for 1974–1994 were obtained from tree ring (n=93) cellulose from Manitoba and Saskatchewan (Ehleringer et al. 1998). Moose (n=59) and wolf (n=47) bone collagen samples for 1961–2004 were drilled from Isle Royale (Lake Superior, USA) specimens using a handheld Dremel microdrill. All available Isle Royale wolf specimens were sampled; moose specimens were selected indiscriminately from all samples available (more than 4000) to mimic field collection. Fifty micrograms of samples were decalcified in 0.5 N HCl for 1–2 days at 4°C and were then rinsed and dried. Samples were lipid extracted in five rinses of a 2 : 1 chloroform and methanol solution, with sonication of each rinse for 0.5 hours. For stable isotope analysis, collagen samples (1.0 mg) were weighed into precombusted tin capsules. Stable carbon isotope ratios were measured using an elemental analyser coupled with a mass spectrometer (Europa Hydra 20/20) at the University of California Davis Stable Isotope Facility. The standard deviation for replicates of a gelatin standard analysed with the collagen samples was <0.2‰ for carbon.
Since most plants in boreal ecosystems assimilate atmospheric CO2 through the C3 photosynthetic pathway, our Isle Royale δ13C records are not complicated by changes in the relative abundances of C3 and C4 plants (Ehleringer & Monson 1993). We intentionally chose a tree species (i.e. jack pine) from a mainland location to reduce the confounding effect of elevation on carbon isotope discrimination (Sparks & Ehleringer 1997). Consequently, the variation exhibited in the jack pine chronology used here is likely to be less (i.e. more conservative) than would be expected for Isle Royale jack pine, where elevation changes (up to 238 m) exceed those of the mainland sites (Ehleringer et al. 1998).
Moose do not prefer jack pine as browse species, but this is of negligible consequence to our analysis because the range in δ13C values of plants that Isle Royale moose consume (−20 to −30‰; Tischler 2004) encompasses the δ13C values of the mainland jack pine. Isle Royale moose and wolves are linked through a strong predator–prey interaction; on average, moose comprise approximately 90% of wolf diet (Thurber & Peterson 1993) and kills are well used (i.e. soft tissue, hide, brain and long-bone marrow are all consumed). Based upon conservative estimates of moose kill rates at Isle Royale (Vucetich et al. 2002), a likely minimum of 150–200 moose contribute to a wolf's diet over the course of its lifetime (approx. 5 years; Vucetich & Peterson 2004).
We highlight that the amount of time represented by producer and consumer samples varies depending upon what tissue is analysed. In general, wolf and moose bone collagen δ13C values reflect a longer time span (multiple years) of resource usage than individual tree ring cellulose δ13C values (one tree ring=1 year of atmospheric CO2 assimilation). Two-thirds of all Isle Royale wolves die before the age of 5 years (Vucetich & Peterson 2004) and only 4 of the 47 wolves sampled in this study were certainly older than 5 years. The mean life expectancy at age 1 year for Isle Royale moose is 7.3 years (Peterson 1977) and 22 of the 59 moose sampled in this study were young (less than 2 years), rapidly growing individuals, the remainder were from older individuals (mean age 12.3 years). To make consumer and producer tissue records comparable, we smooth the jack pine tree ring δ13C data using a 5-year running average, a timeframe that is equivalent to the average turnover time of bone collagen (Hobson & Clark 1992) and wolf life expectancy. Note that Hobson & Clark's (1992) analysis of 13C turnover in tissues involved the use of growing animals, and therefore likely underestimates carbon turnover time in adults. We also explored the effects of 8- to 10- and 12-year running averages because of the older ages of a portion of the moose specimens.
(c) Observational study—La Brea and Great Basin
We compared trends (approx. 12 000–30 000 of 14C years before present (kyr ago BP)) in the concentration of atmospheric CO2 to δ13C values of late Pleistocene Great Basin (Arizona, Utah, Nevada) pack rat midden limber pine (Pinus flexilis) needle cellulose (n=31; Van de Water et al. 1994) and La Brea tar pit (southern California) juniper (Juniperus sp.) wood cellulose (n=11; Ward et al. 2005), and La Brea bison (Bison antiquus, n=31) and dire wolf (Canis dirus, n=73) bone collagen (Coltrain et al. 2004). Atmospheric CO2 concentrations for 12–30 kyr ago BP (n=120) were obtained from ice core records (Neftel et al. 1988; Staffelbach et al. 1991; Marchal et al. 1999) and are plotted with the plant and animal records to graphically demonstrate the synchrony of atmospheric CO2 changes with plant and animal δ13C chronologies.
We intentionally chose a carnivore inferred to have wide dietary breadth in order to examine the relationship between variance in δ13C values and trophic level in a complex ancient food web. While bison likely contributed to dire wolf diet, they were not the only megafaunal prey available to La Brea carnivores. Isotopic food web reconstructions for La Brea mammalian megafauna (Coltrain et al. 2004) show that dire wolf diet was variable, and potentially included all of the abundant grazing and browsing herbivore species at the tar pits (e.g. horse, bison, camel, ground sloth, mastodon). The cranial morphology and levels of tooth breakage in La Brea dire wolves also suggest that they were either generalized predators of large prey or scavengers (Binder et al. 2002).
We compare δ13C values of co-occurring La Brea fauna and juniper trees, but due to the paucity of the juniper dataset we also include δ13C values from an extensive Great Basin limber pine dataset (Van de Water et al. 1994). The juniper data are included solely for visual comparison and are not included in statistical analyses. La Brea bison were grazers (Coltrain et al. 2004) and likely did not consume juniper or limber pine. Therefore, our study does not involve directly interacting plants and animals, but instead focuses on how organismal δ13C records reflect atmospheric changes at the regional scale.
Juniper and limber pine were directly dated by accelerator mass spectrometry 14C analysis. Van de Water et al. (1994) normalized the limber pine δ13C values to modern sea level, but concluded that latitudinal corrections were not necessary. Bone collagen dates reflect the age of the pit from which they were collected. Pit ages in kyr ago BP were 61 and 67 (12 kyr ago BP), 13 (15 kyr ago BP), 3 (15 kyr ago BP), 60 and 91 (26–28 kyr ago BP) and 77 (28–33 kyr ago BP). Thus, for statistical analyses (limber pine data only) and graphical presentation, the limber pine and juniper data were binned according to the age ranges of the La Brea tar pits. Herbivore δ13C data for pits 3, 60, 61, 67 and 91 were from Coltrain et al. (2004); dire wolf δ13C data from pits 3, 60 and 91 were from Coltrain et al. (2004). Collagen δ13C data from the remaining pits are presented here for the first time.
Fossil bone collagen extraction and preparation for the new δ13C data presented here followed the methods in Fox-Dobbs et al. (2006). In brief, approximately 120 mg bone samples were crushed to a coarse powder, continuously rinsed with solvents (petroleum ether and acetone, 24 hours each) in a soxhlet extractor to remove tar, and then decalcified as above. The collagenous residue was gelatinized in 0.01 M HCl at 57°C for 12 hours and then passed across a 1.5 μm glass-fibre filter, with retention and lyophilization of the filtrate. Carbon isotope analysis was as previously described for the Isle Royale samples and done at the University of California Davis Stable Isotope Facility.
Standard deviations of the mean δ13C values were calculated for 12 and 15 kyr ago BP samples for each trophic level; means for other time periods were not analysed statistically due to small sample sizes. Homogeneity of variance tests (α=0.05) were used to test differences in equality of variance between trophic levels by sample age.
3. Results
(a) Experimental
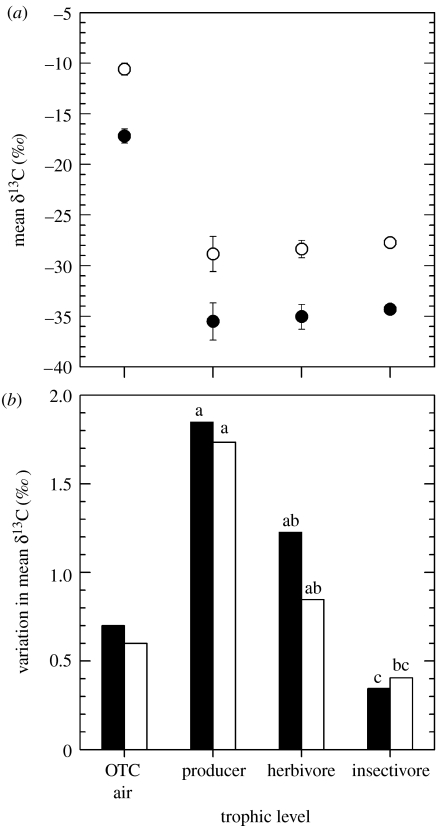
The heavy and light CO2 additions resulted in significantly different OTC air, leaf, caterpillar and soldier bug δ13C values (figure 1). Standard deviations of mean insectivore δ13C values were significantly less variable than herbivore values for the light treatment (F1,8=12.7, p=0.03, n=5) and less variable than producers for both treatments (heavy, F1,8=18.3, p=0.016, n=5; light, F1,8=28.9, p=0.007, n=5; figure 1).
Figure 1.
δ13C values for experimental growth environment and across trophic levels; symbols and bar fills correspond to relatively heavy (open) or light (closed) CO2 treatment sources. (a) Points (mean±1 s.d.) indicate δ13C values for CO2 of open top chamber (OTC) air and tissues at each trophic level. Note OTC air treatment difference is reflected at each trophic level. (b) Bars indicate standard deviations of mean δ13C values measured for CO2 of OTC air and tissues at each trophic level. Bars labelled with different lower case letters are significantly different.
Producer and herbivore C : N ranged between 21.6–21.8 (n=10) and 4.8–5.2 (n=10), respectively, and did not differ between treatments (producers, t8=0.11, p=0.91; herbivores, t8=1.25, p=0.25). This result indicates that the gross forage quality for herbivores and prey for insectivores did not likely differ across treatments.
Isotope discrimination values (mean ±1 s.d.) for producers (heavy=18.3±2.0‰, n=5; and light =18.3±2.6‰, n=5) did not differ across treatments (t8=0.04, p=0.97), indicating equivalent gas exchange and photosynthetic activity between treatments. Trophic shifts (mean±1 s.d.) for herbivores (heavy=0.4±1.2‰, n=5; and light=0.4±2.0‰, n=5) and insectivores (heavy=0.6±0.8‰, n=5; and light=0.7±1.3 ‰, n=5) did not differ across treatments (t8=0.02, p=0.98; and t8=0.14, p=0.89, respectively). These fractionation results associated with biochemical discrimination against 13C fall within typical variation ranges for C3 plants (Dawson et al. 2002) and trophic shifts for animals (McCutchan et al. 2003).
(b) Observational
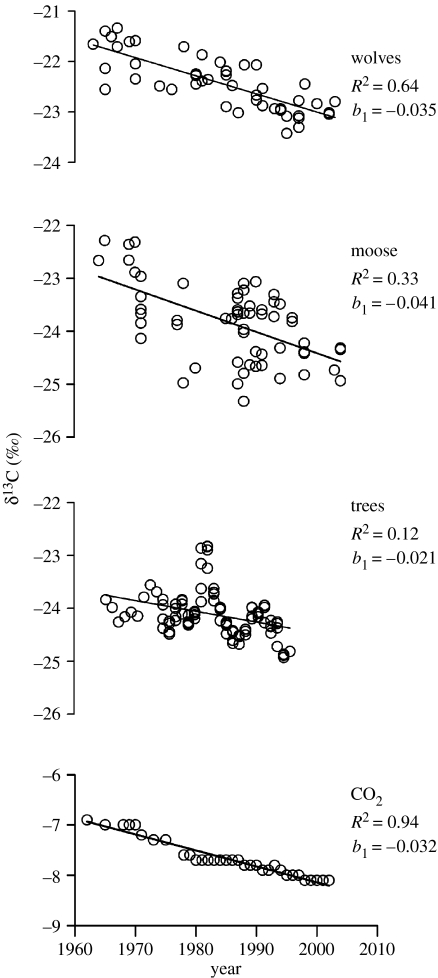
Least squares regression analysis indicated that δ13C chronologies for each trophic level were significantly related to time, reflecting the decline in δ13C values observed in atmospheric CO2 (p<0.01 for each trophic level). Examination of numerical measures of leverage for the wolf, moose and jack pine data did not reveal cases with excessive influence. Atmospheric carbon dioxide has increased 31% since pre-industrial times, from 280 parts per million by volume (ppmv) to more than 370 ppmv today, and is currently increasing at an annual rate of approximately 0.76% due to anthropogenic inputs (Karl & Trenberth 2003). Fossil fuel and biomass combustion release isotopically light (relative to atmospheric CO2 δ13C values) CO2 into the atmosphere, which explains the observed depletion trend in the δ13C chronologies (figure 2; Keeling et al. 2005).
Figure 2.
The relationship between year and δ13C values for global atmospheric CO2, North American jack pine (P. banksiana) tree ring cellulose, and Isle Royale moose (A. alces) and wolf (C. lupus) bone collagen. δ13C data are reported in per mil (‰) relative to Pee Dee Belemnite (PDB) limestone standard. Each panel includes individual δ13C values (open symbols) and a simple linear regression line for the relationship between time and δ13C values. Jack pine data are a 5-year running average (see §2). Note increasing trend in correlation from trees to moose to wolves.
Accepting the atmospheric δ13C depletion trend as a factual environmental signal, we consider the R2 value a suitable indicator of signal-to-noise ratio at each trophic level (figure 2). The δ13C chronology R2 value is 5× higher for wolves than the 5-year smoothed tree rings; 2.5×times higher for moose than the 5-year smoothed tree rings; and the slope of the wolf regression is closest to the slope exhibited for atmospheric CO2 regression (figure 2). An 8- and 9-year smoothing interval lowered the tree ring R2 to 0.10 and 0.11, respectively. With 10-year smoothing, the tree ring R2 remained at 0.12 (the same as in the 5-year smoothing) and the 12-year smoothing raised the R2 slightly to 0.14. Slope coefficients for these larger smoothing intervals ranged from −0.018 to −0.021, which were not significantly different from the tree ring slope of −0.021 found for the 5-year smoothing interval.
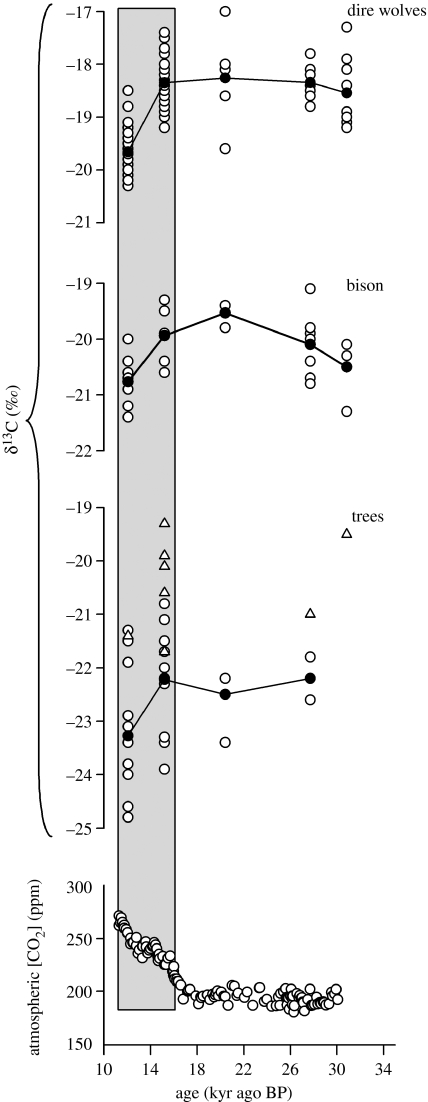
There is a noteworthy decrease in the collagen δ13C values of fauna (herbivore and carnivore) from the La Brea tar pits during the last glacial–interglacial transition (approx. 15 to 12 kyr ago BP; shaded box figure 3). A similar shift in δ13C values is recorded with greater noise (i.e. increased variation) in the δ13C values of contemporaneous Great Basin limber pine trees. These shifts record a past event of major change in the global CO2 concentration of the atmosphere (figure 3; see §4 below).
Figure 3.
The relationship between year and δ13C values for Great Basin pack rat midden limber pine needle (P. flexilis; tree circle symbols) and La Brea tar pit juniper wood (Juniperus sp.; tree triangle symbols) cellulose, and La Brea bison (B. antiquus) and dire wolf (C. dirus) bone collagen δ13C data are reported in per mil (‰) relative to PDB limestone standard. Each panel includes individual δ13C values (open symbols) with lines connecting mean δ13C values (closed symbols) between time periods. We also include ice core-derived atmospheric CO2 concentrations (Neftel et al. 1988; Staffelbach et al. 1991; Marchal et al. 1999). The plant and animal δ13C values are plotted versus 14C age (kyr ago BP), and the CO2 concentrations are versus ice core gas age (kyr ago BP). Shaded box highlights the rapid post-last glacial maximum (LGM; 12–15 kyr ago BP) decrease in plant and animal δ13C values and the concurrent increase in atmospheric CO2 concentration.
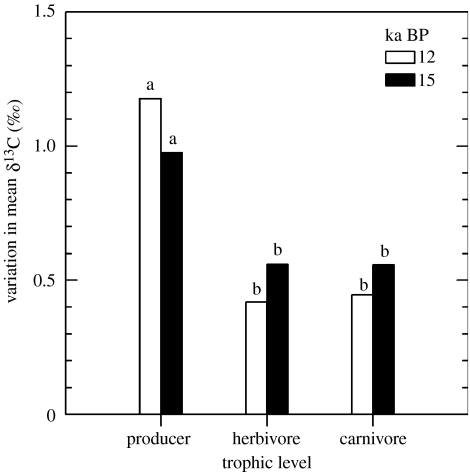
The 12 and 15 kyr ago BP herbivore and carnivore tissue samples exhibited significantly less δ13C variation than the Great Basin limber pine trees (F2,55=11.33, p<0.001 and F2,31=3.31, p=0.025 for 12 and 15 kyr ago BP samples, respectively; figure 4).
Figure 4.
δ13C values for palaeontological tissues from producers (Great Basin P. flexilis and La Brea Juniperus sp.), herbivore (La Brea B. antiquus) and carnivore (La Brea C. dirus). Bars indicate one standard deviation of mean δ13C values measured for cellulose of producers and bone collagen of herbivores and carnivores; bar fill corresponds to sample age in 14C thousands of years before present (kyr ago BP). Bars labelled with different lower case letters are significantly different.
4. Discussion
The experimental results (figure 1) and observational analyses (figures 2–4) provide independent evidence indicating that changes in the δ13C value and concentration of atmospheric CO2 propagate up a food chain with an increasing signal-to-noise ratio at higher trophic levels. We regard the experiment as a proof of concept analysis whose purpose was to verify a pattern that is probably capable of exploitation in a useful manner. The illustration of this potentially useful pattern is novel and was subsequently found to exist in two natural, observational investigations.
Forage quality and insect feeding rate may interact (Coviella & Trumble 1999; Barbehenn et al. 2004) and both can influence results in stable isotope studies (Focken & Becker 1998; Focken 2001). Given that no difference was detected in producer and primary consumer C : N ratios between treatments in the experimental study, and that consumers were allowed to feed ad libitum, it is unlikely that undetected differences in gross forage nutrition or feeding rate influenced our results. When the tree ring data (figure 2) are not smoothed with multi-year running averages (i.e. 5, 8–10, 12 years), their R2 decreases to 0.08 (i.e. 8×lower than that of wolves and 4×lower than that of moose). Hence, even when conservatively accounting for the effects of unequal time represented by producer and consumer tissues, and Isle Royale topography, consumer tissues still reflect the environmental trend better than producer tissues.
Carbon isotope discrimination values for producers in the experiment did not differ across treatments indicating that the treatment δ13C difference explains the δ13C differences exhibited at each trophic level. Variation in carbon isotope discrimination of producers is caused by combined genetic and environmental factors that influence leaf-level gas exchange through morphological and functional plant responses. Consequently, numerous factors (e.g. soil moisture, humidity, irradiance, temperature, nitrogen availability, leaf size, age, and thickness, stomatal density, canopy height, gender, altitude and genotype) lead to between-individual differences in the isotope ratios of primary producer tissues of the same species (reviewed in Dawson et al. 2002). Although the improved signal-to-noise ratio pattern (figures 2 and 3) at upper trophic levels compared with producers had been considered theoretically plausible (figure 1), it was considered biologically unlikely because consumer diet change, body condition, variable tissue turnover rates and migration were mechanisms thought to obscure isotopic patterns by creating substantial variation in stable isotope ratios of individual herbivores and carnivores (Gannes et al. 1997; Dawson et al. 2002; Long et al. 2005). For example, variable diet combinations for Isle Royale moose (e.g. aquatic and terrestrial components; Tischler 2004) and wolves (e.g. moose and beaver, Castor canadensis), and individual physiology (e.g. starvation, lactation) can lead to significant between-individual differences in isotope ratios.
However, our findings provide evidence that top consumers act as ecological integrators, linking lower pathways in space and time. That is, producers incorporate elements over time, averaging isotopic fluctuations across their environment. Herbivores sample multiple producers, averaging across their spatial range, and predators aggregate variation in prey. As a result, higher trophic level species provide higher signal-to-noise ratio for trends in isotope chronologies, such as the δ13C value of atmospheric CO2 (figures 2 and 3). Among animal ecophysiologists and ecologists using stable isotope methods this logic is loosely referred to as trophic averaging and temporal or spatial integration. Although trophic integration of atmospheric isotopic change is well documented in some cases (e.g. 14C in Bada et al. 1990), to our knowledge this is the first experimental test and observational investigation of the patterns of integration.
While our experimental results demonstrate support for a general concept, we also consider the ecological relevance of the design (Bernardo 1998; Morin 1998). The experimental difference in δ13C treatments (6.6‰) may not reflect the current or recent ecological range. Although changes in atmospheric δ13C of a similar magnitude have been inferred over geological time scales (Mora et al. 1996), recent δ13C trends show less change over the past two centuries. Atmospheric δ13C records measured from ice cores exhibit approximately 1.5‰ depletion over the past 200 years, typically attributed to the combustion of isotopically light fossil fuels (Trudinger et al. 1999). Considering our results with this historical perspective, δ13C chronologies developed from higher trophic position animals would arguably detect similar trends based on the lower variance (0.3–0.4‰; figure 1b) at the secondary consumer level. Producer tissue, in contrast, would be less likely to exhibit the historical atmospheric depletion given that the variability observed in their tissue values (1.7–1.8‰; figure 1b) exceeds the net depletion (1.5‰) seen in other studies (Trudinger et al. 1999; Sugawara et al. 2003).
The integrating effect by secondary consumers is a neglected means of developing improved proxies of ecosystem change given ample museum collections and the recent advances in isotopic measurements. Moreover, consumer tissues, in contrast to producer tissues, are abundant in the fossil record (e.g. teeth, bone, chitin) and reliable stable isotope values are routinely measured in biogenic tissues that have been preserved for millions of years (e.g. Cerling et al. 1997; Hedges et al. 2004; McFadden & Higgins 2004; Asara et al. 2007). Notably, bone collagen has been isolated from fossil bones 68 Myr old (Schweitzer et al. 2007).
Tissue and organism metabolic rates are important considerations in selecting archival tissues for developing isotopic proxies of environmental change. Mass-specific metabolic rate decreases exponentially with increasing mass (Schmidt-Nielsen 1983). Thus, carbon turnover in the tissues of smaller organisms will be swifter than in tissues of more massive organisms. Tissue type, due to differences in tissue metabolisms, is also critically linked to turnover rates. For example, bone, muscle, liver and blood plasma are four tissues that turn over at relatively very slow, slow, medium and fast rates, respectively (Fry 2006). Consequently, consumption of a mix of species of different sizes and/or tissues with variable metabolic rates results in the ingestion of a mix of carbon isotope signals. We would expect then that small organisms and metabolically fast tissues to rapidly equilibrate to isotope changes in their environment, whereas larger organisms and metabolically slow tissues will exhibit a lag in isotopic response time. Smaller animals and metabolically fast tissues may be more variable and gain less resolution with higher trophic levels. In contrast, larger organisms and metabolically slow tissues essentially increase temporal integration, which may result in more resolution with higher trophic levels.
The La Brea and Great Basin fossil case study (figure 3) illustrates how the δ13C chronologies of consumers from multiple trophic levels record a past event of major ecosystem change. Specifically, patterns in La Brea consumer chronologies likely reflect how the rapid increase (from approx. 190 to 250 ppmv) in atmospheric CO2 concentration after the LGM (Neftel et al. 1988; Staffelbach et al. 1991; Marchal et al. 1999) affected C3 plant photosynthetic physiology. Previous studies (Leavitt & Danzer 1992; Van de Water et al. 1994) have linked the decrease in producer δ13C values between 15 and 12 kyr ago BP (i.e. shaded box in figure 3) to increased discrimination against 13C during photosynthesis at higher CO2 concentrations. Substrate limitation (i.e. carbon starvation due to low atmospheric CO2 concentrations) appears to have been prevalent in LGM trees from western North America (for a detailed discussion, see Van de Water et al. 1994; Ward et al. 2005). Change in the δ13C value of atmospheric CO2 probably does not explain the figure 3 patterns (shaded box) because in producer and consumer tissues the isotopic signal generated by the increase in CO2 concentration (approx. 1‰ decrease) exceeds the concurrent change in the δ13C value of atmospheric CO2 at the glacial–interglacial transition (approx. 0.25 ‰ increase; Leuenberger et al. 1992; Smith et al. 1999).
Our results are the first to demonstrate how the glacial–interglacial shift in plant δ13C values was recorded in primary (bison) and secondary (dire wolf) consumer tissues from the same geographical region. Trends in top predator chronologies that are also present in primary consumer chronologies probably reflect climate or vegetation changes, not dietary shifts. La Brea dire wolves and bison are equally suitable for detecting baseline isotopic changes, which is remarkable considering the difference in dietary breadth of an opportunistic carnivore versus an obligate grazer. A decrease in the δ13C values of fossil collagen from late Pleistocene European herbivores (horse, bison, elk) is similar in magnitude and timing to that of the La Brea fauna, indicating that the response of C3 plants to changing CO2 concentrations was globally synchronous (Richards & Hedges 2003; Stevens & Hedges 2004). The findings of our La Brea and Great Basin case study have implications for future palaeoclimatic and palaeoenvironmental reconstructions, since spatial and temporal patterns in plant tissue δ13C, δ15N and δ18O values can be driven by climatic factors (Koch 1998). For example, trends in plant water use efficiency (Van de Water et al. 1994; Dawson et al. 2002) are correlated with soil moisture content and ambient temperature patterns (Ehleringer & Monson 1993). Therefore, changes in plant tissue δ13C values due to varying aridity would probably propagate through food webs, in which case the higher signal-to-noise ratio in top predator chronologies would better reflect large-scale climate change.
We note that rising variance of ecological parameters has been identified as foreshadowing ecological regime shifts (Carpenter & Brock 2006). However, Carpenter & Brock (2006) discuss that changes in variance due to impending regime shifts may be difficult to distinguish from other drivers of variance such as exogenous noise that affects ecosystems. If rising variance is indeed a leading indicator of ecological transition then, given the results of our study, stable isotope ratio time series from herbivore and carnivore populations may provide chronologies that are sensitive to major ecological transitions, but less affected by other noisy ecosystem components.
In conclusion, our results indicate that there is greater justification for selecting higher trophic position species in research explicitly interested in developing proxy environmental records and inferring habitat parameters from stable isotope chronologies. There are, however, important caveats to this recommendation, namely considering diet breath and home range. Animals with home ranges that cover broad geographical regions or cross important ecophysiological boundaries will be inappropriate proxies for understanding local environmental change. Care should be taken to discern the degree of omnivory in focal consumers if inferring environmental variables from stable isotopes is the main objective. Consumers with relatively narrow diets and limited ranges, such as are found on Isle Royale, will probably reflect environmental changes better than consumers in complex food webs. In particular, species feeding on both producers using different photosynthetic modes, or on a mix of foods derived from marine, freshwater and terrestrial sources should be avoided. Plants with different modes of photosynthesis exhibit contrasting carbon isotope ratios (Griffith 1991; Dawson et al. 2002), and foods derived from marine and freshwater sources often exhibit contrasting isotopic composition (Chisholm et al. 1982). Yet, contrary to previous expectation, the analyses presented here show that informative inferences about environmental variables based on carbon isotope chronologies are possible with species exhibiting moderate diet breadth and consumer-derived isotopic values can show environmental trends with significantly less noise than producer values.
Acknowledgments
Allyson Eller and Krista McGuire helped with the experiment. Richard Honrath, David Karowe, Nancy Tuchman, Jed Sparks, Christoph Vogel and Peter Curtis provided experimental advice and loaned supplies. Robert Vande Kopple, C. Anthony Sutterley, Richard Spray and the University of Michigan Biological Station staff provided logistical support. John Harris and the staff of the George C. Page museum facilitated sampling of La Brea specimens. Mike Grant and Jennifer Eikenberry helped conduct mass spectrometer analysis. We thank Amy Schrank, Steve Bertman, Alan Talhelm and two anonymous referees for useful comments on drafts of this manuscript. Supported by a Biosphere Atmosphere Research & Training fellowship (NSF IGERT grant 9972803) and U.S. Environmental Protection Agency Greater Research Opportunities fellowship (EPA GRO grant F5F71445) to J.K.B. and grants to P.L.K. from NSF (OPP-0352564) and to R.O.P. and J.A.V. from Isle Royale National Park (CA-6310-9-8001), NSF (DEB-0424562) and Earthwatch, Inc. This research complied with current United States law.
Footnotes
The first two authors contributed equally to this manuscript.
References
- Asara J.M, Schweitzer M.H, Freimark L.M, Phillips M, Cantley L.C. Protein sequences from mastodon and tyrannosaurus rex revealed by mass spectrometry. Science. 2007;316:280–285. doi: 10.1126/science.1137614. doi:10.1126/science.1137614 [DOI] [PubMed] [Google Scholar]
- Bada J.L, Peterson R.O, Schimmelmann A, Hedges R.E.M. Moose teeth as monitors of environmental isotopic parameters. Oecologia. 1990;82:102–106. doi: 10.1007/BF00318540. doi:10.1007/BF00318540 [DOI] [PubMed] [Google Scholar]
- Barbehenn R.V, Karowe D.N, Spickard A. Effects of elevated atmospheric CO2 on the nutritional ecology of C3 and C4 grass-feeding caterpillars. Oecologia. 2004;140:86–95. doi: 10.1007/s00442-004-1572-9. doi:10.1007/s00442-004-1572-9 [DOI] [PubMed] [Google Scholar]
- Bernardo J. The logic, value and necessity of grounding experiments in reality: an essential link in the inferential chain back to nature. In: Resetarits W.J, Bernardo J, editors. Experimental ecology: issues and perspectives. Oxford University Press; New York, NY: 1998. pp. 370–393. [Google Scholar]
- Binder W.J, Thompson E.N, Van Valkenburgh B. Temporal variation in tooth fracture among Rancho La Brea dire wolves. J. Vert. Paleontol. 2002;22:423–428. doi:10.1671/0272-4634(2002)022[0423:TVITFA]2.0.CO;2 [Google Scholar]
- Carpenter S.R, Brock W.A. Rising variance: a leading indicator of ecological transition. Ecol. Lett. 2006;9:311–318. doi: 10.1111/j.1461-0248.2005.00877.x. doi:10.1111/j.1461-0248.2005.00877.x [DOI] [PubMed] [Google Scholar]
- Cerling T.E, Harris J.M, MacFadden B.J, Leakey M.J, Quade J, Eisenmann V, Ehleringer J.R. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. doi:10.1038/38229 [Google Scholar]
- Chisholm B.S, Nelson D.E, Schwarcz H.P. Stable-carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Science. 1982;216:1131–1132. doi: 10.1126/science.216.4550.1131. doi:10.1126/science.216.4550.1131 [DOI] [PubMed] [Google Scholar]
- Ciais P, Tans P.P, Trolier M, White J.W.C, Francey R.J. A large northern hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2. Science. 1995;269:1098–1102. doi: 10.1126/science.269.5227.1098. doi:10.1126/science.269.5227.1098 [DOI] [PubMed] [Google Scholar]
- Ciais P, Cuntz M, Scholze M, Mouillot F, Peylin P, Gitz V. Remarks on the use of 13C and 18O isotopes in atmospheric CO2 to quantify biospheric carbon fluxes. In: Flanagan L.B, Ehleringer J.R, Pataki D.E, editors. Stable isotopes and biosphere-atmosphere interactions: processes and biological controls. Elsevier; New York, NY: 2005. pp. 235–267. [Google Scholar]
- Coltrain J.B, Harris J.M, Cerling T.E, Ehleringer J.R, Dearing M, Ward J, Allend J. Rancho La Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal southern California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;205:199–219. doi:10.1016/j.palaeo.2003.12.008 [Google Scholar]
- Coviella C.E, Trumble J.T. Effects of elevated atmospheric carbon dioxide on insect plant interactions. Conserv. Biol. 1999;13:700–712. doi:10.1046/j.1523-1739.1999.98267.x [Google Scholar]
- Dawson T.E, Mambelli S, Plamboeck A.H, Templer P.H, Tu K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002;33:507–559. doi:10.1146/annurev.ecolsys.33.020602.095451 [Google Scholar]
- DeNiro M.J, Epstein S. Mechanism of carbon fractionation associated with lipid synthesis. Science. 1977;197:261–263. doi: 10.1126/science.327543. doi:10.1126/science.327543 [DOI] [PubMed] [Google Scholar]
- de Ruiter P.C, Wolters V, Moore J.C, Winemiller K.O. Food web ecology: playing jenga and beyond. Science. 2005;309:68–71. doi: 10.1126/science.1096112. doi:10.1126/science.1096112 [DOI] [PubMed] [Google Scholar]
- Drake B.G, Leadley P.W, Arp W.J, Nassiry D, Curtis P.S. An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Funct. Ecol. 1989;3:363–371. doi:10.2307/2389377 [Google Scholar]
- Ehleringer J.R, Monson R.K. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 1993;24:411–439. doi:10.1146/annurev.es.24.110193.002211 [Google Scholar]
- Ehleringer J.R, Brooks J.R, Flanagan L. Oak Ridge National Laboratory Distributed Active Archive Center; Oak Ridge, TN: 1998. BOREAS TE-05 tree ring and carbon isotope ratio data. See http://www.daac.ornl.gov. [Google Scholar]
- Falkowski P, et al. The global carbon cycle: a test of our knowledge of earth as a system. Science. 2000;290:291–296. doi: 10.1126/science.290.5490.291. doi:10.1126/science.290.5490.291 [DOI] [PubMed] [Google Scholar]
- Focken U. Stable isotopes in animal ecology: the effect of ration size on the trophic shift of C and N isotopes between feed and carcass. Isot. Environ. Health Stud. 2001;37:199–211. doi: 10.1080/10256010108033296. doi:10.1080/10256010108033296 [DOI] [PubMed] [Google Scholar]
- Focken U, Becker K. Metabolic fractionation of stable carbon isotopes: implications of different proximate compositions for studies of the aquatic food webs using δ13C data. Oecologia. 1998;115:337–343. doi: 10.1007/s004420050525. doi:10.1007/s004420050525 [DOI] [PubMed] [Google Scholar]
- Fox-Dobbs K, Stidham T.A, Bowen G.J, Emslie S.D, Koch P.L. Dietary controls on extinction versus survival among avian megafauna in the Late Pleistocene. Geology. 2006;34:685–688. doi:10.1130/G22571.1 [Google Scholar]
- Francey R.J, Allison C.E, Etherideg D.M, Trudinger I.G, Leuenberger M, Langenfelds R.L, Michel E, Steele L.P. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B. 1999;51:170–193. doi:10.1034/j.1600-0889.1999.t01-1-00005.x [Google Scholar]
- Fry B. Springer; NewYork, NY: 2006. Stable isotope ecology. [Google Scholar]
- Gannes L.Z, O'Brien D.M, Martínez del Rio C. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology. 1997;78:1271–1276. [Google Scholar]
- Griffith H. Applications of stable isotope technology in physiological ecology. Funct. Ecol. 1991;5:254–269. doi:10.2307/2389263 [Google Scholar]
- Hedges R.E.M. On bone collagen—apatite–carbonate isotopic relationships. Int. J. Osteoarcheol. 2003;13:66–79. doi:10.1002/oa.660 [Google Scholar]
- Hedges R.E.M, Stevens R.E, Richards M.P. Bone as a stable isotope archive for local climatic information. Quatern. Sci. Rev. 2004;23:959–065. doi:10.1016/j.quascirev.2003.06.022 [Google Scholar]
- Hilderbrand G.V, Farley S.D, Robbins C.T, Hanley T.A, Titus K, Servheen C. Use of stable isotopes to determine diets of living and extinct bears. Can. J. Zool. 1996;74:2080–2088. [Google Scholar]
- Hobson K.A, Clark R.G. Assessing avian diet using stable isotopes I: turnover of 13C in tissues. Condor. 1992;94:181–188. doi:10.2307/1368807 [Google Scholar]
- Hobson K.A, Schwarcz H.P. The variation in δ13C values in bone collagen for two wild herbivore populations: implications for palaeodiet studies. J. Archaeol. Sci. 1986;13:101–106. doi:10.1016/0305-4403(86)90001-4 [Google Scholar]
- Hoppe K.A, Paytan A, Chamberlain P. Reconstructing grassland vegetation and paleotemperatures using carbon isotope ratios of bison tooth enamel. Geology. 2006;34:649–652. doi:10.1130/G22745.1 [Google Scholar]
- Karl T.R, Trenberth K.E. Modern global climate change. Science. 2003;302:1719–1723. doi: 10.1126/science.1090228. doi:10.1126/science.1090228 [DOI] [PubMed] [Google Scholar]
- Keeling C.D, Bollenbacher A.F, Whorf T.P. Trends: a compendium of data on global change. Carbon Dioxide Information Analysis Center/National Laboratory, U.S. Department of Energy; Oak Ridge, TN: 2005. Monthly atmospheric 13C/12C isotopic ratios for 10 SIO stations.http://cdiac.ornl.gov/trends/trends.htm [Google Scholar]
- Kennedy D. Climate change and climate science. Science. 2004;304:1565. doi: 10.1126/science.304.5677.1565. doi:10.1126/science.304.5677.1565 [DOI] [PubMed] [Google Scholar]
- Koch P.L. Isotopic reconstruction of past continental environments. Annu. Rev. Earth Planet. Sci. 1998;26:573–613. doi:10.1146/annurev.earth.26.1.573 [Google Scholar]
- Kohn M.J, Cerling T.E. Stable isotope compositions of biological apatite. Rev. Mineral. Geochem. 2002;48:455–488. [Google Scholar]
- Leavitt S.W, Danzer S.R. δ13C variations in C3 plants over the past 50,000 years. Radiocarbon. 1992;34:783–791. [Google Scholar]
- Leng M.J. ISOtopes in Quaternary PALaeoenvironmental reconstruction (ISOPAL) Quatern. Sci. Rev. 2004;23:739–741. doi:10.1016/j.quascirev.2003.06.008 [Google Scholar]
- Leuenberger M, Siegenthaler U, Langway C.C. Carbon isotope composition of atmospheric CO2 during the last ice-age from an Antarctic ice core. Nature. 1992;357:488–490. doi:10.1038/357488a0 [Google Scholar]
- Long E.S, Sweitzer R.A, Diefenbach D.R, Ben-David M. Controlling for anthropogenically induced atmospheric variation in stable carbon isotope studies. Oecologia. 2005;146:148–156. doi: 10.1007/s00442-005-0181-6. doi:10.1007/s00442-005-0181-6 [DOI] [PubMed] [Google Scholar]
- Macko S.A, Lee W.Y, Parker P.L. Nitrogen and carbon isotope fractionation by two species of marine amphipods: laboratory and field studies. J. Exp. Mar. Biol. Ecol. 1982;63:145–149. doi:10.1016/0022-0981(82)90028-4 [Google Scholar]
- Marchal O, Stocker T.F, Joos F, Indermühle A, Blunier T, Tschumi J. Modelling the concentration of atmospheric CO2 during the Younger Dryas climate event. Clim. Dynam. 1999;15:341–354. doi:10.1007/s003820050286 [Google Scholar]
- McCutchan J.H, Jr., Lewis W.M, Jr., Kendall C, McGrath C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 2003;102:378–390. doi:10.1034/j.1600-0706.2003.12098.x [Google Scholar]
- McFadden B.J, Higgins P. Ancient ecology of 15-million-year-old browsing mammals within C3 plant communities from Panama. Oecologia. 2004;140:169–182. doi: 10.1007/s00442-004-1571-x. doi:10.1007/s00442-004-1571-x [DOI] [PubMed] [Google Scholar]
- Mora C.I, Driese S.G, Colarusso L.A. Middle to Late Paleozoic atmospheric CO2 levels from soil carbonate and organic matter. Science. 1996;271:1105–1107. doi:10.1126/science.271.5252.1105 [Google Scholar]
- Morin P.J. Realism, precision, and generality in experimental ecology. In: Resetarits W.J, Bernardo J, editors. Experimental ecology: issues and perspectives. Oxford University Press; New York, NY: 1998. pp. 50–70. [Google Scholar]
- Nakicenovic N, Swart R. Cambridge University Press; Cambridge, UK: 2000. Emmission scenarrios. [Google Scholar]
- Neftel A, Oeschger H, Staffelbach T, Stauffer B. CO2 record in the Byrd ice core 50,000–5,000 years BP. Nature. 1988;331:609–611. doi:10.1038/331609a0 [Google Scholar]
- Ostrom P.H, Colunga-Garcia M, Gage S.H. Establishing pathways of energy flow for insect predators using stable isotope ratios: field and laboratory evidence. Oecologia. 1997;109:108–113. doi: 10.1007/s004420050064. doi:10.1007/s004420050064 [DOI] [PubMed] [Google Scholar]
- Peterson, R.O.Wolf ecology and prey relationships on Isle Royale US Natl Park Services and Science Monograph Series 11. Washington, DC: National Park Service.
- Rayner P.J, Enting I.G, Francey R.J, Langenfelds R. Reconstructing the recent carbon cycle from atmospheric CO2, δ13C and O2/N2 observations. Tellus B. 1999;51:213–232. doi:10.1034/j.1600-0889.1999.t01-1-00008.x [Google Scholar]
- Richards M.P, Hedges R.E.M. Variations in bone collagen δ13C and δ15N values of fauna from Northwest Europe over the last 40,000 years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003;193:261–267. doi:10.1016/S0031-0182(03)00229-3 [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1983. Scaling: why is animal size so important? [Google Scholar]
- Schweitzer M.A, Suo Z, Avci R, Asara J.M, Allen M.A, Arce F.T, Horner J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science. 2007;316:277–280. doi: 10.1126/science.1138709. doi:10.1126/science.1138709 [DOI] [PubMed] [Google Scholar]
- Smith J.H, Fischer H, Wahlen M, Mastroianni D, Deck R. Dual modes of the carbon cycle since the Last Glacial Maximum. Nature. 1999;400:248–250. doi: 10.1038/22291. doi:10.1038/22291 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos M.A, Tonn W.M, Wassenaar L.I. Effects of lipid extraction on stable carbon and nitrogen isotope analyses of fish tissues: potential consequences for food web studies. Ecol. Freshw. Fish. 2004;13:155–160. doi:10.1111/j.1600-0633.2004.00056.x [Google Scholar]
- Sparks J.P, Ehleringer J.R. Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects. Oecologia. 1997;109:362–367. doi: 10.1007/s004420050094. doi:10.1007/s004420050094 [DOI] [PubMed] [Google Scholar]
- Staffelbach T, Stauffer B, Sigg A, Oeschger H. CO2 measurements from polar ice cores: more data from different sites. Tellus B. 1991;43:91–96. doi:10.1034/j.1600-0889.1991.t01-1-00003.x [Google Scholar]
- Stevens R.E, Hedges R.E.M. Carbon and nitrogen stable isotope analysis of northwest European horse bone and tooth collagen, 40,000 BP–present: Palaeoclimatic interpretations. Quatern. Sci. Rev. 2004;23:977–991. doi:10.1016/j.quascirev.2003.06.024 [Google Scholar]
- Sugawara S, Kawamura K, Aoki S, Nakazawa T, Hashida G. Reconstruction of past variations of δ13C in atmospheric CO2 from its vertical distribution observed in the firn at Dome Fuji, Antarctica. Tellus B. 2003;55:159–169. doi:10.1034/j.1600-0889.2003.00023.x [Google Scholar]
- Thurber J.M, Peterson R.O. Effects of population density and pack size on the foraging ecology of gray wolves. J. Mamm. 1993;74:789–889. doi:10.2307/1382426 [Google Scholar]
- Tischler, K. B. 2004 Aquatic plant nutritional quality and contribution to moose diet at Isle Royale National Park. MS thesis, Michigan Technological University, Houghton, MI.
- Trudinger C.M, Enting I.G, Francey R.J, Etheridge D.M, Rayner P.J. Long-term variability in the global carbon cycle inferred from a high precision CO2 and δ13C ice-core record. Tellus B. 1999;51:233–248. doi:10.1034/j.1600-0889.1999.t01-1-00009.x [Google Scholar]
- Van de Water P.K, Leavitt S.W, Betancourt J.L. Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial–interglacial cycle. Science. 1994;264:239–243. doi: 10.1126/science.264.5156.239. doi:10.1126/science.264.5156.239 [DOI] [PubMed] [Google Scholar]
- Vucetich J.A, Peterson R.O. Long-term population and predation dynamics of wolves on Isle Royale. In: Macdonald D, Sillero-Zubiri C, editors. Biology and conservation of wild canids. Oxford University Press; Oxford, UK: 2004. pp. 281–292. [Google Scholar]
- Vucetich J.A, Peterson R.O, Schaefer C.L. The effect of prey and predator densities on wolf predation. Ecology. 2002;83:3003–3013. [Google Scholar]
- Ward J.K, Harris J.M, Cerling T.E, Wiedenhoeft A, Lott M.J, Dearing M.D, Coltrain J.B, Ehleringer J.R. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc. Natl Acad. Sci. USA. 2005;102:690–694. doi: 10.1073/pnas.0408315102. doi:10.1073/pnas.0408315102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.B, Bowen G.J, Cerling T.E, Ehleringer J.R. Stable isotopes as one of nature's ecological recorders. Trends Ecol. Evol. 2006;21:408–415. doi: 10.1016/j.tree.2006.04.002. doi:10.1016/j.tree.2006.04.002 [DOI] [PubMed] [Google Scholar]