Abstract
The position of the earliest-derived living molluscs, the Polyplacophora (chitons) and shell-less vermiform Aplacophora, remains highly contentious despite many morphological, developmental and molecular studies of extant organisms. These two groups are thought to represent either a basal molluscan grade or a clade (Aculifera) sister to the ‘higher’ molluscs (Conchifera). These incompatible hypotheses result in very different predictions about the earliest molluscs. A new cladistic analysis incorporating both Palaeozoic and extant molluscs is presented here. Our results support the monophyly of Aculifera and suggest that extant aplacophorans and polyplacophorans both derive from a disparate group of multivalved molluscs in two major clades. Reanalysis of the critical Ordovician taxon ‘Helminthochiton’ thraivensis shows that this animal lacks a true foot despite bearing polyplacophoran-like valves. Its position within our phylogenetic reconstruction indicates that many fossil ‘polyplacophorans’ in the order Palaeoloricata are likely to represent footless stem-group aplacophorans. ‘H.’ thraivensis and similar forms such as Acaenoplax may be morphological stepping stones between chitons and the shell-less aplacophorans. Our results imply that crown-group molluscan synapomorphies include serial repetition, the presence of a foot, a mineralized scleritome and a creeping rather than worm-like mode of life.
Keywords: Polyplacophora, Helminthochiton thraivensis, Aplacophora, Aculifera
1. Introduction
The shape of the molluscan family tree has been debated for 200 years. Evidence from embryology, palaeontology, molecular sequences and anatomy often yield contradictory results (Ghiselin 1988; Salvini-Plawen & Steiner 1996; Giribet et al. 2006). A resolution of this debate would clarify not merely the internal phylogeny of the Mollusca, but, by determining the character-set plesiomorphic for molluscs, would improve our understanding of the phylogeny of the Lophotrochozoa as a whole (Conway Morris & Caron 2007). The great morphological disparity between extant molluscan groups underlies much of this phylogenetic confusion, and despite an extensive fossil record, fossil molluscan taxa have not for the most part provided the ‘transitional’ morphologies required to fill in these gaps.
Aplacophorans (vermiform molluscs) and polyplacophorans (chitons) are widely accepted as the earliest-diverged living groups of molluscs. The two predominant general models for molluscan phylogeny differ fundamentally in the placement of these groups (see Haszprunar 1996) and have different implications for the character set of the molluscan common ancestor. Both of these models place the remaining ‘higher’ molluscan groups within a single clade (Conchifera), although the monophyly of this assemblage has also occasionally been questioned (Lindberg & Ponder 1996; Giribet et al. 2006).
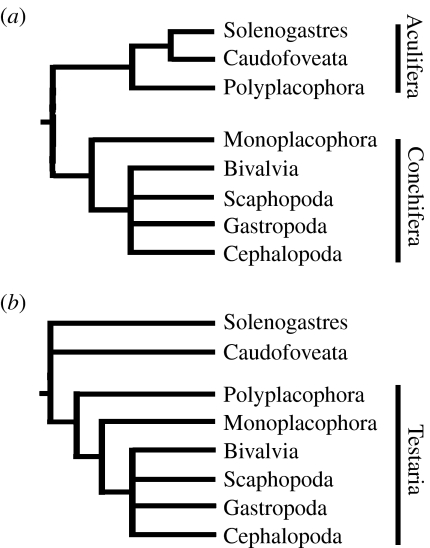
The ‘aculiferan’ model of molluscan phylogeny (figure 1a) divides the group into two subphyla: Conchifera, the ‘shell-bearing’ molluscs, and Aculifera, ‘spiny’ molluscs including Aplacophora and Polyplacophora, named for their calcareous armature or spiculi, and evidence for an exterior cuticula (e.g. Scheltema 1993, 1996). In this model, aplacophorans, comprising the epifaunal Neomeniomorpha (Solenogastres) and the infaunal Chaetodermomorpha (Caudofoveata), form a sister group to the chitons within Aculifera. This model implies that the most recent common ancestor of the crown-group Mollusca had a dorsal valve(s), a locomotory foot and some degree of serial repetition (Lindberg & Ghiselin 2003).
Figure 1.
Generalized topologies of two major competing models of molluscan phylogeny. (a) Aculiferan and (b) testarian models.
The alternative ‘testarian’ model (figure 1b) has the aplacophorans basal to the Testaria, a clade in which the polyplacophorans form a sister group to the Conchifera (e.g. Salvini-Plawen 1985; Haszprunar 2000). In this model, Aculifera is a grade rather than a clade. The differences between the two aplacophoran groups have also raised questions about the monophyly of the Aplacophora (Salvini-Plawen 1980), and adherents of the testarian model have typically interpreted aplacophorans as a paraphyletic group at the base of the molluscan tree. This model implies a vermiform ancestral mollusc, lacking both a foot and serial repetition.
Cladistic studies of living molluscs, using both molecular (e.g. Passamaneck et al. 2004; Giribet et al. 2006) and morphological (e.g. Salvini-Plawen & Steiner 1996; Haszprunar 2000) datasets, have failed to settle the question of these two competing models. Anatomical studies have been unduly influenced by the effects of proximate out-groups on the internal topology of molluscan trees; a testarian model arises from analyses with annelid-like forms as sister to the Mollusca, while the aculiferan model is associated with a turbellarian-like sister group (see Haszprunar 1996). Molecular studies present intriguing alternative topologies, but do not resolve the placement of aplacophoran groups relative to other molluscan classes (Passamaneck et al. 2004; Giribet et al. 2006).
From the divisive literature on this topic, it is clear that the gross morphology of the ancestral mollusc cannot be divined from anatomical studies of living molluscs. The failure of molecular phylogenetic techniques to resolve this problem conclusively may reflect a rapid radiation of the group with relatively little time separating the divergences in question. In view of this lack of resolution, we consider morphological data from the fossil record, and the otherwise undocumented character combinations that it can reveal, to be a critical tool for the study of the interrelationships of these groups. Available data on fossil aculiferan-like animals have been significantly expanded by the recent description of new forms (e.g. Sutton et al. 2001; Vendrasco et al. 2004; Conway Morris & Caron 2007) and the reinterpretation of existing taxa (e.g. Scheltema et al. 2003; Vinther & Nielsen 2005; Caron et al. 2006), showing a wide range of diversity in girdle armature and shell arrangements that may shed light on this early divergence of molluscan clades. The case for the integration of these data into phylogenetic models is thus increasingly pressing.
Although phylogenetic analyses have been included in the initial discussions of some of these organisms (e.g. Vendrasco et al. 2004; Conway Morris & Caron 2007), they have been limited in scope, in terms of both characters used and taxa included. No work has yet been done to synthesize new palaeontological information in toto and to apply it to the problems of higher-level molluscan phylogeny. In this study, we use cladistic methods to analyse molluscan phylogeny using a combination of evidence from well-preserved fossil and living molluscs, with the aim of testing competing phylogenetic models.
2. Material and methods
(a) Taxon selection
The class Polyplacophora has a fossil record extending to the lower Ordovician. Its members have traditionally been divided into the orders Palaeoloricata and Neoloricata, the former being diagnosed by the absence of modern shell features. This concept has led to Palaeoloricata being treated as a ‘bucket’ taxon used to contain a range of polyplacophoran-like fossils. The system was recently revised by Sirenko (2006) with a more thorough approach to shell characterization in fossil forms, but this non-cladistic classification excluded several Palaeoloricata sensu lato. Recent discoveries of articulated and exceptionally preserved material have expanded our knowledge of the animals on which ‘palaeoloricate’ valves were borne, and demonstrated character combinations not seen in extant molluscs. These include the 17-plated multiplacophorans Strobilepis and Polysaccos (Vendrasco et al. 2004), and the vermiform Acaenoplax (Sutton et al. 2001, 2004).
Fossil polyplacophorans are typically represented by isolated valves. Articulated fossils, while relatively rare (Dell'Angelo et al. 2003), are more informative, and thus more significant for phylogenetic studies. All polyplacophoran-like articulated Palaeozoic fossils were included in our study after an exhaustive review of the literature, with the exception of two genera that, although represented by articulated material, are too poorly preserved for meaningful characterization. Plasiochiton curiosus is a fragmentary internal mould of intermediate valves (Hoare 2000). Helminthochiton griffithi, although recently redescribed from the holotype (Sigwart 2007), is an external mould of the dorsal anterior valves with no significant detail preserved; its supposed congener ‘Helminthochiton’ thraivensis is included (see §3), but the placement of this latter species in Helminthochiton is not taxonomically meaningful. Two species of Glaphurochiton are known from articulated fossils; as characters were coded at genus level, both Glaphurochiton concinnus and Glaphurochiton carbonarius are equally represented. Selected problematic genera of palaeoloricate or palaeoloricate-like valves (Matthevia, Heloplax, Enetoplax and Cobcrephora) known only from disarticulated material were also included.
Several Palaeozoic fossils whose molluscan affinities are less well established have also been included in the analysis. Machaeridians (e.g. Adrain 1992) are elongate armoured animals from the Lower Palaeozoic that bear two or four rows of imbricating calcitic dorsal valves. Soft parts for these taxa are unknown, but a molluscan affinity has been entertained by several workers (e.g. Dzik 1994); they are represented here by the genera Plumulites, Lepidocoleus and Turrilepas.
A number of exceptionally preserved problematic Cambrian fossils have been interpreted as having molluscan affinities, notably Halkieria, Wiwaxia, Odontogriphus and Orthrozanclus. The affinities of these fossils have been the subject of vigorous debate; Halkieria has, for example, been interpreted as being more closely related to brachiopods (e.g. Holmer et al. 2002), and both Odontogriphus and, particularly, Wiwaxia as putative annelids (Butterfield 2006). Nonetheless, recent work (e.g. Scheltema et al. 2003; Vinther & Nielsen 2005; Caron et al. 2006; Conway Morris & Caron 2007) has favoured a molluscan interpretation of these taxa on the basis of homologies of scleritome arrangement and radula morphology, and we have included them in our analysis.
The putative Precambrian mollusc Kimberella (Fedonkin & Waggoner 1997) was excluded as it preserves very few characters codeable in our analysis; in addition, we do not consider its in-group status with respect to the brachiopod out-group to be secure.
Additional representative extant genera were coded from specimen material in museum collections, representing major recognized taxonomic subgroups in the living species of aplacophorans, polyplacophorans and monoplacophorans.
Wherever possible, published descriptions for fossil taxa were supplemented by examination of specimens from the National Museum of Ireland (Natural History) and the Natural History Museum (London); table 1 in the electronic supplementary material lists the sources used for each taxon.
(b) Phylogenetic methods
Forty-four morphological characters (see table 2 in the electronic supplementary material) were coded into a matrix across an in-group comprising 31 taxa (see appendix 1 in the electronic supplementary material). Characters are based on both conchological and soft-tissue anatomy; characters not potentially determinable in fossil material (e.g. those based on embryology) were not used. Morphological features (characters) were considered variable between, but not within, individual genus-level taxa.
The nature of the characters used in our analysis excludes the possibility of coding soft-bodied forms such as annelids or other vermiform taxa that have been proposed as molluscan sister groups. We instead used an out-group comprising three brachiopods: two extant and one fossil. As valve-bearing lophotrochozoan taxa, brachiopods are closely related to the molluscs and possess an acceptable number of characters that can be coded in our scheme. The interpretation of potential homologies between molluscs and brachiopods is partially dependent on the acceptance or rejection of the ‘brachiopod-fold’ hypothesis (Cohen et al. 2003); this theory implies that the ventral valve of the brachiopods is anatomically dorsal. Brachiopod taxa were hence coded twice, and cladistic analyses were repeated using out-group taxa coded under the brachiopod-fold hypothesis and secondly using a more traditional anatomical interpretation (Brusca & Brusca 2003). To test the molluscan affinities of Cambrian taxa under our coding scheme, the analyses were repeated with the four taxa we included considered as part of the out-group (figure 3). The complete dataset was analysed using four out-group permutations, resulting from the alternate (fold/non-fold) sets of brachiopod codings, and inclusion/exclusion of these Cambrian taxa in the out-group.
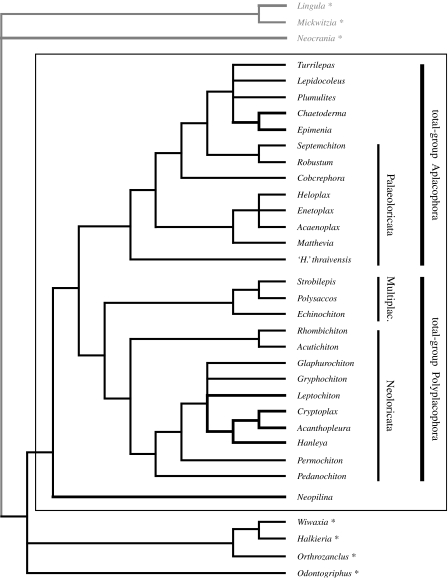
Figure 3.
Strict consensus tree from parsimony analyses showing inferred relationships among Aculifera and related taxa based on 101 most-parsimonious trees generated when brachiopod out-group characters were coded according to the brachiopod-fold hypothesis (Cohen et al. 2003). Lines in bold indicate living taxa; brachiopod (out-group) taxa are in grey; additional fossil out-group taxa are indicated by asterisks. ‘Polyplacophoran’ groups Neoloricata, Palaeoloricata and Multiplacophora (Multiplac.) are labelled; molluscan crown group is indicated by the surrounding box. Unambiguous synapomorphies are as follows (asterisk, maximum consistency index; see electronic supplementary material for character descriptions): Aculifera 22*, 33; total-group Aplacophora 20, 38, 39, 40*; total-group Polyplacophora 4, 15, 16; Halwaxiidae 19, 34*.
Several multistate characters are included in our analysis, which represent transformational series. In each case, it is difficult or impossible to conceive of an evolutionary transition from one end-state to the other without traversing the intermediate state(s). These characters are therefore treated as ordered. As multistate characters have the potential to unduly influence parsimony analysis, all multistate features were down-weighted in proportion to the total number of possible state changes, following the method used by Wills (1997). To ensure integer-value tree lengths, binary characters have a base weight of 6, whereas three-state characters have a reduced weight of 3 and four-state characters have a weight of 2. To evaluate and potentially correct for resulting biases, analyses were run both with and without this a priori weighting scheme.
Data were subject to parsimony analysis using the heuristic search algorithm implemented in PAUP* v. 4.0b10 (Swofford 2002). Eight configurations (two out-group hypotheses, with and without additional Cambrian taxa included in the out-group, and each with and without a priori weighting) were analysed. For each, one hundred randomly seeded TBR branch-swapping replicates were performed in each analysis to determine a set of most-parsimonious trees (MPTs). We investigated distribution of character-state changes as a means of assessing node support.
3. Results
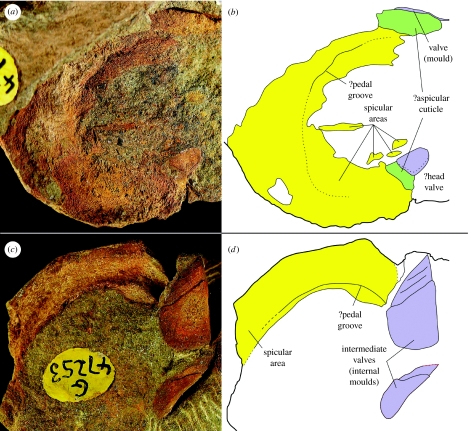
A review of articulated ‘polyplacophoran’ material for the purposes of this study led us to reinvestigate one unexpectedly important taxon ‘H.’ thraivensis (Reed 1911), from the Ordovician of Girvan, Scotland. This species, although included in the analysis of Cherns (2004) and briefly mentioned by other workers (Smith & Hoare 1987; Vendrasco & Runnegar 2004), has not been fully restudied since its original description. Multiple specimens in the collections of the Natural History Museum (London) preserve impressions of the spicular girdle. One three-dimensionally preserved specimen (not illustrated) demonstrates a sub-circular cross section, little wider than the valves. Other compressed specimens (two of which are illustrated in figure 2) include spicular regions that are too expansive to represent only a ventrolateral girdle; these and other specimens examined demonstrate that ‘H.’ thraivensis has a ventrally complete or subcomplete spicular scleritome with no space for a polyplacophoran-like foot. Several specimens display a linear disturbance in a position interpreted as medial prior to compression; this structure may represent a neomeniomorph-like reduced foot. The valves borne by this species are of a typically palaeoloricate morphology, unlike those of Acaenoplax (which also lacks a foot). A full redescription and reclassification of ‘H.’ thraivensis will follow in a future paper. ‘H.’ thraivensis lies outside the range of the polyplacophoran Bauplan as previously conceived, and represents a potential transitional morphology linking aplacophorans with polyplacophoran-like forms. Our phylogenetic reconstruction (see below) and our new interpretation of this species indicate that many palaeoloricate taxa known from isolated plates may represent shelled, but vermiform, molluscs that lack a true foot.
Figure 2.
‘Helminthochiton’ thraivensis (a,b) BMNH G.47246, showing ventral spicular surface, marginally slightly disarticulated, together with head and probably intermediate valve, ×2.9. (c,d) BMNH G.47253, showing (left) ventrolateral surface and (right) dorsolateral surface with internal moulds of intermediate valves, ×2.4.
Heuristic searches with PAUP* on the permutations of the analysis (see above) generated four alternative sets of most-parsimonious trees (MPTs). Acceptance of the brachiopod-fold hypothesis and the down-weighting of multistate characters produced 101 MPTs of minimum length 680 steps (figure 3). Alternative versions of the analysis produced resulting consensus trees with topologies that are not substantially different (see electronic supplementary material). In all cases, including the Cambrian taxa within the out-group has no effect on the resulting topology.
The halwaxiids (Halkieria, Wiwaxia and Orthozanclus) are recovered as a clade in our preferred tree (figure 3). Odontogriphus is a stem-group mollusc or part of a basal molluscan polytomy in all permutations of our analysis. None of these taxa are recovered consistently within the molluscan crown group, and the relationships between them are consistent with the results of Conway Morris & Caron (2007).
Two major in-group clades are recovered in 100% of MPTs in all permutations of the analysis: total-group Polyplacophora and total-group Aplacophora, which together form a monophyletic Aculifera. The total-group Polyplacophora clade includes all neoloricate polyplacophorans with the multiplacophorans in a basal position. The total-group Aplacophora clade recovered by our analyses includes machaeridians, and a number of palaeoloricate polyplacophorans including Acaenoplax and ‘H.’ thraivensis. Unambiguous synapomorphies that support these clades are listed in figure 3.
4. Discussion
The results of this analysis support a monophyletic Aculifera and suggest that aplacophorans are derived from chiton-like ancestors, as shown by the placement of palaeoloricate taxa in the aplacophoran stem group. Although our analysis included only one conchiferan taxon to test the monophyly of total-group Aculifera, the polyplacophoran and aplacophoran clades are each well resolved. Taxa within the total-group Aplacophora show that many fossil ‘chitons’ probably did not have a foot.
The critical taxon ‘H.’ thraivensis, the preserved specimen of which shows it had polyplacophoran-like valves but lacked a well-developed foot, is a sister to the remaining total-group aplacophorans; this transitional morphology links the dorsoventrally flattened chitons and the vermiform aplacophorans (figure 2). Septemchiton also resolves in the aplacophoran stem group; other septemchitonids (e.g. Carnicoleus) have valves that meet ventrally and cannot have possessed a functional foot. This observation lead Dzik (1994) to suggest that septemchitonids may have been ancestral to aplacophorans, an inference that we consider prescient. Our analyses imply that all palaeoloricate polyplacophoran taxa within the total-group aplacophoran clade lacked a fully developed foot. This result has serious implications for the interpretation of any disarticulated polyplacophoran material, as the presence or absence of a foot is unlikely to be evident from shell valves alone.
The recovery of a monophyletic Neoloricata is in agreement with the non-cladistic taxonomic studies of Sirenko (1997, 2006). Within this clade, the derived positions of the extant taxa with respect to the Palaeozoic fossils, and indeed the relative positions within the extant taxa, are compatible with neontological studies (e.g. Okusu et al. 2003; Sirenko 2006). We consider this a corroboration of the validity of our analysis and in particular the application of down-weighting multistate characters, since this topology is not resolved in unweighted analysis (see electronic supplementary material).
The results from our analyses differ fundamentally from the topology recovered in the only previous cladistic analysis including palaeoloricate fossils (Cherns 2004). The earlier analysis was primarily concerned with the relationships among taxa known from disarticulated valves; it was by necessity based on fewer characters than the present study and did not include aplacophorans or problematic Cambrian taxa. Some of the results of Cherns' (2004) analysis seem problematic (for instance, the placement of the multiplacophoran Strobilepis as a sister group to the extant Chiton); however, it does concur with our analysis at least in the recovery of a heloplacid clade (Acaenoplax, Heloplax and Enetoplax). The cladistic analysis of Conway Morris & Caron (2007) is concerned with broader lophotrochozoan phylogeny; the molluscan portion of their tree, while poorly resolved, is in agreement with our findings.
The machaeridian genera in our analysis resolve close to the aplacophorans; they do not however unambiguously form a clade, possibly representing a grade of relatively derived members of the aplacophoran stem group. This suggested position remains speculative in the continued absence of fossilized machaeridian soft tissues.
Our analyses include only two representative living genera of aplacophorans and hence do not directly test the monophyly of living Aplacophora. In view of the disparity of total-group Aplacophora implied in our phylogeny, and especially by the newly identified ‘H.’ thraivensis, it seems plausible that the two aplacophoran groups Neomeniomorpha and Chaetodermomorpha had separate origins within this diverse assemblage.
The heterogeneous nature of the Palaeoloricata is evident in trees resulting from our analyses; as suggested by Vendrasco et al. (2004) these fossils do not represent a natural group, but are stem forms to extant clades. Most palaeoloricate in our analysis resolve as stem-group aplacophorans, while the multiplacophorans and the allied Echinochiton resolve as stem-group polyplacophorans. The Palaeoloricata is clearly non-monophyletic and we contend that the use of this taxon should be discontinued.
Our analyses do not test the monophyly of the Conchifera, as the only conchiferan included is the extant monoplacophoran Neopilina. Partial sequence data for a monoplacophoran led Giribet et al. (2006) to suggest a sister-group relationship linking Polyplacophora and Monoplacophora, ‘Serialia’. In their analysis, aplacophorans appeared more closely related to scaphopods and cephalopods, in a clade sister to the remaining Mollusca; Aplacophora, Testaria and the Conchifera are not recovered. These results, while intriguing, are not supported by morphological homologies (Nielsen et al. 2007), run counter to all morphologically based phylogenetic analyses, ours included, and are not congruent with other molecular phylogenies (e.g. Rosenberg et al. 1997; Passamaneck et al. 2004). A resolution of the discrepancies between molecular and morphological signals is clearly still required, but is beyond the scope of this paper.
The topologies of all of our recovered trees support the aculiferan rather than the testarian model of molluscan evolution. This debate is at the core of molluscan phylogeny: what are the synapomorphies of the Mollusca, and what did the most recent common ancestor of the Mollusca look like? Our results imply a character set for this animal that includes seven- to eightfold serial repetition, the presence of valves and a foot, and a creeping rather than worm-like mode of life. The consideration of informative fossil taxa in phylogenetic analyses provides insights into the disparity and evolutionary history of groups that cannot be obtained from solely neontological studies.
Acknowledgments
This project was funded by the Royal Society (UK) and Royal Irish Academy Research Exchange Scheme. The authors thank G. Giribet and a second anonymous reviewer for suggestions that substantially improved this paper. L. Allcock and G. Dyke offered useful comments on earlier versions of this manuscript. N. Butterfield and L. Cherns provided stimulating discussion on this work.
Supplementary Material
Supplementary tables, appendix, figure and references
References
- Adrain J.M. Machaeridian classification. Alcheringa. 1992;16:15–32. [Google Scholar]
- Brusca R.C, Brusca G.J. 2nd edn. Sinauer Associates; Sunderland, MA: 2003. Invertebrates. [Google Scholar]
- Butterfield N.J. Hooking some stem-group ‘worms’: fossil lophotrochozoans in the Burgess Shale. Bioessays. 2006;28:1161–1166. doi: 10.1002/bies.20507. doi:10.1002/bies.20507 [DOI] [PubMed] [Google Scholar]
- Caron J.-B, Scheltema A, Schander C, Rudkin D. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature. 2006;442:159–162. doi: 10.1038/nature04894. doi:10.1038/nature04894 [DOI] [PubMed] [Google Scholar]
- Cherns L. Early Palaeozoic diversification of chitons (Polyplacophora Mollusca) based on new data from the Silurian of Gotland, Sweden. Lethaia. 2004;37:445–456. doi:10.1080/00241160410002180 [Google Scholar]
- Cohen B.L, Holmer L.E, Lüter C. The brachiopod fold: a neglected body plan hypothesis. Palaeontology. 2003;46:59–65. doi:10.1111/1475-4983.00287 [Google Scholar]
- Conway Morris S, Caron J.-B. Halwaxiids and the early evolution of the lophotrochozoans. Science. 2007;315:1255–1258. doi: 10.1126/science.1137187. doi:10.1126/science.1137187 [DOI] [PubMed] [Google Scholar]
- Dell'Angelo B, Gallorini H, Taviani M. First Neogene record of an articulated polyplacophoran. J. Paleontol. 2003;77:1193–1194. doi:10.1666/0022-3360(2003)077<1193:FNROAA>2.0.CO;2 [Google Scholar]
- Dzik, J. 1994 Machaeridia, chitons, and conchiferan molluscs of the Mojcza Limestone. In Ordovician carbonate platform of the Holy Cross Mountains (eds J. Dzik, E. Olempska, & A. Pisera). Palaeontol. Pol.53, 213–252.
- Fedonkin M.A, Waggoner B.M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature. 1997;388:868–871. doi:10.1038/42242 [Google Scholar]
- Ghiselin M.T. The origin of molluscs in light of molecular evidence. Oxford Surv. Evol. Biol. 1988;5:66–95. [Google Scholar]
- Giribet G, Okusu A, Lindgren A.R, Huff S.W, Schrödl M, Nishiguchi M.K. Evidence for a clade composed of molluscs with serially repeated structures: monoplacophorans are related to chitons. Proc. Natl Acad. Sci. USA. 2006;103:7723–7728. doi: 10.1073/pnas.0602578103. doi:10.1073/pnas.0602578103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haszprunar G. The Mollusca: coelomate turbellarians or mesenchymate annelids? In: Taylor J.D, editor. Origin and evolutionary radiation of the Mollusca. Oxford University Press; Oxford, UK: 1996. pp. 3–28. [Google Scholar]
- Haszprunar G. Is the Aplacophora monophyletic? A cladistic point of view. Am. Malacol. Bull. 2000;15:115–130. [Google Scholar]
- Hoare R.D. Considerations on Paleozoic Polyplacophora including the description of Plasiochiton curiosus n. gen. and sp. Am. Malacol. Bull. 2000;15:131–137. [Google Scholar]
- Holmer L.E, Skovsted C.B, Williams A. A stem group brachiopod from the Lower Cambrian: support for a Micrina (halkieriid) ancestry. Palaeontology. 2002;45:875–882. doi:10.1111/1475-4983.00265 [Google Scholar]
- Lindberg D.R, Ghiselin M.T. Fact, theory and tradition in the study of molluscan origins. Proc. Calif. Acad. Sci. 2003;54:663–686. [Google Scholar]
- Lindberg D.R, Ponder W. An evolutionary tree for the Mollusca: branches or roots? In: Taylor J.D, editor. Origin and evolutionary radiation of the Mollusca. Oxford University Press; Oxford, UK: 1996. pp. 67–75. [Google Scholar]
- Nielsen C, Haszprunar G, Ruthensteiner B, Wanninger A. Early development of the aplacophoran mollusc Chaetoderma. Acta Zool. 2007;88:231–247. doi:10.1111/j.1463-6395.2007.00270.x [Google Scholar]
- Okusu A, Schwabe E, Eernisse D.J, Giribet G. Towards a phylogeny of chitons (Mollusca Polyplacophora) based on combined analysis of five molecular loci. Org. Div. Evol. 2003;4:281–302. doi:10.1078/1439-6092-00085 [Google Scholar]
- Passamaneck Y.J, Schander C, Halanych K.M. Investigation of molluscan phylogeny using large-subunit and small-subunit nuclear rRNA sequences. Mol. Phylogenet. Evol. 2004;32:25–38. doi: 10.1016/j.ympev.2003.12.016. doi:10.1016/j.ympev.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Reed F.R.C. A new fossil from Girvan. Geol. Mag. 1911;8:337–340. [Google Scholar]
- Rosenberg G, Tillier S, Tillier A, Kuncio G.S, Hanlon R.T, Masselot M, Williams C.J. Ribozomal RNA phylogeny of selected major clades in the Mollusca. J. Moll. Stud. 1997;63:301–309. doi:10.1093/mollus/63.3.301 [Google Scholar]
- Salvini-Plawen L.v. A reconsideration of systematics in Mollusca. Malacologia. 1980;19:247–278. [Google Scholar]
- Salvini-Plawen L.v. Early evolution and the primitive groups. In: Trueman E.R, Clarke M.R, editors. The mollusca. Evolution. vol. 10. Academic Press; New York, NY: 1985. pp. 59–150. [Google Scholar]
- Salvini-Plawen L.v, Steiner G. Synapomorphies and plesiomorphies in higher classification of Mollusca. In: Taylor J.D, editor. Origin and evolutionary radiation of the mollusca. Oxford University Press; Oxford, UK: 1996. pp. 29–51. [Google Scholar]
- Scheltema A.H. Aplacophora as progenetic aculiferans and the coelomate origin of mollusks as the sister taxon of Sipuncula. Biol. Bull. 1993;184:57–78. doi: 10.2307/1542380. doi:10.2307/1542380 [DOI] [PubMed] [Google Scholar]
- Scheltema A.H. Phylogenetic position of the Sipuncula, Mollusca and the progenetic Aplacophora. In: Taylor J.D, editor. Origin and Evolutionary Radiation of the Mollusca. Oxford University Press; Oxford, UK: 1996. pp. 53–58. [Google Scholar]
- Scheltema A.H, Kerth K, Kuzirian A.M. Original molluscan radula: comparisons among Aplacophora, Polyplacophora, Gastropoda, and the Cambrian fossil Wiwaxia corrugata. J. Morph. 2003;257:219–245. doi: 10.1002/jmor.10121. doi:10.1002/jmor.10121 [DOI] [PubMed] [Google Scholar]
- Sigwart J.D. The Irish fossil Polyplacophora. Ir. J. Earth Sci. 2007;25:27–38. [Google Scholar]
- Sirenko B.I. The importance of the development of articulamentum for taxonomy of chitons (Mollusca Polyplacophora) Ruthenica. 1997;7:1–24. [Google Scholar]
- Sirenko B.I. New outlook on the system of chitons (Mollusca: Polyplacophora) Venus. 2006;65:27–49. [Google Scholar]
- Smith A.G, Hoare R.D. Paleozoic Polyplacophora: a checklist and bibliography. Occas. pap. Calif. Acad. Sci. 1987;146:1–71. [Google Scholar]
- Sutton M.D, Briggs D.E, Siviter D.J, Siviter D.J. An exceptionally preserved vermiform mollusc from the Silurian of England. Nature. 2001;410:461–463. doi: 10.1038/35068549. doi:10.1038/35068549 [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E, Siviter D.J, Siviter D.J. Computer reconstruction and analysis of the vermiform mollusc Acaenoplax hayae from the Herefordshire Lagerstätte (Silurian England), and implications for molluscan phylogeny. Palaeontology. 2004;47:293–318. doi:10.1111/j.0031-0239.2004.00374.x [Google Scholar]
- Swofford, D. W. 2002 PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland, MA: Sinauer Associates.
- Vendrasco M.J, Runnegar B.N. Late Cambrian and Early Ordovician stem group chitons (Mollusca: Polyplacophora) from Utah and Missouri. J. Paleontol. 2004;78:675–689. doi:10.1666/0022-3360(2004)078<0675:LCAEOS>2.0.CO;2 [Google Scholar]
- Vendrasco M.J, Wood T.E, Runnegar B.N. Articulated Palaeozoic fossil with 17 plates greatly expands disparity of early chitons. Nature. 2004;429:288–291. doi: 10.1038/nature02548. doi:10.1038/nature02548 [DOI] [PubMed] [Google Scholar]
- Vinther J, Nielsen C. The Early Cambrian Halkieria is a mollusc. Zool. Scripta. 2005;34:81–89. doi:10.1111/j.1463-6409.2005.00177.x [Google Scholar]
- Wills M. A phylogeny of recent and fossil Crustacea derived from morphological characters. In: Fortey R.A, Thomas R.H, editors. Arthropod relationships. Chapman & Hall; London, UK: 1997. pp. 189–209. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables, appendix, figure and references