Abstract
Morphological and biometrical analyses of the partial hand IPS18800 of the fossil great ape Hispanopithecus laietanus (=Dryopithecus laietanus), from the Late Miocene (about 9.5 Ma) of Can Llobateres (Catalonia, Spain), reveal many similarities with extant orang-utans (Pongo). These similarities are interpreted as adaptations to below-branch suspensory behaviours, including arm-swinging and clambering/postural feeding on slender arboreal supports, due to an orang-like double-locking mechanism. This is confirmed by the long and highly curved phalanges of Hispanopithecus. The short and stout metacarpals with dorsally constricted heads, together with the dorsally extended articular facets on proximal phalanges, indicate the persistence of significant degrees of palmigrady. A powerful grasping capability is indicated by the great development of basal phalangeal tubercles, the marked insertions for the flexors on phalangeal shafts and the large pits for the collateral ligaments. The morphology of the Hispanopithecus long bones of the hand indicates a unique positional repertoire, combining orthogrady with suspensory behaviours and palmigrade quadrupedalism. The retention of powerful grasping and palmigrady suggests that the last common ancestor of hominids might have been more primitive than what can be inferred on the basis of extant taxa, suggesting that pronograde behaviours are compatible with an orthograde bodyplan suitable for climbing and suspension.
Keywords: Hominoidea, fossil primates, palaeobiology, hand, locomotion, evolution
1. Introduction
Ape hands reflect a compromise between manipulative and locomotor selection pressures, albeit more closely resembling the ‘true hands’ of humans than the ‘foot-hands’ of other primates (Napier 1993). Besides differences in carpal, metacarpal and phalangeal morphology, the hands of extant apes and humans significantly differ in proportions. Human hands are shorter and display relatively longer thumbs, presumably due to the removal of locomotor selection pressures with the advent of bipedalism (Alba et al. 2003). The morphology and proportions of the fossil hand bones from the stem ape Proconsul (Napier & Davis 1959; Begun et al. 1994), interpreted as a palmigrade quadruped with powerful grasping abilities (Ward 1993), indicate that a short hand with a relatively long thumb is the primitive condition from which the elongated hands of the orthograde living apes must have evolved. Postcranial remains of Pierolapithecus are indicative of an orthograde bodyplan in spite of the lack of suspensory adaptations (Moyà-Solà et al. 2004, 2005). This taxon, interpreted as a stem great ape on the basis of cranial anatomy (it combines a derived great ape facial pattern with a primitive, more prognathic profile with low and posterior glabella; Moyà-Solà et al. 2004), therefore indicates that a combination of orthograde climbing and pronograde palmigrady is likely to be ancestral for hominoids as a whole. While this suggests that suspensory adaptations are homoplastic between hylobatids and hominids (Moyà-Solà et al. 2004, 2005), the question remains as to whether these derived hominid features are truly homologous between orang-utans and African apes (having been inherited from a common great ape ancestor), or instead they are homoplastic (having independently arisen in each of these groups).
The postcranial remains of the fossil great ape Hispanopithecus laietanus Villalta & Crusafont 1944 (Primates, Hominidae) from Can Llobateres (Late Miocene, MN10, about 9.5 Ma; Moyà-Solà & Köhler 1996; Köhler et al. 2001), formerly included within the genus Dryopithecus, allow us to test the hypothesis of whether suspensory adaptations are homologous between African great apes and orang-utans. Hispanopithecus, variously interpreted as an early pongine (Moyà-Solà & Köhler 1993, 1995; Köhler et al. 2001) or hominine (Begun et al. 1997), represents the first simultaneous evidence of an orthograde bodyplan combined with suspensory adaptations in the hominoid fossil record. Here we focus on morphological and morphometric analyses of the H. laietanus partial hand from Can Llobateres, which includes several complete and partial long bones (metacarpals and phalanges), in order to infer the positional repertoire of this taxon. This is the first time that Hispanopithecus (or Dryopithecus s.l.) metacarpals and complete proximal phalanges are described in detail and interpreted from a functional and evolutionary viewpoint.
2. Materials and methods
(a) Measurements and comparative sample
Maximum length and transverse (mediolateral and dorsopalmar) diameters of both metacarpals and phalanges (proximal and intermediate or middle) were measured at the base, midshaft and head/trochlea to the nearest 0.1 mm in both Hispanopithecus and the extant comparative sample. A detailed explanation of these measurements, previously employed by other investigators (e.g. Inouye 1992), is shown in the electronic supplementary material, figure 10. Curvature (in degrees) was computed using the included angle method (Jungers et al. 1997). Adult body mass (in kg) for individual specimens, taken from museum records, was also employed for the comparative sample. Measurements and estimates from the H. laietanus long bones of the hand were made by the senior author (S.A.), whereas most of the measurements from the extant comparative sample were taken by D.M.A. or kindly provided by Esteban Sarmiento. This sample included individuals from the following genera: Pan (both Pan paniscus and Pan troglodytes); Gorilla (both Gorilla gorilla and Gorilla beringei); Pongo (including the two extant subspecies); Homo (i.e. Homo sapiens); and Papio (Papio cynocephalus).
(b) Statistical techniques
When comparing the relationship between two given metrical variables (y and x) across a broad sample of taxa differing in size, size-scaling effects must be taken into account by computing variables of relative size. In many instances, simple shape ratios (y/x) or bivariate comparisons (y versus x) do not adequately reflect relative size, because in many instances different variables tend to vary allometrically (according to a nonlinear relationship) instead of isometrically (in a directly proportional way). Allometric variation can be depicted using the so-called allometric equation y=bxk (Gould 1966; Klingenberg 1998), which is often logarithmically transformed (ln y=ln b+k ln x), in order to linearize the relationship between the two variables being compared. In this paper, allometric regressions, computed by means of linear regression with ln-transformed data, were employed for removing size-scaling effects when comparing different taxa. The term ‘allometric regression’ is hence employed throughout the paper to refer to ‘linear regression on the basis of ln-transformed data’. Natural logarithms (ln) were used for transforming the raw measurements, whereas least-squares linear regression was employed as the line-fitting method. Static, mixed-sex adult allometry was employed in all instances for each living genus separately.
Manual proportions and robusticity of the long bones of the hand were assessed by means of bivariate allometric comparisons and multivariate discriminant (canonical) analyses. Multivariate analyses included measurements from all the manual rays for which Hispanopithecus measurements or reliable estimations are available (table 1) except for distal phalanges (which are of uncertain attribution). Bivariate comparisons were restricted to manual ray IV, although similar results would be obtained for other rays. Intrinsic manual ray proportions were assessed by means of an allometric regression of phalangeal length (proximal+intermediate phalanges) versus metacarpal length. Robusticity of metacarpal and proximal phalanx was evaluated by means of separate allometric regressions of base and head/trochlea area (computed as the product between the mediolateral width and the dorsopalmar height) versus bone length. Finally, relative lengths of metacarpal and proximal phalanx were calculated by means of separate allometric regressions of bone length versus body mass.
Table 1.
Measurements of the several long bones of the hand (metacarpals and phalanges) of the H. laietanus IPS18800 partial hand from Can Llobateres. (All measurements are given in millimetres (mm). Values between parentheses are estimates. Abbreviations: MC, metacarpal; PP, proximal phalanx; MP, intermediate phalanx; L, length; B, base; MS, midshaft; H, head (in metacarpals)/trochlea (in phalanges); ML, mediolateral breadth; DP, dorsopalmar height.)
| MC2 | MC3a | MC4 | MC5a | PP2b | PP3 | PP4 | PP5c | MP2 | MP3d | MP4e | MP5 | DP2 | DP4 | DP5f | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | 75.2 | (72.7) | 65.5 | — | (53.2) | 59.6 | 62.7 | (49.6) | 31.4 | 40.0 | (41.3) | 33.6 | 16.0 | 19.1 | 16.6 |
| BML | 12.6 | 11.4 | 10.8 | 11.0 | (13.0) | 13.3 | 14.1 | 12.4 | 11.3 | 14.3 | 13.1 | 12.1 | 8.4 | 9.3 | 8.1 |
| BDP | 14.9 | 13.4 | 13.0 | 11.0 | (10.1) | 11.9 | 12.0 | 10.3 | 8.3 | 9.3 | 10.9 | 9.0 | 6.1 | 6.6 | (5.9) |
| MSML | 7.3 | (8.6) | 7.8 | (7.4) | (11.0) | 11.7 | 11.5 | (10.4) | 8.5 | 9.6 | 9.7 | 9.4 | 3.5 | 4.5 | 3.6 |
| MSDP | 6.6 | (8.5) | (7.5) | (6.5) | (6.3) | 7.3 | 7.3 | (5.9) | 4.8 | 5.7 | 7.0 | 5.3 | 3.8 | 3.9 | 3.5 |
| HML | 12.5 | — | 12.1 | — | 9.0 | 9.6 | 9.8 | — | 7.8 | (9.3) | 9.2 | 8.4 | 4.1 | 5.5 | 4.6 |
| HDP | 13.5 | — | 15.3 | — | 7.6 | 7.8 | 8.2 | — | 5.7 | 6.3 | 6.0 | 5.5 | 3.7 | 3.8 | 3.5 |
Metacarpal head lacking.
Lacking a small fraction of the proximal articular facet.
Trochlear area missing.
Lacking most of the ulnar part of the trochlea.
Some fragments of the central diaphysis missing.
Lacking a fragment of the base at the palmar side.
With regard to multivariate analyses, manual overall proportions were assessed by means of a discriminant analysis of bone lengths, whereas metacarpal and phalangeal robusticity was evaluated on the basis of two different analyses including bone length as well as transverse diameters. Hispanopithecus was classified on the basis of Mahalanobis distances to group centroids, whereas cluster diagrams were plotted using Euclidean distances on the basis of group centroids and the Hispanopithecus discriminant scores for all four available discriminant functions. Statistical computations were made by means of the statistical package SPSS v. 14.0, and graphics were plotted using Excel 2000 and PAST v. 1.54.
3. Results
(a) Reconstruction and morphological description
The reconstruction of the partial hand IPS18800 from Can Llobateres 2 is shown in figure 1 (see table 1 for measurements), except for five associated sesamoids (not shown). The morphology of the fourth proximal phalanx, as compared to Sivapithecus and Pongo, among other taxa, is further depicted in greater detail in figure 2, whereas the morphology of the fourth metacarpal is shown in figure 3. All the manual remains of IPS18800 were found associated, but non-articulated, with one another in a very restricted space of approximately 0.02 m2, with no repeated elements, so that we can be confident that the remains belong to the right hand of a single individual. There is more scatter with regard to the cranium IPS18000 and the other postcranial bones of IPS18800 due to carnivore activity. However, given the lack of repeated postcranial elements, it is reasonable to assume that all these remains belong to the same adult male individual.
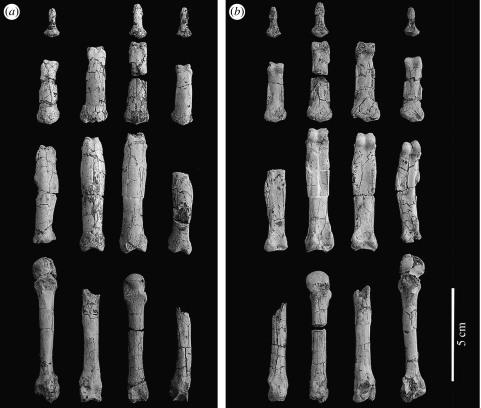
Figure 1.
Reconstruction of the H. laietanus partial hand IPS18800 from Can Llobateres 2, (a) in dorsal and (b) palmar view.
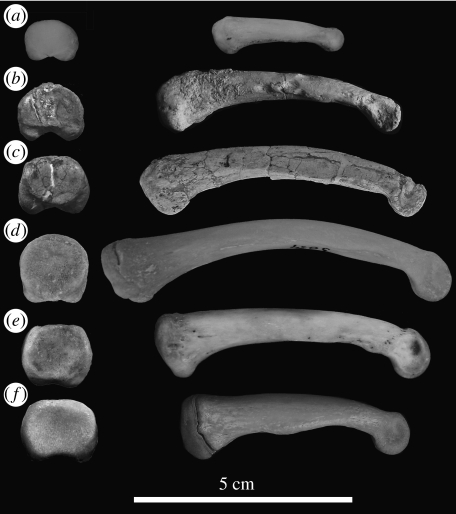
Figure 2.
Proximal and ulnar views of the fourth proximal phalanx in selected fossil and extant taxa: (a) Papio sp., (b) Sivapithecus, (c) Hispanopithecus, (d) Pongo, (e) Pan and (f) Gorilla. All the depicted specimens come from the right side (the Sivapithecus one being a mirror image of a cast from the left side).
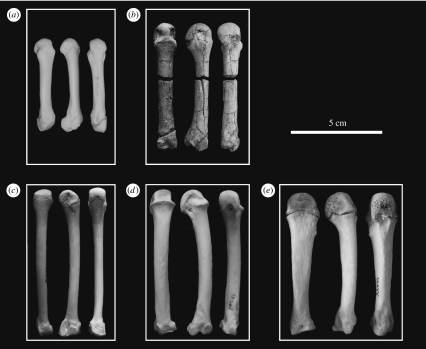
Figure 3.
Dorsal, radial and palmar views of the fourth metacarpal in selected fossil and extant taxa: (a) Papio, (b) Hispanopithecus, (c) Pongo, (d) Pan and (e) Gorilla.
In this partial hand, all manual digits except the first one are more or less completely preserved. The fourth metacarpal is broken into two fragments (figures 1 and 3), which cannot be glued together due to sediment infilling and minimal distortion. However, no fragment of diaphysis is lacking, so that metacarpal length can be readily measured. The reconstruction employed here differs from the previous one (Moyà-Solà & Köhler 1996; Moyà-Solà et al. 1999) by discarding putative thumb fragments and by the new ray assignment of the third and fourth proximal phalanges. In the new reconstruction, the fourth proximal phalanx is longer than the third one, as is common among extant orang-utans (especially males; Susman 1979). As noted by the latter author, ‘when proximal phalanx IV exceeds III in length, the former bone is also more asymmetrical. In this case the third phalanx takes on a pattern normally seen in II’, although ‘the overall robusticity of proximal phalanx III is still greater than that of IV’ (Susman 1979, p. 225). Accordingly, the manual ray attributions of the third and fourth proximal phalanges employed here are justified by several lines of evidence, including: (i) the congruence between articular facets of fourth metacarpal and fourth proximal phalanx, (ii) the greater shaft robusticity of third versus fourth proximal phalanx (143 versus 133%), according to Susman's criterion (Susman 1979, footnote 2), and (iii) the greater development of the radial side of the base in the third proximal phalanx (where the second dorsal interosseous insert) and the ulnarly favoured basal asymmetry in the fourth one (displaying a more protruding ulnar tubercle for insertion of the fourth interosseous; figures 1, 2 and 4). In spite of these compelling anatomical arguments, in order to avoid any potential bias due to manual ray assignment of proximal phalanges III and IV, statistical comparisons were carried out by taking into account the two possible assignments.
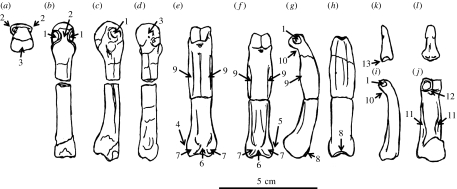
Figure 4.
Line drawing of selected bones from the H. laietanus partial hand IPS18800 from Can Llobateres, showing the main anatomical features discussed in the text: (a) fourth metacarpal in distal, (b) dorsal, (c) radial and (d) palmar views, (e) fourth proximal phalanx in palmar view, (f) third proximal phalanx in palmar, (g) radial and (h) dorsal views, third intermediate phalanx in (i) radial and (j) palmar views, possible fourth distal phalanx in (k) radial and (l) palmar views. Characters: 1, enlarged pits for the collateral ligaments; 2, marked dorsal constriction of metacarpal heads; 3, smooth palmar surface of metacarpal heads; 4, enlarged ulnar tubercle; 5, protruding radial tubercle; 6, deep groove for channelling the long flexor tendons; 7, well-developed palmar tubercles; 8, dorsal extension of the proximal articular surface; 9, strong and distally positioned flexor sheath ridges; 10, palmarly bent trochlea; 11, well-developed insertions for the superficial flexors; 12, conspicuous muscular insertions at the palmar side only; 13, larger palmar lips.
The functionally most relevant morphological features of the Hispanopithecus partial hand are described below. The metacarpals are short and stout (figures 1 and 3), with subcircular (nearly circular) diaphyseal cross-sections and without strong muscular impressions. The pits for the attachment of the collateral ligaments are well developed and very dorsally placed, causing a marked dorsal constriction of the metacarpal head unlike that of any extant great ape (Lewis 1977; Susman 1979). In contrast, palmarly, the metacarpal heads do resemble extant great apes in being smooth (non-fluted), i.e. with no trace of palmar grooves (Lewis 1977). Unlike the metacarpals, the proximal phalanges are long, slender and very curved (figures 1 and 2), with an average included angle of 73° (69° the second, 78° the third and 71° the fourth), which is higher than in Hylobates and Ateles (approx. 50–60°), but fully comparable to Pongo (approx. 75°; Jungers et al. 1997; Richmond & Whalen 2001). Moreover, articular surfaces on the bases of the proximal phalanges are laterally restricted and almost circular (figure 2), more closely resembling those of orang-utans, albeit extending slightly at the midline onto the dorsal aspect of the shaft (figures 2 and 4). In contrast, these articular facets are laterally and palmarly surrounded by well-defined ridges (figure 2), with expanded areas for the insertion of the collateral ligaments and interossei muscles. The proximal phalanges further display strongly developed palmar tubercles, and distally positioned, strong flexor sheath ridges that are laterally and even palmarly expanded (causing a palmar concavity; figures 1 and 4). Distally, articular surfaces of proximal phalanges are relatively small, albeit with well-developed pits for the collateral ligaments of the proximal interphalangeal joint (figures 2 and 4). The trochleae are high and palmarly bent (figures 2 and 4), with a deep and narrow trochlear groove. Intermediate phalanges also display strong muscular impressions, with deep insertions for the superficial flexors (producing developed ridges at the proximal half of the shaft), and laterally and palmarly voluminous areas for the attachment of collateral ligaments and other interphalangeal joint structures (figure 4). Trochleae, on the other side, are relatively small, with conspicuous muscular insertions at the distal part of the shaft on the palmar side only (figure 4). The curvature of the intermediate phalanges is particularly marked at the distal shaft, causing a palmarly bent trochlea (figure 4). Finally, distal phalanges are different from those of extant hominoids by several features, including larger palmar lips as compared with dorsal ones (figure 4), so that the articular surface does not face palmarly as in living forms (Begun et al. 1994).
(b) Morphometric comparisons
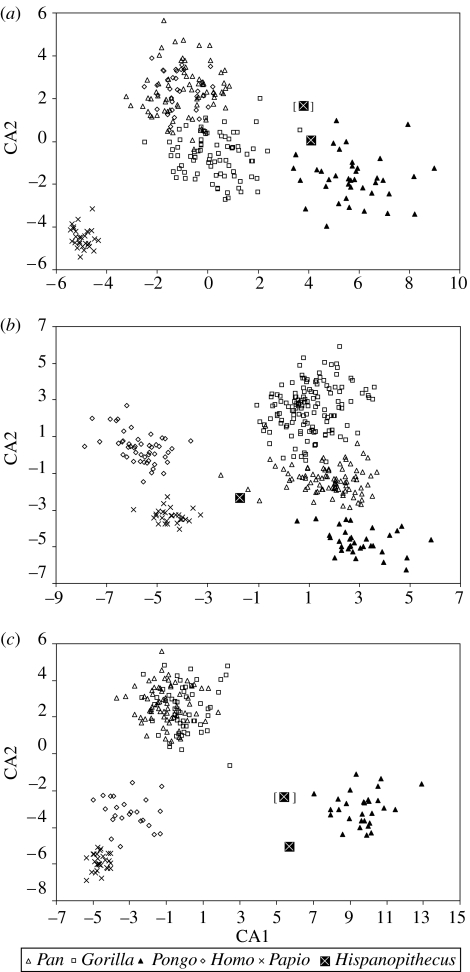
Descriptive statistics for the extant comparative sample are reported in the electronic supplementary material, table 3. Three different discriminant (canonical) analyses were performed with extant hominid genera and baboons (figures 5 and 6; see also electronic supplementary material, tables 2 and 4), in order to evaluate, on the basis of the long bones of the hand, overall manual proportions (only length measurements included), as well as hand robusticity (separately for metacarpals and phalanges, and including both length and transverse diameters). These analyses discriminate very well among the extant genera (98% of original cases correctly classified). On the basis of overall manual proportions, Hispanopithecus is classified as an orang-utan, irrespective of ray assignment of the third and fourth proximal phalanges. When metacarpal robusticity is taken into account, Hispanopithecus fails to cluster with orang-utans, showing instead a greater similarity with baboons. In contrast, as far as phalangeal robusticity is concerned, Hispanopithecus is classified again with orang-utans. This holds even when the third and fourth proximal phalanges are taken for one another, and indicates that, in spite of overall manual proportions resembling orang-utans, the long bones of the hand of Hispanopithecus are not completely orang-like. Its greatest similarities with Pongo lie in the phalangeal region, whereas the metacarpals still retain primitive proportions.
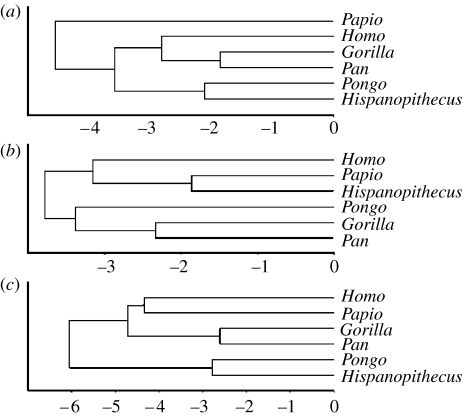
Figure 5.
Results of the three discriminant analyses, displayed by means of UPGMA (Unweighted Pair Group Method with Arithmetic Mean) clusters based on Euclidean distances computed from group centroids (extant genera) and discriminant scores (Hispanopithecus) for the four canonical axes (see electronic supplementary material, table 2): (a) overall manual proportions, (b) metacarpal robusticity and (c) phalangeal robusticity.
Figure 6.
Bivariate plot of second versus first canonical axes (discriminant functions) for the three discriminant analyses: (a) overall manual proportions, (b) metacarpal robusticity and (c) phalangeal robusticity. The results for Hispanopithecus by interchanging third and fourth proximal phalanges are displayed within brackets.
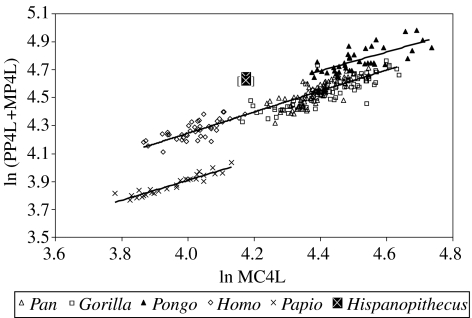
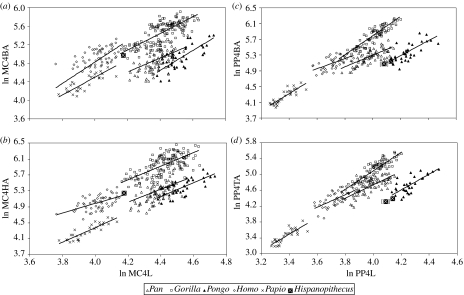
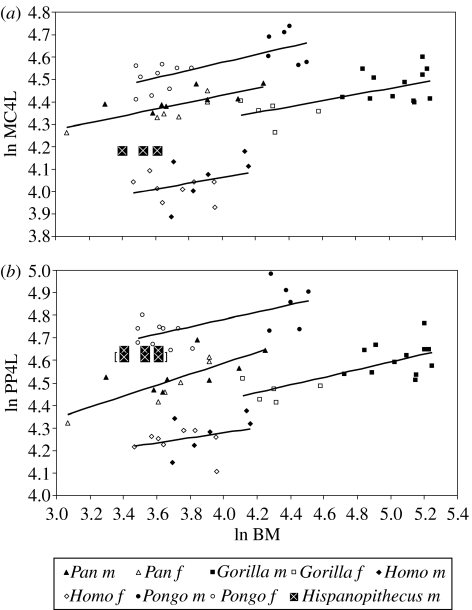
With regard to allometric bivariate comparisons (regressions reported in the electronic supplementary material, table 5), when the intrinsic manual ray proportions are taken into account (figure 7), baboons display shorter phalanges relative to metacarpals than hominids, but orang-utans differ from African apes (and humans) by displaying even longer phalanges, not only on absolute terms, but also at equal metacarpal lengths. Hispanopithecus clearly departs from the hominine regression, but displays a position that would be expected for an orang-utan with absolutely shorter metacarpals, irrespective of the attribution of the third and fourth proximal phalanges. This indicates that the former taxon also displays long phalanges relative to the metacarpals. With regard to metacarpal and phalangeal robusticity (figure 8), gorillas are always the most robust great apes and orang-utans the least. Interestingly, Hispanopithecus displays a different pattern for metacarpals and phalanges, with the former being considerably robust (similar to African apes), but phalanges being comparatively very slender (fully comparable to those of orang-utans, which holds irrespective of manual ray attribution of the third and fourth proximal phalanges). Finally, when bone length relative to body mass is evaluated (figure 9), the same patterns are obtained for metacarpals and phalanges: humans display the shortest manual rays relatively and orang-utans the longest, with African apes displaying intermediate values. Relative metacarpal and phalangeal length in Hispanopithecus can be evaluated by taking into account published estimates of body mass in this taxon (Moyà-Solà & Köhler 1996, their table 1). Four different estimates, ranging from 30 to 37 kg (mean value, 34 kg) were derived by Moyà-Solà & Köhler (1996) on the basis of postcranial measurements of the femur, tibia and lumbar vertebrae, which are directly related to weight-bearing and therefore display a high correlation with body mass. Interestingly, Hispanopithecus displays a remarkably different pattern in metacarpals as compared with phalanges; whereas relative metacarpal length is very low (lower than in all extant great apes, gorillas included), proximal phalanx length relative to body mass is very high (almost in the range of female orang-utans in both relative and absolute grounds). These results do not significantly vary when uncertainty in body mass estimation is taken into account (figure 9), and hold irrespective of manual ray assignment of the third and fourth proximal phalanges.
Figure 7.
Intrinsic manual ray IV proportions displayed as an allometric bivariate plot of phalangeal versus metacarpal length in hominines, orang-utans and baboons. The results for Hispanopithecus by interchanging third and fourth proximal phalanges are displayed within brackets. Abbreviations: MC, metacarpal; PP, proximal phalanx; MP, intermediate phalanx; L, length.
Figure 8.
Robusticity of metacarpal and proximal phalanx IV displayed as allometric bivariate plots of articular area versus bone length in extant hominids and baboons: (a) robusticity of metacarpal base, (b) robusticity of metacarpal head, (c) robusticity of proximal phalanx base and (d) robusticity of proximal phalanx trochlea. The results for Hispanopithecus by interchanging third and fourth proximal phalanges are displayed within brackets. Abbreviations: MC, metacarpal; PP, proximal phalanx; B, base; H, head; T, trochlea; L, length; A, area.
Figure 9.
Relative metacarpal and phalangeal length in manual ray IV displayed as allometric bivariate plots of bone length versus body mass in extant hominids and baboons: (a) relative metacarpal length and (b) relative proximal phalanx length. The several points corresponding to Hispanopithecus do not represent different individuals, but different size estimates. The results for Hispanopithecus by interchanging third and fourth proximal phalanges are displayed within brackets. Black symbols, males; open symbols, females. Abbreviations: MC, metacarpal; PP, proximal phalanx; BM, body mass.
4. Discussion
(a) Functional interpretation
Several morphological features of the Hispanopithecus long bones of the hand indicate powerful grasping capabilities. Thus, the strong flexor sheath ridges along the shafts of proximal and intermediate phalanges result from the presence of powerful flexors, which is further confirmed by the presence of strongly developed tubercles at the base of proximal phalanges. These tubercles would have reinforced the deep groove of the fibrocartilaginous glenoid plate that channels the long flexor tendons along the base of the proximal phalanges, in their course from the metacarpophalangeal joint to the flexor sheaths (Susman 1979), thus preventing them from dislocation (Rose 1986; Nakatsukasa et al. 2003). This contrasts with the smooth palmar surface of the metacarpal heads, which indicates that, if present at all, periarticular sesamoids would have only minimally contributed to the thickening of the lateral margins of the glenoid plate for providing additional channelling of the powerful flexors during extreme extension (Lewis 1977). This permits us to infer that dorsiflexed postures at the metacarpophalangeal joint would not have been so hyperextended as in committed palmigrade and digitigrade terrestrial forms, where large sesamoids are regularly present, in association with a well-developed fluting of the metacarpal heads. In contrast to the smooth palmar surface, the metacarpal heads of Hispanopithecus display a marked dorsal constriction. A similar constriction is present, albeit to a lesser extent, in Nacholapithecus, Proconsul and some cebids such as Alouatta (Rose et al. 1996), in which the general morphology of the metacarpal head has been related to ‘grasping hand use during predominantly pronograde quadrupedal activities’ (Rose et al. 1996, p. 10). The latter authors interpret the dorsal origin of the collateral ligaments as permitting an increased range of abduction–adduction and/or axial rotation at the metacarpophalangeal joint, albeit without compromising stability by becoming taut at flexed postures. The pronounced dorsal constriction of the Hispanopithecus metacarpal heads, however, probably results, at least in part, from the great development of the pits for insertion of the collateral ligaments of the metacarpophalangeal joint. According to Begun et al. (1994), well-developed insertion areas for the collateral ligaments at the metacarpophalangeal and interphalangeal joints would reflect the great significance of transversely oriented (mediolateral) bending stresses. These stresses might have been huge in a form such as Hispanopithecus, with relatively short metacarpals but very long phalanges, due to the unique combination of palmigrady and suspension (see later). Moreover, since the collateral ligaments of the metacarpophalangeal joints become taut at flexed postures, the large and dorsally positioned pits for their insertion at the metacarpal heads would have ensured enhanced resistance against lateral stresses during climbing and/or suspension, further providing a secure and powerful grasp during palmigrade quadrupedalism.
The significance of palmigrady in Hispanopithecus is confirmed by the dorsal extension of the proximal articular facets of the proximal phalanges and by the length and robusticity of the metacarpals, which unlike phalanges are quite robust and relatively shorter than in extant great apes. The robusticity of metacarpals depends to a large extent on their length relative to body mass, those of orang-utans being the longest and the least robust, since they do not habitually support weight-bearing compressive stresses. On the contrary, the short and stout metacarpals of Hispanopithecus are indicative of palmigrade quadrupedalism, most closely resembling those of stem hominoids such as Proconsul. The proximal articular facets on the proximal phalanges of Hispanopithecus, albeit somewhat elliptical (broader than higher), display a more circular contour than those of middle-sized Sivapithecus (Rose 1986), thus more closely resembling orang-utans (figure 2). The relatively wider proximal articular facet, together with its greater dorsal extension, suggests a higher significance of palmigrady at the expense of suspensory behaviours in Sivapithecus. In the cluster analysis of overall manual proportions on the basis of discriminant functions, Hispanopithecus clusters with orang-utans, and these two taxa further resemble each other by the great degree of phalangeal elongation relative to metacarpals. When body mass is taken into account, Hispanopithecus only resembles orang-utans by the relative length of the phalanges, whereas the metacarpals are remarkably short, which explains why the length of phalanges relative to metacarpals is even greater in Hispanopithecus. This further explains the different patterns of metacarpal and phalangeal robusticity displayed by Hispanopithecus in both multivariate and bivariate comparisons. The pronounced orang-like slenderness of the Hispanopithecus phalanges is thus attributable to the high degree of phalangeal elongation, most similar to that of orang-utans when allometric comparisons are taken into account. This must be interpreted as a feature functionally related to orthograde suspensory behaviours such as arm-hanging and swinging, which can be also inferred from the palmar concavity on the shafts and tall trochleae of proximal phalanges (Begun 1993).
Suspension is further indicated by a degree of phalangeal curvature that is higher than in Sivapithecus (approx. 50°; Richmond & Whalen 2001), but fully comparable to extant orang-utans. If our ray attribution of the phalanges is correct, Hispanopithecus also displays a fourth proximal phalanx longer than the third one, indicating an orang-like ulnar shift of the main axis of the hand, which has been related to grasping vertical supports during climbing (Susman 1979), and might merely reflect a high commitment to arboreality in this relatively large-bodied taxon. Strong muscular insertions on the intermediate phalanges just proximal to the trochlea are restricted to the palmar side of the shaft, unlike in Nacholapithecus, where both palmar and dorsal insertions are present (Nakatsukasa et al. 2003), suggesting a higher emphasis on flexion over extension in Hispanopithecus. This, combined with the considerable length and curvature of the phalanges, and the relatively small but palmarly bent trochleae of the intermediate phalanges, suggests that this taxon would have displayed an orang-like ‘double-locking mechanism’. When the hand is held in a double-locked position in orang-utans, the tips of the fingers are tucked into the skin-fold present where the finger meets the palm, so that with further flexion, the locked fingers are rolling into the palm, thus permitting to securely grasp slender vertical supports (Napier 1993, p. 27 and his figs. 10 and 11; see also Napier 1960, p. 651 and his fig. 3b and his fig. 3 in plate I).
(b) Palaeobiological reconstruction and evolutionary inferences
The partial hand of H. laietanus displays a combination of two sets of features that cannot be found in any living or known fossil catarrhine: (i) the short and stout metacarpals, with a generalized catarrhine morphology, which are indicative of palmigrady on horizontal supports and (ii) the elongated, curved and slender phalanges, which together with other characters indicate suspensory behaviours. Our results hence allow us to infer that Hispanopithecus displayed a unique locomotor repertoire, combining vertical climbing with both suspensory orthograde behaviours and some kind of pronograde palmigrady with powerful grasping capabilities. Orang-utans employ an enormous diversity of different positional modes: vertical climbing and suspensory orthograde behaviours are most frequent, but they also occasionally use above-branch quadrupedalism (as do Pan spp.) and, uniquely among extant apes, pronograde suspensory positional behaviour (in particular, torso-pronograde suspensory locomotion; Thorpe & Crompton 2006; see their appendices A and B for further details on pronograde suspensory behaviours in orang-utans). Hispanopithecus would presumably have displayed a similarly diverse positional repertoire, already employing suspensory behaviours (such as clambering) for travelling and feeding on slender branches, but with a much greater emphasis on above-branch pronograde quadrupedalism (at the expense of arm-swinging) when moving through horizontal or slightly inclined arboreal supports. Powerful grasping palmigrady would have been employed in the case of moderately large branches, whereas a more standard palmigrady (albeit with markedly ulnarly deviated hand postures) would have been performed on larger ones.
While the elongated and slender phalanges of Hispanopithecus are best interpreted as derived characters, the short and stout metacarpals are most probably a primitive retention. A priori, phalangeal lengthening seems most important for suspensory behaviours (being essential for the hook grasp and the double-locking mechanism), but metacarpal lengthening would be further advantageous. This indicates that the retention of short and stout metacarpals in Hispanopithecus must be also functionally interpreted, resulting from the action of some additional selection pressure, related to palmigrady, that would be acting against the lengthening of the palm. The hand anatomy of Hispanopithecus thus reflects a functional compromise between the biomechanical demands of suspensory and quadrupedal behaviours, which are the two dominant locomotor modes that can be inferred for this taxon.
Whether the reported phalangeal similarities to orang-utans are synapomorphic or homoplastic is difficult to determine, given current uncertainties on the phylogenetic position of Hispanopithecus and the scarcity of fossil hominoid postcranial remains. Several workers have previously stressed the preponderant role of homoplasy in hominoid evolution (Larson 1998; Young 2003), with most similarities found between the brachiating atelines and hylobatids with the exclusion of great apes (Young 2003). The documented manual similarities of Hispanopithecus with extant orang-utans, even including an ulnar shift of the main axis of the hand, support previous proposals that this taxon is an early member of the Pongo clade (Moyà-Solà & Köhler 1993, 1995, 1996; Köhler et al. 2001). However, even if Hispanopithecus is alternatively interpreted as an early hominine (Begun et al. 1997), its hand morphology indicates that the last common ancestor of living great apes and humans must have been more primitive than inferred on the basis of extant taxa, by retaining palmigrade adaptations subsequently lost in several subclades independently. Thus, while the short manual rays of the stem hominid Pierolapithecus confirm that the long-handed pattern of hylobatids and living great apes is the homoplastic result of parallel evolution from orthograde but non-suspensory ancestors (Moyà-Solà et al. 2004, 2005), Hispanopithecus further suggests that suspensory adaptations, to some degree, also evolved independently between pongines and hominines.
The uniqueness of the Hispanopithecus locomotor repertoire further suggests that the evolution of positional behaviours in hominoids has occurred in a mosaic fashion. Proconsul would illustrate the initial stage, characterized by tail loss (Nakatsukasa et al. 2004), which probably occurred once grasping was sufficiently powerful to entirely support the balancing function. Pierolapithecus documents a more derived pattern, in which ulnocarpal articulation had been lost as an adaptation to vertical climbing (Moyà-Solà et al. 2004) in spite of the retention of palmigrady. Hispanopithecus reflects an even more derived pattern, in which palmigrade behaviours remain significant, but suspensory adaptations have been already selected. The adaptive reasons underlying the evolution of suspensory behaviours in hominoids have been related to their large body size, permitting them to have access to food resources located in the periphery of the canopy (Hunt 1992). Powerful grasping palmigrady would have permitted a large-bodied tailless primate such as Proconsul to maintain balance during above-branch quadrupedalism (Kelley 1997), but not to exploit the feeding niche that includes the slenderest branches at the crown's periphery, due to its relatively large body mass. Among extant great apes, the almost strictly arboreal orang-utans have circumvented these limitations by more heavily relying on below-branch arm-hanging and swinging, whereas chimpanzees and gorillas have become secondarily adapted to terrestrial travel between feeding sites (although they still employ suspensory behaviours when on the trees). These considerations, together with the mosaic nature of the Hispanopithecus locomotor repertoire, suggest that suspensory behaviours progressively replaced palmigrady during great ape evolution, until the latter behaviour was definitely abandoned as a significant component of their positional behaviour in the ancestors of the surviving lineages. Anatomically, this abandonment would have implied the selection for longer palms relative to body mass (with a concomitant reduction of thumb length relative to the rest of the hand), due to suspensory-related selection pressures.
Recognizing the mosaic nature of locomotor evolution in hominoids further provides new insights into the interpretation of other fossil great apes such as Sivapithecus. Reported pronograde features in the postcranial skeleton of this taxon (Richmond & Whalen 2001) have proved difficult to reconcile with its orang-like cranial morphology, leading to the so-called ‘Sivapithecus dilemma’ (Pilbeam & Young 2001). Available postcranial evidence for the latter taxon suggest a combination of palmigrady and powerful grasping with some amount of vertical climbing and minimal orthograde clambering (Madar et al. 2002). The morphology of the proximal phalanges and the metacarpophalangeal joint certainly indicate a higher emphasis of suspensory behaviours in Hispanopithecus than in Sivapithecus. In addition, the morphology of several other anatomical regions in Hispanopithecus, including lumbar vertebrae and femur (Moyà-Solà & Köhler 1996; Köhler et al. 2001), indicates that palmigrade adaptations do not necessarily preclude the existence of an orthograde bodyplan with suspensory adaptations, by implication also in other Miocene hominoids, even though this combination is unknown among extant members of this group.
Acknowledgments
We are indebted to the several curators who have granted us access to extant skeletal material (W. van Neer, B. Engesser and A. Malgosa) and to E. Sarmiento for kindly making available to us his database of hand measurements. We thank D. Pilbeam and two anonymous reviewers for making helpful comments and suggestions on a previous version of the manuscript. This paper has been possible thanks to the support of the Generalitat de Catalunya (predoctoral fellowship 2006 FI 00065 to S.A., postdoctoral grant 2005 BP-B1 10253 to D.M.A., and SOMHI project) and National Science Foundation (project RHOI-Hominid-NSF-BCS-0321893).
Supplementary Material
Descriptive statistics of the several long bones of the hand in the extant comparative primate sample
Numerical results of the multivariate analyses
Allometric regressions
Schematic depiction of right fourth manual ray of Homo sapiens in palmar and lateral views, indicating the points employed for taking the measurements
Main results from the three discriminant analyses, including centroids for extant taxa and discriminant scores for Hispanopithecus
References
- Alba D.M, Moyà-Solà S, Köhler M. Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. J. Hum. Evol. 2003;44:225–254. doi: 10.1016/s0047-2484(02)00207-5. doi:10.1016/S0047-2484(02)00207-5 [DOI] [PubMed] [Google Scholar]
- Begun D.R. New catarrhine phalanges from Rudabánya (Northeastern Hungary) and the problem of parallelism and convergence in hominoid postcranial morphology. J. Hum. Evol. 1993;24:373–402. doi:10.1006/jhev.1993.1028 [Google Scholar]
- Begun D.R, Teaford M.F, Walker A.C. Comparative and functional anatomy of Proconsul phalanges from the Kaswanga Primate Site, Rusinga Island, Kenya. J. Hum. Evol. 1994;26:89–165. doi:10.1006/jhev.1994.1008 [Google Scholar]
- Begun D.R, Ward C.V, Rose M.D. Events in hominoid evolution. In: Begun D.R, Ward C.V, Rose M.D, editors. Function, phylogeny and fossils: miocene hominoid evolution and adaptation. Plenum Press; New York, NY: 1997. pp. 389–415. [Google Scholar]
- Gould S.J. Allometry and size in ontogeny and phylogeny. Biol. Rev. 1966;41:587–640. doi: 10.1111/j.1469-185x.1966.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Hunt K.D. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am. J. Phys. Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. doi:10.1002/ajpa.1330870108 [DOI] [PubMed] [Google Scholar]
- Inouye S.E. Ontogeny and allometry of African ape manual rays. J. Hum. Evol. 1992;23:107–138. doi:10.1016/0047-2484(92)90103-G [Google Scholar]
- Jungers W.L, Godfrey L.R, Simons E.L, Chatrath P.S. Phalangeal curvature and positional behavior in extinct sloth lemurs (Primates, Paleopropithecidae) Proc. Natl Acad. Sci. USA. 1997;94:11 998–12 001. doi: 10.1073/pnas.94.22.11998. doi:10.1073/pnas.94.22.11998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. Paleobiological and phylogenetic significance of life history in Miocene hominoids. In: Begun D.R, Ward C.V, Rose M.D, editors. Function, phylogeny and fossils: miocene hominoid evolution and adaptation. Plenum Press; New York, NY: 1997. pp. 173–208. [Google Scholar]
- Klingenberg C.P. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol. Rev. 1998;73:79–123. doi: 10.1017/s000632319800512x. doi:10.1017/S000632319800512X [DOI] [PubMed] [Google Scholar]
- Köhler M, Moyà-Solà S, Alba D.M. Eurasian hominoid evolution in the light of recent Dryopithecus findings. In: de Bonis L, Koufos G.D, Andrews P, editors. Phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press; Cambridge, UK: 2001. pp. 192–212. [Google Scholar]
- Larson S.G. Parallel evolution in the hominoid trunk and forelimb. Evol. Anthropol. 1998;6:87–99. doi:10.1002/(SICI)1520-6505(1998)6:3<87::AID-EVAN3>3.0.CO;2-T [Google Scholar]
- Lewis O.J. Joint remodelling and the evolution of the human hand. J. Anat. 1977;123:157–201. [PMC free article] [PubMed] [Google Scholar]
- Madar S.I, Rose M.D, Kelley J, MacLatchy L, Pilbeam D. New Sivapithecus postcranial specimens from the Siwaliks of Pakistan. J. Hum. Evol. 2002;42:705–752. doi: 10.1006/jhev.2002.0554. doi:10.1006/jhev.2002.0554 [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M. Recent discoveries of Dryopithecus shed new light on evolution of great apes. Nature. 1993;365:543–545. doi: 10.1038/365543a0. doi:10.1038/365543a0 [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M. New partial cranium of Dryopithecus Lartet, 1863 (Hominoidea, Primates) from the upper Miocene of Can Llobateres, Barcelona, Spain. J. Hum. Evol. 1995;29:101–139. doi:10.1006/jhev.1995.1049 [Google Scholar]
- Moyà-Solà S, Köhler M. A Dryopithecus skeleton and the origins of great-ape locomotion. Nature. 1996;379:156–159. doi: 10.1038/379156a0. doi:10.1038/379156a0 [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M, Rook L. Evidence of hominid-like precision grip capability in the hand of the Miocene ape Oreopithecus. Proc. Natl Acad. Sci. USA. 1999;96:313–317. doi: 10.1073/pnas.96.1.313. doi:10.1073/pnas.96.1.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M, Alba D.M, Casanovas-Vilar I, Galindo J. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science. 2004;306:1339–1344. doi: 10.1126/science.1103094. doi:10.1126/science.1103094 [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M, Alba D.M, Casanovas-Vilar I, Galindo J. Response to comment on “Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain”. Science. 2005;308:203. doi: 10.1126/science.1103094. doi:10.1126/science.1108433 [DOI] [PubMed] [Google Scholar]
- Nakatsukasa M, Kunimatsu Y, Nakano Y, Takano T, Ishida H. Comparative and functional anatomy of phalanges in Nacholapithecus kerioi, a Middle Miocene hominoid from northern Kenya. Primates. 2003;44:371–412. doi: 10.1007/s10329-003-0051-y. doi:10.1007/s10329-003-0051-y [DOI] [PubMed] [Google Scholar]
- Nakatsukasa M, Ward C.V, Walker A, Teaford M.F, Kunimatsu Y, Ogihara N. Tail loss in Proconsul heseloni. J. Hum. Evol. 2004;46:777–784. doi: 10.1016/j.jhevol.2004.04.005. doi:10.1016/j.jhevol.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Napier J. Studies of the hands of living primates. Proc. Zool. Soc. Lond. 1960;134:647–657. [Google Scholar]
- Napier J. Princeton University Press; Princeton, NJ: 1993. Hands. [Google Scholar]
- Napier J.R, Davis P.R. The fore-limb skeleton and associated remains of Proconsul africanus. Fossil. Mamm. Afr. 1959;16:1–77. [Google Scholar]
- Pilbeam D.R, Young N.M. Sivapithecus and hominoid evolution: some brief comments. In: de Bonis L, Koufos G.D, Andrews P, editors. Phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press; Cambridge, UK: 2001. pp. 349–364. [Google Scholar]
- Richmond B.G, Whalen M. Forelimb function, bone curvature and phylogeny of Sivapithecus. In: de Bonis L, Koufos G.D, Andrews P, editors. Phylogeny of the Neogene hominoid primates of Eurasia. Cambridge University Press; Cambridge, UK: 2001. pp. 326–348. [Google Scholar]
- Rose M.D. Further hominoid postcranial specimens from the Late Miocene Nagri Formation of Pakistan. J. Hum. Evol. 1986;15:333–367. doi:10.1016/S0047-2484(86)80016-1 [Google Scholar]
- Rose M.D, Nakano Y, Ishida H. Kenyapithecus postcranial specimens from Nachola, Kenya. Afr. Stud. Monogr. Suppl. 1996;24:3–56. [Google Scholar]
- Susman R.L. Comparative and functional morphology of hominoid fingers. Am. J. Phys. Anthropol. 1979;50:215–236. doi: 10.1002/ajpa.1330500211. doi:10.1002/ajpa.1330500211 [DOI] [PubMed] [Google Scholar]
- Thorpe S.K.S, Crompton R.H. Orangutan positional behavior and the nature of arboreal locomotion in Hominidea. Am. J. Phys. Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. doi:10.1002/ajpa.20422 [DOI] [PubMed] [Google Scholar]
- Ward C.V. Partial skeleton of Proconsul nyanzae from Mfangano Island, Kenya. Am. J. Phys. Anthropol. 1993;92:291–328. doi: 10.1002/ajpa.1330900106. doi:10.1002/ajpa.1330920306 [DOI] [PubMed] [Google Scholar]
- Young N.M. A reassessment of living hominioid postcranial variability: implications for ape evolution. J. Hum. Evol. 2003;45:441–464. doi: 10.1016/j.jhevol.2003.09.001. doi:10.1016/j.jhevol.2003.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive statistics of the several long bones of the hand in the extant comparative primate sample
Numerical results of the multivariate analyses
Allometric regressions
Schematic depiction of right fourth manual ray of Homo sapiens in palmar and lateral views, indicating the points employed for taking the measurements
Main results from the three discriminant analyses, including centroids for extant taxa and discriminant scores for Hispanopithecus