Abstract
Despite increasing evidence that marine predators associate with mesoscale eddies, how these marine features influence foraging movements is still unclear. This study investigates the relationship of at-sea movements of king penguins to mesoscale eddies using oceanographic remote sensing and movement data from 43 individual trips over 4 years. Simultaneous satellite measurements provided information on gradients of sea surface temperature and currents associated with eddies determined from altimetry. Penguins tended to swim rapidly with currents as they travelled towards foraging zones. Swimming speed indicative of foraging occurred within mesoscale fronts and strong currents associated with eddies at the Polar Front. These results demonstrate the importance of mesoscale eddies in directing foraging efforts to allow predators to rapidly get to rich areas where high concentrations of prey are likely to be encountered. When returning to the colony to relieve the incubating partner or to feed the chick, the birds followed a direct and rapid path, seemingly ignoring currents.
Keywords: king penguin, foraging, mesoscale eddies, marine currents, fronts, oceanographic features
1. Introduction
The open ocean is a heterogeneous environment that is characterized by a variety of physical features. The complexity of this environment results in patchy production over a very broad range of scales in both space and time. Pelagic predators such as seabirds and turtles are reported to actively use particular oceanic features to travel (Weimerskirch et al. 2000; Luschi et al. 2003; Gaspar et al. 2006) and search efficiently for their food supply (Nevitt et al. 1995). Owing to the patchiness in marine resources, these mechanisms are expected to have a crucial importance on the foraging strategy of marine predators.
Although they range widely over the open ocean, predators are thought to concentrate their foraging activity according to mesoscale features such as eddies where prey biomass is likely to be elevated (Nel et al. 2001; Polovina et al. 2006; Ream et al. 2005). Mesoscale eddies are suspected to be of primary importance for pelagic ecosystems because they serve as centres of biological production (Strass et al. 2002). Recent work suggests the importance of eddies in providing foraging opportunities for flying birds (Nel et al. 2001; Weimerskirch et al. 2004) as well as for other predators such as sea turtles (Ferraroli et al. 2004) although other uses have been reported that are life-stage dependent (Hays et al. 2006).
To date, no study has investigated how penguin species use this dynamic environment to travel and search for profitable patches. These diving seabirds face different environmental constraints than dynamic soaring seabirds, in that they have to deal with the high cost of swimming when compared with the low cost of soaring and gliding (Costa 1991). As a result, their foraging range is restricted during the breeding season (Wilson 1995). King penguins (Aptenodytes patagonicus) are one of the major avian predators of the Southern Ocean in terms of population size and prey consumption (Woehler 1995). During the breeding season, these diving birds mainly exploit the Polar Front, where they feed on pelagic fishes (Bost et al. 1997; Guinet et al. 1997; Charassin & Bost 2001; Sokolov et al. 2006). The Polar Front is a productive circumpolar boundary that corresponds to the northernmost limit of the Antarctic waters (Park et al. 1993). As it is also a dynamic front that is characterized by the presence of many mesoscale eddies (Park et al. 2002; Kostianoy et al. 2004), the behaviour of king penguins offers an ideal opportunity to explore how a swimming predator uses mesoscale eddies to forage.
Firstly, our aim was to assess whether king penguins forage preferentially in mesoscale frontal zones defined by sea surface temperature (SST) gradients, as has been shown for other long-range marine predators (Sims & Quayle 1998). Secondly, we wanted to investigate how movement en route to the Polar Front oriented to current. Here, we examined king penguin movement patterns by investigating swimming speed and direction relative to currents associated with eddies. We predicted that penguins would swim in the same direction as current en route to foraging spots as a mechanism to reduce travel cost.
2. Material and methods
(a) Datasets
King penguins nesting on Possession Island, Crozet Archipelago were fitted with platform transmitter terminals (PTTs; model Kiwisat 101, Sirtrack Ltd., Havelock North, New Zealand) each austral summer (December to March) from 2002 to 2005. A total of 43 breeding king penguins were tracked. The transmission interval was 45 s with an on/off interval of 6 h. The on/off interval was programmed to ensure reception of uplinks during the time of the day that has previously been determined as corresponding to high penguin at-sea activity (Charrassin et al. 2002). To ensure that breeding was not disturbed, birds were equipped before departure to sea just after they had been relieved by their partner at the nest. The PTTs weighed 208 g, i.e. approximately 1.8% of body weight, and were hydrodynamically streamlined. They were fitted to the lower back to reduce drag, with the antennae positioned posteriorly (Bannasch et al. 1994). The devices were fixed to the back feathers with cyanoacrylate glue (Loctite 401) and secured with cable ties. Antenna-induced effect of satellite transmitters may have deleterious effects on the hydrodynamics of swimming (Wilson et al. 2004). In our study, the trip duration of brooding penguins instrumented with PTT was increased only by 9%. The meal size of penguins fitted with PTTs was not different from those of control birds (C. A. Bost 2005, personal observation). Likewise, no differences in breeding failure were reported among control and instrumented birds. Location data were analysed using ELSA software (Argos CLS, Toulouse, France) and custom-written software Crozarg. The accuracy of Argos locations depends on the number of uplinks received. The daily frequency of retained uplinks varied between 8 and 15. Therefore, almost all categories of Argos locations (A, B, 0, 1, 2 and 3, but not Z) were included in the analyses. Swimming speed was conservatively estimated by assuming a straight-line direction and at constant speed between two successive locations (Weavers 1992). As speed errors could come from Argos location inaccuracies (Hays et al. 2001), location errors were filtered such that locations indicating a travelling speed higher than 14 km h−1 were rejected. This value corresponds to the greatest swimming speed recorded in a king penguin (Kooyman & Davis 1987).
Simultaneous oceanographic data were obtained from satellite measurements. We used 9 km resolution satellite-derived SST from advanced very high resolution radiometer and moderate-resolution imaging spectroradiometer. Although both SST data are available as a daily product, the radiometer does not penetrate cloud cover. Therefore, we used weekly averages as clouds diminished. This temporal resolution was sufficient for studying mesoscale dynamics. To identify mesoscale fronts, we applied a moving window across the grid of SST values. This window permitted slope calculations within a 5×5 neighbourhood calculated by
| (2.1) |
where x, y and T indicate longitude; latitude; and SST values, respectively. A low-pass filter (Gaussian method on a 3×3 moving window) was then applied to the new grid of slope values to remove the high-frequency noise and thus smoothed the frontal structures. We considered the surface signature of a Polar Front to be between the 4 and 5°C isotherms (Park et al. 1993). Associated with this surface approximation of the Polar Front, the SST gradients reliably reveal the presence of mesoscale fronts (Kostianoy et al. 2004).
Weekly maps of gridded sea level anomalies (SLA) at 0.3° resolution on a Mercator projection were used for the periods of penguin tracking. These maps were made by merging datasets from T/P, ERS-1/2, Jason and Envisat satellite altimeters (Ducet et al. 2000). The SLA data were distributed by the archiving, validation and interpretation of satellite oceanographic project of the collecte, localization, satellite (CLS/Centre National d'Études Spatiales, France) for the Ssalto program. Despite possibilities of error due to differences in ground tracks and orbit repeat periods among the several altimeter instruments and to interpolation in the grid construction, merged data have the advantage of resolving mesoscale oceanic processes beyond the capability of a single instrument (Fu et al. 2003). Geostrophic currents (0.3° resolution) were computed from SLA as follows:
| (2.2) |
| (2.3) |
where g=980 cm s−2; f=2Ω sin Φ; Ω=7.29×10−5 radians s−1; Φ is the latitude; and H is sea level anomaly
(b) Analyses
For each penguin trip, we identified three phases using running averages over five consecutive swimming speeds and compared these running averages with the mean swimming speed for the whole trip. In doing so, we assumed that king penguins would decrease their swimming speed to actively forage, i.e. they would decrease their horizontal (displacements) activity while they increased their vertical (diving) activity (in accordance with Bost et al. 1997; Pütz et al. 1999; Charrassin et al. 2002). These three phases were defined as the following. (i) The transit phase, indicating the travel between the colony and the central phase, where the running averages of swimming speeds were higher than the mean speed of the whole trip. (ii) The central phase, which began at the first episode of slowing down, where the running averages were below the mean speed. Slowing-down episodes in the central phase were frequently separated by speed-up episodes, corresponding to running averages above the overall mean speed. (iii) The return phase, where running averages of swimming speed were higher than the overall mean speed, followed the last slowing-down episode of the central phase. Penguins in incubating and chick-rearing stages were pooled for this analysis. Since slowing-down episodes of the central phase represented intense foraging activity (Bost et al. 1997), they were compared statistically with episodes of speeding up in the central phase and also with transit and return phases. To tie these behaviours in with oceanographic data, corresponding analyses were performed using oceanographic parameters (SST, SST gradient and currents from SLA) from measurements taken closest to the penguin's at-sea locations. Bearing deviations between penguin and underlying current directions, varying from 0° to 360°, were also investigated. A bearing deviation from 0° to 90° and from 270° to 360° signified similar directions for a penguin and the current (positive rheotaxis), while a bearing deviation from 90° to 270° corresponded to opposing directions (negative rheotaxis; Ream et al. 2005). Similar (0°–90° and 270°–360°) directions suggested that currents assisted the directional movements of the penguins whereas opposing (90°–270°) directions suggested opposing forces. Net king penguin speed and movement are defined by parameters (east–west) and (north–south), and currents by (east–west) and (north–south), with the total vectors estimated as. Net penguin speed was the resultant of swimming and underlying current speeds. Thus, we calculated the real swimming speed vector (in terms of amplitude and direction) of penguins by and . We used circular statistics to estimate mean length (ML) of mean swimming and current vectors, and also direction confidence interval (CI) from bootstrap method for each phase.
3. Results
(a) King penguin trips and mesoscale fronts
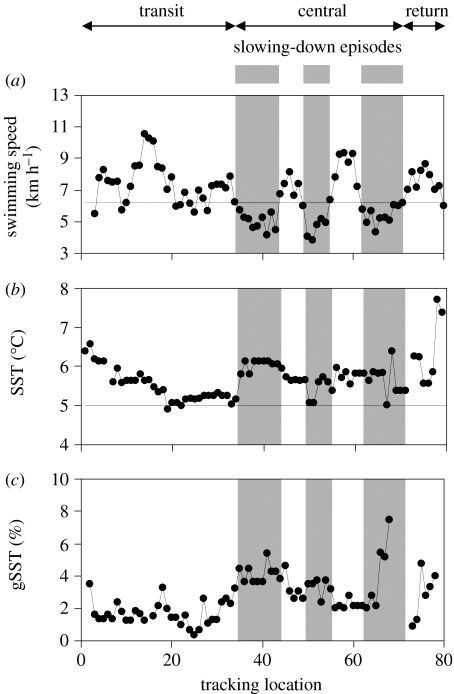
For all trips, penguins tended to slow down when they encountered the steepest SST gradient (table 1). Steep SST gradients are indicators of mesoscale fronts, where physical and biological processes occur. This relationship suggests that penguins were slowing down to forage at these mesoscale fronts while the rest of the trip, i.e. transit and return phases and also speed-up episodes during the central phase, tended to be associated with shallower SST gradients. These successive phases are detailed for one typical trip (figure 1) where the several slowing-down episodes were clearly associated with steeper SST gradients.
Table 1.
Sea surface temperature gradients (gSST, means ±s.e.) for all locations of the 43 king penguin trips.
| trip phases | gSST (%) | U-test (versus slowing-down episodes) |
|---|---|---|
| transit | 2.13±1.28 | p<0.001 |
| central | ||
| slowing-down episodes | 2.95±1.80 | |
| speed-up episodes | 2.65±1.61 | p=0.023 |
| return | 2.19±1.36 | p<0.001 |
Figure 1.
Parameters for a king penguin track with (a) penguin swimming speed from running average over five positions (horizontal line indicates the mean speed for the whole trip), (b) sea surface temperature, SST (horizontal line indicates northern limit of the Polar Front) and (c) sea surface temperature gradients (gSST). Grey bars indicate slowing-down episodes during the central phase, defined as swimming speeds below the mean. Slowing-down episodes correspond to bouts of intense foraging (Bost et al. 1997).
(b) King penguin movements and mesoscale currents
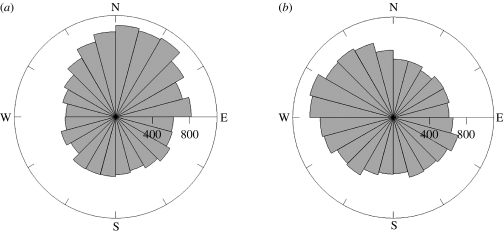
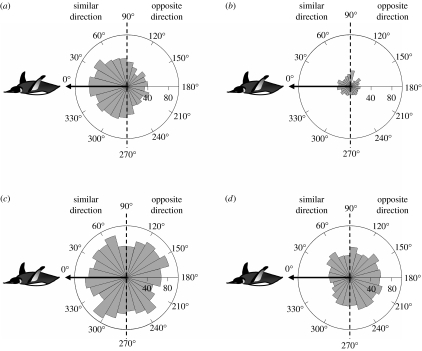
Frequencies of estimated mesoscale current directions in the whole area, defined by tracking bird ranges, show a slight northeastward direction during the transit and return phases (between Crozet Island and the Polar Front), and a slight westward direction during the central phase (within the Polar Front; figure 2). The mesoscale current situation is highly changing due to eddies, variable in both time and amplitude. Currents encountered along the tracks were significantly stronger during slowing-down episodes than during the rest of trip (table 2). Bearing deviations during the transit phase indicate that birds tended to swim in a similar direction as the current (bird direction, CI=170°–178°, ML=0.52; current direction, CI=174°–185°, ML=0.33; 0° and 180° being north and south direction, respectively; Watson test U2=0.21, p>0.1; table 3 and figure 3). This trend disappeared during slowing-down episodes characteristic of the central phase, where birds had no preferred direction (bird direction, CI=169°–214°, ML=0.09), and suggesting that birds no longer swam with the current (CI=128°–150°, ML=0.16). Speed-up episodes of the central phase, where penguins left a foraging place to another one, indicate that birds tended to swim with currents as in the transit phase (bird direction, CI=170°–189°, ML=0.4; current direction, CI=157°–199°, ML=0.17; U2=0.07, p>0.1). During the return phase, penguins tended to swim against the current (bird direction, CI=345°–353°, ML=0.51; current direction, CI=144°–172°, ML=0.14; U2=0.47, p<0.001). Currents speeds were at least one order of magnitude lower than penguin swimming speeds, suggesting that they could easily swim against the current (table 2). Swimming speeds during the transit phase and speed-up episodes of the central phase were similar, while they were faster on average during the return phase.
Figure 2.
Frequencies of directions of marine currents, estimated from satellite altimetry, in the areas prospected by king penguins (a) during transit and return phases and (b) during the central phase. Limits of the areas are defined by tracking bird ranges.
Table 2.
Means ±s.e. of penguin swimming and underlying current speeds for all locations of the 43 king penguin trips.
| trip phases | swimming speed (km h−1) | U-test (versus slowing-down episodes) | current speed (km h−1) | U-test (versus slowing-down episodes) |
|---|---|---|---|---|
| transit | 5.8±4.0 | p<0.001 | 0.23±0.14 | p<0.001 |
| central | ||||
| slowing-down episodes | 3.9±3.6 | 0.31±0.18 | ||
| speed-up episodes | 5.7±4.1 | p<0.001 | 0.23±0.13 | p<0.001 |
| return | 6.4±4.8 | p<0.001 | 0.24±0.14 | p<0.001 |
Table 3.
Trip proportion presenting similar penguin–current directions (i.e. bearing deviation between 270° and 90°) from the mean bearing deviation per phase for each of the 43 king penguin trips.
| trip phases | penguin–current similar directions (%) |
|---|---|
| transit | 74 |
| central | |
| slowing-down episodes | 53 |
| speed-up episodes | 63 |
| return | 39 |
Figure 3.
Frequencies of bearing deviations between penguin and underlying current direction for all locations of the 43 king penguin trips during (a) transit phase, (b) speed-up and (c) slowing-down episodes of the central phase and (d) return phase. Arrows indicate the referential swimming direction of penguins and the dotted lines separate similar (270°–90°) and opposing (90°–270°) directions.
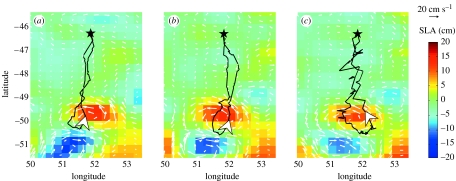
Figure 4 illustrates penguin movements in relation to currents. These journeys of three different seabirds corresponded to a situation where an eddy remained static for at least one month. The birds exhibited similar trip patterns in response to a similar mesoscale pattern of marine circulation: southward transits and northward returns were in straight lines between the colony and an eddy while once at the edge of this anticyclonic eddy the three trips were clockwise, i.e. in a similar direction as the prevailing current.
Figure 4.
SLA and geostrophic currents with successive tracks from three different penguins (a) first, 28 January 2004 to 10 February 2004 (b) second, 2 February 2004 to 24 February 2004 and (c) third, 11 February 2004 to 25 February 2004. The star indicates the position of Crozet Archipelago. Arrows give trip directions.
4. Discussion and conclusions
There is increasing evidence that a wide variety of marine vertebrates aggregate in association with mesoscale eddies (e.g. Nel et al. 2001). However, very few studies go further than documenting an empirical association. Because eddies are characterized by strong gradients in water mass properties, especially SST gradients in the Polar Front (Kostianoy et al. 2004), it is relevant to assess quantitative associations between penguin activity (travelling and foraging) and such gradients (SST gradients and currents). Our analysis suggests that penguin swimming behaviour relative to the current changes at mesoscale fronts, where penguins are known to increase their diving activity and food intake (Bost et al. 1997; Charassin & Bost 2001). The changes were especially marked at the periphery of eddies, suggesting that the foraging activity occurred more at the edge of eddies than the centre (Nel et al. 2001; Weimerskirch et al. 2004; Polovina et al. 2006). Besides the broad strategy of king penguins to go to the Polar Front, these results confirm the importance of considering the influence of finer-scale physical parameters. In particular, frontal meanders of the Polar Front created by eddies give rise to mesoscale convergence and divergence. These features can concentrate zooplankton and micronekton (Pakhomov & Fronemann 2000), preyed upon by fish such as myctophids, which opportunistically forage on mesozooplankton (Pakhomov et al. 1996) and presumably attract king penguins as well as others upper-trophic predators (Hyrenbach et al. 2006). Eddy structures are beneficial to predators. Upwelling occurs at the centre, whereas convergence (horizontal concentration of productivity and preys) occurs at the edge of cyclonic (cold-core) eddy. The edge of anticyclonic (warm-core) eddy presents an elevation of water masses, leading to a more accessible concentration of prey at shallow depths (Polovina et al. 2006). Several foraging predators including penguins have been shown to forage in association with thermoclines, strong physical discontinuities that concentrate potential prey items (Charrassin & Bost 2001). Were thermoclines to occur at shallower depths, this would be beneficial to penguins since shallower dives would be required for prey capture. Despite their ephemeral nature, these mesoscale fronts may provide useful feeding grounds for top predators, particularly in pelagic systems where prey patches are overdispersed (Nel et al. 2001; Weimerskirch 2007). Furthermore, these mesoscale features are relatively common within the Polar Front and may be particularly important for predators such as king penguins, which are not able to cover vast distance during the constraints of the breeding season. Although transitory eddies and associated ephemeral fronts dominate the Polar Front, some marked static physical features can nevertheless occur. Since birds tend to travel to similar locations to forage, even over vast distances, persisting eddies may be ecologically relevant. These longer term productive features are likely to constitute favourable and above all predictable feeding grounds for highly constrained predators like penguins. Persisting eddies can have their own production and food chain development in contrast to usual transitory features that simply aggregate planktonic organisms (Palacios et al. 2006).
Recently, studies on marine predator movements have particularly emphasized the mechanical influence of currents with respect to their deflecting action, forcing animals to drift away from the most suitable route (Gaspar et al. 2006; Girard et al. 2006). Turtles were thus reported to be ‘caught’ by the circulation, concentrating their activity on swimming vertically rather than horizontally to forage (Luschi et al. 2003). While these findings concerned predators with a swimming speed of the same order of magnitude as the current speed, our results are focusing on a situation where penguin movements are substantially faster than the ambient currents (by a factor of 10 at least). In this situation, the predator movements are indeed less impacted by the deflecting action of currents. This important difference was due to the following reasons. Firstly, their trips under breeding constraints were made with a sustained swimming speed comparable with breeding Antarctic fur seals (1–8 km h−1 from Boyd et al. 1995) and higher than most long-range foraging predators such as turtles (0.7–3.5 km h−1 from Luschi et al. 1998; Polovina et al. 2004; Ferraroli et al. 2004, 2006; Girard et al. 2006), tuna (approx. 1.5 km h−1 from Block et al. 1998) and non-breeding seals (0.3–2.2 km h−1 from Ream et al. 2005; Austin et al. 2006). Secondly, the currents in the Polar Frontal zone are weaker than that in northern fronts that constitute the major portion of the antarctic circumpolar current (Park et al. 2002) and also weaker than that in most regions where animal movements have been studied (Luschi et al. 2003; Ferraroli et al. 2004; Polovina et al. 2006).
Despite penguin swimming speed substantially greater than current speed, our study shows that king penguins shifted their movement behaviour in relation to currents through the distinct phases of their trips. Importantly, penguin movements appeared to be related to currents as they commute from the colony to foraging places, but are unrelated during actual foraging. Our results support the hypothesis, also suggested for turtles, that penguins may take advantage of current flow to increase their speed without increasing energy cost (Hays et al. 1999). Breeding penguins leave the colony after several weeks of fasting and they have to rapidly rebuild their own energetic reserves before stocking food for their offspring during chick rearing (Kooyman et al. 1992). Once penguins slowed down to forage, swimming direction no longer correlates with current direction, despite stronger currents at the periphery of eddies. This behavioural shift is probably due to the increase in foraging effort (Bost et al. 1997; Charassin & Bost 2001), and also due to the optimal exploitation of the mesoscale fronts until the benefit from staying in this area either drops below some threshold or they replenish their reserves. During the return phase, where diving rate and food intake decrease drastically (Bost et al. 1997; Charrassin et al. 2002), penguins came back rapidly to the colony owing to the breeding constraints, i.e. partner relieve on egg or chick, or chick feeding, effectively swimming up stream to do so. Since they used mainly southward currents while travelling towards the Polar Front, the penguins had to face these currents when they came back, which explain the higher proportion of opposing currents encountered during the return.
Mesoscale eddies are thus suggested to be particularly important for king penguins through (i) the creation of mesoscale fronts that are potential feeding grounds in the vicinity of the Polar Front and (ii) providing king penguins with either a faster means of reaching their goal or a directional cue to get there. By contrast to relatively slow marine turtles, affected by the drifting action of strong currents (Gaspar et al. 2006; Girard et al. 2006), faster penguins highly constrained in space and time may take advantage of these currents as an aid to progress rapidly to favourable fronts associated with the mesoscale eddy activity. Despite certain technical limitations on the availability of physical measurements from the surface (e.g. cloudiness), remote sensing provides undoubtedly valuable information on the relationship between at-sea animal activity and its environment. Furthermore, mechanistic studies are still required to better understand the causal biophysical interaction and the link between lower and upper trophic system constituents that take place at these mesoscale eddies (Hyrenbach et al. 2006). The present study provides new insights into the influence of eddy activity on the behaviour of marine predators, particularly in high latitudes. It highlights the role that mesoscale features play in marine ecosystems as well as the lack of information on mechanisms driving the biological production and prey aggregation associated with these features. Further studies will examine diving and feeding activity associated with eddies during king penguin foraging trips. In this mesoscale context, the role of foraging experience through tracking successive trips of the same birds should be especially promising.
Acknowledgments
This study was approved by the ethics committee of the French Polar Institute (IPEV). We thank P.-M. Theveny for helping with data analysis and L. G. Hasley, H. Weimerkirch, J.-B. Charrassin, Y. Cherel and D. Pinaud for their helpful comments on an earlier version of the manuscript. Special thanks to A. Pape for his help with the English. This study was carried out in the framework of GICC and REMIGE (ANR 2005 Biodiv-11) programs. The IPEV and the TAAF provided financial and logistical support. We thank Gabrielle Nevitt and two anonymous referees for their suggestive and helpful comments that substantially improve the manuscript.
Supplementary Material
Penguin tracking and weekly mesoscale situation (geostrophic currents and sea level anomaly, high in red low in blue). Black points are travelling and white points are foraging. The three different penguins exhibited similar trips relatively to the well-marked anticyclonic eddy
References
- Austin D, Bowen W.D, McMillan J.I, Iverson S.J. Linking movement, diving, and habitat to foraging success in a large marine predator. Ecology. 2006;87:3095–3108. doi: 10.1890/0012-9658(2006)87[3095:lmdaht]2.0.co;2. doi:10.1890/0012-9658(2006)87[3095:LMDAHT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bannasch R, Wilson R.P, Culik B. Hydrodynamic aspects of design and attachment of a back-mounted device in penguins. J. Exp. Biol. 1994;194:83–96. doi: 10.1242/jeb.194.1.83. [DOI] [PubMed] [Google Scholar]
- Block B.A, Dewar H, Farwell C, Prince E.D. A new satellite technology for tracking the movements of Atlantic bluefin tuna. Proc. Natl Acad. Sci. USA. 1998;95:9384–9389. doi: 10.1073/pnas.95.16.9384. doi:10.1073/pnas.95.16.9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost C.A, et al. Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar. Ecol. Prog. Ser. 1997;150:21–33. [Google Scholar]
- Boyd I.L, Reid K, Bevan R.M. Swimming speed and allocation of time during the dive cycle in Antarctic fur seals. Anim. Behav. 1995;50:769–784. doi:10.1016/0003-3472(95)80137-5 [Google Scholar]
- Charrassin J.B, Bost C.A. Utilization of the oceanic habitat by king penguins over the annual cycle. Mar. Ecol. Prog. Ser. 2001;221:285–297. [Google Scholar]
- Charrassin J.B, Le Maho Y, Bost C.A. Seasonal changes in the diving parameters of king penguins (Aptenodytes patagonicus) Mar. Biol. 2002;141:581–589. doi:10.1007/s00227-002-0843-4 [Google Scholar]
- Costa D.P. Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: implications for life history patterns. Am. Zool. 1991;31:11–130. [Google Scholar]
- Ducet N, Le Traon P.Y, Reverdin G. Global high resolution mapping of ocean circulation from TOPEX/Poseidon and ERS-1/2. J. Geophys. Res. 2000;105:19 477–19 498. doi:10.1029/2000JC900063 [Google Scholar]
- Ferraroli S, Georges J.Y, Gaspar P, Le Maho Y. Endangered species: where leatherback turtles meet fisheries. Nature. 2004;429:521–522. doi: 10.1038/429521a. doi:10.1038/429521a [DOI] [PubMed] [Google Scholar]
- Fu L.-L, Stammer D, Leben R.R, Chelton D.B. Improved spatial resolution of ocean surface topography from the T/P-Jason-1 altimeter mission. EOS Trans. Am. Geophys. Union. 2003;84:241. doi:10.1029/2003EO260002 [Google Scholar]
- Gaspar P, Georges J.Y, Fossette S, Lenoble A, Ferraroli S, Le Maho Y. Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc. R. Soc. B. 2006;273:2697–2702. doi: 10.1098/rspb.2006.3623. doi:10.1098/rspb.2006.3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C, Sudre J, Benhamou S, Roos D, Luschi P. Homing in green turtles Chelonia mydas: oceanic currents act as a constraint rather than as an information source. Mar. Ecol. Prog. Ser. 2006;322:281–289. [Google Scholar]
- Guinet C, Koudil M, Bost C.A, Durbec J.P, Georges J.Y, Mouchot M.C, Jouventin P. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Mar. Ecol. Prog. Ser. 1997;150:11–20. [Google Scholar]
- Hays G.C, Luschi P, Papi F, del Seppia C, Marsh R. Changes in behaviour during the internesting period and postnesting migration for Ascension Island green turtles. Mar. Ecol. Prog. Ser. 1999;180:263–273. [Google Scholar]
- Hays G.C, Åkesson S, Godley B.J, Luschi P, Santidrian P. The implications of location accuracy for the interpretation of satellite tracking data. Anim. Behav. 2001;61:1035–1040. doi:10.1006/anbe.2001.1685 [Google Scholar]
- Hays G.C, Hobson V.J, Metcalfe J.D, Righton D, Sims D.W. Flexible foraging movements of leatherback turtles across the North Atlantic Ocean. Ecology. 2006;87:2647–2656. doi: 10.1890/0012-9658(2006)87[2647:ffmolt]2.0.co;2. doi:10.1890/0012-9658(2006)87[2647:FFMOLT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hyrenbach K.D, Veit R.R, Weimerskirch H, Hunt G.L., Jr Seabirds associations with mesoscale eddies: the subtropical Indian Ocean. Mar. Ecol. Prog. Ser. 2006;324:271–279. [Google Scholar]
- Kooyman G.L, Davis R.W. Diving behavior and performance, with special reference to penguins. In: Croxall J.P, editor. Seabirds feeding ecology and role in marine ecosystems. Cambridge University Press; Cambridge, UK: 1987. pp. 63–75. [Google Scholar]
- Kooyman G.L, Cherel Y, Le Maho Y, Croxall J.P, Thorson P.H, Ridoux V, Kooyman C.A. Diving behaviour and energetics during foraging cycles in king penguins. Ecol. Monogr. 1992;62:143–163. doi:10.2307/2937173 [Google Scholar]
- Kostianoy A.G, Ginzburg A.I, Frankignoulle M, Dellile B. Fronts in the Southern Indian Ocean as inferred from satellite sea surface temperature data. J. Mar. Syst. 2004;45:55–73. doi:10.1016/j.jmarsys.2003.09.004 [Google Scholar]
- Luschi P, Hays G.C, del Seppia C, Marsh R, Papi F. The navigational feats of green turtles migrating from Ascension Island investigated by satellite telemetry. Proc. R. Soc. B. 1998;265:2279–2284. doi: 10.1098/rspb.1998.0571. doi:10.1098/rspb.1998.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschi P, Sale A, Mencacci R, Hughes G.R, Lutjeharms J.R.E, Papi F. Current transport of leatherback sea turtles (Dermochelys coriacea) in the ocean. Proc. R. Soc. B. 2003;270(Suppl.):129–132. doi: 10.1098/rsbl.2003.0036. doi:10.1098/rsbl.2003.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel D.C, Lutjeharms J.R.E, Pakhomov E.A, Ansorge I.J, Ryan P.G, Klages N.T.W. Exploitation of mesoscale oceanographic features by grey-headed albatross Thalassarche chrysostoma in the Southern Indian Ocean. Mar. Ecol. Prog. Ser. 2001;217:15–26. [Google Scholar]
- Nevitt G.A, Veit R.R, Kareiva P. Dimethyl sulphide as a foraging cue for Antarctic Procellariiform seabirds. Nature. 1995;376:680–682. doi:10.1038/376680ao [Google Scholar]
- Pakhomov E.A, Fronemann P.W. Composition and spatial variability of macrozooplankton and micronekton within the Antarctic Polar Frontal Zone of the Indian Ocean during austral summer 1997. Polar Biol. 2000;23:410–419. doi:10.1007/s003000050462 [Google Scholar]
- Pakhomov E.A, Perissinotto R, McQuaid C.D. Prey composition and daily rations of myctophid fishes in the Southern Ocean. Mar. Ecol. Prog. Ser. 1996;134:1–14. [Google Scholar]
- Palacios D.M, Bograd S.J, Foley D.G, Schwing F.B. Oceanographic characteristics of biological hot spots in the North Pacific: a remote sensing perspective. Deep Sea Res. II. 2006;53:250–269. doi:10.1016/j.dsr2.2006.03.004 [Google Scholar]
- Park Y.H, Gambéroni L, Charriaud E. Frontal structure, water masses, and circulation in the Crozet Basin. J. Geophys. Res. 1993;98:12 361–12 385. [Google Scholar]
- Park Y.H, Pollard R.T, Read J.F, Leboucher V. A quasi-synoptic view of the frontal circulation in the Crozet Basin during the Antares-4 cruise. Deep Sea Res. II. 2002;49:1823–1842. doi:10.1016/S0967-0645(02)00014-0 [Google Scholar]
- Polovina J.J, Balazs G.H, Howell E.A, Parker D.M, Seki M.P, Dutton M.P. Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish. Oceanogr. 2004;13:36–51. doi:10.1046/j.1365-2419.2003.00270.x [Google Scholar]
- Polovina J.J, Uchida I, Balazs G.H, Howell E.A, Parker D.M, Dutton M.P. The Kuroshio extension bifurcation region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep Sea Res. II. 2006;53:326–339. doi:10.1016/j.dsr2.2006.01.006 [Google Scholar]
- Pütz K, Ropert-Coudert Y, Charrassin J.B, Wilson R.P. Foraging areas of King penguins Aptenodytes patagonicus breeding at Possession Island, southern Indian Ocean. Mar. Ornithol. 1999;27:77–84. [Google Scholar]
- Ream R.R, Sterling J.T, Loughlin T.R. Oceanographic features related to northern fur seal migratory movements. Deep Sea Res. II. 2005;52:823–843. doi:10.1016/j.dsr2.2004.12.021 [Google Scholar]
- Sims D.W, Quayle V.A. Selective foraging behaviour of basking sharks on zooplankton in a small-scale front. Nature. 1998;393:460–464. doi:10.1038/30959 [Google Scholar]
- Sokolov S, Rintoul S.R, Wienecke B. Tracking the Polar Front south of New Zealand using penguin dive data. Deep Sea Res. I. 2006;53:591–607. doi:10.1016/j.dsr.2005.12.012 [Google Scholar]
- Strass V.H, Naveira Garabato A.C, Pollard R.T, Fischer H.I, Hense I, Allen J.T, Read J.F, Leach H, Smetacek V. Mesoscale frontal dynamics: shaping the environment of primary production in the Antarctic Circumpolar Current. Deep Sea Res. II. 2002;49:3735–3769. doi:10.1016/S0967-0645(02)00109-1 [Google Scholar]
- Weavers B.W. Seasonal foraging ranges and travels at sea of little blue penguins Eudyptula minor, determined by radiotracking. Emu. 1992;91:302–307. [Google Scholar]
- Weimerskirch H. Are seabirds foraging for unpredictable resources? Deep Sea Res. II. 2007;54:211–223. doi:10.1016/j.dsr.2.2006.11.013 [Google Scholar]
- Weimerskirch H, Guionnet T, Martin J, Shaffer S.A, Costa D.P. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. B. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. doi:10.1098/rspb.2000.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimerskirch H, Le Corre M, Jacquemet S, Potier M, Marsac D.P. Foraging strategy of a top predator in tropical waters: great fregatebirds in the Mozambique Channel. Mar. Ecol. Prog. Ser. 2004;275:297–308. [Google Scholar]
- Wilson R.P. Foraging ecology. In: Williams T.D, editor. The penguins. Oxford University Press; Oxford, UK: 1995. pp. 81–106. [Google Scholar]
- Wilson R.P, Kreye J.M, Lucke K, Urquhart H. Antennae on transmitters on penguins: balancing energy budgets on the high wire. J. Exp. Biol. 2004;207:2649–2662. doi: 10.1242/jeb.01067. doi:10.1242/jeb.01067 [DOI] [PubMed] [Google Scholar]
- Woehler E.J. Consumption of Southern Ocean resources by penguins. In: Dann P, Norman I, Reilly P, editors. The penguins, ecology and management. Surrey Beatty and Sons; Chipping Norton, UK: 1995. pp. 267–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Penguin tracking and weekly mesoscale situation (geostrophic currents and sea level anomaly, high in red low in blue). Black points are travelling and white points are foraging. The three different penguins exhibited similar trips relatively to the well-marked anticyclonic eddy