Abstract
Recently, the size of the active stem cell pool has been predicted to scale allometrically with the adult mass of mammalian species with a 3/4 power exponent, similar to what has been found to occur for the resting metabolic rate across species.
Here we investigate the allometric scaling of human haemopoietic stem cells (HSCs) during ontogenic growth and predict a linear scaling with body mass. We also investigate the allometric scaling of resting metabolic rate during growth in humans and find a linear scaling with mass similar to that of the haemopoietic stem cell pool.
Our findings suggest a common underlying organizational principle determining the linear scaling of both the stem cell pool and resting metabolic rate with mass during ontogenic growth within the human species, combined with a 3/4 scaling with adult mass across mammalian species. It is possible that such common principles remain valid for haemopoiesis in other mammalian species.
Keywords: haemopoietic stem cells, haemopoiesis, reticulocytes, human ontogeny, allometric scaling
1. Introduction
Circulating blood cells have a finite lifespan and are continuously being replaced. Blood cell production in the bone marrow is carefully controlled to cope with the requirements of daily living while output can be increased under conditions of higher demand such as infection or bleeding. Production of blood cells depends on a pool of HSCs that have the dual capability of self-renewal and differentiation into all types of circulating cellular elements (Morrison et al. 1995; Dick 2003; Reya et al. 2001). The number of HSCs appears to be conserved across species within the range 11 000–22 000 (Abkowitz et al. 2002; Gordon et al. 2002; McCarthy 2003). It is thought that, at any time, only a fraction of these cells are actively contributing to blood cell formation, a feature which was given an allometric explanation recently (Dingli & Pacheco 2006).
Allometric scaling (Huxley 1932) pervades throughout biology and has provided many fundamental insights into the nature of basic organizational principles of living systems (Schmidt-Nielsen 1984). A paradigmatic example of allometric scaling is provided by the basal metabolic rate, which has been shown to follow an allometric exponent of 3/4 for a mass range spanning 27 orders of magnitude (West et al. 2002). Basal metabolism is clearly related to the haemopoietic system since haemoglobin, exclusively transported by red blood cells, is the main carrier of oxygen throughout the organism, thereby ensuring an adequate supply for metabolic needs. Therefore, it is natural to expect that the haemopoietic system also satisfies an allometric scaling relation. Indeed, we have recently shown that in adult mammals, the size of the active stem cell pool, NSC, scales with adult mass M as Nsc=N0M3/4 across five orders of magnitude (Dingli & Pacheco 2006). Consequently, our results show that the active stem cell pool and basal metabolic rate scale with the same exponent. Similarly, one expects that, during ontogenic growth, the active stem cell pool also scales allometrically with mass, in line with what has been found for the basal metabolic rate (West et al. 2001). This is the purpose of the present work in which we investigate the allometric scaling of the active stem cell pool during ontogenic growth in humans.
(a) Allometric scaling across mammals
The active HSCs, Nsc, replicate at a rate that scales with mass in adult mammals (Dingli & Pacheco 2006) which also specifies the cell metabolic rate of the organism (in vivo), Bc. The active HSCs produce all blood cells including reticulocytes, RT (circulating red blood cell precursors). Reticulocytes mature in a characteristic time τ that is ultimately (West et al. 2002) determined by Bc: , an assumption which is also consistent with the Hayflick hypothesis (Hayflick & Moorhead 1961), as discussed in Dingli & Pacheco (2006). As a result, one may write the fundamental equation relating the rate of replication of the active stem cell compartment and reticulocyte production
| (1.1) |
The coefficient A represents the set of (exponential) amplification stages which couple stem cell replication with reticulocyte formation. Such an architecture of amplification and differentiation (Dingli et al. 2007a) is assumed to remain constant both across species as well as during ontogenic growth (Dingli & Pacheco 2006). Given that , we conclude that Nsc∼RT. Experimental data for RT as a function of adult mass M across species lead to RT∼M3/4, and therefore, Nsc∼M3/4(Dingli & Pacheco 2006).
2. Material and methods
The growth rate of healthy boys and girls has been recorded for many years and is a standard component in health care monitoring of the paediatric population (Goldson & Reynolds 2006). The age-specific mass, m(t) (in kg) and height, H(in m) were determined by taking the fiftieth centile for both sexes as the most representative from standard curves (Goldson & Reynolds 2006). The resting metabolic rate, B(t) (in kJ d−1), was determined from validated functions that require the mass and height of the child as input (WHO 1985). In the case of males 3 or more and less than 10 years of age, B(t)=95×m(t)+2071, while for females of the same age range, B(t)=94×m(t)+2088. On the other hand, for males 10 or more but less than 18 years of age, B(t)=[16.6×m(t)+77×H+572]×4.18 and for females of the same age group, B(t)=[7.4×m(t)+482×H+217]×4.18 (WHO 1985).
Age- and gender-specific blood volumes were estimated according to established criteria (Raes et al. 2006). The total circulating reticulocyte count, RT, was calculated as the product of the age-specific red blood cell concentration per litre (RBC), age (mass)-specific blood volume (BV) and age-specific reticulocyte percentages (RT; Castriota-Scanderbeg et al. 1992) (RBC×BV×RT). The extreme lower and upper limits for the reticulocyte counts were calculated. The values plotted in figure 1 correspond to the mean reticulocyte counts.
Figure 1.
Scaling of the mean reticulocyte count (RTD) with body mass during ontogenic growth in humans. Data for girls are shown with open circles, whereas data for boys are shown with solid circles. The scaling of RTD with mass is well approximated (in both cases, correlation coefficient is greater than 0.999) by a linear relation (straight dashed and solid lines, respectively), as opposed to a 3/4 scaling, illustrated by the curved lines (dashed and solid lines, respectively). Such an intra-species allometric relation with exponent 1 contrasts with the allometric exponent 3/4 obtained for inter-species scaling for mammals spanning a mass range of five orders of magnitude.
3. Results
(a) Expansion of the active haemopoietic stem cell pool with human growth
During human growth, the demands on the haemopoietic system increase commensurate with the higher oxygen requirements. Nonetheless, the fundamental relation above, equating stem cell replication rate with reticulocyte maturation should remain valid during ontogenic growth under the assumption that the hierarchical organization of cell division and differentiation that links the active HSCs with reticulocytes remains invariant from birth into adulthood. Moreover, one expects that holds at all times. In other words, the relation Nsc∼RT should remain valid during growth (Dingli et al. 2007a), irrespective of whether Bc remains constant during growth, as assumed by West et al. (2001; see below) or varies (Glazier 2005 and references therein).
The mean number of circulating reticulocytes RT as a function of mass was determined as detailed in §2 and is shown in figure 1 for females (open circles) and males (solid circles), respectively. As expected, RT increases with age and mass to meet the increasing demands for oxygen transport and blood volume expansion. Interestingly, the absolute reticulocyte output in the adult is less than 10-fold higher of what is produced by a five-month-old baby. The data show a dependence which may be well approximated by a linear relationship between RT and mass (solid and dashed lines constitute linear fits to the solid and open circles, respectively). This dependence on mass during growth within a single species (humans) contrasts with that observed in adults across mammalian species, where a 3/4 allometric scaling was found (Dingli & Pacheco 2006). In other words, intra-species allometric scaling of the stem cell pool is different from its inter-species counterpart. The stem cell pool grows proportional to body mass until it reaches a maximum in adulthood. From our previous estimates across species, an adult human has an active pool of Nsc≈385 stem cells contributing to haemopoiesis (Dingli & Pacheco 2006). This allows us to fix the scaling constant and write Nsc(t)=(385/M)m(t)=5.5m(t), for the size of the active stem cell pool at any time, where M (≈70 kg) is the human adult mass. For instance, a 4 kg baby is estimated to satisfy Nsc≈22.
(b) Implications for other growth processes
Recently, a universal model of ontogenic growth has been proposed (West et al. 2001). Inherent to this model is the assumption that the resting metabolic rate (B) of animals across species, found to scale as B∼M3/4(with M being the adult mass of the species; West et al. 2002), can be extrapolated to that of a single species during ontogenic growth, that is B(t)∼m(t)3/4. Moreover, it has also been assumed that Bc remains constant during growth, a feature which one expects to be qualitatively valid (Glazier 2005; West et al. 1999) in spite of observed quantitative deviations (Glazier 2005).
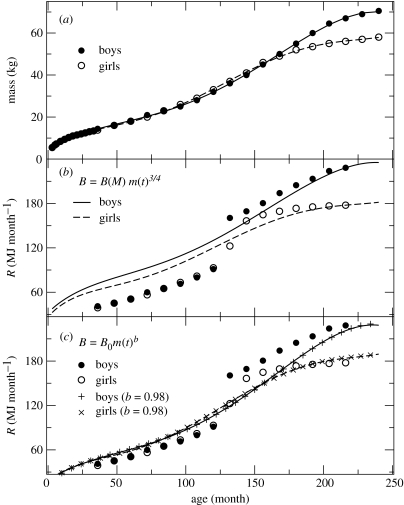
As is well known (Brody 1964), the fraction of energy expenditure devoted to body maintenance increasingly dominates body energy requirements up to adulthood, when body requirements are maximal and used almost exclusively for maintenance. Therefore, in children, a fraction of the daily energy consumption is spent to maintain tissues while the other fraction is used to build new tissues in the process of growth. We have compiled detailed data for the age dependence of mass in humans, the data being plotted in figure 2a. Solid circles depict the results for boys, whereas open circles show the results for girls. Solid and dashed lines are the result of lowest-order polynomial fits to the two sets of data, respectively, which accurately follow the experimental age dependence (in both cases, the quality of fits was excellent with correlation coefficients greater than 0.999). Given m(t) we compute B(t) following West et al. (2001) by first extrapolating the inter-species formula B(t)=BMm(t)3/4 to human growth. The results are shown in figure 2b, with solid (boys) and dashed (girls) lines, in which the constant BM was adjusted to match adult metabolic rates for both sexes. Experimental data are shown with symbols. Clearly, the curves account only for the variability of B(t) after puberty, being incapable of matching the energy expenditure during childhood. Indeed, good agreement for large values of B precludes a similar agreement for the lower values and vice versa. This fact has subtle consequences, as we show below. Note that in this argument, we are not considering the jumps in the metabolic rate which are well known to occur in many species during growth (Brody 1964). Moreover, these ‘jumps’ are also known to be species specific, without following any universal pattern.
Figure 2.
Ontogenic growth model for humans. Experimental data are represented with open circles for girls and solid circles for boys. (a) Experimental data for age dependence of mass. Solid and dashed lines correspond to polynomial fits to data and illustrate the quality of fit. (b) We test the assumption of West et al. of extrapolating to intra-specific resting metabolic rate during growth the 3/4 scaling obtained for inter-specific data. Clearly, this assumption is unable to account simultaneously for the low and high regimes of B(t). The Χ2 values for boys and girls are 55.4 and 30.4, respectively. (c) Introducing data from (a) (and their time derivatives) in equation (1.2) leads to the age-dependent solution for B(t) depicted with solid and dashed lines. Comparison with experimental data shows that these solutions of equation (1.2) account better for the overall age dependence of B(t), supported by the associated Χ2 values for boys and girls, which now become 21.6 and 9.8, respectively. A nonlinear least squares fit to these lines leads to nearly linear scaling exponents (b=0.98) for the rest metabolic rate as a function of mass during growth (+ and ×), in agreement with our prediction for the mass scaling of the active stem cell pool.
Let us now deduce the best possible scaling for B(t). To this end, we start from the balance equation proposed by West et al. (2001)
where Nc(t) is the number of cells at time t; Bc(t) is the cell metabolic rate at time t; and Ec is the average metabolic energy cost to produce a new cell and the sum extends to all cell types. We follow West et al. (2001) and assume that Bc does not depend on t; multiplying both terms by the cell mass mc and rearranging leads to1
| (1.2) |
When growth stops dm(t)/dt=0, m=M and Bc=B0M−1/4 (West et al. 2002). Using West et al. (2001) estimates for the different constants (mc=3×10−12 kg, Ec=2.1×10−5 J, Bc=9.77×10−6 J month−1) and introducing the experimental age-dependent curves for m(t) and the numerically computed derivatives dm(t)/dt, we obtain the age-dependent curves for B(t) shown in figure 2c with solid and dashed lines, respectively. The results qualitatively follow the trend of the data for boys and girls (solid and open circles): they start close to the lower metabolic regime at the early stages of post-natal life and smoothly approach the higher metabolic regimes as adulthood is reached. The associated quality of the fits improves correspondingly (figure 2). This suggests that the balance equation of West et al. (2001) provides an overall rationale for the growth process. Under this premise, we can now obtain the mass scaling of B(t) by fitting the lines in figure 2c with an allometric relation of the type B(t)=B0m(t)α. In both cases we obtain excellent fits with associated exponents αboys=αgirls=0.98 (+ and × symbols in figure, respectively, with associated correlation coefficients greater than 0.999). This ‘linear’ scaling of basal metabolic rate with mass during single species ontogenic growth correlates well with our predictions for the corresponding scaling of Nsc and restores the overall consistency for both intra- and inter-species allometric scaling relations. During growth, both the basal metabolic rate of humans and the size of their active stem cell pool exhibit an overall linear scaling with mass, although across adult mammals both quantities are found to follow a 3/4 power law allometric scaling.
4. Discussion
Childhood is a time for growth in preparation for adult life and during this period, many changes occur in body anatomy and physiology. In this report we explored the use of allometric scaling relations to investigate how the active haemopoietic stem cell pool increases with age and body mass during growth. The size of this pool is critically important not only in health but also in disease. Indeed, the risk of developing haemopoietic tumours is in part dependent on the size of this stem cell pool, since current evidence suggests that some cases of leukaemia and the myeloproliferative disorders arise by the acquisition of mutations in HSCs (Reya et al. 2001; Dick 2005; Wang & Dick 2005). This does not exclude the possibility that cells downstream of the HSCs pool can acquire stem cell-like properties due to mutations. In this sense, a small pool is susceptible to significant stochastic effects (Dingli et al. 2007b). Consequently, correct modelling of the cumulative risk of this group of neoplasms depends on an accurate assessment of the size of the pool of cells and how this changes with time.
There is a fundamental relationship between the size of the active stem cell pool and mass. Across species, Nsc∼M3/4 in mature mammals (Dingli & Pacheco 2006). During ontogenic growth, we find that the scaling relationship for humans is isometric, Nsc∼m(t). Interestingly, in pelagic animals, similar isometric scaling during ontogeny has been found recently by Glazier (2006).
The 3/4 exponent across species has been rationalized by West et al. (1999) as a surface to volume ratio in a hierarchical biological world in four dimensions. With respect to Nsc, the same 3/4 power scaling across mammals correlates well with microenvironmental models of stem cell heterogeneity (Uchida et al. 1993) or with its organization in niches within the bone marrow (Moore & Lemischka 2006), which may reflect common organizational principles. On the other hand, the study of Castriota-Scanderbeg et al. (1992) shows that the concentration of reticulocytes in human blood remains fairly constant during the lifetime of an individual, and hence one would expect an isometric scaling of reticulocyte production with mass. Our results confirm this point and suggest that, for humans, both resting metabolic rate and size of active stem cell pool exhibit the same (isometric) scaling during growth. Across mammals, resting metabolic rate and size of active stem cell pool also exhibit the same (3/4) scaling with mass. The difference of the scaling exponents reflects potentially important aspects of mammalian haemopoiesis. For instance, a 3/4 scaling for Nsc would predict that a newborn baby would have an active stem cell pool with the same size of that of an adult cat with the same weight, i.e. approximately 40 active stem cells (Dingli & Pacheco 2006). This is manifestly different from the prediction of our relation, approximately 22, i.e. half of the value expected based on a 3/4 relation. Despite the qualitative nature of the estimates, given the lack of quantitative confidence intervals, the impact of such a factor of 2 is significant. Taking into consideration the distinct metabolic rates of the two species, the implications of these different estimates concerning reticulocyte (and blood) production are considerable, let alone development of acquired HSCs disorders. Given our results in humans, the study of the active HSCs pool during growth in other mammals may provide insightful clues towards our understanding of the common principles behind the evolution of haemopoiesis.
Allometric considerations have additional implications for studies on haemopoiesis. Modelling haemopoiesis in the mouse is convenient but perhaps not completely relevant when applied to higher mammals: one stem cell is the absolute minimum required for haemopoiesis and in the typical laboratory mouse, this is sufficient to maintain blood cell production for the duration of the murine lifetime (Dingli & Pacheco 2006).
Our estimate that haemopoiesis is maintained by a small group of active stem cells, perhaps as low as 22 in a newborn has implications for gene therapists. Such a small number of cells present an obstacle because the stem cells that are actually transduced with the therapeutic vectors have to be selected to contribute to haemopoiesis. Thus, not only must the vectors used for therapy efficiently transduce non-dividing cells (since most HSCs are in G0), but positive selection will also be necessary to ensure that these modified cells actually contribute to haemopoiesis.
The present results, combined with those in Dingli & Pacheco (2006), provide a unified picture of the size of the active stem cell pool contributing to haemopoiesis during the lifetime of a human. These observations open the way for more quantitative investigations of dynamical processes in haemopoiesis, where stochastic effects (Dingli et al. 2007b) may prove increasingly important, given the small number of active stem cells which contribute to haemopoiesis during ontogeny.
Acknowledgments
We wish to thank Arne Traulsen for many insightful discussions and comments on this manuscript. This work was supported in part by Mayo Foundation (D.D.) and FCT Portugal (J.M.P.). Part of this work was carried out during a visit of J.M.P. to the Program for Evolutionary Dynamics that is supported by Jeffrey Epstein.
Endnotes
Assuming that B(t)∼mα, West equation leads to a differential equation for m(t)which may be written in the general form dm(t)/dt=amα−bmβ. L. von Bertalanffy (1957 and references therein) has studied the sensitivity of this equation to the exponents α and β; also Banavar et al. 2002.
References
- Abkowitz J.L, Catlin S.N, McCallie M.T, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. doi:10.1182/blood-2002-03-0822 [DOI] [PubMed] [Google Scholar]
- Banavar J.R, Damuth J, Maritan A, Rinaldo A. Ontogenetic growth: modelling universality and scaling. Nature. 2002;420:626. doi: 10.1038/420626a. doi:10.1038/420626a [DOI] [PubMed] [Google Scholar]
- Brody S. Hafner Press; Darien, CT: 1964. Bioenergetics and growth. [Google Scholar]
- Castriota-Scanderbeg A, Pedrazzi G, Mercadanti M, Stapane I, Butturini A, Izzi G. Normal values of total reticulocytes and reticulocyte subsets in children and young adults. Haematologica. 1992;77:363–364. [PubMed] [Google Scholar]
- Dick J.E. Stem cells: self-renewal writ in blood. Nature. 2003;423:231–233. doi: 10.1038/423231a. doi:10.1038/423231a [DOI] [PubMed] [Google Scholar]
- Dick J.E. Acute myeloid leukemia stem cells. Ann. N. Y. Acad. Sci. 2005;1044:1–5. doi: 10.1196/annals.1349.001. doi:10.1196/annals.1349.001 [DOI] [PubMed] [Google Scholar]
- Dingli D, Pacheco J.M. Allometric scaling of the hematopoietic stem cell pool across mammals. PLoS ONE. 2006;1:e2. doi: 10.1371/journal.pone.0000002. doi:10.1371/journal.pone.0000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D, Traulsen A, Pacheco J.M. Compartmental architecture and dynamics of hematopoiesis. PLoS ONE. 2007a;2:e345. doi: 10.1371/journal.pone.0000345. doi:10.1371/journal.pone.0000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D, Traulsen A, Pacheco J.M. Stochastic dynamics of hematopoietic tumor stem cells. Cell Cycle. 2007b;6:441–446. doi: 10.4161/cc.6.4.3853. [DOI] [PubMed] [Google Scholar]
- Glazier D.S. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. Camb. Philos. Soc. 2005;80:611–662. doi: 10.1017/S1464793105006834. doi:10.1017/S1464793105006834 [DOI] [PubMed] [Google Scholar]
- Glazier D.S. The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. BioScience. 2006;56:325–332. doi:10.1641/0006-3568(2006)56[325:TPLINU]2.0.CO;2 [Google Scholar]
- Goldson E, Reynolds A. Child development and behavior. In: Hay W.W, Levin M.J, Sondheimer J.M, Deterding R.R, editors. Current pediatric diagnosis and treatment. McgrawHill; New York, NY: 2006. pp. 66–101. [Google Scholar]
- Gordon M.Y, Lewis J.L, Marley S.B. Of mice and men…and elephants. Blood. 2002;100:4679–4680. doi: 10.1182/blood-2002-08-2517. doi:10.1182/blood-2002-08-2517 [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. doi:10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- Huxley J.S. Dial Press; New York, NY: 1932. Problems of relative growth. [Google Scholar]
- McCarthy K.F. Marrow frequency of rat long-term repopulating cells: evidence that marrow hematopoietic stem cell concentration may be inversely proportional to species body weight. Blood. 2003;101:3431–3435. doi: 10.1182/blood-2002-10-3026. doi:10.1182/blood-2002-10-3026 [DOI] [PubMed] [Google Scholar]
- Moore K.A, Lemischka I.R. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. doi:10.1126/science.1110542 [DOI] [PubMed] [Google Scholar]
- Morrison S.J, Uchida N, Weissman I.L. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. doi:10.1146/annurev.cb.11.110195.000343 [DOI] [PubMed] [Google Scholar]
- Raes A, Van Aken S, Craen M, Donckerwolcke R, Vande Walle J. A reference frame for blood volume in children and adolescents. BMC Pediatr. 2006;6:3. doi: 10.1186/1471-2431-6-3. doi:10.1186/1471-2431-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison S.J, Clarke M.F, Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. doi:10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; New York, NY: 1984. Why is animal size so important? [Google Scholar]
- Uchida N, Fleming W.H, Alpern E.J, Weissman I.L. Heterogeneity of hematopoietic stem cells. Curr. Opin. Immunol. 1993;5:177–184. doi: 10.1016/0952-7915(93)90002-a. doi:10.1016/0952-7915(93)90002-A [DOI] [PubMed] [Google Scholar]
- von Bertalanffy L. Quantitative laws in metabolism and growth. Q. Rev. Biol. 1957;32:217–231. doi: 10.1086/401873. doi:10.1086/401873 [DOI] [PubMed] [Google Scholar]
- Wang J.C, Dick J.E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. doi:10.1016/j.tcb.2005.07.004 [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H, Enquist B.J. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. doi:10.1126/science.284.5420.1677 [DOI] [PubMed] [Google Scholar]
- West G.B, Brown J.H, Enquist B.J. A general model for ontogenetic growth. Nature. 2001;413:628–631. doi: 10.1038/35098076. doi:10.1038/35098076 [DOI] [PubMed] [Google Scholar]
- West G.B, Woodruff W.H, Brown J.H. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc. Natl Acad. Sci. USA. 2002;99(Suppl. 1):2473–2478. doi: 10.1073/pnas.012579799. doi:10.1073/pnas.012579799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 1985 Energy and protein requirements: report of joint FAO/WHO/UNO expert consultation. World Health Organization Tech. Rep. Ser., vol. 724, pp. 1–206. [PubMed]