Abstract
While the facilitation–competition paradigm under the stress-gradient hypothesis has received recent attention, its rigorous testing is yet to be explored. Most of the studies have considered a switch in the net interactions from competition to facilitation with increasing environmental stress as primary evidence supporting the hypothesis, though few studies examined changes in interaction along a full range of a stress gradient. Here, we have conceptualized possible variations in the patterns of change in interaction strength along such gradient. Based on this, we empirically evaluated the temporal shift in the interaction between two marine sessile animals, goose barnacles (Capitulum mitella) and mussels (Septifer virgatus), under multiple stress factors. The net effect of goose barnacles on mussel survivorship was positively related to the total stress gradient encompassing two stress factors, physical disturbance and thermal stress, while no negative value occurred even under mild conditions. When the two stress factors were treated separately, however, the net effect demonstrated apparently different patterns: monotonic increase with physical disturbance versus a quasi-asymptotic pattern (no change over a wide range) with thermal stress. These variable situations have not previously been recognized in this discipline, and the present study emphasizes the importance of an integrative and mechanistic approach to testing and deciphering the facilitation–competition paradigm.
Keywords: facilitation, competition, environmental stress, net effect, stress-gradient hypothesis, species interaction
1. Introduction
Two opposite processes, facilitation and competition, have been recognized as key drivers in a wide range of natural communities (Bruno et al. 2003; Kawai & Tokeshi 2006). The relationship between the harshness of environmental stress and the relative importance of facilitation and competition has been conceptually formalized in the ‘stress-gradient hypothesis’, predicting that the net negative competitive effects are more important under relatively benign environmental conditions, whereas positive facilitative effects are more important under harsher conditions (Bertness & Callaway 1994; Callaway & Walker 1997; Brooker & Callaghan 1998). Note that the term ‘stress’ in this hypothesis should be interpreted to mean any environmental factor, the extreme conditions of which have some negative effects on the organisms involved (e.g. heat/desiccation, nutrient level, osmotic pressure, disturbance and grazing, as suggested by Bertness & Callaway (1994)), whereas plant ecologists tend to restrict its reference to some conditions such as desiccation and high temperature. The generality of this hypothesis has recently been under vigorous debate, particularly among plant researchers (Maestre et al. 2005, 2006; Lortie & Callaway 2006). The lack of a consistent approach for testing this hypothesis makes it difficult to achieve an integrated view of the relationships between stress gradients and species interaction. Most of the previous studies examined species interactions at only two levels of a stress factor (e.g. Pugnaire & Luque 2001), amounting at most to an estimation of a linear relationship. Clearly, assessment of species interaction under all possible levels of stress is necessary for a better understanding of the relationship (Maestre & Cortina 2004; Brooker et al. 2006; Lortie & Callaway 2006).
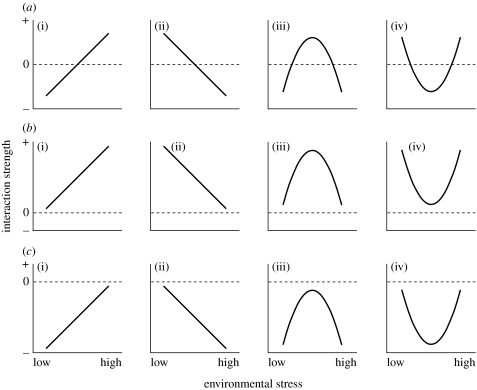
Conceptually, if a whole range of a stress gradient is taken into account, a system may show a facilitation–competition shift (figure 1a), facilitation-only (figure 1b) or competition-only (figure 1c), with either monotonic increase/decrease (cases (i) and (ii) of figure 1) or non-monotonic changes with a peak in the intermediate levels of stress (cases (iii) and (iv) of figure 1). For identifying these variable relationships, a shift in interaction must be examined along a sufficiently wide range of stress gradients covering both benign and severe conditions for target species. For example, if interaction was examined only along the left half of the stress gradient (under benign conditions) in case a(i), the relationship would be indistinguishable from case c(i). Likewise, the right half of case a(i) cannot be distinguished from case b(i). Moreover, the half parts of cases a(iii) and a(iv) have the same patterns as in cases a(i) and a(ii). Thus, researchers should be aware of potential misrepresentation and misinterpretation due to an inadequate assessment of interaction within narrow ranges (Belcher et al. 1995; Kadmon 1995; Foster 1999).
Figure 1.
Schematic showing the possible relationships between an environmental stress gradient and the strength of species interaction: (a) facilitation–competition shift, (b) facilitation-only and (c) competition-only, with (i) monotonic increase, (ii) monotonic decrease, (iii) positively peaked and (iv) negatively peaked.
In addition to this ‘range of coverage’ problem for a single stress factor, there is an issue of multiple environmental factors operating simultaneously when one deals with the facilitation–competition paradigm. In natural communities, two or more environmental factors often act at the same time as stresses for a species and may have separate and/or combined effects on the performance of that species, leading to variable relationships as depicted in figure 1. Despite this, most of the previous studies have dealt with the relationship between only one stress factor and net species interaction (e.g. Callaway et al. 2002; Maestre & Cortina 2004). Kawai & Tokeshi (2004) demonstrated that on a moderately wave-exposed intertidal rocky shore of Amakusa, southwestern Japan, the goose barnacle Capitulum mitella positively influenced the survivorship and growth of the mussel Septifer virgatus through amelioration of thermal stress and of physical disturbance caused by wave action. As the magnitude of these stress factors varies temporally in different ways and the tolerance of Septifer may differ depending on the factors, Septifer is expected to respond to such stress factors differently. Therefore, two different temporal stress gradients for Septifer must exist. This implies that the net effect of Capitulum on Septifer may probably have different relationships with different stress gradients. Furthermore, there is no obvious common unit for these stress factors, making it difficult to compare their harshness even for one species. One possibility is to estimate the harshness of environmental stress by the performance (e.g. mortality, growth or productivity) of each target species, not by the direct measurement of environmental conditions themselves (Grime 1977; Underwood 1989; Goldberg et al. 1999; Parker et al. 1999; Hastwell & Facelli 2003; Lortie & Callaway 2006).
The present study has aimed at an integrative and mechanistic approach to testing the stress-gradient hypothesis, using the Capitulum–Septifer pair as a model system. We have assessed temporal variations in (i) the combined and separate effects of two stress factors (thermal stress and physical disturbance) for Septifer and (ii) the net effect of Capitulum on Septifer. This represents the first attempt to tease apart the effects of multiple stress gradients in animal–animal interactions. In order to achieve this goal, we have employed a series of transplant experiments involving artificial patches separately controlling the effects of different stress factors.
2. Material and methods
The study was conducted on a moderately wave-exposed rocky shore (32°31′ N, 130°02′ E) of the Amakusa Shimoshima Island, southwestern Japan. A series of artificial patch transplant experiments involving artificial shade or transparent physical barriers were carried out in the upper intertidal zone (2.0–2.5 m above mean lower low water (MLLW)) during February–December 2003. Artificial shade reduced thermal stress only (caused by solar radiation), while transparent barriers reduced physical disturbance only (hydrodynamic forces caused by wave actions; see below). Experiments were conducted six times during the study period, each lasting four weeks.
In each experiment a total of 32 artificial patches were prepared and assigned to the following four treatments (eight replicates each): (i) Septifer-only, (ii) Septifer with artificial shade, (iii) Septifer with transparent physical barrier, and (iv) mixed (Septifer with Capitulum). Middle-sized Septifer (25–40 mm in shell length, the maximum anterior–posterior axis) and Capitulum (10–20 mm in rostral–tergal (R–T) length the length between the rostrum and the tip of the tergum) were collected from the upper intertidal habitats near the transplant site. For the Septifer-only, the Septifer with artificial shade and the Septifer with transparent physical barrier treatments, 10 Septifer were placed in the centre of a 10×10×1 cm ceramic tile and allowed to attach to the tile in the aquarium. For the mixed patches, 16 Capitulum were glued in a circle onto a ceramic tile using water-resistant epoxy resin, and 10 Septifer were placed within the Capitulum-circle and allowed to adjust their positions and attach to the tile and to one another by byssal threads. These tiles were kept in a laboratory running seawater aquarium for one week to allow firm attachment and acclimatization. Each artificial patch except the barrier treatment was covered with a predator exclusion cage (10×10×5 cm) constructed of 5×5 mm stainless mesh. This successfully excluded the predatory snails Morula musiva and Thais clavigera. The barrier patch was covered with a small cage (7×6×1.5 cm) made of 5×5 mm stainless mesh for preventing Septifer from being dislodged or damaged by wave action, but not protected from thermal stress. The shaded treatment involved coverage by a 10×10 cm double-layered canopy of white plastic screen (0.5×0.5 mm mesh) attached to the top of the cage to reduce solar radiation, but not physical disturbance. After all the mussels had attached firmly, the tiles (patches) were taken to the field and randomly placed on rock surfaces using stainless steel screws on a slightly sloping flat rock in the upper intertidal. Patch positions were randomized at the start of each experiment and all patches were removed at the end of each experiment. The mortality of Septifer was monitored for an experimental period of four weeks; this experimental period was considered appropriate, as the thermal stress fluctuated on an approximately monthly basis at this site (Arakaki & Tokeshi 2006). Parts of this experiment (Septifer-only and mixed treatments) were identical with those in our previous work (Kawai & Tokeshi 2006).
The harshness of stress factors for Septifer was quantified by the performance (i.e. mortality) of Septifer in the barrier treatment (thermal stress only), the shaded treatment (physical disturbance only) and the Septifer-only treatment (combined stress). The net effect of Capitulum was estimated as the difference in the performance (survivorship, the reciprocal of mortality) of Septifer between the mixed situation with Capitulum and the single situation using the log response ratio, lr (Gurevitch & Hedges 2001). The effect of each stress factor on the mortality of Septifer was tested using a three-way ANOVA, with physical disturbance and thermal stress as fixed factors and time as a random factor. This was based on the results that neither physical disturbance nor thermal stress had a significant negative effect on Septifer under the mixed situation with Capitulum throughout the year (see §3). To evaluate the relationship between the stress gradients expressed as temporal variation in the mortality of Septifer and the net effect of Capitulum, regression analysis was conducted. All statistical analyses were performed with the SPSS v. 11.5.1 package (SPSS, Inc.).
3. Results
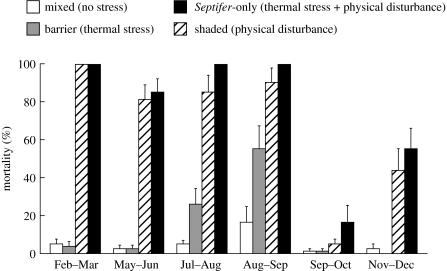
Stress factors for Septifer showed large temporal variations (0–100% mortality; figure 2). Mortality in the Septifer-only treatment was low (approx. 15%) in early autumn and intermediate (approx. 50%) in early winter, but was very high (90–100%) in all other seasons. In contrast, almost all Septifer survived in the mixed treatment with Capitulum, indicating no stress for mussel survivorship. Two stress factors showed different temporal patterns; physical disturbance was very strong all year round, except from early autumn to early winter, while thermal stress was substantial only in midsummer. There was no interaction between the two stress factors, suggesting that these stresses had additive effects on the mortality of Septifer throughout the year (table 1).
Figure 2.
Mortality of Septifer in the transplant experiments for six experimental periods (n=8 for each treatment). Values are untransformed means+1 s.e. Stress factors involved are shown in parentheses.
Table 1.
Results of a three-way ANOVA on the mortality of Septifer in a series of transplant experiments, with physical disturbance and thermal stress as fixed factors and time as a random factor. (Data were arcsine (square root) transformed before the analysis.)
| source of variation | d.f. | F | p |
|---|---|---|---|
| physical disturbance (p) | 1,5 | 25.444 | 0.004 |
| thermal stress (t) | 1,5 | 4.945 | 0.077 |
| time | 5,5.36 | 2.034 | 0.219 |
| p×t | 1,5 | 0.001 | 0.979 |
| p×time | 5,5 | 14.608 | 0.005 |
| t×time | 5,5 | 1.647 | 0.299 |
| p×t×time | 5,168 | 1.650 | 0.150 |
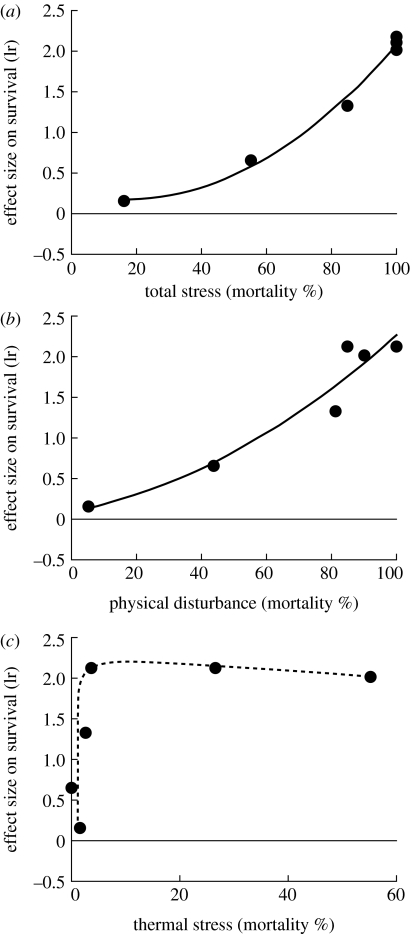
The net effect of Capitulum had a monotonically increasing relationship with the total stress gradient encompassing physical disturbance and thermal stress (figure 3a). However, no effect showed significantly negative values even under the mildest condition. Considering the net effect along two different stress gradients separately, it showed different patterns with different stress gradients (figure 3b,c). The net effect positively scaled with the harshness of physical disturbance (figure 3b), indicating a pattern similar to the total stress gradient (figure 3a). In contrast, the net effect had a quasi-asymptotic relationship with thermal stress (figure 3c), departing from the pattern observed for the total stress gradient (figure 3a).
Figure 3.
Relationships between the stress gradients and the net effect of Capitulum on the survivorship of Septifer. (a) Total stress expressed as the mortality of Septifer in the Septifer-only treatment versus the net effect of Capitulum (y=0.229−0.008x+0.00027x2, R2=0.99, p=0.0008). (b) Physical disturbance expressed as the mortality of Septifer in the shaded treatment versus the net effect of Capitulum (y=0.093+0.008x+0.00014x2, R2=0.93, p=0.0202). (c) Thermal stress expressed as the mortality of Septifer in the barrier treatment versus the net effect of Capitulum (a dashed line fitted by eye).
4. Discussion
Conceptualization of possible relationships under the stress-gradient hypothesis (figure 1) is considered useful, as the hypothesis lacks an integrated view of what patterns are to be expected and tested. Recent reviews (Maestre et al. 2005; Lortie & Callaway 2006) evaluating the predictability of the stress-gradient hypothesis have considered a significant switch in the net interactions from competition to facilitation with increasing environmental stress as primary evidence supporting the hypothesis. However, it should be pointed out that, without examining the patterns of change in interaction along the stress gradient, differences among cases a(i), a(iii) and a(iv) of figure 1, for example, cannot be detected. Furthermore, changes in interaction strength without a facilitation–competition shift (figure 1b,c) are also possible. Indeed, our study has experimentally demonstrated a monotonically increasing relationship between the total stress gradient for Septifer covering a wide range (16–100% mortality in average per month) and the net effect of Capitulum on Septifer in the positive range of interaction (figure 3a). The strength of the facilitative effect of Capitulum on Septifer survival increased with increasing environmental stress on the temporal scale, while competitive effects never appeared even under mild conditions, coincident with case b(i) in figure 1.
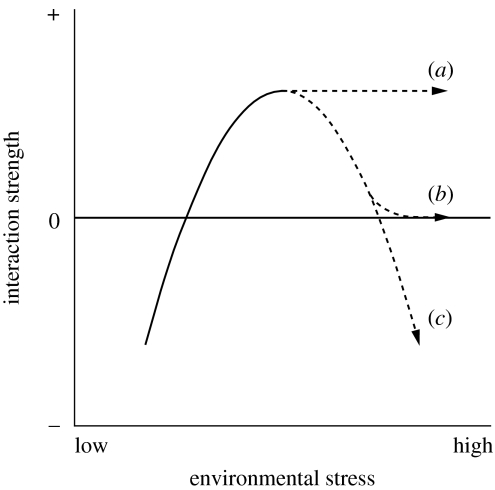
Liancourt et al. (2005) suggested that a species having both low tolerance to a particular environmental stress and a strong competitive-response ability is likely to benefit from other species without receiving competitive effects. Septifer with a faster growth rate (15 mm yr−1 in shell length; Morton 1995) and low tolerance of both thermal stress and physical disturbance may fit this picture. In addition, a potentially low competitive ability of Capitulum with a slow growth rate (2 mm yr−1 in rostral–carinal length; Nakamura & Tanaka 1995) may also have contributed to the present observation. However, it is still uncertain whether such a monotonically increasing relationship within the positive range (case b(i) of figure 1) is common among natural communities. Callaway et al. (2002), in one of the few studies where sufficient data were gathered for this type of analysis, found an asymptotic relationship in alpine plant communities. The importance of the facilitative effect of plants on neighbours increased towards an asymptote with environmental harshness (left half of case a(iii) of figure 1). Brooker et al. (2006) demonstrated another case in which heather had a facilitative effect on pine saplings through mitigation of deer browsing impact, particularly at the intermediate levels of grazing, resulting in a hump-backed relationship between the facilitative effect and grazing pressure (case b(iii) of figure 1). In this circumstance, the potential for heather to hide the saplings was reduced in areas with high deer density due to increased foraging. In general, the importance and/or intensity of facilitative effects may reach an asymptote (figure 4a) or even decline (figure 4b) with increasing environmental stresses, probably because facilitators are less successful in ameliorating stress factors and promoting the survival of facilitated species under very severe environmental conditions (Michalet et al. 2006). Furthermore, if stress factors coincide with resource factors for both the facilitator and the facilitated species, such as water for plants, competitive effects may manifest under extremely severe conditions (Tielbörger & Kadmon 2000; Maestre & Cortina 2004; Michalet 2007; figure 4c). In addition, different main stress factors among studies (e.g. disturbance, this study; low temperature, Callaway et al. 2002; grazing, Brooker et al. 2006) may also lead to variation in the shape of the relationship. More empirical and theoretical work examining not only the switch in interaction but also the whole shape of relationship under various stress factors will be needed for a comprehensive testing of the stress-gradient hypothesis.
Figure 4.
Schematic showing possible variation in the relationship between an environmental stress gradient and the strength of species interaction under harsh environmental conditions. Facilitative effect may (a) reach an asymptote, (b) decline to a neutral level or (c) turn into a negative value (competitive effect) with increasing environmental stress.
In our study site, two stress factors, physical disturbance and thermal stress, had a significant influence on the performance of Septifer, showing different temporal stress gradients (figure 2). Considering these two stress gradients separately, the net effect along the gradient of thermal stress revealed a quite different pattern from that along the gradient of physical disturbance (figure 3b,c). The net effect of Capitulum involves two different stress-ameliorating functions, amelioration of thermal stress and of physical disturbance (Kawai & Tokeshi 2004), suggesting that the gradient of each stress factor particularly thermal stress in this case may not solely explain the shift of the net effect. These results suggest that consideration of the net effect along just one particular stress gradient may be misleading.
Most of the previous studies have dealt with only one environmental condition such as precipitation or temperature as an indicator of environmental harshness (e.g. Tielbörger & Kadmon 2000; Callaway et al. 2002; Maestre & Cortina 2004; Kikvidze et al. 2005). Although Maestre & Cortina (2004) used variation in precipitation among sites as a spatial stress gradient, the survivorship of Pistacia without tussock grass neighbours did not show a monotonic change with the precipitation level. Other factors could possibly have had a large influence on shrub survival, suggesting that examining the relationship between the net effect and precipitation would not be sufficient for testing the stress-gradient hypothesis. Non-monotonic relationships between the net effect and one stress gradient revealed in the study by Maestre & Cortina (2004) and in this study (figure 3c) may imply the involvement of other stress factor(s).
Several kinds of stress factors usually influence facilitated species and the net effect of facilitators may also include multiple habitat-modifying functions as well as competitive effects. Consequently, for a rigorous testing of the stress-gradient hypothesis, the relationship between the net effect encompassing multiple components and the total stress gradient should be examined. We suggest that the performance of organisms, such as productivity, growth and survivorship, under the single-species situation may be used as a surrogate for the harshness of the combined stresses. However, it should be noted here that sensitivity to environmental stresses often differs among these performance measures. For quantifying stress gradients, growth or productivity with potentially high sensitivity to stress factors may be effective under benign environmental conditions, while survivorship with relatively lower sensitivity may be adequate under harsher conditions. Improvement in our understanding of the relationship between species interaction and stress gradients through an integrative and mechanistic approach as used in this and our previous studies (Kawai & Tokeshi 2006) is considered crucial for making reliable predictions about the possible changes in community structure under variable environmental conditions, particularly in the scenario of ongoing climate changes (Harley et al. 2006).
Acknowledgments
This experiment was carried out under the guidance for Animal Experiments in the Faculty of Science, Kyushu University and the Law (No. 105) and Notification (No. 6) of the Japanese Government.
We thank Minako, Aya and Taka'aki for their help with fieldwork, and Masako, Teruo, Kentaro, Kazuko and other members of the Amakusa Marine Biological Laboratory for various logistical support, encouragement and advice throughout this study. We are also grateful to two anonymous reviewers for their valuable comments on earlier drafts of this manuscript. This research was partly supported by the twenty-first century COE programme from the Ministry of Education, Culture, Sports, Science and Technology, the Kyushu University P & P programme, the Fujiwara Natural History Foundation (a research grant to T.K.) and the Japan Society for the Promotion of Science (‘Grant-In-Aid’ nos. 05J05862 to T.K., 14255013 and 14340246 to M.T.).
References
- Arakaki S, Tokeshi M. Short-term dynamics of tidepool fish community: diel and seasonal variation. Environ. Biol. Fish. 2006;76:221–235. doi:10.1007/s10641-006-9024-5 [Google Scholar]
- Belcher J.W, Keddy P.A, Twolan-Strutt L. Root and shoot competition intensity along a soil depth gradient. J. Ecol. 1995;83:673–682. doi:10.2307/2261635 [Google Scholar]
- Bertness M.D, Callaway R. Positive interactions in communities. Trends Ecol. Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. doi:10.1016/0169-5347(94)90088-4 [DOI] [PubMed] [Google Scholar]
- Brooker R.W, Callaghan T.V. The balance between positive and negative plant interactions and its relationship to environmental gradients: a model. Oikos. 1998;81:196–207. doi:10.2307/3546481 [Google Scholar]
- Brooker R.W, Scott D, Palmer S.C.F, Swaine E. Transient facilitative effects of heather on Scots pine along a grazing disturbance gradient in Scottish moorland. J. Ecol. 2006;94:637–645. doi:10.1111/j.1365-2745.2006.01129.x [Google Scholar]
- Bruno J.F, Stachowicz J.J, Bertness M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003;18:119–125. doi:10.1016/S0169-5347(02)00045-9 [Google Scholar]
- Callaway R.M, Walker L.R. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- Callaway R.M, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. doi:10.1038/nature00812 [DOI] [PubMed] [Google Scholar]
- Foster B.L. Establishment, competition and the distribution of native grasses among Michigan old-fields. J. Ecol. 1999;87:476–489. doi:10.1046/j.1365-2745.1999.00366.x [Google Scholar]
- Goldberg D.E, Rajaniemi T, Gurevitch J, Stewart-Oaten A. Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology. 1999;80:1118–1131. [Google Scholar]
- Grime J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977;111:1169–1194. doi:10.1086/283244 [Google Scholar]
- Gurevitch J, Hedges L.V. Meta-analysis: combining the results of independent experiments. In: Scheiner S.M, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd edn. Oxford University Press; Oxford, UK: 2001. pp. 347–369. [Google Scholar]
- Harley C.D.G, Hughes A.R, Hultgren K.M, Miner B.G, Sorte C.J.B, Thomber C.S, Rodriguez L.F, Tomanek L, Williams S.L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. doi:10.1111/j.1461-0248.2005.00871.x [DOI] [PubMed] [Google Scholar]
- Hastwell G.T, Facelli J.M. Differing effects of shade-induced facilitation on growth and survival during the establishment of a chenopod shrub. J. Ecol. 2003;91:941–950. doi:10.1046/j.1365-2745.2003.00832.x [Google Scholar]
- Kadmon R. Plant competition along soil moisture gradients: a field experiment with the desert annual Stipa capensis. J. Ecol. 1995;83:253–262. doi:10.2307/2261564 [Google Scholar]
- Kawai T, Tokeshi M. Variable modes of facilitation in the upper intertidal: goose barnacles and mussels. Mar. Ecol. Prog. Ser. 2004;272:203–213. [Google Scholar]
- Kawai T, Tokeshi M. Asymmetric coexistence: bidirectional abiotic and biotic effects between goose barnacles and mussels. J. Anim. Ecol. 2006;75:928–941. doi: 10.1111/j.1365-2656.2006.01111.x. doi:10.1111/j.1365-2656.2006.01111.x [DOI] [PubMed] [Google Scholar]
- Kikvidze Z, Pugnaire F.I, Brooker R.W, Choler P, Lortie C.J, Michalet R, Callaway R.M. Linking patterns and processes in alpine plant communities: a global study. Ecology. 2005;86:1395–1400. doi:10.1890/04-1926 [Google Scholar]
- Liancourt P, Callaway R.M, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. doi:10.1890/04-1398 [Google Scholar]
- Lortie C.J, Callaway R.M. Re-analysis of meta-analysis: support for the stress-gradient hypothesis. J. Ecol. 2006;94:7–16. doi:10.1111/j.1365-2745.2005.01066.x [Google Scholar]
- Maestre F.T, Cortina J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc. R. Soc. B. 2004;271(Suppl.):S331–S333. doi: 10.1098/rsbl.2004.0181. doi:10.1098/rsbl.2004.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre F.T, Valladares F, Reynolds J.F. Is the change of plant–plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol. 2005;93:748–757. doi:10.1111/j.1365-2745.2005.01017.x [Google Scholar]
- Maestre F.T, Valladares F, Reynolds J.F. The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: further insights from arid environments. J. Ecol. 2006;94:17–22. doi:10.1111/j.1365-2745.2005.01089.x [Google Scholar]
- Michalet R. Highlighting the multiple drivers of change in interactions along stress gradients. New Phytol. 2007;173:3–6. doi: 10.1111/j.1469-8137.2006.01949.x. doi:10.1111/j.1469-8137.2006.01949.x [DOI] [PubMed] [Google Scholar]
- Michalet R, Brooker R.W, Cavieres L.A, Kikvidze Z, Lortie C.J, Pugnaire F.I, Valiente-Banuet A, Callaway R.M. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. doi:10.1111/j.1461-0248.2006.00935.x [DOI] [PubMed] [Google Scholar]
- Morton B. The population dynamics and reproductive cycle of Septifer virgatus (Bivalvia, Mytilidae) on an exposed rocky shore in Hong Kong. J. Zool. 1995;235:485–500. [Google Scholar]
- Nakamura R.K.G, Tanaka M. Effects of aggregation on growth and survival of the intertidal stalked barnacle, Capitulum mitella. Benthos Res. 1995;49:29–37. [Google Scholar]
- Parker E.D, et al. Stress in ecological systems. Oikos. 1999;86:179–184. doi:10.2307/3546584 [Google Scholar]
- Pugnaire F.I, Luque M.T. Changes in plant interactions along a gradient of environmental stress. Oikos. 2001;93:42–49. doi:10.1034/j.1600-0706.2001.930104.x [Google Scholar]
- Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- Underwood A.J. The analysis of stress in natural populations. Biol. J. Linn. Soc. 1989;37:51–78. [Google Scholar]