Abstract
Apoptosis plays critical role in diabetic cardiomyopathy and endoplasmic reticulum stress (ERS) is one of intrinsic apoptosis pathways. For previous studies have shown that endoplasmic reticulum become swell in diabetic myocardium and ERS was involved in diabetes mellitus and heart failure, this study aimed to demonstrate whether ERS was induced in myocardium of streptozocin (STZ)-induced diabetic rats. We established type 1 diabetic rat model with STZ intraperitoneal injection, used echocardiographic evaluation, hematoxylin-eosin staining and the terminal deoxynucleotidyl transferase-mediated DNA nick-end labeling staining to identify the existence of diabetic cardiomyopathy and enhanced apoptosis in the diabetic heart. We performed immunohistochemistry, Western blot and real time PCR to analysis two hallmarks of ERS, glucose regulated protein78 (Grp78) and Caspase12. We found both Grp78 and Caspase12 had enhanced expression in protein and mRNA levels in diabetic myocardium than normal rat’s, and Caspase12 was activated in diabetic heart. Those results suggested that ERS was induced in STZ-induced diabetic rats’ myocardium, and ERS-associated apoptosis took part in the pathophysiology of diabetic cardiomyopathy.
Keywords: diabetic cardiomyopathy, endoplasmic reticulum stress, apoptosis, Grp78, Caspase12
Introduction
In humans and animal models of diabetes, a heart muscle-specific disease in the absence of any vascular pathology has been described, termed diabetic cardiomyopathy [1, 2]. The pathogenesis of diabetic cardiomyopathy is a chronic and complex process that is attributed to abnormal cellular metabolism and defects in organelles such as myofibrils, mitochondria, sarcolemma, and endoplasmic reticulum (ER) [3–7]. However, the mechanisms of diabetic cardiomyopathy are not fully known, and appropriate approaches to minimize these risks are still being explored. Probable candidates to explain this heart disease include autonomic abnormalities, metabolic disorders, abnormal enzyme function, and interstitial fibrosis [8–10]. Apoptosis, a regulated, energy-dependent, cell suicide mechanism was also been reported to play a critical role in the development of diabetic cardiomyopathy [3, 11, 12].
As an organelle involved in the intrinsic pathway of apoptosis [13], the ER serves several important functions and participates in the folding of secretory and membrane proteins. Various conditions can disturb ER’s functions and result in endoplasmic reticulum stress (ERS), these conditions include ischemia, hypoxia, gene mutation, elevated protein synthesis, and exposure to free radicals. Several signaling pathways are initiated to cope with ERS, which were designated as the unfolded protein response (UPR) [14]. One major pathway of UPR is up-regulate expression of ER-localized molecular chaperons, such as glucose regulated protein 78 (Grp78), which can contribute to repair unfolded proteins. When ERS become increased or UPR is compromised, cell apoptosis can be led through known ways including activating Caspase12, which is a representative caspase implicated in the cell death-executing mechanisms relevant to ERS [15].
Evidences have demonstrated that apoptosis initiated by the ERS was involved in pathogenesis of diabetes and heart failure [16–18], and ultrastructural analysis found ER was swelling in diabetic model’s myocardium [19, 20], but the relationship between ERS and diabetic cardiomyopathy is unknown.
Materials and Methods
Animals
Thirty male Wistar rats from animal center of Shandong University (8 weeks of age; mean body weight 280 ± 10 g) were used. The rats were housed by cage with free access to normal rat diet and tap water of the center. They were maintained under conditions of standard lighting (alternating 12 h light/dark cycle), temperature (22 ± 0.5°C) and humidity (60 ± 10%) for at least 1 week before the experiments. All experiments were carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee of Shandong University. Twenty rats were given a single intraperitoneal dose (65 mg/kg) of STZ (S0130, Sigma-Aldrich, MO) while the rest 10 rats were given a same dosage of saline. Diabetes was confirmed by measuring the venous circulating plasma concentrations of glucose. Seven days post-STZ injection, blood samples were obtained from the rat-tail vein and the glucose concentration was determined with an automatic analyzer (One Touch SureStep Meter, LifeScan, CA). Nineteen rats reached the diabetic rat standard of a fasting blood glucose level higher than 300 mg/dl, while the normal value in control healthy rats injected with saline ranges from 90 to 130 mg/dl. Two STZ-diabetic rats were died from infection at the time of 2 and 10 weeks. At the time of 16 weeks post-saline or STZ injection, 17 STZ-diabetic rats were left, while the control group rats were all survived.
Echocardiographic evaluation
At 16 weeks of study, before sacrifice, each rat was anaesthetized with Ketamine HCl (50 mg/kg) and Xylazine (10 mg/kg), placed in the left lateral decubitus position. Chest of the rat was shaved and a layer of acoustic coupling gel was applied to the thorax, two-dimensional and M-mode echocardiography was performed using a commercially available 12-MHz linear-array transducer system and echocardiogram machine (Sonos 5500, HP, MA). M-mode recordings were obtained of the left ventricle at the level of mitral valve in the parasternal view using two-dimensional echocardiographic guidance in both the short and long axis views. Pulsed wave Doppler was used to examine mitral diastolic inflow from the apical four chamber view. For each measurement, data from three consecutive cardiac cycles were averaged. All measurements were made from digital images captured at the time of the study by use of inherent analysis software. The results are in Table 1.
Table 1.
The sequence for each primer and product length
| sequence | length | |
|---|---|---|
| Grp78 | F5' TCAGCCCACCGTAACAAT 3' | 275 bps |
| R5' CAAACTTCTCGGCGTCAT 3 | ||
| Caspase12 | F5' GGAAGGTAGGCAAGAGT 3' | 179 bps |
| R5' GTAGAAGTAGCGTGTCATA 3' | ||
| GAPDH | F5' GTCGGTGTCAACGGATTTG 3' | 397 bps |
| R5' ACAAACATGGGGGCA TCAG 3' |
The primers’ sequence and product length designed for Grp78, Caspase12 and GAPDH.
Histopathology, TUNEL stain and immunohistochemistry
Two days after echocardiographic evaluation, rats were anaesthetized again, hearts were removed from the animals, sliced transversely, 8 diabetic and 5 control hearts were fixed in 4% neutral buffered formalin and then paraffin-embedded for light microscopic evaluation. Nine diabetic and 5 control hearts were frozen at −80°C for Western-blot and real time PCR use. Experimental procedures were adhered to the guidelines of Shandong University’s Code for the Care and Use of Animals for Scientific Purposes. All studies were performed with observer masked to study groups to which the animals had been assigned. Hearts embedded in paraffin were sectioned at 5 µm and studied with hematoxylin-eosin stain, a TUNEL assay kit, and immunohistochemistry. Sections were examined using light microscopy, and analyzed with a computer-assisted color image analysis system (Image-ProPlus 5.1, Media Cybernetics, MD).
Assessment for apoptosis was conducted using a commercially available TUNEL assay kit (code number KGA 703, Keygen Biotechnology, China). Briefly, sections were deparaffinized, digested with proteinase K (20 µg/ml), at room temperature for 15 min, and soaked in phosphate buffer saline (PBS) for 5 min. Each section was covered with a terminal deoxynucleotidyl transferase (TDT) enzyme solution containing 45 µl equilibration buffer, 1 µl biotin-11-dUTP, 4 µl TDT enzyme and incubated for 1 h at 37°C in a humidified chamber. The sections were immersed in stop buffer to terminate the enzymatic reaction and then gently rinsed with PBS. 50 µl streptavidin-horseradish peroxidase (HRP) solution containing 0.25 µl streptavidin-HRP and 49.75 µl PBS was then applied to each section and incubated at room temperature for 30 min in the dark. Slides were washed in PBS, exposed for 5–7 min to DAB + chromogen (code number ZLI-9032, Zhongshan Golden Bridge Biotechnology, China). Then slides were rinsed in water and counterstained with hematoxylin. Then the sections were examined using light microscopy. Sections incubated with PBS, instead of TDT enzyme solution, served as the negative controls.
Tissue expression of Caspase12 and Grp78 was assessed immunohistochemically using a polyclonal anti-Caspase12 (code number sc1611, Santa Cruz Biotechnology, CA) and a monoclonal anti-Grp78 antibody (code number sc1050, Santa Cruz Biotechnology, CA). After deparaffinization, endogenous peroxidase activity was quenched with 30% methanol and 0.3% hydrogen PBS. Then the slides were boiled in citrate buffer with microwaves. After blocking nonspecific binding with 5% bovine serum albumin, the slides were incubated with primary antibodies overnight at 4°C (dilutions for each antibody: anti-Caspase12 1:200; anti-Grp78 1:400). The following day the sections were thoroughly washed in PBS and incubated with a peroxidase-conjugated polymer which carries antibodies to rat (1:100) and goat (1:200) immunoglobulin (code number ZB-2307, ZB-2306 Zhongshan Golden Bridge Biotechnology, China) for 30 min. After rinsing with PBS, the sections were exposed for 7 min to DAB + chromogen. The slides were rinsed in water and counterstained with haematoxylin. The immunohistochemical staining of the samples was performed at different times but by the same technical personnel. Sections incubated with PBS, instead of the primary antiserum, served as the negative controls.
RNA expression analysis
Total RNA was extracted from normal and diabetic rat hearts by the single-step acid-guanidium-phenol chloroform method [21] using Trizol reagent (code number 15596-026, CA) according to the manufacturer’s instructions. RNA was quantified by UV spectrophotometry at 260/280 nm and diluted to 1 µg/µl in diethyl pyrocarbamate (DEPC)-treated water. RNA samples were analyzed by formaldehyde-agarose gel electrophoresis, and integrity was confirmed by visualization of 18S and 28S rRNA bands. First-strand cDNA was synthesized in a 40 µl total volume by using oligo (dT) 12–18 primer and M-MLV reverse-transcriptase (code number m1701, Promega, WI). To reduce pipetting variation, a master RT mixture was prepared and added to each of the different RNA samples. Briefly, reverse-transcriptase was performed in a 40 µl volume mixture made up of 6 µl of total RNA, 2 µl Olig (dT), 8 µl of reverse-transcriptase buffer, 2 µl of 2.5 mM dNTP mixture, 2 µl M-MLV reverse-transcriptase, and 20 ml DEPC-treated water. The condition was incubated at 37°C for 1 h, heated at 95°C for 5 min. The cDNA were stored at −20°C for use. Sequence for each primer and product are in Table 2.
Table 2.
Transmitral Doppler flow velocity recordings in normal (n = 10) and diabetic (n = 17) rats at 1st and 16th week
| 1st week |
16th week |
|||
|---|---|---|---|---|
| Normal | Diabetic | Normal | Diabetic | |
| E | 53.25 ± 5.57 | 52.24 ± 5.62 | 55.34 ± 5.67 | 30.45 ± 7.56*# |
| A | 27.34 ± 6.57 | 31.09 ± 5.65 | 28.45 ± 6.67 | 53.45 ± 6.87*# |
| E/A | 2.11 ± 0.31 | 1.76 ± 0.13 | 2.05 ± 0.45 | 0.61 ± 0.06*# |
| Eat | 28.15 ± 6.45 | 27.89 ± 5.89 | 27.45 ± 6.34 | 16.95 ± 5.45*# |
| Edt | 48.34 ± 6.59 | 47.23 ± 7.12 | 48.98 ± 7.01 | 52.48 ± 7.14 |
| EF | 61.34 ± 7.76 | 61.57 ± 4.42 | 60.56 ± 5.10 | 38.05 ± 7.76*# |
| FS | 27.39 ± 3.19 | 27.18 ± 4.67 | 28.16 ± 4.08 | 16.31 ± 5.27*# |
Results shown are mean ± S.D. E: peak early transmitral filling velocity during early diastole; A: peak transmitral atrial filling velocity during late diastole; Eat: acceleration time of E-wave; Edt: deceleration time of E-wave; EF: ejection fraction; FS: fractional shortening.
p<0.05 compared to 1st week data.
p<0.05 compared to normal rats.
Real time PCR was carried out using the real time detection system (ABI Prism 7000, CA) with a real time PCR master mix (code number: QPK-201,Toyobo, Japan), which contains SYBR-Green I as fluorescent dye enabling real-time detection of PCR products, the protocol was according to the manufacturer. A 50 µl volume mixture made up of: sense primer 1 µl (10 µM), antisense primer 1 µl (10 µM), real time PCR master mix 25 µl, (DEPC)-treated water 18 µl and cDNA 5 µl. Cycling conditions were 95°C for 3 min, followed by 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s. For quantification, the mRNA of Grp78, Caspase12 gene was normalized to the mRNA of the internal standard gene glyceraldehyde phosphate dehydrogenase (GAPDH).
Western blot
Protein from diabetic and normal rat hearts was extracted for western-blot use. The tissue samples were homogenized in a lysis buffer (0.1 mol/l NaCl, 0.01 M Tris-HCl PH 7.5, 1 mM EDTA, and 1 µg/ml Aprotinin), then the homogenates were centrifuged at 7000 g for 15 min at 4°C. The supernatants were used as protein samples. We used a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) to determine each sample’s protein concentration, then performed Western blot analysis with a 5% acrylamide stacking gel and a 14% acrylamide resolving gel, 60 µg of protein was subjected to gel electrophoresis. Proteins from the gel to nitrocellulose membranes (code number LC2006, Invitrogen, CA) was performed for 50 min at 120 V. Nonspecific protein binding to the nitrocellulose membrane was reduced by pre-incubating the membrane with blocking buffer (5% nonfat dry milk, 2.7 mM KCl, 137 mM NaCl, 8 mM NaHPO4, 1.4 mM KPO4 and 0.1% Tween 20) for 2 h at room temperature. Then incubation with the monoclonal antibody anti-β actin (1:1,000 diluted in blocking buffer, Zhongshan Golden Bridge Biotechnology, China), anti-Grp78 (1:500) anti-Casepase12 (1:1,000) was performed overnight at 4°C, then incubation with secondary antibody (anti-rabbit, anti-rat and anti-goat IgG conjugated to HRP, in blocking buffer 1:1,000, Zhongshan Golden Bridge Biotechnology, China) lasted 1 h, also at room temperature. The reaction was visualized by chemiluminescence (Enhanced Chemiluminescent Kit, ECL, Amersham). The film was scanned with an imaging densitometer (fluorchem HD IS-9900, Alpha, CA) and the optical density was quantified using Multi-Analyst software.
Statistics
All the results are expressed as mean ± SD. The individual groups were tested for differences by using one-way ANOVA repeated measurements followed by independent samples t test. Differences were considered as being statistically significant at p<0.05.
Results
Experimental animals
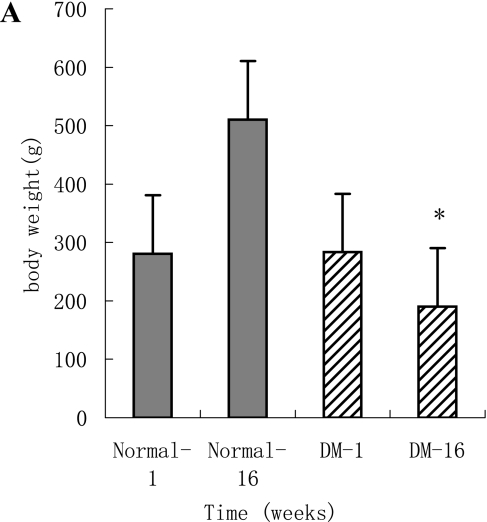
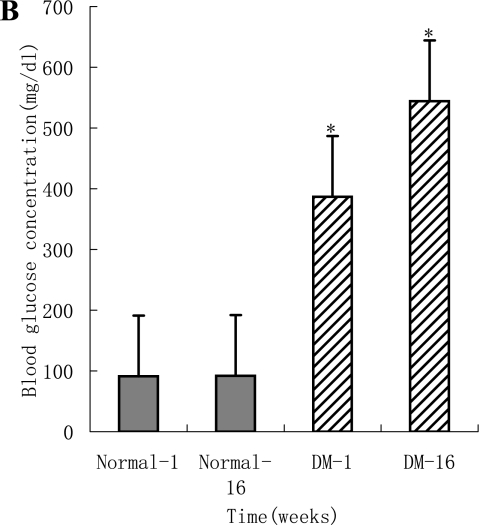
The body weight and blood glucose levels are shown in Fig. 1A and B. Left ventricular systolic function parameters, fractional shortening (%) and ejection fraction of the diabetic animals were significantly reduced compared to their 1st week levels and normal animals at 16 weeks (Table 2). Left ventricular diastolic function variables expressed by the E-wave (early diastolic filling, early peak velocity) and A-wave (late atrial filling, atrial peak velocity) differed significantly in diabetic animals compared to their 1st week levels and normal animals at 16 weeks. A significant decrease was found in the E-wave velocity, increase in the A-wave velocity and significant decrease in the E/A ratio after 16 weeks in the diabetic groups, with a significant decrease in the fractional shortening and ejection fraction. The altered cardiac diastolic performance is thought to result from a reduced cardiac compliance.
Fig. 1.
A: Body weight in the diabetic rats was significantly decreased at 16 weeks compared to their 1 week levels and normal rats (p<0.05). The body weight of normal rats increased at 16 weeks compared to their 1 week levels (p<0.05). B: Blood glucose levels in the diabetic animals have shown a significant raise at 1 week after diabetes induction compared to the normal rats (p<0.05). A significant increase in the blood glucose was observed in diabetic rats at 16 weeks.
Haematoxylin-eosin, TUNEL and histological studies
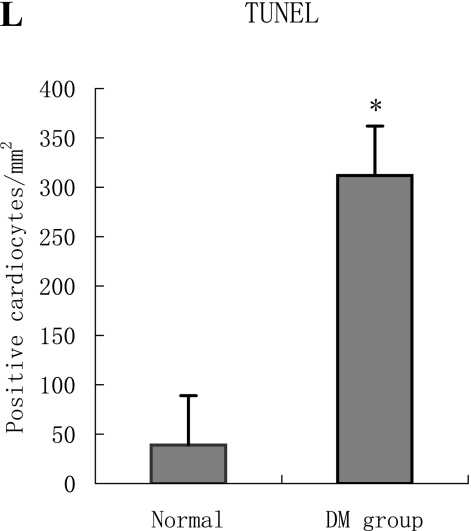
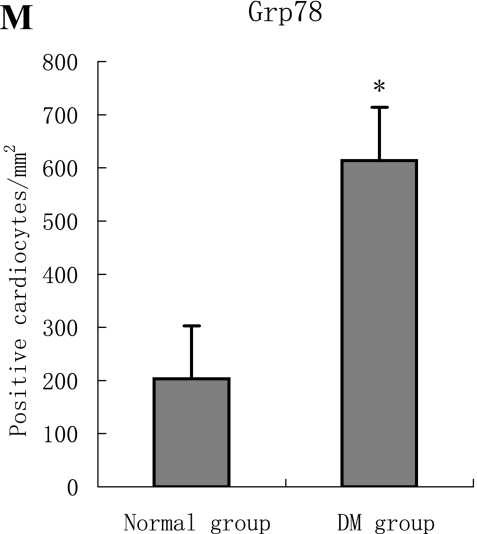
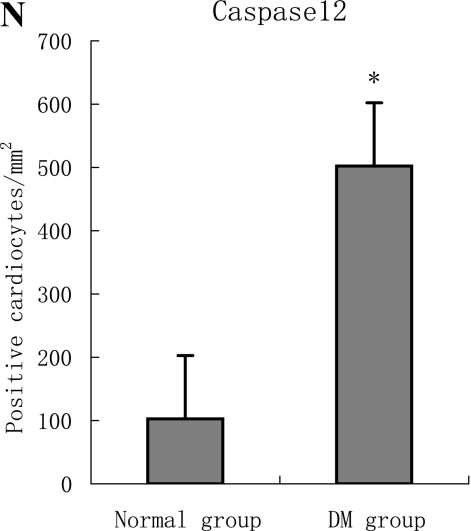
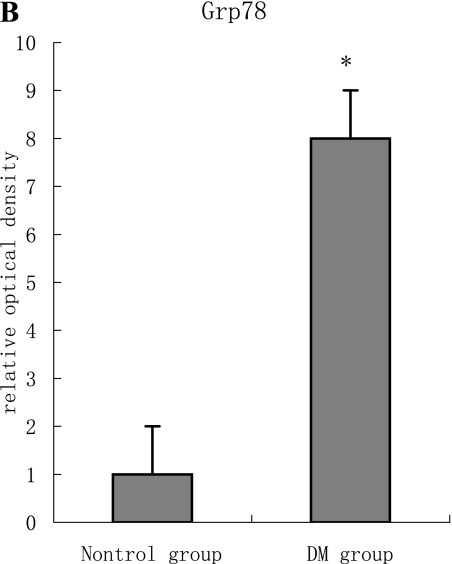
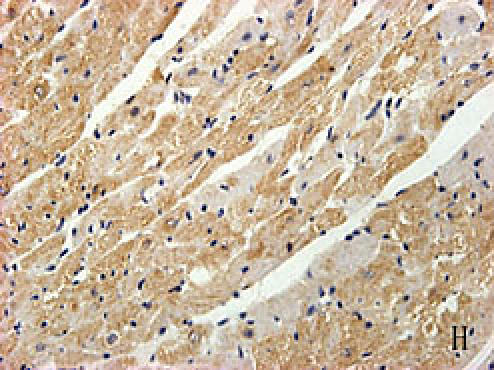
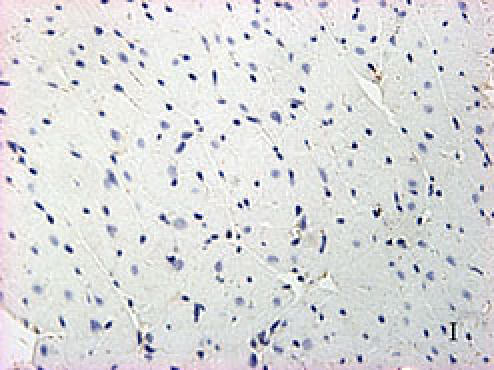
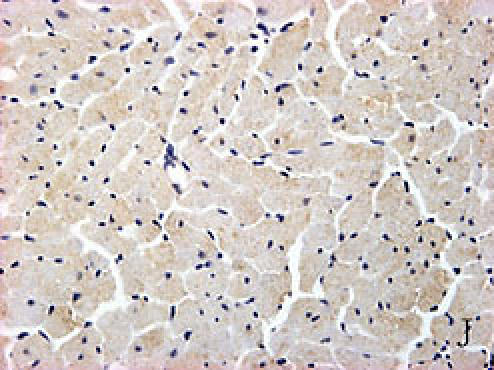
With hematoxylin-eosin and TUNEL staining, the characteristics of diabetic cardiomyopathy and more apoptotic cardiocytes and endothelial cells were found in diabetic group. Correspondingly, the two ERS hallmarks, Grp78 and Caspase12, in the diabetic heart showed strong immunoreactivity than normal group. (Fig. 2 A–N)
Fig. 2.
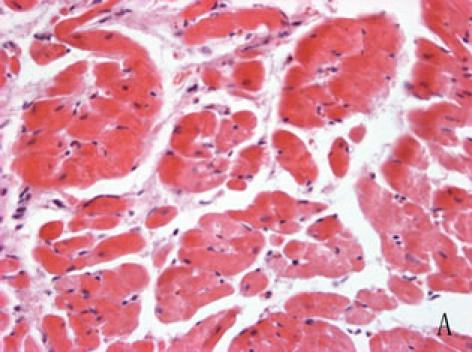
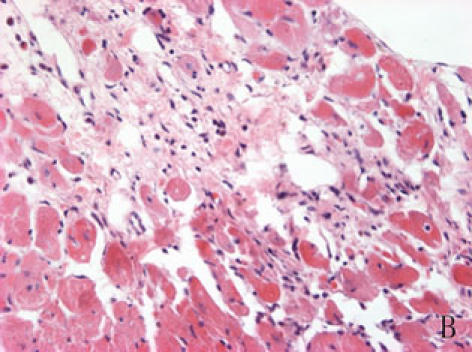
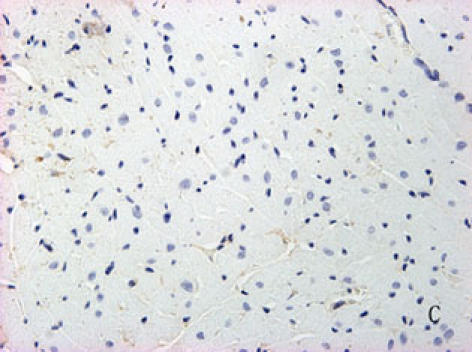
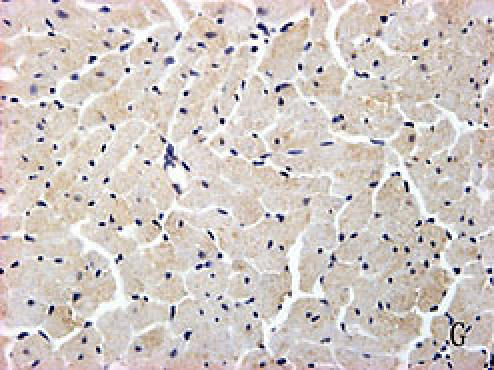
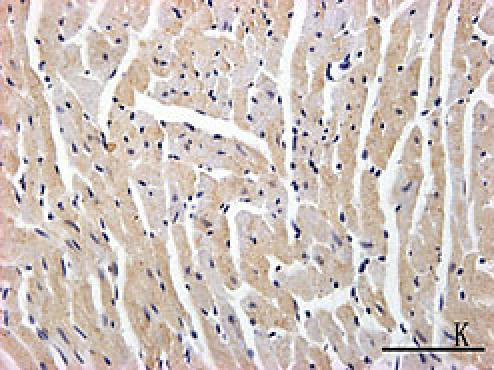
A, B: The normal (A) and diabetic (B) myocardium were stained with haematoxylin-eosin, diabetic cardiac muscle fibers were disorder and many of them were collapsed, diabetic myocardium showed fibrosis and extensive focal coalescent areas of ischemic myocyte degeneration in the subendocardial, subepicardial region and papillary muscles of the myocardium.
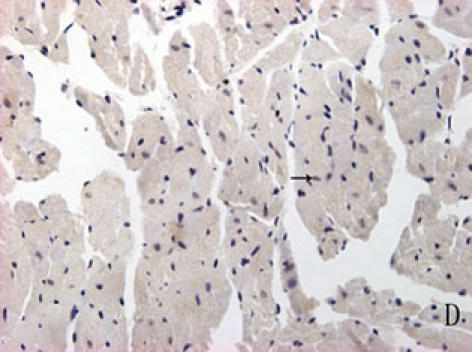
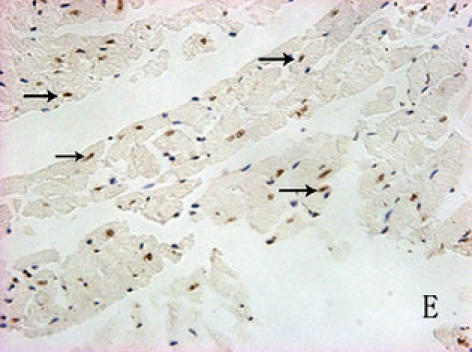
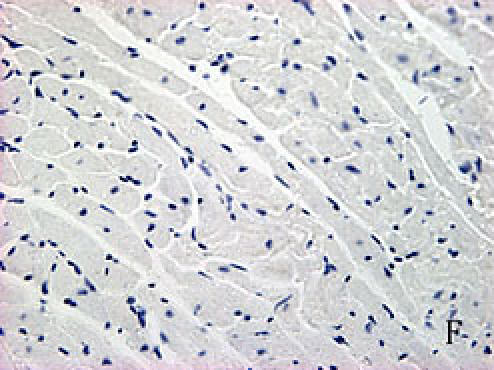
C, D, E: Diabetic heart (E) showed more TUNEL-positive cardiocytes and endothelial cells than normal (D) heart (p<0.05). C was negative control.
F, G, H, I, J, K, L, M, N: Immunoreactive Grp78 and Caspase12 of sections from diabetic heart (H: Grp78; K: Caspase12) and normal heart (G: Grp78; J: Caspase12) were shown. The diabetic heart showed strong immunoreactivity for Grp78 (p<0.05) and Caspase12 (p<0.05). F (Grp78) and I (Caspase12) were negative control. Bar = 50 µm.
mRNA expression
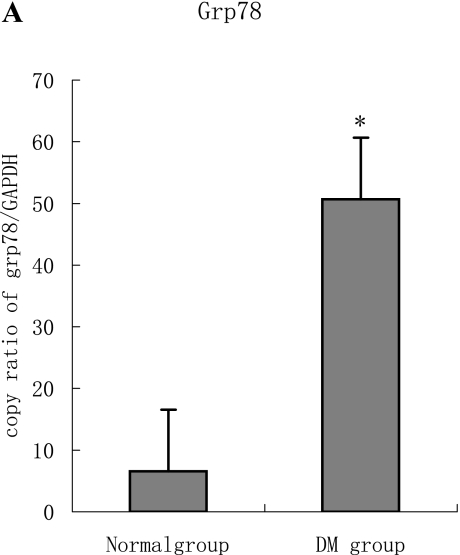
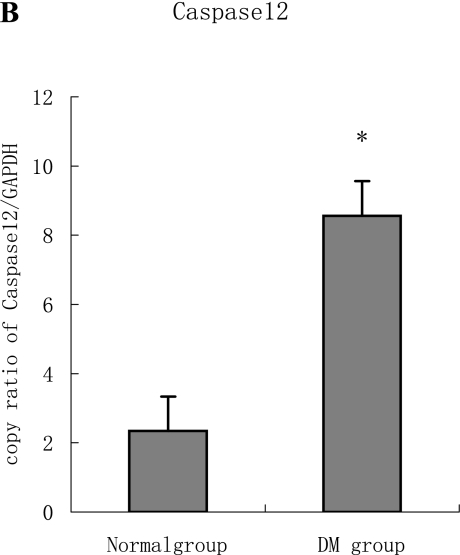
PCR analysis performed on total RNA showed that Grp78 and Caspase12 mRNA in both diabetic and normal hearts were easily detectable. However, the Grp78 (or Caspase12)/GAPDH densitometric ratio in the diabetic group was higher than normal group. (Fig. 3 A–B)
Fig. 3.
A, B: The expression of both Grp78 and Caspase12 mRNA in the diabetic heart increased significantly compared to the normal heart tissue. Furthermore, the two targets were regulated in the same manner at mRNA level, which are statistically significant difference (p<0.05).
Western blot
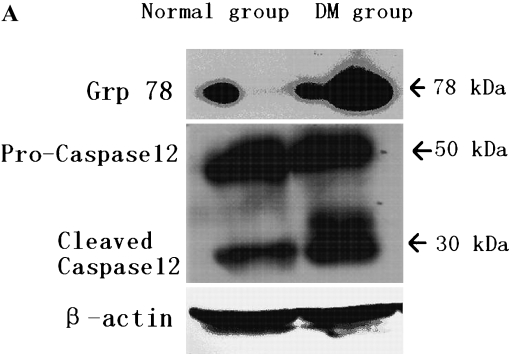
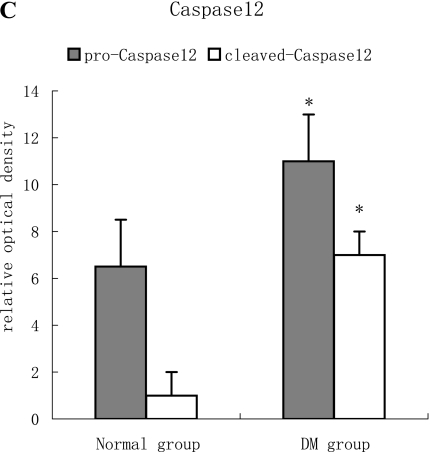
Representative results of Western blot analysis are shown in Fig. 4. With an antibody against Caspase12, samples of normal rat myocardium showed a band with a molecular weight of 50 kDa, and a weak band with a molecular of 30 kDa were also found. This means that there was a slight Caspase12 activation even in the normal state. Two bands at 30 kDa in the diabetic rats could be found as the result of more pro-Caspase12 was activated. (Fig. 4 A–C)
Fig. 4.
A, B, C: With antibody against Grp78, a weak band was detectable in samples of normal rats myocardium, but those of diabetic rats showed a single strong band with a molecular weight of 78 kDa (p<0.05). With an antibody against Caspase12, samples of normal rat myocardium showed a band with a molecular weight of 50 kDa, and a weak band with a molecular of 30 kDa was also found. While in diabetic rats’ myocardium, the pro- and cleaved-Caspase12 with a molecular weight between 50–30 kDa was obviously found. With quantitative analysis, we found that more Caspase12 were activated in diabetic heart (p<0.05).
Discussion
Diabetic cardiomyopathy is characterized by both systolic and diastolic dysfunction [22]. In the current study, we established type 1 diabetic rat model and used echocardiography to identify the existence of diabetic cardiomyopathy in them. The result of TUNEL stain suggested that more cardiocytes and endothelial cells were apoptosis in the diabetic hearts. We demonstrated that Grp78 has enhanced expression in the diabetic heart, which suggested that ERS was induced in our experimental paradigm. Caspase12, an ER-associated caspase, had strong immunogenicity and more cleaved fragment in the diabetic heart, which indicated that ERS-associated apoptosis pathway was activated. This is the first study that implicates ER-stress plays a role in the pathophysiology of diabetic cardiomyopathy.
Cell death, as a comprehensive consequence of abnormal cellular metabolism and gene expression in response to hyperglycemia, has been considered to be the important cause of diabetic cardiomyopathy. Because myocytes rarely proliferate in adult cardiac muscles, the loss of cardiocytes would eventually lead to compromised cardiac function. Loss of endothelial cells will lead vascular to dysfunction and aggravate the ischemia of the heart. Apoptosis of cardiocytes and endothelial cells has been observed in the heart of patient with diabetes and in STZ-induced diabetic rat [10, 11]. Exposing to high glucose also induced the two types of cells to death in vitro [23, 24]. Recent study showed high glucose could increase apoptosis of cultured lens epithelial cells through ERS pathway [25], and in current study, the cleaved Caspase12 enhanced expression also indicated ERS-associated apoptosis was activated in diabetic myocardium. Indeed, besides hyperglycemia, diabetic heart experiences many other conditions that can invoke ERS, such as increased oxidative stress, hypoxia, homocysteine, lipid deposition [26], and increased synthesis of secretory proteins [27–29]. Recent study showed in hypertrophic and failing heart after aortic constriction, ERS was induced by angiotensin II, which was also increased in diabetic heart [1, 2, 18]. Previous studies have shown the ER was swelling in diabetic myocardium, which gave ultrastructural evidence for the ERS in diabetic heart [19, 20].
As one of intrinsic pathways of apoptosis, ERS implicates several proteins including the transcription factor GADD153/CHOP (growth arrest and DNA damage, also called CHOP) [30], the JNK-pathway [31], and the ER-resident cysteine protease, Caspase12. Nakagawa et al. [15] demonstrated that Caspase12 mediated apoptosis was a specific apoptosis pathway of ER, and apoptosis that occurred as a result of membrane- or mitochondrial-targeted signals did not activate Caspase12. Grp78, an ER chaperone protein, is a well-established hallmark of ERS. Increased Grp78 was reported in ERS-associated apoptosis of pancreatic β cells [15], renal proximal tubular cells [32], cardiocytes in heart failure [19], and endothelial cells [33]. In current study, enhanced expression of Grp78 was also found in apoptosis of diabetic myocardium.
In summary, this study provided the first evidence that Grp78 increased and Caspase12 is activated in the diabetic heart. This meant ERS was involved in the pathology of diabetic cardiomyopathy, and ERS-associated apoptosis acted roles in diabetic heart failure. It will be a potential therapeutic target for diabetic cardiomyopathy.
References
- 1.Avogaro A., Vigilide Kreutzenberg S., Negut C., Tiengo A., Scognamiglio R. Diabetic cardiomyopathy: a metabolic perspective. Am. J. Cardiol. 2004;93:13A–16A. doi: 10.1016/j.amjcard.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Picano E. Diabetic Cardiomyopathy: The importance of being earliest. JACC. 2003;42:454–457. doi: 10.1016/s0735-1097(03)00647-8. [DOI] [PubMed] [Google Scholar]
- 3.Dyntar D., Sergeev P., Klisic J., Ambuhl P., Schaub M.C., Donath M.Y. High glucose alters cardiomyocyte contacts and inhibits myofibrillar formation. J. Clin. Endocrinol. Metab. 2006;91:1961–1967. doi: 10.1210/jc.2005-1904. [DOI] [PubMed] [Google Scholar]
- 4.Feuvray D. Diabetic cardiomyopathy. Arch. Mal. Coeur. Vaiss. 2004;97:261–265. [PubMed] [Google Scholar]
- 5.Tappia P.S., Asemu G., Aroutiounova N., Dhalla N.S. Defective sarcolemmal phospholipase C signaling in diabetic cardiomyopathy. Mol. Cell. Biochem. 2004;261:193–199. doi: 10.1023/b:mcbi.0000028756.31782.46. [DOI] [PubMed] [Google Scholar]
- 6.Ligeti L., Szenczi O., Prestia C.M., Szabo C., Horvath K., Marcsek Z.L., van Stiphout R.G., van Riel N.A., Op den Buijs J., Van der Vusse G.J., Ivanics T. Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int. J. Mol. Med. 2006;17:1035–1043. [PubMed] [Google Scholar]
- 7.Pereira L., Matthes J., Schuster I., Valdivia H.H., Herzig S., Richard S., Gomez A.M. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 8.Galicka Latala D., Konduracka E., Kuzniewski M., Fedak D., Sieradzki J. Myocardial dysfunction, neuropathy and nephropathy in long standing type 1 diabetic patients. Przegl. Lek. 2005;62:1451–1454. [PubMed] [Google Scholar]
- 9.An D., Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1489–1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 10.Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol. Cell. Biochem. 2004;261:187–191. doi: 10.1023/b:mcbi.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- 11.Cai L., Kang Y.J. Cell death and diabetic cardiomyopathy. Cardiovasc. Toxicol. 2003;3:219–228. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S., Rodrigues B. Cardiac cell death in early diabetes and its modulation by dietary fatty acids. Biochim. Biophys. Acta. 2006;1761:1148–1162. doi: 10.1016/j.bbalip.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Ferri K.F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 14.Bernales S., Papa F.R., Walter P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 16.Nakatani Y., Kaneto H., Kawamori D., Yoshiuchi K., Hatazaki M., Matsuoka T.A., Ozawa K., Ogawa S., Hori M., Yamasaki Y., Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 17.Hayden M.R., Tyagi S.C., Kerklo M.M., Nicolls M.R. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6:287–302. [PubMed] [Google Scholar]
- 18.Okada K., Minamino T., Tsukamoto Y., Liao Y., Tsukamoto O., Takashima S., Hirata A., Fujita M., Nagamachi Y., Nakatani T., Yutani C., Ozawa K., Ogawa S., Tomoike H., Hori M., Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 19.Bhimji S., Godin D.V., McNeill J.H. Myocardial ultrastructural changes in alloxan-induced diabetes in rabbits. Acta. Anat. 1986;125:195–200. doi: 10.1159/000146161. [DOI] [PubMed] [Google Scholar]
- 20.Jackson C.V., McGrath G.M., Tahiliani A.G., Vadlamudi R.V., McNeill J.H. A functional and ultrastructural analysis of experimental diabetic rat myocardium. Manifestation of a cardiomyopathy. Diabetes. 1985;34:876–883. doi: 10.2337/diab.34.9.876. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J. Am. Coll. Cardiol. 2006;48:1548–1551. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Fiordaliso F., Bianchi R., Staszewsky L., Cuccovillo I., Doni M., Laragione T., Salio M., Savino C., Melucci S., Santangelo F., Scanziani E., Masson S., Ghezzi P., Latini R. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J. Mol. Cell Cardiol. 2004;37:959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Do Y., Carling D., Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 25.Mulhern M.L., Madson C.J., Danford A., Ikesugi K., Kador P.F., Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest. Ophthalmol. Vis. Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 26.Shiota G., Tsuchiya H. Pathophysiology of NASH: Insulin resistance, free fatty acids and oxidative stress. J. Clin. Biochem. Nutr. 2006;38:127–132. [Google Scholar]
- 27.Ganguly P.K. Role of atrial natriuretic peptide in congestive heart failure due to chronic diabetes. Can. J. Cardiol. 1991;7:275–280. [PubMed] [Google Scholar]
- 28.Siebenhofer A., Ng L.L., Plank J., Berghold A., Hodl R., Pieber T.R. Plasma N-terminal pro-brain natriuretic peptide in type 1 diabetic patients with and without diabetic nephropathy. Diabet. Med. 2003;20:535–539. doi: 10.1046/j.1464-5491.2003.00948.x. [DOI] [PubMed] [Google Scholar]
- 29.Beer S., Golay S., Bardy D., Feihl F., Gaillard R.C., Bachmann C., Waeber B., Ruiz J. Increased plasma levels of N-terminal brain natriuretic peptide (NT-proBNP) in type 2 diabetic patients with vascular complications. Diabetes Metab. 2005;31:567–573. doi: 10.1016/s1262-3636(07)70232-x. [DOI] [PubMed] [Google Scholar]
- 30.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R.T., Remotti H., Stevens J.L., Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Holbrook N.J. Elevated gadd153/chop expression and enhanced c-Jun N-terminal protein kinase activation sensitizes aged cells to ER stress. Exp. Gerontol. 2004;39:735–744. doi: 10.1016/j.exger.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Yoneda T., Imaizumi K., Oono K., Yui D., Gomi F., Katayama T., Tohyama M. Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 33.Ohse T., Inagi R., Tanaka T., Ota T., Miyata T., Kojima I., Ingelfinger J.R., Ogawa S., Fujita T., Nangaku M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006;70:1447–1455. doi: 10.1038/sj.ki.5001704. [DOI] [PubMed] [Google Scholar]