Abstract
We have proposed that diacylglycerol hydroperoxide-induced unregulated signal transduction causes oxidative stress-related diseases. In this study, we investigated which molecular species of diacylglycerol hydroperoxide activated human peripheral neutrophils. All diacylglycerol hydroperoxides, diacylglycerol hydroxides, and diacyglycerols tested in the present study induced superoxide production by neutrophils. The ability to activate neutrophils among molecular species containing the same fatty acid composition was as follows; diacylglycerol hydroperoxide>diacylglycerol hydroxide≥diacylglycerol. The diacylglycerol hydroperoxide composed of linoleate was a stronger activator for neutrophils than that composed of arachidonate. 1-Palmitoyl-2-linoleoylglycerol hydroperoxide (PLG-OOH) was the strongest stimulator for neutrophils. We reconfirmed that PLG-OOH activated protein kinase C (PKC) in neutrophils. PLG-OOH induced the phosphorylation of p47phox, a substrate of PKC and a cytosolic component of NADPH oxidase, in neutrophils, as did N-formyl-methionyl-leucyl-phenylalanine or 4β-phorbol-12β-myristate-13α-acetate. Moreover, the time course of p47phox phosphorylation was comparable to that of superoxide production. These results suggest that PLG-OOH activated intracellular protein kinase C. PLG-OOH, produced via an uncontrolled process, can act as a biological second messenger to cause inflammatory disease from oxidative stress.
Keywords: 1-palmitoyl-2-linoleoylglycerol hydroperoxide, neutrophil, oxidative stress, protein kinase C, p47phox
Introduction
Lipid oxidation products are formed by free radicals in vivo [1–4]. Recently, various lipid oxidation products (such as phosphatidylserine hydroperoxide, cardiolipin hydroperoxide, 4-hydroxy-2-nonenal and 8-isoprostane) have attracted much attention as signaling molecules [5–11]. For example, phosphatidylserine hydroperoxide [5, 11] and cardiolipin hydroperoxide [5, 6] participate in the apoptotic process. We have previously reported that diacylglycerol (DAG) hydroperoxide (DAG-OOH), mainly dilinoleoylglycerol hydroperoxide, activated rat brain protein kinase C (PKC) [12] as strongly as 4β-phorbol-12β-myristate-13α-acetate (PMA), a powerful PKC activator [13]. DAG-OOH would be formed via the hydrolysis of phospholipid hydroperoxide by the action of phospholipase C (PLC) [14]. Oxidized phospholipids are formed by the oxidation of membrane phospholipids, since lipids are vulnerable to free radical-induced oxidation [15]. This process is unregulated since it does not mediate a receptor in the biomembrane. PMA induces various diseases, such as cancer, via PKC activation [16, 17], but it is not a physiological molecule. Therefore, DAG-OOH is expected to work as a physiological PKC activator, which induces oxidative stress-related diseases (such as cancer, inflammatory disease, autoimmune disease, and atherosclerosis) [15].
We have also reported that 1-palmitoyl-2-linoleoylglycerol hydroperoxide (PLG-OOH) induced superoxide (O2·−) production by human peripheral neutrophils [18]. Since other molecular species of DAG-OOH can be formed in the biological systems, it is important to confirm which molecular species of DAG-OOH activates neutrophils. O2·− is produced by human neutrophils via both PKC-dependent and -independent pathways [19, 20]. Therefore, we examined which pathway occurred in DAG-OOH-stimulated PMNs. PKC inhibitors, such as chelerythrine, staurosporine, and H-7, suppressed the production of O2·− from neutrophils activated by DAG-OOH [18], but this is indirect evidence. Since p47phox, a cytosolic component of NADPH oxidase, is phosphorylated by PKC when O2·− is generated from neutrophils, the detection of phosphorylated p47phox is direct evidence for PKC activation in neutrophils [21]. If phosphorylation of p47phox is observed in DAG-OOH-stimulated neutrophils, it indicates the activation of PKC in neutrophils by DAG-OOH.
In the present study, we investigated which molecular species of DAG-OOH activate human peripheral neutrophils and the phosphorylation of p47phox in neutrophils stimulated by DAG-OOH.
Materials and Methods
Materials
1-Palmitoyl-2-linoleoyl-phosphatidylcholine, 1-stearoyl-2-linoleoyl-phosphatidylcholine, 1-palmitoyl-2-arachidonoyl-phosphatidylcholine, 1-stearoyl-2-arachidonoyl-phosphatidylcholine, 1,2-dioleoylglycerol (OOG), and 1-oleoyl-2-acetylglycerol (OAG) were purchased from Funakoshi Co. Ltd. (Tokyo, Japan). PMA, N-formyl-methionyl-leucyl-phenylalanine (fMLP), bacillus cereus PLC (type IX), and soybean lipoxygenase (type 1B) were obtained from Sigma Chemical Co. (St. Louis, MO). 2-Methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo [1,2-a] pyrazin-3-one (MCLA) was obtained from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Chelex®100 was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA). [32P]-Orthophosphoric acid was purchased from NEN Life Science Products, Inc. (Boston, MA). Solvents and other reagents were of the highest grade available. Rabbit polyclonal anti-p47phox antibody was from Dr. Naoki Okamura (Hiroshima University School of Medicine) [22].
Preparation of various molecular species of diacylglycerol, their hydroperoxides and their hydroxides
Various molecular species of DAG, DAG-OOH and DAG hydroxide (DAG-OH) were prepared as previously reported [18] with some modifications. Phosphatidylcholine (PC) was purified using reversed-phase HPLC prior to use. PC (6 µg) was oxidized with lipoxygenase (2.6 mg) in 0.2 M borate buffer treated with Chelex 100 (pH 9, 20 ml) containing 3 mM sodium deoxycholate for 20 min by mixing vigorously at room temperature under air. If necessary, sodium borohydride was added to the reaction mixture to reduce the phosphatidylcholine hydroperoxide (PC-OOH) after the reaction was over. Solid phase extraction was performed with a Sep-Pak C18 cartridge (10 g, Waters Co., Milford, MA), which was pretreated with 100 ml of 100 µM EDTA aqueous solution. The reaction mixture containing PC-OOH or PC hydroxide (PC-OH) was applied to the cartridge after it was equilibrated with 100 ml of water. PC-OOH or PC-OH was eluted with 100 ml of methanol after washing with 200 ml of water. PC-OOH or PC-OH was purified with reversed-phase HPLC after the eluate was concentrated with a rotary evaporator, and it was filtered with a 0.22 µm filter (MILLEX®-GP; Millipore Co., Bedford, MA). The PC, PC-OOH, or PC-OH was hydrolyzed by PLC in 50 mM Tris-HCl (pH 7.4) containing 40% methanol at 37°C. The reaction was monitored with HPLC. The DAG, DAG-OOH or DAG-OH formed was extracted with chloroform/methanol (2/1, by volume) three times. After the concentration of the collected lower layer using a rotary evaporator, DAG, DAG-OOH or DAG-OH was purified by HPLC with a CAPCELPAK C18 column (type: UG120, 20 × 250 mm, 5 µm, Shiseido Co. Ltd., Tokyo, Japan) and methanol/2-propanol (=17/3, by volume) as the mobile phase at a flow rate of 10 ml/min. Their elution times were as follows; 15.8 min (PLG), 20.5 min (1-stearoyl-2-linoleoylglycerol; SLG), 15.5 min (1-palmytoyl-2-arachidonoylglycerol; PAG), 19.0 min (1-stearoyl-2-arachidonoylglycerol; SAG), 14.5 min (1,3-dilinoleoylglycerol; 1,3-LLG), 9.3 min (PLG-OOH), 10.5 min (SLG-OOH), 9.3 min (PAG-OOH), 10.5 min (SAG-OOH), 8.5 min (1,3-LLG-OOH), 9.3 min (PLG hydroxide; PLG-OH), 10.3 min (SLG-OH), 9.3 min (PAG-OH) and 10.8 min (SAG-OH). DAG was monitored at 215 nm. DAG-OH or DAG-OOH was monitored at 234 nm. The concentration of DAG-OOH was determined using a hydroperoxide-specific, isoluminol chemiluminescence assay [23]. The absence of hydroperoxide in DAG-OH and DAG was also confirmed using this assay. The concentration of DAG-OH was estimated from its absorbance at 234 nm using an assumed molar extinction coefficient, 28,000 M−1cm−1 [24]. The concentration of DAG was calculated from their weights.
Measurement of MCLA-dependent chemiluminescence
Blood was drawn from healthy volunteers. Neutrophils were prepared as described previously [18]. A glass cuvette containing neutrophils (5.0 × 104 cells) and 2 µmol of MCLA in 1,990 µl of Hanks’ balanced salt solution containing 1.3 mM calcium ion and 0.9 mM magnesium ion was incubated at 37°C for 3 min under air. Thereafter, 10 µl of a stimulator, such as 800 µM or 1.6 mM DAG, DAG-OOH, or DAG-OH, or 200 µM fMLP dissolved in methanol, was added to the reaction mixture using a microsyringe. MCLA-dependent luminescence (O2·− production [25]) was recorded using a luminescence reader (type: BLR-301, Aloka Co. Ltd., Tokyo, Japan).
Detection of phosphorylated p47phox
Neutrophils were suspended in calcium ion, phosphate-free buffer (10 mM HEPES, 137 mM sodium chloride, 5.4 mM potassium chloride, 5.6 mM D-glucose, 0.8 mM magnesium chloride, and 0.025% BSA, pH 7.4) at 1.0 × 107 cells/ml [26]. [32P]-Orthophosphate was added to the neutrophil suspension at 100 µCi/107 cells and the cells were incubated at 37°C for 60 min with gentle mixing every 10 min. After 3 washes, calcium ion, phosphate-free buffer was added to the pellet (1.0 × 107 cells/ml).
Neutrophils (1.0 × 107 cells/ml × 300 µl) were suspended in phosphate-free buffer (695 µl; 10 mM HEPES, 137 mM sodium chloride, 5.4 mM potassium chloride, 1.26 mM calcium chloride, 5.6 mM D-glucose, 0.8 mM magnesium chloride, and 0.025% BSA, pH 7.4) [26]. After preincubation for 1 min at 37°C, the reaction was started by adding 5 µl of stimulator dissolved in methanol (1.6 mM PLG-OOH, 200 µM fMLP or 640 nM PMA). The reaction was stopped by centrifugation (12,000 rpm × 10 s) at the times described in the figure legends, and the supernatant was removed. Two hundred microliters of lysis buffer (200 µM phenylmethylsulfonyl fluoride, 3 µM leupepsin, 2 µM pepstatin, 1 µM staurosporine, 100 nM calysurin A, 100 µM o-vanadate, 150 mM NaCl, and 1% NP-40 in 50 mM Tris-HCl (pH 8); protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany) plus staurosporine) was added to the pellet and mixed vigorously for 1 min. After centrifugation, 5 µl of rabbit polyclonal anti-p47phox antibody was added to the supernatant and the solution was rotated at 4°C for 1.5 h. Eighty microliters of 10% protein A sepharose CL-4B in NET gel buffer (Tris buffer containing NP-40, EDTA, and gelatin) was added and the solution was rotated at 4°C for 1 h. After 3 washes of the precipitate with lysis buffer, 40 µl of SDS-PAGE sample buffer was added and the samples were boiled at 100°C for 5 min. Electrophoresis was performed with a 10% polyacrylamide gel. A low molecular weight marker (Bio-Rad Laboratories, Inc., Herculus, CA) was used. Phosphorylated protein was detected using autoradiography.
Results
Synthesis of various molecular species of DAG, DAG-OOH, and DAG-OH
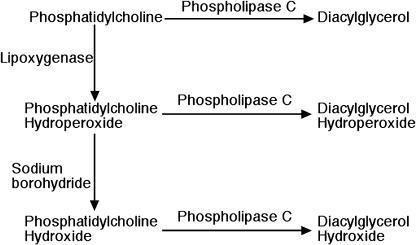
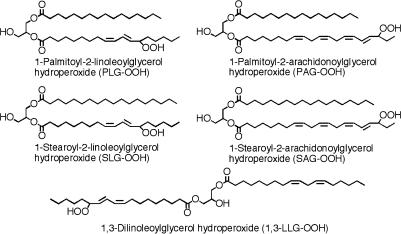
We first synthesized various molecular species of DAG-OOH to investigate which molecular species of DAG-OOH activate neutrophils. PC-OOH was formed by the oxidation of PC with lipoxygenase and it was hydrolyzed with PLC to form DAG-OOH, as described in the “Materials and Methods” section (Fig. 1). 1,3-LLG-OOH was synthesized by autoxidation of 1,3-LLG with a-tocopherol. The structures of various molecular species of DAG-OOH used in the present study are shown in Fig. 2. Various molecular species of DAG-OH were synthesized via the hydrolysis of PC-OH, which was formed by the reduction of PC-OOH with sodium borohydride, with PLC (Fig. 1). Various molecular species of DAG were synthesized by the hydrolysis of PC with PLC (Fig. 1).
Fig. 1.
Procedures for the synthesis of diacyglycerol, diacylglycerol hydroperoxide, and diacylglycerol hydroxide.
Fig. 2.
Structures of various molecular species of diacylglycerol hydroperoxide used in the present study.
Activation of neutrophils by various molecular species of DAG, DAG-OOH, or DAG-OH
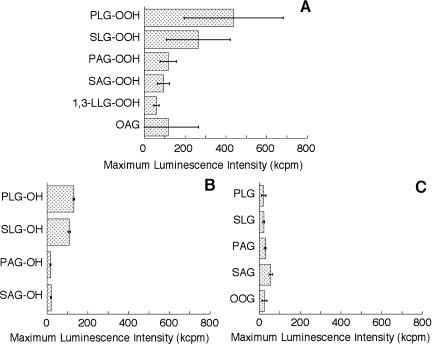
The activation of neutrophils by various molecular species of DAG, DAG-OOH, or DAG-OH (4 µM each) was investigated (Fig. 3). Activation of neutrophils was evaluated using maximum MCLA-dependent luminescence intensity, which represents the maximum rate of O2·− formation. No light emission was observed when only methanol was added to the neutrophil suspension (data not shown). The DAG-OOH or DAG-OH composed of linoleate hydroperoxide (hydroxide) was a more powerful activator of neutrophils than that composed of arachidonate hydroperoxide (hydroxide) (Fig. 3A, B). The activation of neutrophils by 1,3-LLG-OOH was weaker than that by any 1,2-DAG-OOH, as reported previously [18]. The difference in the fatty acid composition of DAG species, including 1,2-dioleoylglycerol (OOG), which is widely used as a PKC activator, did not affect the maximum luminescence intensity (Fig. 3C). PLG-OOH was the strongest activator of neutrophils among the various DAG, DAG-OOH, and DAG-OH species tested in the present study. Moreover, the activation of neutrophils by PLG-OOH was significantly stronger than that by 1-oleoyl-2-acetylglycerol (OAG), which is generally used as a stimulator of cells (Fig. 3A). Based on these findings, PLG-OOH was used in the subsequent experiments. The ability to activate neutrophils among molecular species containing the same fatty acid composition was as follows; DAG-OOH>DAG-OH≥DAG. This order did not change when 8 µM of stimulator was used (data not shown). No significant difference between the activation of PKC by DAG-OOH and that by DAG-OH in vitro has been reported [12]. Further studies are needed to elucidate why different results were obtained in vitro versus ex vivo.
Fig. 3.
Activation of neutrophils by diaclyglycerol hydroperoxides (A), diacylglycerol hydoxides (B), or diaclyglycerols (C). The maximum MCLA-dependent luminescence intensity was measured as the rate of superoxide anion formation from neutrophils (2.5 × 104 cells/ml). The concentration of each stimulator used here was 4 µM. One micromolar MCLA was used in this experiment. Results are expressed as means ± standard deviations (n = 5). Abbreviations used in the figures are as follows; PAG, 1-palmitoyl-2-arachidonoylglycerol; PLG, 1-palmitoyl-2-linoleoylglycerol; SAG, 1-stearoyl-2-arachidonoylglycerol; SLG, 1-stearoyl-2-linoleoylglycerol; 1,3-LLG, 1,3-dilinoleoylglycerol; OAG, 1-oleoyl-2-acetylglycerol; OOG, 1,2-dioleoylglycerol; -OOH, hydroperoxide; -OH, hydroxide.
Phosphorylation of p47phox in neutrophils by the stimulation of DAG-OOH
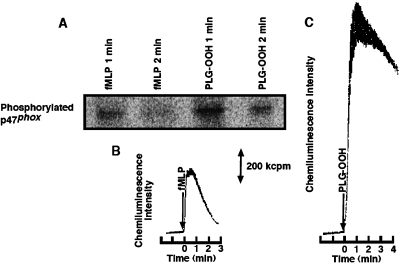
The activation of PKC in neutrophils by DAG-OOH was reconfirmed. p47phox is a cytosolic factor of NADPH oxidase, and known as a substrate of PKC [21]. When only methanol (0.5% to solution) was added to the neutrophil suspension, p47phox was not phosphorylated after 2 min at 37°C (Fig. 4). p47phox was phosphorylated in 8 µM PLG-OOH-stimulated neutrophils, as in PMA (3 nM)-stimulated or fMLP (1 µM)-stimulated neutrophils (Fig. 4).
Fig. 4.
Phosphorylation of p47phox in neutrophils (3 × 106 cells/ml) activated by various stimulators. Lane 1; Methanol (0.5% of solution) was used in the control experiment (2 min). Lane 2; Three nanomolar 4β-phorbol-12β-myristate-13α-acetate (PMA) was used, and stimulation was stopped at 5 min. Lane 3; One micromolar N-formyl-methionyl-1-leucyl-phenylalnine (fMLP) was used, and stimulation was stopped at 2 min. Lane 4; Eight micromolar 1-palmitoyl-2-linoleoylglycerol hydroperoxide (PLG-OOH) was used, and stimulation was stopped at 2 min.
The phosphorylated p47phox at 1 min after stimulation decreased at 2 min in both fMLP (1 µM)-stimulated and PLG-OOH (8 µM)-stimulated neutrophils (Fig. 5A). This change was comparable to that in MCLA-dependent luminescence intensity (1 µM fMLP; Fig. 5B, 8 µM PLG-OOH; Fig. 5C). These results suggest that p47phox was phosphorylated by PKC in PLG-OOH-stimulated neutrophils. In fact, the production of O2·− was inhibited by serine/threonine kinase inhibitors and PKC inhibitors, such as staurosporine, H-7, and chelerythrine [18].
Fig. 5.
Time course of p47phox phosphorylation in neutrophils (3 × 106 cells/ml) stimulated by 1 µM N-formyl-methionyl-1-leucyl-phenylalnine (fMLP) or 8 µM 1-palmitoyl-2-linoleoylglycerol hydroperoxide (PLG-OOH) (A). Time-dependent change in MCLA-dependent luminescence from 1 µM fMLP-stimulated neutrophils (2.5 × 104 cells/ml) (B) and 8 µM PLG-OOH-stimulated neutrophils (2.5 × 104 cells/ml) (C).
Discussion
The activation of human neutrophils by various molecular species of DAG, DAG-OOH, and DAG-OH was investigated in the present study. In order of the amount of PC in rat liver, the species ranked as follows; SAPC (16.1%)>SLPC (14.8%)>PLPC (14.1%)>PAPC (9.7%) [27]. In terms of the amount of fatty acid in the phospholipids of mammalian cells, fatty acid ranked as follows; palmitate (33%)>oleate (25%)>stearate (12%)>linoleate (10%)>arachidonate (8%) (human neutrophil phospholipids) [28], palmitate (33%)>arachidonate (19%)>stearate (16%)>oleate (13%)>linoleate (7%) (human coronary artery phospholipids) [29], and linoleate (25%)> stearate (24%)>palmitate (17%)>arachidonate (16%) (rat heart phosphatidylcholine at 100 days old) [30]. Almost all phospholipids in mammalian cells have a saturated fatty acid at the sn-1 position and an unsaturated fatty acid at the sn-2 position. Oleate is not easily oxidized by free radicals. Therefore, diacylglycerols with palmitate or stearate at the sn-1 position and linoleate or arachidonate at the sn-2 position were used in the present study. Although all molecular species of DAG, DAG-OOH, and DAG-OH containing these fatty acids activated human neutrophils, PLG-OOH was the most potent activator.
Human neutrophils have PKCα and PKCδ [31]. We also detected these PKC isoforms in neutrophils in the Western blot analysis (data not shown). Recently, it was found that DAG-OOH activated various PKC isoforms in vitro, but only PKCα and PKCδ were activated by DAG-OOH more strongly than by DAG (our unpublished data). These PKC isoforms may be involved in the activation of neutrophils by PLG-OOH.
Calcium, phospholipid-independent activity of bovine brain PKC is increased by oxidation of the regulatory domain of PKC by hydrogen peroxide or periodate [32, 33]. Even PKC in cells (PKCγ in N/N1003A cells [34] and PKCδ in COS-7 cells [35]) was oxidized by hydrogen peroxide and was activated. Oxidation of PKC by PLG-OOH might cause neutrophil activation in the present study. However, 8 µM PC-OOH, linoleate hydroperoxide, and hydrogen peroxide did not induce production of O2·− by human neutrophils in our previous study [18]. Moreover, we reconfirmed the activation of PKC in neutrophils by PLG-OOH using p47phox phosphorylation in the present study. Therefore, PLG-OOH might activate PKC in neutrophils directly. PKCδ is activated via tyrosine phosphorylation by high concentrations (mM) of hydrogen peroxide [36, 37]. Further study is needed to clarify whether tyrosine residues of PKCδ in neutrophils are phosphorylated by PLG-OOH stimulation.
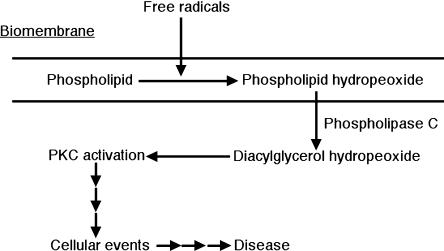
It has been suggested that oxidative stress causes disease, cancer, and aging through the accumulation of oxidative damage, including the oxidation of DNA, lipids, and proteins [15]. However, there is little direct evidence that oxidative damage is the cause of various diseases, although much indirect evidence has been reported. We previously proposed that DAG-OOH, formed by the actions of PLC after the oxidation of biomembrane phospholipids by free radicals [14], activates PKC, and causes diseases such as cancer, since this signal is not regulated by receptors (Fig. 6) [12, 16]. In the present study, we confirmed that PLG-OOH activates intracellular PKC. This result suggests that our proposal occurs in biological systems.
Fig. 6.
Proposed mechanism for free radical-induced unregulated signal transduction.
The activation of neutrophils is involved in inflammatory diseases (for example; acute inflammatory liver injury [38] and chronic inflammatory airway disease [39]). PKC is activated under oxidative stress [40] (for example; cardiac reperfusion [41], diabetic nephropathy [42] and ischemia reperfusion of skeletal muscle [43]). In some cases, PLG-OOH may activate PKC.
In summary, various molecular species of DAG, DAG-OOH and DAG-OH were synthesized. All the DAG-OOHs tested in the present study activated neutrophils, but PLG-OOH was the most potent activator. When PLG-OOH stimulated neutrophils, O2·− was produced via the phosphorylation of p47phox by PKC. These results suggest that PLG-OOH activates PKC in cells. Therefore, PLG-OOH, produced via an uncontrolled process, can act as a second messenger in the progression from oxidative stress-induced damage to inflammatory disease (Fig. 6).
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government.
Abbreviations
- DAG
diacylglycerol
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- 1,3-LLG
1,3-dilinoleoylglycerol
- MCLA
2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo [1,2-a]pyrazin-3-one
- O2·−
superoxide
- OAG
1-oleoyl-2-acetylglycerol
- OOG
1,2-dioleoylglycerol
- -OH
hydroxide
- -OOH
hydroperoxide
- PAG
1-palmitoyl-2-arachidonoylglycerol
- PC
phosphatidylcholine
- PKC
protein kinase C
- PLC
phospholipase C
- PLG
1-palmitoyl-2-linoleoylglycerol
- PMA
4β-phorbol-12β-myristate-13α-acetate
- SAG
1-stearoyl-2-arachidonoylglycerol
- SLG
1-stearoyl-2-linoleoylglycerol
References
- 1.Mashima R., Onodera K., Yamamoto Y. Regioisometric distribution of cholesteryl linoleate hydroperoxides and hydroxides in plasma from healthy humans provides evidence for free radical-mediated lipid peroxidation in vivo. J. Lipid Res. 2000;41:109–115. [PubMed] [Google Scholar]
- 2.Awad J.A., Morrow J.D., Takahashi K., Roberts L.J. Identificationi of non-cyclopxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J. Biol. Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 3.Uchida K. Protein-bound 4-hydroxy-2-nonenal as a marker of oxidative stress. J. Clin. Biochem. Nutr. 2005;36:1–10. [Google Scholar]
- 4.Itabe H. Searching for oxidized low-density lipoproteins in vivo. J. Clin. Biochem. Nutr. 2005;37:1–8. doi: 10.3164/jcbn.11-00020R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan V.E., Borisenko G.G., Tyurina Y.Y., Tyurin V.A., Jiang J., Potapovich A.I., Kini V., Amoscato A.A., Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Nomura K., Imai H., Koumura T., Kobayashi T., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida K. Cellular response to bioactive lipid peroxidation products. Free Radic. Res. 2000;33:731–737. doi: 10.1080/10715760000301251. [DOI] [PubMed] [Google Scholar]
- 8.Morrow J.D., Roberts L.J. The isoprostanes: unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 9.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 10.Huber J., Boechzelt H., Karten B., Surboeck M., Bochkov V.N., Binder B.R., Sattler W., Leitinger N. Oxidized cholesteryl linoleates stimulate endotherial cells to bind monocytes via the extracellular signal-regulated kinase 1/2 pathway. Arterioscler. Thromb. Vasc. Biol. 2002;22:581–586. doi: 10.1161/01.atv.0000012782.59850.41. [DOI] [PubMed] [Google Scholar]
- 11.Matsura T., Togawa A., Kai M., Nishida T., Nakada J., Ishibe Y., Kojo S., Yamamoto Y., Yamada K. The presence of oxidized phosphatidylserine on Fas-mediated apoptotic cell surface. Biochim. Biophys. Acta. 2005;1736:181–188. doi: 10.1016/j.bbalip.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Takekoshi S., Kambayashi Y., Nagata H., Takagi T., Yamamoto Y., Watanabe K. Activation of protein kinase C by oxidized diacylglycerols. Biochem. Biophys. Res. Commun. 1995;217:654–660. doi: 10.1006/bbrc.1995.2824. [DOI] [PubMed] [Google Scholar]
- 13.Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 14.Kambayashi Y., Takekoshi S., Watanabe K., Yamamoto Y. Phospholipase C-dependent hydrolysis of phosphatidylcholine hydroperoxides to diacylglycerol hydroperoxides and its reduction by phospholipid hydroperoxide glutathione peroxidase. Redox Rep. 2002;7:29–33. doi: 10.1179/135100002125000154. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 3rd Ed. Oxford University Press; New York: 1999. [Google Scholar]
- 16.Nishizuka Y. Studies and prospectives of the protein kinase C family for cellular regulation. Cancer. 1989;63:1892–1903. doi: 10.1002/1097-0142(19890515)63:10<1892::aid-cncr2820631005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 18.Yamamoto Y., Kambayashi Y., Ito T., Watanabe K., Nakano M. 1,2-Diacylglycerol hydroperoxides induce the generation and release of superoxide anion from human polymorphonuclear leukocytes. FEBS Lett. 1997;412:461–464. doi: 10.1016/s0014-5793(97)00823-5. [DOI] [PubMed] [Google Scholar]
- 19.Bass D.A., Gerard C., Olbrantz P., Wilson J., McCall C.E., McPhail L.C. Priming of the respiratory burst of neutrophils by diacylglecerol. Independence from activation or translocation of protein kinase C. J. Biol. Chem. 1987;262:6643–6649. [PubMed] [Google Scholar]
- 20.Watson F., Robinson J., Edwards S.W. Protein kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J. Biol. Chem. 1991;266:7432–7439. [PubMed] [Google Scholar]
- 21.Segal A.W., Heyworth P.G., Cockcroft S., Barrowman M.M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985;316:547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi M., Saeki S., Yamane H., Okamura N., Ishibashi S. Hyperphosphorylated p47-phox lost the ability to activate NADPH oxidase in guinea pig neutrophils. Biochem. Biophys. Res. Commun. 1995;216:203–208. doi: 10.1006/bbrc.1995.2611. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y., Brodsky M.H., Baker J.C., Ames B.N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal. Biochem. 1987;160:7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]
- 24.Chan H.W., Levett G. Autoxidation of methyl linoleate. Separation and analysis of isomeric mixtures of methyl linoleate hydroperoxides and methyl hydroxylinoleates. Lipids. 1977;12:99–104. doi: 10.1007/BF02532979. [DOI] [PubMed] [Google Scholar]
- 25.Nakano M. Detemination of superoxide radical and singlet oxygen based on chemiluminescecne of luciferin analogs. Methods Enzymol. 1990;186:585–591. doi: 10.1016/0076-6879(90)86154-n. [DOI] [PubMed] [Google Scholar]
- 26.El Benna J., My-Chan Dang P., Gaudry M., Fay M., Morel F., Hakim J., Gougerot-Pocidalo M.-A. Phosphorylation of the respiratory burst oxidase subunit p67phox during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J. Biol. Chem. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 27.Patton G.M., Fasulo J.M., Robins S.J. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J. Lipid Res. 1982;23:190–196. [PubMed] [Google Scholar]
- 28.Pratt V.C., Watanabe S., Bruera E., Mackey J., Clandinin M.T., Baracos V.E., Field C.J. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementatioin. Br. J. Cancer. 2002;87:1370–1378. doi: 10.1038/sj.bjc.6600659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luostarinen R., Boberg M., Saldeen T. Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis. 1993;99:187–193. doi: 10.1016/0021-9150(93)90021-l. [DOI] [PubMed] [Google Scholar]
- 30.Novák F., Tvrzická E., Hamplová B., Kolár F., Nováková O. Postnatal development of phospholipids and their fatty acid profile in rat heart. Mol. Cell. Biochem. 2006;293:23–33. doi: 10.1007/s11010-006-2215-8. [DOI] [PubMed] [Google Scholar]
- 31.Kent J.D., Sergeant S., Burns D.J., McPhail L.C. Identification and regulation of protein kinase C-delta in human neutrophils. J. Immunol. 1996;157:4641–4647. [PubMed] [Google Scholar]
- 32.Gopalakrishna R., Anderson W.B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. USA. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopalakrishna R., Anderson W.B. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch. Biochem. Biophys. 1991;285:382–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- 34.Lin D., Takemoto D.J. Oxidative activation of protein kinase Cγ through the C1 domain. Effects on GAP junction. J. Biol. Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 35.Umada-Kajimoto S., Yamamoto T., Matsuzaki H., Kikkawa U. The complex formation of PKCδ through its C1- and C2-like regions in H2O2-stimulated cells. Biochem. Biophys. Res. Commun. 2006;341:101–107. doi: 10.1016/j.bbrc.2005.12.161. [DOI] [PubMed] [Google Scholar]
- 36.Konishi H., Tanaka M., Takemura Y., Matsuzaki H., Ono Y., Kikkawa U., Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohmori S., Shirai Y., Sakai N., Fujii M., Konishi H., Kikkawa U., Saito N. Three distinct mechanisms for translocation and activation of the δ subspecies of protein kinase C. Mol. Cell. Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeschke H., Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 39.Louis R., Djukanovic R. Is the neutrophil a worthy target in severe asthma and chronic obstructive pulmonary disease? Clin. Exp. Allergy. 2006;36:563–567. doi: 10.1111/j.1365-2222.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 40.Gopalakrishna R., Jaken S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 41.Churchill E.N., Szweda L.I. Translocation of δPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005;439:194–199. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee H.B., Yu M.-R., Yang Y., Jiang Z., Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14:S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 43.Huda R., Vergara L.A., Solanki D.R., Sherwood E.R., Mathru M. Selective activation of protein kinase C delta in human neutrophils following ischemia reperfusion of skeletal muscle. Shock. 2004;21:500–504. doi: 10.1097/01.shk.0000124029.92586.5a. [DOI] [PubMed] [Google Scholar]