Abstract
This study aimed to investigate the preventive effects of green tea fractions (GTFs) on rat model of oxygen-induced retinopathy (OIR). Neonatal Sprague-Dawley rats were exposed to daily cycles of 80% oxygen (20.5 h), ambient air (0.5 h), and progressive return to 80% oxygen (3 h) until postnatal day 12 (P12), then the rats were placed in ambient air until P18. The green tea was fractionated by DM-A50, DM-W, M-B, and M-W. The rats were treated once daily from P6 to P17 by gastric gavage of GTFs (0.05 or 0.01 g/ml) or distilled water (DW) at 50 µl/10 g body weight. On P18, the rats were sacrificed and the retinal samples were collected. The retinal neovascularization (NV) was scored and avascular areas (AVAs) were measured as a % of total retinal area (%AVAs) in ADPase stained retinas. The NV scores in 0.01 g/ml M-W were significantly lower than those in DW. The %AVAs in 0.05 g/ml DM-A50 and in 0.05 g/ml and 0.01 g/ml M-W were significantly lower than those in DW. There were less catechins, and less caffeine in M-W fraction compared with other GTFs, suggesting components of green tea except for catechins and caffeine might suppress the neovascularization in rat model of OIR.
Keywords: oxygen-induced retinopathy, green tea, neovascularization, retinopathy of prematurity
Introduction
Retinal neovascularization in retinopathy of prematurity (ROP) is a major cause of vision loss [1, 2]. The treatment of ROP is of increasing importance as survival rates of premature babies increase [3–6]. Rat model of oxygen-induced retinopathy (OIR) provides a good experimental tool for studying vascular changes in ROP [7]. By using the rat model of OIR, previous studies have shown that high oxygen concentration inhibits vascular growth in the retina and subsequent normal oxygen concentration in the air promotes neovascularization (NV) [7]. In an angiogenic process in the retina, numerous angiogenic factors including fibroblast growth factor (FGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), angiopoietins, platelet-derived growth factor (PDGF), tumor necrosis factor (TNF) and interleukins related to retinal angiogenesis have been identified [1].
Green tea is widely consumed in the eastern world. Studies have shown that green tea possess diverse pharmacological properties that include anti-oxidative, anti-inflammatory, anti-carcinogenic, anti-arteriosclerotic and anti-bacterial effects [8–11]. Several previous studies demonstrated the inhibitory effects of green tea on NV in the vascular changes of the eye. Of interest, Cao and Cao [12] demonstrated that drinking tea inhibited VEGF-induced corneal NV in mice. More recently, Minami et al. [13] reported the green tea extracts suppressed NV in the rat model of OIR.
Most abundant components in green tea are catechins [10]. In addition, green tea includes other various components such as caffeine, theanine, proteins, vitamins, minerals and pigments [14–16]. However, there are few studies which investigated what components in the green tea play an important role in the prevention of NV in ROP. The purpose of the study was to examine which green tea components inhibit oxygen-induced retinal NV. For this purpose, we investigated the preventive effects of green tea fractions (GTFs) on retinal neovascularization in the rat model of OIR. Also, we analyzed components in the GTFs to identify the specific probable components which could expert beneficial effects on the prevention of NV in the rat model of OIR.
Materials and Methods
All experiments were performed in accordance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the institutional committee of animal care and use at our institution.
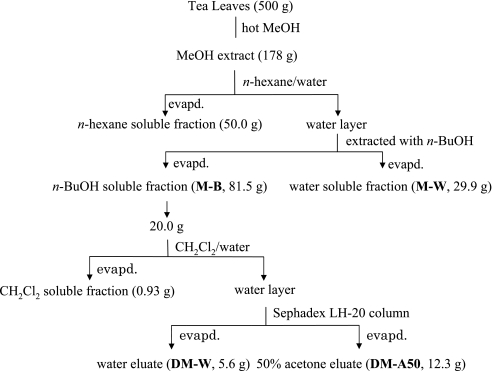
Exraction and fractionation of green Tea (Fig. 1)
Fig. 1.
Extraction and fractionation of green tea
The Yuukisaibai-cha (Yabukita 80%, Kurasawa 10%, others 10%) purchased from Nakai-hotoen Co. Ltd. (Kyoto, Japan) was used as green tea leaves (the leaves of Camellia sinensis L.). The dried tea leaves (500 g) were extracted with hot MeOH (5 l) for 2 h and the methanolic extract was evaporated to give a residue (178 g). This methanolic extract was dissolved in water (1.7 l) and extracted with n-hexane (2 l, 3 times), then n-butanol (BuOH) (2 l, 3 times). The n-hexane and water layers were evaporated to give the n-hexane soluble fraction (50.0 g) and the water soluble fraction (M-W, 29.9 g), respectively. The n-BuOH layer was evaporated to give a residue (M-B, 81.5 g). M-B (20.0 g) was partitioned between dichloromethane (CH2Cl2) (400 ml) and water (400 ml). The CH2Cl2 layer was evaporated to give the CH2Cl2 soluble fraction (0.93 g). The water layer was concentrated and subjected to Sephadex LH-20 column chromatography eluting with water and then 50% acetone. The fractions eluted with water and 50% acetone evaporated to give the water eluate (DM-W, 5.6 g) and the 50% acetone eluate (DM-A50, 12.3 g), respectively.
Analysis of catechins and caffeine in each fraction
(+)-Catechin, (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), and caffeine were purchased from Funakoshi Co. Ltd. (Tokyo, Japan).
The high performance liquid chromatography (HPLC) analyses were performed using an LC-10ADVP system with SPD-10AVP UV-VIS detector and LCsolution software (Shimadzu, Kyoto, Japan). Chromatographic conditions: column, Shiseido Capcellpak C18 (4.6 mm i.d. × 100 mm, 3 µm); mobile phase, (A) 0.1% H3PO4 containing 0.1% CH3CN and 5% DMF, (B) CH3CN; 0 min, A:B (99:1), after 40 min, A:B (83:17); flow rate, 1.0 ml/min; detector, 280 nm, column temp., 40°C.
Analysis of L-theanine in each fraction
L-Theanine, amino acids mixture standard solution, Type ANII and Type B were purchased Wako Pure Chemical Industries, Ltd (Osaka, Japan).
The thin layer chromatography (TLC) was carried out on Merck plates precoated with Kieselgel 60F254 developing with EtOAc-n-PrOH-AcOH-H2O (40:40:1:30). The spot of theanine (Rf 0.55) was detected by Ninhidrin Spray (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Analysis of amino acids in the M-W fraction
Amino Acids in the M-W Fraction were analyzed with Hitachi L-8800A amino acid analyzer. The data from M-W (10.1 mg in 1 ml of 0.02 M hydrochloric acid) were compared with those from the standard solutions.
Animal model
Sprague-Dawley neonatal rats (Clea Japan Co., Tokyo, Japan) were used for the experiment. The neonatal rats were randomly divided into distilled water (DW), DM-A50, DM-W, M-B, or M-W treatment group in room-air or oxygen-exposed condition. For each experiment, 8 to 14 neonatal rats were kept with a mother rat in each cage. Mother rats were rotated between the hyperoxia and room air environments every two days within the experimental period. Retinal NV was induced in neonatal rats by our standard protocol [7, 17]. The oxygen-exposed group rats were exposed from birth to day 12 (P12) to daily cycles of 80% oxygen (20.5 h), room air (0.5 h) and progressive return to 80% oxygen (3 h). On P12, the rats were placed in room air until P18.
Treatment schedule
Four kinds of GTFs (DM-A50, DM-W, M-B, or M-W; 0.05 g/ml or 0.01 g/ml) were used. DM-W and M-W were dissolved in distilled water (DW), DM-A50 and M-B were dissolved in striped corn oil (Tama Biochemical Co. Ltd., Tokyo). Those were given by gastric gavage to animals in constant volume of 50 µl/10 g pretreatment body weight once daily from P6 to P17. Body weight was measured every 2 days from P0 to P18.
Retina processing
On P18, the rats were sacrificed by intraperitoneal injection of 0.3 ml of sodium pentobarbital (50 mg/ml) and the retinal samples were fixed, stained for adenosine diphosphatase (ADPase), and flatmounted as previously described [7]. Retinal NV was scored using the method of Hasebe et al. [17]. Avascular areas (AVAs) were measured in ADPase stained retinas. Digital images of the mounted ADPase stained retinas were obtained using a camera and scanner (Nikon, Tokyo, Japan). Avascular and total retinal areas were measured using NIH image software (National Institutes of Health, Bethesda, MD). The mean percentages of AVAs in the total retinal area (%AVAs) were calculated.
Statistical analysis
Statistical analysis was performed by using Welch's t test. All data are expressed as a mean ± standard error (SE). Differences were considered significant when p<0.05.
Results
Analysis of green tea fractions
The concentration of EGC, EGCG, ECG and EC was high in both DM-A50 and M-B fractions. Levels of these components in DM-A50 were nearly twice higher when compared to those in M-B fraction. In contrast, these components were not present very much in both DM-W and M-W fractions. High levels of caffeine were found in DM-W and M-B fractions (Table 1). The spot of theanine was distinctly in M-W. Although the week spot of theanine were detected in M-B and DM-W, no spot of theanine was detected in DM-A50.
Table 1.
| DM-A50 | DM-W | M-B | MW | |
|---|---|---|---|---|
| Caffeine | 0.4 | 6.7 | 19.6 | 1.1 |
| (−)-EGC | 57.9 | — | 32.1 | 1 |
| (+)-Catechin | 1.1 | 0.3 | 0.8 | 0.04 |
| (−)-EC | 6.8 | 0.6 | 4.1 | 0.1 |
| (−)-EGCG | 18.9 | — | 10.7 | — |
| (−)-ECG | 10.7 | — | 6.6 | — |
The contents of major amino acids in M-W were shown as follows: L-theanine 8.1%, L-glutamic acid 0.91%, L-glutamine 0.76%, L-aspartic acid 0.46%, L-serine 0.24%, glycine 0.17%, L-threonine 0.12%, L-asparagine 0.12%, L-arginine 0.08%.
Body weight change
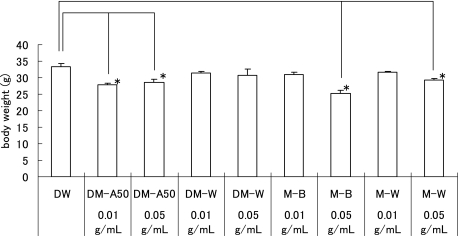
The body weights of oxygen-exposed rats on P18 of DM-A50, M-B and M-W in 0.05 g/ml-treated groups and DM-A50 in 0.01 g/ml-treated groups were significantly lower than these of DW (p<0.05, Fig. 2) in the oxygen-exposed rats. The body weights of room-air raised rats (RA control) on P18 of DM-A50, DM-W, and M-B 0.05 g/ml-treated groups, and M-B and M-W in 0.01 g/ml-treated groups were significantly lower than those of DW (p<0.05, data not shown).
Fig. 2.
Body weight of the oxygen-exposed rats on P18. Data represents mean ± SE. The body weights on P18 of DM-A50, M-B and M-W in 0.05 g/ml-treated group and DM-A50 in 0.01 g/ml-treated group were significantly lower than these of DW (*p<0.05, n = 5–31).
Effect of GTFs treatment on OIR
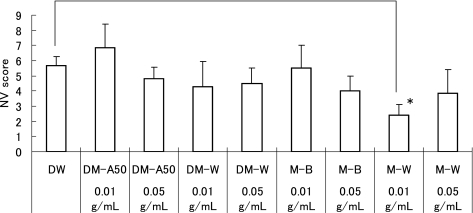
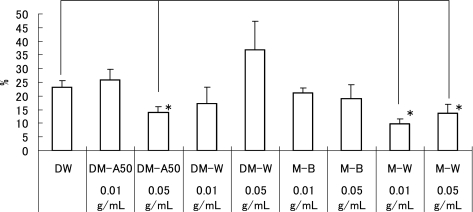
In all retinal samples from the RA control, normal retinal vascular developments were observed. Therefore, the NV scores and the %AVA were 0 in all of the RA control receiving oral DW (n = 31). A typical sample of flatmounted retinas in the oxygen-exposed rats are shown in the Fig. 3. The NV scores and the % AVA were 5.7 ± 0.6 and 23 ± 2% in the oxygen-exposed rats receiving oral DW (n = 31, p<0.001), respectively. The oxygen-exposed retinas exhibited neovascular changes and retained avascular areas, regardless with GTFs treatment. In the RA control, both NV scores and %AVAs did not differ among the GTFs treatment groups (data not shown). In the oxygen-exposed rats, the NV scores were 4.8 ± 0.8 in 0.05 g/ml DM-A50 (n = 10), 6.9 ± 1.6 in 0.01 g/ml DM-A50 (n = 7), 4.5 ± 1.0 in 0.05 g/ml DM-W (n = 8), 4.3 ± 1.7 in 0.01 g/ml DM-W (n = 7), 4.0 ± 1.0 in 0.05 g/ml M-B (n = 5), 5.5 ± 1.5 in 0.01 g/ml M-B (n = 6), 3.9 ± 1.6 in 0.05 g/ml M-W (n = 7), and 2.4 ± 0.7 in 0.01 g/ml M-W (n = 7), respectively (Fig. 4). The NV scores in 0.01 g/ml M-W treated group was significantly lower than those in DW (p<0.05). The %AVAs were 14 ± 2% in 0.05 g/ml DM-A50, 26 ± 4% in 0.01 g/ml DM-A50, 37 ± 10% in 0.05 g/ml DM-W, 17 ± 6% in 0.01 g/ml DM-W, 19 ± 5% in 0.05 g/ml M-B, 21 ± 2% in 0.01 g/ml M-B, 14 ± 3% in 0.05 g/ml M-W and 10 ± 2% in 0.01 g/ml M-W, respectively (Fig. 5). The %AVAs in 0.05 g/ml DM-A50 and 0.05 g/ml and 0.01 g/ml M-W treated groups were significantly lower than those in DW (p<0.05).
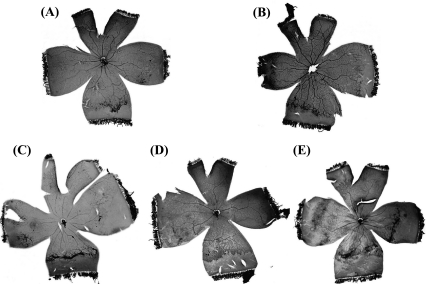
Fig. 3.
The typical samples of flatmounted ADPase stained retinas in OIR. (A) 0.05 g/ml DM-A50 treated group. NV score is 6 %AVAs is 10.62%. (B) 0.05 g/ml DM-W treated group. NV score is 3 %AVAs is 16.39%. (C) 0.05 g/ml M-B treated group. NV score is 7 %AVAs is 26.18%. (D) 0.05 g/ml M-W treated group. NV score is 5 %AVAs is 23.28%. (E) DW group. NV score is 13 %AVAs is 32.05%.
Fig. 4.
NV score in the oxygen-exposed rat retinas. In 0.01 g/ml M-W treated group, NV score is significantly lower than DW. The data represent mean ± SE (*p<0.05, n = 5–31).
Fig. 5.
%AVAs in the oxygen-exposed rat retinas. In 0.05 g/ml DM-A50 and 0.05 g/ml and 0.01 g/ml M-W treated groups the %AVAs are significantly lower than those in DW. The data represent mean ± SE (p<0.05, n = 5–31).
Discussion
The present study demonstrated that the oxygen-exposed retinas exhibited neovascular changes and retained avascular areas in the rat model of OIR, regardless of GTFs treatment.
The neonatal rat retina is incompletely vascularized at birth, providing a model for the human premature infant retina and for retinopathy of prematurity [7, 18, 19]. In the present experiment, both NV scores and %AVAs were extremely higher in the oxygen exposed retinas as compared to those in the RA control. These findings confirmed that our rat model of OIR is a suitable model for the present study [17].
Green tea extracts reportedly decreased NV in a rat model of OIR [13]. It is possible that some factors in green tea may prevent NV in an OIR. However, there are no studies which investigated the exact potent antiangiogenic components in green tea in rat model of OIR. Therefore, we hypothesized that some components of green tea could prevent OIR. Indeed, a significant reduction in the severity of abnormal retinal NV was observed in the eyes treated with 0.01 g/ml M-W (Fig. 4). In addition, both doses of M-W and higher dose, e.g. 0.05 g/ml, of DM-A50 had lesser, but significant preventive effect on %AVAs (Fig. 5).
Green tea contains a number of constituents, including catechins, proteins, amino acids, vitamins, minerals and pigments [14–16]. Of these constituents, the main ingredients of green tea extract, catechins, have powerful beneficial effects in various pathophysiological condition [10, 11, 16]. Higher dose of DM-A50 which contains abundant catechins (Table 1) significantly decreased %AVAs (Fig. 5), suggesting that high dose of catechins might have some preventive effect on NV. Epigallocatechin gallate (EGCG), a principal polyphenolic component of green tea, is considered key to these healthful qualities. Sartippour et al. [20] reported that EGCG inhibited protein and mRNA expression of VEGF by human breast cancer cells in vitro. On the contrary, purified EGCG reportedly had no effect on NV in the OIR rats model [13]. One of reasons for these different effects of EGCG may be a dual function of anti-oxidant and pro-oxidant potentials of EGCG [21]. Our study demonstrated that DM-A50 contains high concentration of various catechins including EGC, ECG, EC as well as EGCG. Therefore, it is difficult to identify a specific component of catechins which could exert preventive effect on NV in OIR, although we can not exclude the possibility of synergetic effects of various components contained in the DM-A50.
On the other hand, the analysis of components in the M-W fraction showed that the contents of each catechin were only negligible in the M-W fraction (Table 1), but the M-W fraction contains high amount of theanine, caffeine, (−)-EGC and (−)-EC. Theanine is a major amino acid in green tea [10, 14]. Theanine comprises 1–2% of the dry weight of tea leaves, makes up approximately 50% of the amino acids in tea [14]. Theanine also protects normal cells from damage by these drugs via antioxidant activity [14]. Taken together, the evidences suggest that components other than catechins play an important role in the prevention of OIR rat receiving the M-W fraction. A potential beneficial component in the M-W fraction may be theanine. Further work on the role of theanine in the prevention of OIR is warranted.
GTFs can be given by ad libitum access to drinking water or to sucking mother’s milk containing GTFs. Catechins from green tea are rapidly absorbed and detected in plasma [22, 23]. The addition of milk to tea does not interfere with catechin absorption [16, 24–27], but milk may affect the antioxidant potential of tea, depending upon milk fat content, milk volume added, and the method used to assess this parameter [16, 24, 26]. We chose gastric gavage method to provide accurate amount of GTFs to neonatal rats in this experiment. As for the dosage of GTFs, 0.5 g/ml DM-A50 in distilled water was lethal for the neonatal rats in our preliminary study. Therefore, we selected 0.05 g/ml and 0.01 g/ml of GTFs for this experiment. DM-A50 and higher dose of M-B and M-W significantly decreased body weight of the rats on P18. Most animals receiving higher dose of the GTFs had mild diarrhea.
The inhibition of NV by GTFs did not show significant dose dependence within the range of the dose used in our study. There are many reports of the dose-dependent effect of green tea, whereas the inhibition of carcinogenesis by green tea polyphenol fraction was not dose-dependent in rats [28]. These results indicate that effects of green tea extract fraction are not necessarily dose-dependent. Since only two doses of each GTF were given to neonatal rats in this experiment, we can not draw any conclusion on the dose dependency of GTFs’ effect on the NV in this experimental setting.
A limitation of our study is that we do not have any data on the other components except catechins, caffeine and theanine which could have influenced our results. Another limitation is that we did not use purified individual components of green tea for the comparison of their potency in OIR model. However, a previous study demonstrated that modulation of biological expression by green tea extracts can not be attributed to the action of a single tea catechin, but rather is due to the effects of a complex mixture [13].
Retinopathy of prematurity continues to be a major cause of blindness in children [2]. Increased survival of extremely low birth infants due to advances in antenatal and neonatal care has resulted in a population of infants at high risk of developing ROP [3–6]. Prevention of retinopathy of prematurity minimizes the likelihood of blindness. The present results showed that gastric administration of both M-W and DM-A50 fractions derived from green tea substantially ameliorated NV in the rat model of OIR. The finding suggests that M-W fraction of green tea is a potent inhibitor of oxygen-induced NV. If these effects are paralleled in human neonates, M-W supplementation of GTFs during oxygen therapy might be beneficial in decrease the risk of developing retinopathy of prematurity. Moreover, our results provide possibility that maternal dietary supplementation with green tea fractions during the last term of pregnancy and throughout the duration of litter suckling could protect retinopathy of prematurity. Since consumption of long-term green tea has already been proven safe in humans, the present data indicate that clinical trials of green tea fraction such as M-W fraction for proliferative retinopathy should be implemented expeditiously.
Acknowledgments
The authors thank Ms. E. Ozawa for her technical assistance.
References
- 1.Das A., McGuire P.G. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Steinkuller P.G., Du L., Gilbert C., Foster A., Collins M.L., Coats D.K. Childhood blindness. J. AAPOS. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 3.Gong A., Anday E., Boros S., Bucciarelli R., Burchfield D., Zucker J., Long W. One-year follow-up evaluation of 260 premature infants with respiratory distress syndrome and birth weights of 700 to 1350 grams randomized to two rescue doses of synthetic surfactant or air placebo. American Exosurf Neonatal Study Group I. J. Pediatr. 1995;126:S68–74. doi: 10.1016/s0022-3476(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 4.Valentine P.H., Jackson J.C., Kalina R.E., Woodrum D.E. Increased survival of low birth weight infants: impact on the incidence of retinopathy of prematurity. Pediatrics. 1989;84:442–445. [PubMed] [Google Scholar]
- 5.Schalij-Delfos N.E., Cats B.P. Retinopathy of prematurity: the continuing threat to vision in preterm infants. Dutch survey from 1986 to 1994. Acta Ophthalmol. Scand. 1997;75:72–75. doi: 10.1111/j.1600-0420.1997.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibson D.L., Sheps S.B., Uh S.H., Schechter M.T., McCormick A.Q. Retinopathy of prematurity-induced blindness: birth weight-specific survival and the new epidemic. Pediatrics. 1990;86:405–412. [PubMed] [Google Scholar]
- 7.Reynaud X., Dorey C.K. Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest. Ophthalmol. Vis. Sci. 1994;35:3169–3177. [PubMed] [Google Scholar]
- 8.Zhao B. The health effects of tea polyphenols and their antioxidant mechanism. J. Clin. Biochem. Nutr. 2006;38:59–68. [Google Scholar]
- 9.Crespy V., Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 10.Cooper R., Morre D.J., Morre D.M. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J. Altern. Complement. Med. 2005;11:521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R., Morre D.J., Morre D.M. Medicinal benefits of green tea: part II. review of anticancer properties. J. Altern. Complement. Med. 2005;11:639–652. doi: 10.1089/acm.2005.11.639. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y., Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 13.Minami M., Hasebe Y., Nakanishi-Ueda T., Iwai S., Ueda T., Dorey C.K., Oguchi K., Yasuhara Y., Koide R. Inhibition of Oxygen-induced Retinal Neovascularization in the Neonatal Rat by Green Tea Extract. J. Clin. Biochem. Nutr. 2003;33:23–31. [Google Scholar]
- 14.L-theanine. Monograph. Altern. Med. Rev. 2005;10:136–138. [PubMed] [Google Scholar]
- 15.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera C., Artacho R., Gimenez R. Beneficial effects of green tea? a review. J. Am. Coll. Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 17.Hasebe Y., Thomson L.R., Dorey C.K. Pentoxifylline inhibition of vasculogenesis in the neonatal rat retina. Invest. Ophthalmol. Vis. Sci. 2000;41:2774–2778. [PubMed] [Google Scholar]
- 18.Ricci B. Oxygen-induced retinopathy in the rat model. Doc. Ophthalmol. 1990;74:171–177. doi: 10.1007/BF02482606. [DOI] [PubMed] [Google Scholar]
- 19.Penn J.S., Tolman B.L., Henry M.M. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest. Ophthalmol. Vis. Sci. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 20.Sartippour M.R., Shao Z.M., Heber D., Beatty P., Zhang L., Liu C., Ellis L., Liu W., Go V.L., Brooks M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 21.Kanadzu M., Lu Y., Morimoto K. Dual function of (−)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Lett. 2005;241:250–255. doi: 10.1016/j.canlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Lee M.J., Li H., Yang C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 23.Rietveld A., Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J. Nutr. 2003;133:3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- 24.Leenen R., Roodenburg A.J., Tijburg L.B., Wiseman S.A. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur. J. Clin. Nutr. 2000;54:87–92. doi: 10.1038/sj.ejcn.1600900. [DOI] [PubMed] [Google Scholar]
- 25.Reddy V.C., Vidya Sagar G.V., Sreeramulu D., Venu L., Raghunath M. Addition of milk does not alter the antioxidant activity of black tea. Ann. Nutr. Metab. 2005;49:189–195. doi: 10.1159/000087071. [DOI] [PubMed] [Google Scholar]
- 26.van het Hof K.H., Kivits G.A., Weststrate J.A., Tijburg L.B. Bioavailability of catechins from tea: the effect of milk. Eur. J. Clin. Nutr. 1998;52:356–359. doi: 10.1038/sj.ejcn.1600568. [DOI] [PubMed] [Google Scholar]
- 27.Hollman P.C., Van Het Hof K.H., Tijburg L.B., Katan M.B. Addition of milk does not affect the absorption of flavonols from tea in man. Free Radic. Res. 2001;34:297–300. doi: 10.1080/10715760100300261. [DOI] [PubMed] [Google Scholar]
- 28.Yamane T., Hagiwara N., Tateishi M., Akachi S., Kim M., Okuzumi J., Kitao Y., Inagake M., Kuwata K., Takahashi T. Inhibition of azoxymethane-induced colon carcinogenesis in rat by green tea polyphenol fraction. Jpn. J. Cancer Res. 1991;82:1336–1339. doi: 10.1111/j.1349-7006.1991.tb01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]