Abstract
The eye is afflicted by chronic vision debilitating neovascular disorders, such as age-related macular degeneration, proliferative diabetic retinopathy and corneal angiogenesis. Photodynamic therapy (PDT) is an innovative, evolving approach for treating neovascular diseases of the eye. PDT refers to the process of activating a light sensitive agent or carrier with non-thermal light to induce chemical reactions that ameliorate a pathological condition. Key components of PDT include a photosensitizer, a colloidal carrier or formulation and a light source. This article summarizes currently available clinical PDTs, desirable features of PDTs and photosensitizers, useful light sources for PDT and investigational nanosystems and colloidal carriers for PDT.
Keywords: Photodynamic therapy, drug delivery, gene delivery, nanotechnology, photosensitizers
Introduction
Therapeutic application of light is ancient. In fact, a review of the historical usage of light therapy reveals a cross-cultural pattern of inclusion in folk medicine, leading to established medical procedures. Throughout ancient Egypt, India and China, light alone, or light in combination with chemical compounds, was used extensively to induce therapeutic effects in various conditions including depigmented skin lesions (vitiligo), psoriasis and neurodermite [1]. Around the turn of the last century, a medical student named Oscar Raab observed that low concentrations of acrydine dye in the presence of light could be lethal to the protozoan paramecium [1]. Raab hypothesized that upon irradiation, acrydine dye converted light energy into chemical energy. The chemical reaction initiated phototoxic effects in the presence of molecular oxygen to kill the paramecia; hence, the term “photodynamic reaction” was coined for the reactions [1]. Such photodynamic reactions are of potential value in reducing undesirable cell proliferation in various disorders, especially endothelial cell or other cell proliferation associated with neovascular or angiogenic disorders including cancers. Photodynamic reactions are the driving forces of photodynamic therapy (PDT) currently used in clinics to treat ocular neovascular disorders, such as age related macular degeneration. The purpose of the present review is to present key advances in light-sensitive delivery systems intended for improved drug and gene therapy in the eye.
Clinical Applications
The application of PDT, as seen in Figure 1, employs a delivery method to localize a photosensitizing compound selectively in the target tissue. For instance, in treating neovascularization or abnormal endothelial proliferation in the back of the eye, a colloidal carrier containing a photosensitizer can be injected intravenously to facilitate the localization of the carrier in the neovascular region due to enhanced permeability and slow clearance of colloidal carriers from such regions [2]. Subsequent activation of the photosensitizing compound via a non-thermal laser light generates highly reactive singlet oxygen (1O2) in order to prompt light-induced cytotoxicity [2]. Damaged neovascular endothelial cells are known to release procoagulant and vasoactive factors through the lipo-oxygenase and cyclo-oxygenase pathways, resulting in platelet aggregation, fibrin clot formation and vasoconstriction [3]. Effective PDT is dependent upon an abundant supply of molecular O2, which ensures its usefulness in living tissue [1]. When treating corneal angiogenesis associated with transplant rejections, destruction of neovascular vessels is often needed [4]. Thus, it can be envisioned that the photosensitizer formulation can be administered either intravenously or as a topical drop, followed by activation. An alternative to the use of photosensitizer as a therapeutic agent is its use as a mediator in facilitating targeted release of drugs, plasmids, or dyes intended for therapy. For instance, a photosensitizer incorporated in a carrier formulation can potentially be activated to disrupt the carrier and release its contents, including therapeutic or diagnostic agents.
Fig. 1.
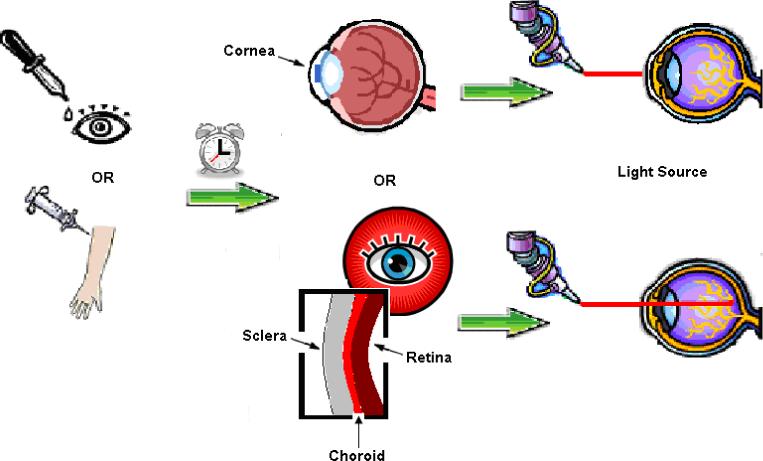
Photodynamic Therapy Mechanism
The most attractive light sensitive delivery system for PDT is capable of controlled photosensitizer or drug release, of targeting specific tissues and of being biocompatible and having limited toxic effects in the body (Box 1).
In recent years, PDT has evolved into a viable treatment for age-related macular degeneration (AMD) as well as cancers. In developed countries, AMD is the leading cause of vision loss [2]. A long-term therapeutic strategy is needed for such blinding diseases as AMD because they are chronic and progressive [5]. Clinical use of PDT is now facilitated through Visudyne®, a FDA approved light sensitive delivery system (Table 1). Visudyne is a liposomal formulation that reduces the risk of vision loss in cases of predominately classic (“wet”) subfoveal choroidal neovascularization due to age-related macular degeneration or subfoveal choroidal neovascularization secondary to pathological myopia, particularly in the absence of occult CNV [6]. The photosensitizer verteporfin, a benzoporphyrin-derivative monoacid ring A, is the principle ingredient of Visudyne®. The finished drug product is a green, lyophilized liposome powder that is reconstituted before intravenous infusion using sterile water for injection. Visudyne® is administered in a two-stage process requiring both the injection of verteporfin, followed by the application of non-thermal red light after a 15 min interval. Liposomal formulation of Visudyne® is selectively retained in neovascular spots of the eye, which allows targeted therapy. The interval between the administration of formulation and light application is the shortest among the clinical applications of PDT listed in Table 1. Such a short interval allows the design of a formulation that minimizes prolonged skin photosensitivity.
Table 1.
FDA Approved Photodynamic Therapies[49]
|
Brand name |
Formulation and light source |
Indication |
Dosing regimen |
|---|---|---|---|
| Visudyne® | Ingredients: 15 mg verteporfin plus lactose, egg phosphatidylglycerol, dimyristoyl phosphatidylcholine, ascorbyl palmitate and butylated hydroxytoluene | CNV treatment: Classic (“wet”) subfoveal choroidal neovascularization due to AMD | Photosensitizer dosage: 6 mg/m2 body surface area dose intravenously (diluted with 5% dextrose for 30 ml volume) |
| Reconstitution: Each vial of Visudyne is mixed with 7 mL of sterile water to provide 7.5 mL containing 2 mg/mL verteporfin. The preparation must be used within 4 h after reconstituted | Classic subfoveal choroidal neovascularization secondary to pathological myopia | Light dosage: Exposure time of 83 seconds at a dose of 50 J/cm2 of neovascular lesion at a light intensity of 600 mW/cm2 | |
| Approved light sources: Coherent Opal Photoactivator laser console and modified Coherent LaserLink adapter, Manufactured by Lumenis, Inc., Santa Clara, CA; Zeiss VISULAS 690s laser and VISULINK PDT/U adapter, Manufactured by Carl Zeiss Inc., Thornwood, NY. | Light application: Exposure to 689±3 nm wavelength (non-thermal red light) using diode laser 15 min after start of injection | ||
| |
|
|
Treatment spot size should be 1000 microns larger than the greatest linear dimension of the lesion on the retina to allow a 500 micron border. The maximum spot size used in clinical trials was 6400 microns. |
| Photofrin® | Ingredients: 75 mg porfimer sodium as a freeze-dried cake or powder | Cancer treatments: Esophageal cancer | Photosensitizer dosage: 2 mg/kg body weight dose intravenously |
| Reconstitution: Each vial of Photofrin is mixed with 31.8 ml of 5% dextrose or 0.9% sodium chloride resulting in a final concentration of 2.5 mg/kg porfimer sodium. The preparation must be protected from bright light and administered immediately | Non-small cell lung cancer | Light dosage: Exposure times of 12.5 min at a dose of 300 joules/cm of tumor length in esophageal cancer | |
| Precancerous lesions in Barrett's Esophagus | Exposure time of 8.3 min at a dose of 200 joules/cm of tumor length in endobronchial cancer | ||
| Approved light source: Cylindrical Optiguide− fiber optic diffusers are used to pass light through the operating channel of an endoscope/bronchoscope. | Light application: Exposure to 630 nm wavelength (non-thermal laser) after 40−50 h interval following injection | ||
| |
|
|
Optional 2nd laser application 96−120 h after injection Important to perform a debridement of residual tumor 2−3 days following each light administration |
| Levulan ® | Ingredients: Plastic tube containing two sealed glass ampules; 1st ampule contains 1.5 ml of solution vehicle comprising alcohol USP (ethanol content = 48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol; 2nd ampule contains 354 mg of aminolevulinic acid HCl (Levulan Kerastick for Topical Solution) as a dry solid. | Actinic keratosis treatment: Minimally to moderately thick actinic keratosis (Grade 1 or 2) of the face and scalp | Photosensitizer dosage: Application of product with Levulan Kerastick |
| Kerastick ® | Reconstitution: N/A Administered using Levulan Kerastick | Light dosage: Exposure time of 16.67 minutes at a dose of 10 J/cm2 | |
| Approved light source: BLU-U Blue Light Photodynamic Therapy Illuminator | Light application: Exposure to 400−450 nm wavelength using blue light after 14−18 h interval following product application |
Likewise, Photofrin® is currently approved by the FDA for the treatment of esophageal cancer and endobronchial non-small cell lung cancers. The active ingredient of Photofrin® is the photosensitizer, porfimer sodium, often referred to as dihematoporphryin ether (DHE). Photofrin®is a freeze-dried cake that requires reconstitution in a vehicle, provided by the manufacturer, before administration. DHE is a widely-used photosensitizer with an established safety profile. In preclinical trials, DHE demonstrated a distinct ability to localize in neovascular tissues for photodynamic therapy of the bladder, laryngeal papillomas, bronchial, cutaneous, and ocular malignancies, and particularly corneal neovascularization [7]. Photosensitizer clearance from several other tissues occurs over 40−72 h following the injection. In patients with corneal neovascularization, intravenous administration of DHE (2 mg/kg), followed by treatment with an argon green laser (514 nm) at 72 h, is effective. Patients experienced positive outcomes with immediate and sustained reduction in corneal neovascularization. Some patients experienced short-term phototoxicity reactions and other adverse effects [7]. Integration of DHE into liposomal or other carriers might further enhance this therapeutic approach.
Furthermore, Levulan® Kerastick® is another form of PDT applied topically followed by light therapy. This therapy has been approved for the treatment of actinic keratoses using aminolevulinic acid HCl as the photosensitizer. Each Levulan® Kerastick® for Topical Solution applicator consists of a plastic tube containing two sealed glass ampules and an applicator tip. The first ampule contains 1.5 ml of solution vehicle, while the second ampule contains 354 mg of aminolevulinic acid.HCl. Both ampules of the applicator are crushed and mixed within a protective cardboard sleeve and cap. Immediately after reconstitution, the solution is applied directly from the applicator tip to the target lesions. Following a 14−18 hour interval, laser light is applied using the FDA-approved BLU-U Blue Light Photodynamic Therapy Illuminator. During light exposure, patients often experience transient stinging or burning at the target site. The use of Levulan® Kerastick® for Topical Solution with any other light device is not recommended. Adverse effects may occur if Levulan® Kerastick® comes into contact with perilesional areas, because exposure to light may result in skin photosensitivity and erythema and/or edema of the lesions. The effect of Levulan® Kerastick® on ocular tissue is unknown, yet it is possible that such an approach might find application to the corneal and conjunctival surfaces of the eye.
In addition to the above FDA-approved approaches for PDT, several clinical trials are now underway to determine the usefulness of PDT as an adjuvant to other therapeutic agents in treating choroidal neovascularization in AMD. Several of these examples are listed in table 2. For example, the therapies being assessed involve Visudyne® in combination with anti-VEGF therapeutics, including Lucentis (a VEGF antibody fragment approved for intravitreal use in treating AMD), Avastin™ (a VEGF antibody approved for colon cancer treatment but shown to be of value in treating AMD after intravitreal injection), and Vatalanib (a receptor tyrosine kinase inhibitor with specifity for VEGF and platelet derived growth factor receptors). Also, Visudyne® is being assessed in combination with steroid drugs including triamcinolone acetonide and dexamethasone for its effectiveness in CNV. Although the motivation behind some of these studies might be better to position therapeutic agents, such as Lucentis and Avastin™ compared to Visudyne®, it will be interesting to see if the combination therapies result in improved vision compared to any of the monotherapies.
Table 2.
Current Clinical Trials: PDT as an Adjuvant to Other Therapeutic Agents
|
Therapeutic Combination |
Comparative Trial |
Application |
|---|---|---|
| Visudyne® / Lucentis (LUV Trial) |
Combination therapy with intravitreal Lucentis and verteporfin PDT versus Intravitreal Lucentis alone |
AMD/ CNV (Phase IV) |
| TAC-PF / Avastin® (VERTACL) |
Triple therapy using intravitreal Avastin®, half fluence verteporfin PDT, and intravitreal triamcinolone acetonide-preservative free (TAC-PF) versus Intravitreal Avastin alone |
AMD/CNV (Phase II) |
| Visudyne® / Avastin® |
Visudyne PDT (low or very low fluence rate) combined with intravitreal injections Avastin versus Intravitreal Avastin alone |
AMD/ CNV (Phase II) |
| Vatalanib / Visudyne® |
Oral Vatalanib (VEGF-receptor tyrosine kinase inhibitor) with Visudyne® PDT versus Oral Vatalanib alone |
AMD/ CNV (Phase II) |
| Reduced Fluence Visudyne-Anti-VEGF-Dexamethasone (RADICAL) | Reduced-fluence Visudyne followed by intravitreal Lucentis (within 2 h) or two regimens of reduced-fluence Visudyne followed by intravitreal Lucentis-Dexamethasone triple therapy (within 2 h) versus Intravitreal Lucentis | AMD/ CNV (Phase II) |
Identifying the growth factors and receptors of diseases such as CNV, may open doors toward treatments devised to block the receptors that stimulate new vessel formation. As an addendum to this research, one study proposed stimulating a complementary therapeutic outcome through co-administering photodynamic therapy, using either a liposomal formulation or polymeric nanoparticles, in conjunction with a compound capable of inhibiting vascular endothelial growth factor (anti-VEGF) [3]. The combined use of both treatments involves the occlusion of CNV lesions using PDT while anti-VEGF therapy alters the progression of the disease and maintains the PDT effect.
Evidence from another study involving AQ4, an antitumor anthracenedione, suggests that AQ4 may potentially be used in the photodynamic therapy of ocular neovascular disorders. In the eye, AQ4 inhibits endothelial cell proliferation and vascular endothelial growth factor secretion [8].
A light-sensitive delivery system protects and decreases the toxicity of a therapeutic agent while improving its stability. In the following discussion, examples of delivery systems that can be used for photosensitizers or other therapeutic agents as a part of PDT are described.
Light Sensitive Delivery Systems
Liposomes
Liposomes possess characteristics that are favorable for ocular photodynamic therapy. Besides their established use as nanocarriers for drug and gene delivery, liposomes can entrap photosensitizers with different physicochemical properties [9, 10]. Visudyne® is the only liposomal photosensitizer that has been clinically approved. Liposomes in Visudyne® therapy are primarily vesicles composed of phospholipids and cholesterol. These liposomes allow accumulation of the photosensitizer in the neovascular tissue of the eye. Further, liposomal formulations are capable of substantially decreasing photosensitizer aggregation, which ameliorates the efficiency of the photosensitizer.
Upon light exposure, the light sensitive liposomes release their content by oxidative degradation of the lipid bilayer. Additional strategies can be used to induce triggered release from liposomes. For example, addition of lipids, such as plasmenylcholine, may be used as liposome membrane constituents. In response to photosensitization at wavelengths between 630 nm and 820 nm, such usage increases membrance permeability of the liposome [9]. In the presence of light, reactive oxygen species attack the plasmenycholine vinyl-ether bond, generating single chain surfactants, such as lysolipids and fatty aldehydes [9]. This results in vesicle membrane defects, leading to liposome leakage. The irradiation wavelength required for drug release is dictated by the absorption spectrum of the liposome-entrapped sensitizer. If an appropriate sensitizer is chosen, liposome oxidation may occur at wavelengths that are non-destructive to the drug or neighboring tissues [11].
The development of a liposomal formulation capable of remaining stable and inert before administration to the body is critical [9]. A notable approach involves liposomes made of dipalmitoyl phosphatidylcholine and N-stearoyl-L-histidine incorporating the dye, haematoporphyrin [9]. These liposomes are stable in the dark and leaky upon photolysis with visible light. In these liposomes, one photosensitizer was bound to the membrane and another encapsulated in the core. In such cases, the membrane-bound photosensitizer initiates triggered release of the liposome contents, and the encapsulated photosensitizer proceeds to occlude ocular lesions with improved efficiency.
Another liposome innovation entails the use of polymerizable lipids for light sensitive delivery. One example is inclusion of bis-substituted photosensitive lipids (e.g., bis-SorbPC or bis-DenPC) in poly (ethylene glycol) liposomes (PEG-liposomes) that allows the light-induced formation of cross-linked polymer networks [12]. Because the liposomes are PEG conjugated, they are sterically stable with increased drug and gene delivery potential. The cross polymerization of domains significantly increases the permeability of the liposome's bilayer. The reactive lipids, bis-SorbPC and bis-DenPC, each have an activated diene group incorporated in the lipid tail near the glycerol backbone or at the end of the hydrocarbon tail [13]. Light-induced destabilization of PEG-liposomes consisting of bis-Sorb PC may be further sensitized to visible light by incorporating cyanine dye into the bilayer wall. A particularly effective sensitizer dye is the green light absorbing 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine (DiIC(18)3) because it has a strong affinity for SorbPC bilayers. Researchers have shown that the addition of ballasted cyanine dyes to liposomes composed of polymerizable lipids render the liposome sensitive to the wavelength of light absorbed by the dye, which is advantageous in comparison to plasmologen liposomes [12, 14, 15]. It is speculated that the dye initiates electron transfer from the dye-excited state of oxygen, yielding superoxide anion, which forms hydrogen peroxide in aqueous media [13]. Oxygen is required to initiate the reaction; however, dye-sensitized polymerization is mediated by the formation of hydroxyl radicals near the lipid bilayer. The reaction is limited to select monomers due to oxygen's ability to inhibit radical polymerization [13]. Properties of cyanine dye, such as absorption, emission, and aggregation, are also affected in the lipid environment. Further investigation of the interaction between lipid bilayer and ballasted cyanine dye may elucidate ways to impede the dye's tendency to aggregate. Different cyanine dyes exhibit maximum absorption in the blue, green or red regions of the visible spectrum, providing the ability to vary the wavelength of activation light based on the therapeutic aim [13].
With respect to gene therapy, cationic liposomes are useful in enhancing gene expression in the target tissue. However, their limitations include: inefficient DNA condensation, inefficient cell surface receptor binding, poor intracellular trafficking, toxicity, and reduced efficiency in the presence of serum. Compared to naked pDNA, pDNA/cationic liposome complexes enhance gene expression in ocular tissues after intravitreal injection [16]. Gene delivery to selected neovascular targets can be potentially enhanced by using light sensitive cationic liposomes or other delivery systems in conjunction with the pDNA. Light-induced destabilization of such liposomes offers a method to couple the temporal and spatial control of light to drug and gene delivery, allowing the development of new therapeutic treatments.
In retrospect, patients receiving Visudyne® PDT often require several treatments, due to persistent inflammation and hypoxia along with the elevation of angiogenic stimuli,including vascular endothelial growth factor (VEGF) [3]. Repeated PDT treatments may lead to cumulative damage of the retinal pigment epithelium (RPE), choriocapillaries, and overlying neurosensory retina [17]. Visudyne® is also not applicable in the treatment of CNV lesions < 50% classic or occult CNV without the classic component [17, 18]. In this regard, Visudyne® has not met the ideal objective of PDT, which is to destroy, extensively, abnormal blood vessels while avoiding damage to the normal surrounding tissues of the eye. Even after including photosensitizer dyes in the gene and drug delivery systems, their toxicity needs further careful evaluation. For instance, liposomes containing porphyrin as the photosensitizer have induced early and extensive endocytoplasmic damage [9].
Polymeric Micelles
Polymeric micelles widely assessed as nanocarriers for drug and gene delivery, offer innovative opportunities for PDT [2]. The core of polymeric micelles can act as a reservoir for hydrophobic photosensitizers intended for photodynamic therapy. Compared to liposomes, polymeric micelles can potentially be smaller, allowing greater tissue-penetrating ability. Micelles ranging in size between 50−100 nm can be readily prepared. Similar to liposomes, micelles can be formed based on principles of self-assembly. Block copolymers can be judiciously used for this purpose. For instance, a di-block copolymer with one hydrophilic segment and a lipophilic segment can self-assemble into a micelle at and above critical micellar concentrations. Alternatively, a diblock copolymer with one positively-charged segment and a neutral segment can interact with a negatively charged material, resulting in the formation of micelle like structures. In order to overcome PDT side effects, such as skin hypersensitivity, micellar delivery systems have been investigated in recent years.
An example of PDT micellar systems entails incorporation of dendritic photosensitizers into micelles. Recently, ionic dendritic porphyrin loaded in PEG-block-poly(L-lysine) micelles has been shown to be a viable delivery system for ophthalmic applications [2]. In this design, the focal core of porphyrin is surrounded by the 3rd generation of poly(benzyl ether) dendrons with peripheral ionic (carboxyl) groups. The framework of ionic dendritic porphyrin prevents the aggregation of porphyrin and ensures effective production of singlet oxygen, even in extremely high concentrations. In general, photosensitizers exhibit large π-conjugation domains and hydrophobic characteristics, causing them to aggregate easily [19]. When photosensitizers are loaded into delivery systems, aggregates form and result in self-quenching of the excited state, which may change the photochemical reaction mechanism from type II to type I. The change in reaction mechanism depletes the yield of singlet oxygen and the efficiency of PDT [19, 20]. Ionic dendritic porphyrin is easily incorporated into polymeric micelles through the electrostatic interaction of the carboxyl groups with the positively charged PEG-block-poly(L-lysine) copolymers. Dendritic porphyrin also has a peak excitation wavelength (λmax) in the 415−430 nm range. In an ophthalmic study using rats, PDT with dendritic porphyrin-loaded PEG-block-poly(L-lysine) micelles was useful in treating choroidal neovascularization (CNV) [2, 19]. Observations revealed that the dendritic porphyrin-loaded micelles specifically accumulated in the CNV sites as early as 15 minutes after injection, peaked at 4 h, and the accumulation was still evident 24 h after the injection. In comparison, free dendritic porphyrin concentrated in CNV sites up to 4 h following injection, but disappeared within 24 h due to its negatively-charged periphery [19]. The application of the laser 4 h following injection resulted in a 60−80% occlusion of the CNV lesions. Approximately 80% occlusion of CNV was maintained 7 days after the treatment, indicating the success of using dendritic porphryin-loaded micelles for PDT. In response to dilution in the body, dendritic porphyrin-loaded micelles are expected to dissociate gradually avoiding damage to the retinal and choroidal vessels while preventing long term light-induced cytotoxicity. When exposed to broadband visible light (377−700 nm) 4 h after treatment, the rats did not suffer any skin damage [2, 19].
Light induced gene transfer may be allocated through the use of dendritic porphyrin coated non-viral vectors. One such technology employs “photochemical internalization”. In this approach, photosensitizers are coated in a polymeric micelle containing the gene of interest. After cellular uptake, the photochemical reaction of the photosensitizer upon irradiation damages the endosomal membrane and triggers endosomal escape of the plasmid DNA/cationic polymer complexes (polyplexes), allowing gene transfection in a light-inducible manner [2, 18, 21-23]. The polyplexes possess the favorable ability to package and protect negatively-charged plasmid DNA. In one such application, pDNA/polycation polyplex was enveloped with the photosensitizer, anionic dendritic phthalocyanine, and functionalized with a nuclear localization signal [24]. The nuclear localization signal enabled the transport of the complexes to the nuclei [25]. Dendritic phthalocyanine has an excitation wavelength of 680 nm, which is longer in comparison to the wavelength of dendritic porphyrin. Quadruplicated cationic peptide, a disulfide-linked cationic peptide containing a nuclear localization signal, was used as the polycation for plasmid DNA condensation. The protocol of the study involved subconjunctival injection of the ternary complexes into the eyes of a rat followed by laser irradiation. Hypothetically, the complexes underwent cellular uptake via endocytosis. Protonation of the carboxyl groups on the dendrimer periphery facilitated the dissociation of dendritic phthalocyanine from the complex and its interaction with the endosomal membrane. The application of light cued the reaction of dendritic phthalocyanine, disrupting the endosomal membrane and releasing the complex into the cytoplasm. As a result, there was appreciable gene expression in the conjunctival tissue at the laser-irradiated site and reduced light-induced cytotoxicity [2].
Currently, most light sensitive systems for drug and gene delivery are administered intravenously; however, techniques to administer PEO-PPO-PEO non-ionic copolymeric micelles through topical eye drops have shown promise in ocular tissues [26]. The study revealed the ability of PEO-PPO-PEO polymeric micelles to deliver plasmid DNA with lacZ gene in vivo [26]. After topical administration three times a day for 2−3 days, enhanced gene expression was observed in the iris, sclera, conjunctiva and lateral muscle of rabbit eyes. The copolymer micelle avoided degradation by the liver, prevented serum/protein interaction and displayed an increased half life (t½) for potentially effective drug delivery [26]. Sensitizing PEO-PPO-PEO micelle systems to visible light and administering them intravenously rather than topically may improve the time interval between administration and localization while providing further control over gene release rates and site-directed expression at predefined periods of time. Further research concerning polymeric micelles with integrated smart functions such as light sensitivity might establish them as viable nanomedicines.
Polymeric micelles have yet to undergo clinical trials. Unfortunately, in vivo use of the application may be limited due to significant light-induced cytotoxicity. Further exploration of polymeric micelles may offer insight into new nanotechnology-based treatment of ophthalmic neovascular diseases.
Vectosomes
Drug and gene delivery to posterior tissues of the eye pose difficulties because the eye is a small and closed organ that receives a tiny fraction of the cardiac output. Based on limitations posed by this formality, vectosomes, vesicles made of VP22, a structural protein of the herpes simplex virus, may be utilized for localized gene therapy of ocular diseases. The protein VP22 contains overlapping domains dictating characteristic subcellular localization patterns such as cytoplasmic accumulation, microtuble binding, and mitotic chromatin binding [27]. Vectosomes are spherical particles of 0.3 to 1 μm diameter that can be easily internalized by cells and remain stable in the cell cytoplasm for weeks. A specific example of vectosomes is light-sensitive complexes that consist of antisense oligonucleotides bound to the C-terminal amino acids of purified VP22 protein [28]. Vectosomes have been observed in retinal layers, specifically in the cytoplasm of RPE cells 24 h after intravitreal injection. Following illumination, vectosomes destabilize and release antisense oligonucleotides allowing their migration from the cytoplasm to the cell nuclei. Although the mechanism is not fully known, a recent study confirmed the feasibility of VP22 light-induced delivery of antisense oligonucleotides in vitro in ocular melanoma cells and in cells of retinal pigment epithelial origin [28]. The mechanism requires the covalent linkage of fluorochrome to either the protein or the antisense oligonucleotide. The presence of fluorochrome, a photosensitizer, is important, based on the assumption that thermal effects resulting from fluorochrome's absorption of light may cause vectosome disruption and antisense oligonucleotide release throughout the cell. Without affecting cell structure or function, vectosomes form well-delineated endsome-like vesicles in cell cytoplasm. Therefore, the intracellular process is more than likely carried out by endocytosis [28]. In theory, when light is applied, fluorochrome absorbs energy of a specific wavelength and in turn releases energy, inducing physical changes of the VP22 protein to release antisense oligonucleotides. Displaying a base sequence complementary to a specific mRNA, antisense oligonucleotides are able to selectively block the production of faulty disease associated proteins [5, 29].
In comparison to cationic lipids and other delivery systems as depicted in Table 4, vectosomes may be a feasible alternative for the transfection of genes because cationic lipids are limited by their potential toxicity and reduced efficiency in the presence of serum. In contrast, VP22 imparts a high rate of transfection and is not affected by serum [28]. When compared to polymeric nanoparticles, vectosomes exhibit the same migration pattern in ocular tissue after intravitreal injection. However, vectosomes are internalized more rapidly by various cell types and remain strictly in the cytoplasm until activated. Vectosome disruption is initiated with white light, and studies have also found that lasers of 488, 532, and 543 nm efficiently induce disruption. Further, vectosomes may be coupled with different fluorophores and tailored for a specific therapeutic aim. Before seeking clinical approval, extensive in vivo studies must be conducted to identify proper light wavelengths and energies in order to prevent harmful damage to the retina [28]. A limitation is that the protein VP22 might be immunogenic. Nevertheless, vectosomes portray beneficial attributes toward advanced ocular drug and gene delivery.
Table 4.
Comparison of Delivery Systems
|
Delivery System |
Advantages |
Disadvantages |
|---|---|---|
| Liposome (e.g., Visudyne) | • Capable of treating CNV lesions >50% classic for clinical use Prevent long term cytotoxicity | • Incapable of treating CNV lesions <50% classic |
| • Particle size of 120−125 nm | • Require reconstitution before intravenous infusion | |
| • Biodegradable | • Require repeated treatments | |
| • Accommodate oxidative degradable lipid or photo-polymerizable lipid and ballasted cyanine dyes Triggered release system | • Accumulate in other parts of the body besides targeted tissue | |
| • Flexible and high loading capacity | • Reduced efficiency in the presence of serum | |
| |
|
• Potential toxicity still remaining |
| Polymeric Micelle | • Effectively occlude CNV lesions in experimental animal studies | • Not internalized rapidly by various cell types |
| • Reduced toxicity | • Require reconstitution before intravenous infusion | |
| • Particle size of 50−100 nm | • Require repeated treatments | |
| • Dissociate gradually avoiding damage to ocular vessels | • Accumulate in other parts of the body besides targeted tissue | |
| • Ability to package pDNA and provide appreciable gene expression to the conjunctival tissue in research | ||
| |
• Effectively occlude CNV lesions in research studies |
|
| Vectosome | • Selectively modulate the expression of a given gene in animal studies | • Mechanism of action and dosage administration not yet physically observed and established |
| • Remain strictly in the cytoplasm until activated | • Antisense oligonucleotides have a very short half life | |
| • Spherical particles of 0.3−1 μm diameter | • Potential skin photosensitivity | |
| • Form well delineated endsomelike vesicles accommodating different fluorophores in cell cytoplasm | ||
| • Triggered release system | ||
| |
• Internalized rapidly by various cell types and not affected by serum |
|
| Hydrogel | • Maintain controlled drug release while protecting encapsulated drug or gene | • Dosage administration not yet physically observed and established |
| • Biodegradable | • Slow response time | |
| • Stimuli-mediated release | • Possible toxic monomers | |
| • Thermosensitive | • Potential sustained skin photosensitivity |
Hydrogels
Hydrogels are a network of hydrophilic polymers that swell in water and maintain their structure. Hydrogels protect the encapsulated drug and allow controlled drug release. Currently, there is significant effort in developing stimuli-sensitive hydrogel based drug delivery systems. In ophthalmic drug delivery, light sensitive hydrogels might be of value [30]. The administration and specified therapeutic aim of hydrogels have yet to be characterized exclusively. Hydrogels may be sensitized to either UV or visible light. For drug and gene delivery to the eye, sensitizing hydrogels to visible light is advantageous. The preparation of visible light-sensitive hydrogels requires the introduction of a light sensitive chromophore into a hydrogel. For instance, trisodium salt of copper chlorophyllin is useful as a photosensitizer in conjunction with poly(N-isopropylacrylamide) hydrogels [31]. However, the monomers used to prepare this hydrogel are not known to be biocompatible [31]. A less toxic formulation for ocular tissues may consist of biodegradable hydrogels of crosslinked hyaluronic acid encapsulating a photosensitizer [30]. In the presence of visible light (approximately 488 nm), the photosensitive molecules absorb light energy, which induce temperature changes in the hydrogel. An increase in temperature alters the swelling behavior of the hydrogel because poly(N-isopropylacrylamide) hydrogels are thermo-sensitive. The temperature increase is proportional to the light intensity and the photosensitizer concentration. Light sensitive hydrogels have a significantly slow response time, which hinders the efficiency of the delivery system. Although the application of light is instantaneous, the conversion of light into thermal energy in order to alter the gel structure is time consuming. Methods of improving the structure and response time of these light sensitive delivery systems is a challenge in drug and gene delivery applications [31].
Nanotechnology in Summary
The evolution of nanoparticles and nanotechnology complements the imperative vision of efficient drug and gene delivery to the eye [32]. In ophthalmology, light sensitive delivery systems are promising functional carriers. Often the target tissues of ocular PDT are located in the posterior segment of the eye. For drug and gene delivery to the posterior segment of the eye, intravitreal, periocular and subretinal injections are superior to topical and systemic routes. Since repeated injections by these routes are inconvenient, steps are still being taken to optimize the efficiency of intravenous photodynamic therapy. Attention is focused on enhancing aspects of the delivery mechanism, such as the particle size, sensitizer type and concentration, administration route, cellular uptake, appropriate light dose and appropriate drug-to-light interval in addition to issues of skin photosensitivity [1]. The nanoparticles previously discussed include liposomes, polymeric micelles, vectosomes, and hydrogels. Each of these nanoparticles may be conveniently utilized as carriers to facilitate improved photodynamic therapy due to their favorable size, enhanced permeability and retention, and high affinity for neovascular tissue. It has been suggested that photosensitizers incorporated in colloidal carriers can directly occlude diseased vascular tissue or rather functionalize carriers to have a triggered release. In the latter case, the photosensitizer mediates targeted delivery of therapeutic agents for definitive treatment of neovascular disorders.
Materials Useful for Light Sensitive Delivery
Photosensitizer
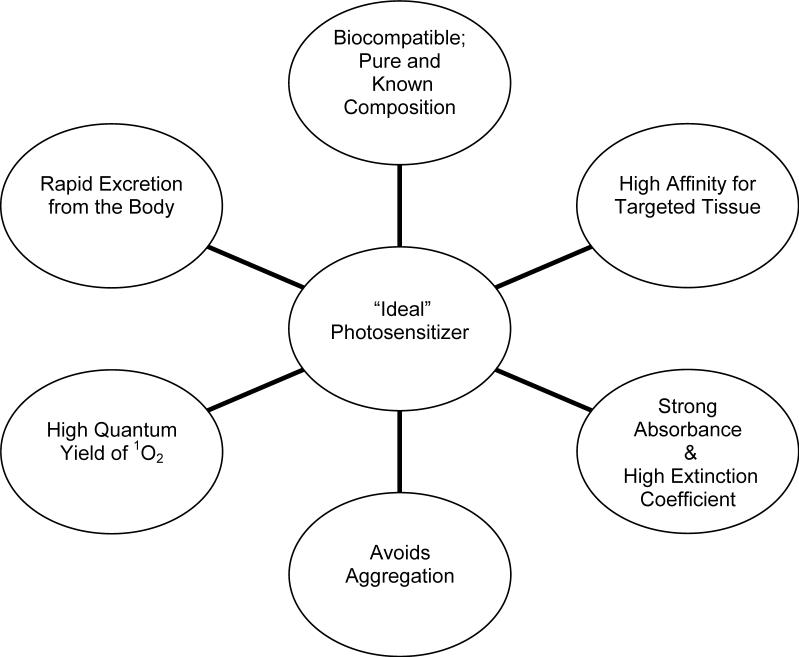
The photosensitizer is the most critical component of photodynamic therapy and its attributes determine the success of PDT in terms of drug and gene therapy. Understanding the photophysical and photochemical properties of a photosensitizer are imperative, for ideal photosensitizers must meet certain criteria. The photosensitizer's absorption wavelength and corresponding extinction coefficient are important [33]. A strong absorbance in the 600−800 nm range with a high extinction coefficient is desirable [1]. At longer wavelengths, photosensitizers are typically more efficient photodynamic agents with higher molar absorption [17]. Photosensitizers are capable of undergoing two separate reactions known as type I reaction and type II reaction. Whether or not a photosensitizer can excite to a higher, energy-rich triplet state determines the success of a light sensitive delivery system. At the ground state in the absence of light, photosensitizers exist with no unpaired electron spin. Exposure to light causes an electron of the photosensitizer to shift to a higher orbit and undergo fast spin inversion, which generates the triplet state containing two unpaired electrons (type I reaction) [1, 34]. In type II reactions, the photosensitizer molecules then collide with molecular O2, and following the transfer of energy the photosensitizer returns to the ground state [1, 35]. Type II reactions are the major mechanism of photochemical tissue damage, for they lead to the production of singlet O2 radicals and, perhaps, superoxide and hydroxyl radicals. Subsequently, highly toxic singlet oxygen is generated with a very short lifetime (<0.04 microsecond) and acting distance (< 0.02 μm) in biological systems [1, 34, 36, 37]. Maintaining sufficient amounts of molecular O2 during the irradiation of target cells ensures continuous production of singlet O2 for effective PDT [1, 34]. In summary, as illustrated in figure 2, an ideal photosensitizer is chemically pure and of known composition; it has high affinity for localization in target tissue; it is only cytotoxic upon photoactivation; it has the ability to generate excited triplet states; it avoids aggregation, which can affect its pharmokinetics and pharmacodynamics; and it is rapidly excreted from the body to minimize systemic toxicity [1, 33, 38].
Fig. 2.
Characteristics of Ideal Photosensitizer
In the midst of progressive research, two generations of photosensitizers have been categorized. First generation photosensitizers, such as Photofrin® and hematoporphyrin derivative (HPD), were employed in early ophthalmic studies [39]. Photofrin® is a purified hematoporphyrin derivative, approved for the treatment of cancer in several countries of the Western world. The use of first generation photosensitizers is limited because of inconsistent tissue penetration and skin photosensitivity [33]. Because Photofrin® only absorbs light up to 640 nm, light of longer wavelengths penetrates further into tissue [34]. Second generation photosensitizers have evolved from the structure of porphyrin, chlorin, purpurin, phtalocyanine or naphtalocyanine. Although the second generation sensitizers have proven to be successful in photodynamic occlusion of sites of ocular neovascularization, the sensitizers have also shown neurological side affects in animal studies [1, 40, 41]. There are, however, some second generation photosensitizers that may be appropriate for clinical use. These include verteporfin (Visudyne®), tin ethyl etiopurpurin, lutetium texaphryin, mono-L-aspartyl chlorine e6, aluminum phthalocyanine tetrasulfonate, optrin and ATX-S10 [1, 17, 42]. Based on the ideal criteria of photosensitizers, evaluation of these sensitizers will further reveal the advantages of each for ophthalmic treatments.
Looking to the future, quantum dots present a prominent platform toward the advancement of ocular light sensitive delivery systems. Quantum dots are semiconductor nanocrystals that may function as photosensitizers when linked to biocompatible delivery systems [43]. In consideration of ophthalmic treatments, quantum dot bioconjugates can be synthesized to localize in target tissue and functionalized to be water soluble [44]. The characteristics and properties of quantum dots are attractive for gene and drug delivery, for quantum dots further exhibit sharp and symmetric emission spectra, high quantum yield (the probability that a photosensitizer will excite to triplet state after absorbing a quantum of light), good chemical and photo-stability, size-tunable fluorescence emission, and strong absorption, due to a large transition dipole moment [43-45]. Not only can the size (1−6 nm) of quantum dots be controlled, but their fluorescence emission may also be tuned from the UV to the near infrared parts of the spectrum, which is quite advantageous compared to first and second generation photosensitizers exhibiting visible emission [43]. Conversely, the outer layer of quantum dots must be thin enough for the transfer of energy between the quantum dot and oxygen. Encapsulation in tri-n-octylphosphine oxine and other such polymers as polyethylene glycol may compromise the photosensitizer characteristics of quantum dots [44]. Therefore, coupling quantum dots with conventional photosensitizers may elevate the efficiency of the delivery system and prove effective for ocular photodynamic therapy. Problems with self aggregation and the lack of degradable processes for final excretion from the body are two additional aspects of quantum dots that are presently being scrutinized by ophthalmologists. A greater understanding of the functionality and set backs of quantum dots is needed. Researchers are currently studying the potential of quantum dots evolving into the next generation of novel photosensitizers for photodynamic therapy [43-45].
Light Source
Among the resources of PDT referred to in Table 3, the light source is a fundamental variable for ophthalmic drug and gene delivery. Early researchers used white light with specific filters before moving toward the more recent use of monochromatic light sources, such as lasers and, presently, light-emitting diodes. When classifying the appropriate light source, control over certain aspects, such as thermal effects, light intensity and light dose should be ascertained [1]. The wavelength of the light source should be based on the absorption spectra of the photosensitizer, which determines the depth of light penetration in tissue. Precise dosimetry over the selected spot ranging as large as 8000 μm is a requirement of ophthalmic light-sensitive delivery, in order to accomplish homogeneous phototoxicity [33]. For use in PDT, lasers are the standard light sources. Argon dye, potassium-titanium-phosphate (KTP) dye, metal vapour lasers, and diode lasers have been used for clinical PDT. In the study employing vectosomes, a 532 nm argon laser was applied through the cornea using a cover-slip and projected on the posterior pole of the retina (10 impacts, 50 μm, 10−15 J/cm2) [28]. In the study of verteporfin (Visudyne®), a diode laser delivered a uniformly-illuminated circular spot using a contact lens via a fiber optic unit coupled to a slit-lamp [33]. As emerging light applicators, light-emitting diodes generate high-energy light of desired wavelengths and they are reliable, portable, and cost efficient [1, 33].
Table 3.
Resources for Ophthalmic Photodynamic Therapy
|
Component |
Functions |
Examples (Reference) |
|---|---|---|
| Delivery System | Reservoir or carrier for photosensitizer, drug, or gene | Liposome (10, 13, 14, 17) |
| Polymeric micelle (3, 18, 19, 23) | ||
| Controlled solute release | ||
| Vectosome (25, 26) | ||
| Targetability | ||
| Hydrogel (28, 29) | ||
| Biocompatibility | ||
| |
Limited toxicity |
|
| Photosensitizer | React to produce highly reactive 1O2 in the presence of light | 1st Generation (30, 31) |
| 2nd Generation (2, 37, 38) | ||
| |
|
Quantum Dots (40−42) |
| Light Source | Activate photosensitizer & plasmid release | White light (2) |
| Dye laser (25) | ||
| Effective within 600−800 nm range | ||
| |
|
Diode laser (2, 30) |
| Gene | Therapeutic agents | Viral vectors (5, 43) |
| Non-viral vectors (5, 44, 45) |
Biological characteristics of the body also affect the choice of an efficient light source. Because tissues have diverse optical properties, the penetrating depth of activating light is limited. Further, the biological tissues limit the intensity of light that can be tolerated without resulting in thermal effects. Light penetration has been defined as most decisive within the 600−800 nm range, coinciding with the absorption spectra of most existing photosensitizers [1]. Located in the retina, RPE, and choroid tissues, the pigments melanin, macular lutein, and hemoglobin might theoretically absorb light in the shorter wavelength range, which, in turn, may increase light absorption and decrease light penetration. This hypothesis remains unsupported because the tissue layers in the area of the retina, RPE and choroid are so thin that they negate the loss of light intensity. Researchers have also observed that light in longer wavelengths results in improved transmission, forming the basis of a positive outlook for the treatment of patients with varying degrees of cataract [33].
Gene Use
The coupling of gene therapy with light sensitive delivery systems, as non-viral vectors, appears to be an ideal method of ensuring long-term therapeutic efficiency, thus reducing the need for repeated treatments. The use of a light sensitive delivery system improves the stability of the gene and decreases its toxicity, while potentially allowing a slow release effect. In addition, the use of non-viral vectors as opposed to viral vectors, despite their lower transfection efficiency, seems even more attractive for gene therapy. Viral vectors are widely used for ocular gene therapy, yet they pose several pertinent risks [5, 46]. Conversely, non-viral vectors exhibit the ability to induce transgene expression without being potentially mutagenic when integrated into the host genome and without inducing significant ocular tissue inflammatory responses. Non-viral vectors also have low toxicity, are safe to handle, and can be produced in large quantities [5, 47, 48].
Future Directions & Unmet Challenges of Light Sensitive Delivery
When tested in clinical trials, the vital challenge of light sensitive delivery systems is the inability to outperform, exclusively, alternative applications that are currently used clinically. The optimization of variables for treatment using light sensitive delivery is not fully developed, hence, clinician resistance toward accepting this method as a mainstream alternative for the therapy of neovascular or angiogenic disorders. The application of light sensitive systems for drug and gene delivery has yet to evolve. Fully efficient and cost-effective avenues warrant further investigation into appropriate photosensitizers, light sources, and application processes, not only from a chemistry standpoint, but a biological perspective as well.
Methods of expanding the treatment of PDT to both categories of CNV, classic and occult, must be considered. In reference to the desirable features of PDT in Box 1 and light-directed delivery systems, improved loading capacity and controlled drug release is needed. For some photosensitizers, sustained skin photosensitivity remains a significant problem. Drugs that are highly selective and require a short drug to light interval also need development. In retrospect, the clinical use of Visudyne® and other FDA-approved photodynamic therapies is an innovative step, perhaps leading to unforeseen and unexpected opportunities for successful treatment of ocular diseases.
Box 1. Desirable Features of Photodynamic Therapy (PDT)
Photosensitizer of viable photophysical and photochemical properties
Limited or no toxicity; Absence of skin photosensitivity
Controlled photosensitizer release at the target site for predetermined rates and for predefined periods of time
Appropriate wavelength of light source (e.g., 600−800 nm) for optimum tissue penetration
Precise dosimetry for light exposure
Short interval between drug administration and light treatment
Abundant supply of molecular oxygen (O2) at the target site
Acknowledgements
The authors acknowledge the Experimental Program to Stimulate Competitive Research (EPSCoR) and the University of Nebraska's Summer Undergraduate Research Program in association with the Office of Student Equity and Multicultural Affairs (OSEMA). This work was also supported in part by the NIH grants R24 EY017045 and R21 EY017360.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pervaiz S, Olivo M. Art and science of photodynamic therapy. Clin Exp Pharmacol Physiol. 2006;33(5−6):551–6. doi: 10.1111/j.1440-1681.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112(3):630–48. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Zuluaga MF, et al. Synergies of VEGF inhibition and photodynamic therapy in the treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48(4):1767–72. doi: 10.1167/iovs.06-1224. [DOI] [PubMed] [Google Scholar]
- 4.Torres PF, Kijlstra A. The role of cytokines in corneal immunopathology. Ocul Immunol Inflamm. 2001;9(1):9–24. doi: 10.1076/ocii.9.1.9.3978. [DOI] [PubMed] [Google Scholar]
- 5.Bloquel C, et al. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58(11):1224–42. doi: 10.1016/j.addr.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Bressler NM, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Invest Ophthalmol Vis Sci. 2000;41(3):624–8. [PubMed] [Google Scholar]
- 7.Sheppard JD, Jr., et al. Argon laser photodynamic therapy of human corneal neovascularization after intravenous administration of dihematoporphyrin ether. Am J Ophthalmol. 2006;141(3):524–529. doi: 10.1016/j.ajo.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Raghava S, Kompella UB. AQ4, an antitumor anthracenedione, inhibits endothelial cell proliferation and vascular endothelial growth factor secretion: Implications for the therapy of ocular neovascular disorders. Eur J Pharmacol. 2007;568(1−3):68–74. doi: 10.1016/j.ejphar.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derycke AS, de Witte PA. Liposomes for photodynamic therapy. Adv Drug Deliv Rev. 2004;56(1):17–30. doi: 10.1016/j.addr.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Zeimer R, Goldberg MF. Novel ophthalmic therapeutic modalities based on noninvasive light-targeted drug delivery to the posterior pole of the eye. Adv Drug Deliv Rev. 2001;52(1):49–61. doi: 10.1016/s0169-409x(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 11.Anderson VC, Thompson DH. Triggered release of hydrophilic agents from plasmalogen liposomes using visible light or acid. Biochim Biophys Acta. 1992;1109(1):33–42. doi: 10.1016/0005-2736(92)90183-m. [DOI] [PubMed] [Google Scholar]
- 12.Mueller A, et al. Visible-Light-Stimulated Destabilization of PEG-Liposomes. Macromolecules. 2000;33:4799–4804. [Google Scholar]
- 13.Clapp PJ, et al. Two-Dimensional Polymerization of Lipid Bilayers: Visible -Light-Sensitized Photoinitiation. Macromolecules. 1997;30(1):32–41. [Google Scholar]
- 14.Bondurant B, et al. Photoinitiated destabilization of sterically stabilized liposomes. Biochim Biophys Acta. 2001;1511(1):113–22. doi: 10.1016/s0005-2736(00)00388-6. [DOI] [PubMed] [Google Scholar]
- 15.Lamparski H, et al. Photoinduced destabilization of liposomes. Biochemistry. 1992;31(3):685–94. doi: 10.1021/bi00118a008. [DOI] [PubMed] [Google Scholar]
- 16.Hashida M, et al. Lipid carrier systems for targeted drug and gene delivery. Chem Pharm Bull (Tokyo) 2005;53(8):871–80. doi: 10.1248/cpb.53.871. [DOI] [PubMed] [Google Scholar]
- 17.Renno RZ, Miller JW. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv Drug Deliv Rev. 2001;52(1):63–78. doi: 10.1016/s0169-409x(01)00195-8. [DOI] [PubMed] [Google Scholar]
- 18.Prasmickaite L, et al. Evaluation of different photosensitizers for use in photochemical gene transfection. Photochem Photobiol. 2001;73(4):388–95. doi: 10.1562/0031-8655(2001)073<0388:eodpfu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Ideta R, et al. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Lett. 2005;5(12):2426–31. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- 20.Grossweiner LI, et al. Type I and type II mechanisms in the photosensitized lysis of phosphatidylcholine liposomes by hematoporphyrin. Photochem Photobiol. 1982;36(2):159–67. doi: 10.1111/j.1751-1097.1982.tb04358.x. [DOI] [PubMed] [Google Scholar]
- 21.Hogset A, et al. Photochemical transfection: a technology for efficient light-directed gene delivery. Somat Cell Mol Genet. 2002;27(1−6):97–113. doi: 10.1023/a:1022979806314. [DOI] [PubMed] [Google Scholar]
- 22.Berg K, et al. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59(6):1180–3. [PubMed] [Google Scholar]
- 23.Hogset A, et al. Photochemical transfection: a new technology for light-induced, site-directed gene delivery. Hum Gene Ther. 2000;11(6):869–80. doi: 10.1089/10430340050015482. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama N, et al. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat Mater. 2005;4(12):934–41. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph C, et al. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278(13):11411–8. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 26.Liaw J, et al. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001;8(13):999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 27.Martin A, et al. Herpes simplex virus tegument protein VP22 contains overlapping domains for cytoplasmic localization, microtubule interaction, and chromatin binding. J Virol. 2002;76(10):4961–70. doi: 10.1128/JVI.76.10.4961-4970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normand N, et al. VP22 light controlled delivery of oligonucleotides to ocular cells in vitro and in vivo. Mol Vis. 2005;11:184–91. [PubMed] [Google Scholar]
- 29.Helene C, Toulme JJ. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- 30.Yui N, et al. Photo-responsive degradation of heterogeneous hydrogels comprising crosslinked hyaluronic acid and lipid microspheres for temporal drug delivery. Journal of Controlled Release. 1993;26:141–145. [Google Scholar]
- 31.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 32.Amrite AC, et al. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47(3):1149–60. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195–214. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 34.Dougherty TJ, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeersch G, et al. Type I and type II photosensitization by the antibacterial drug nalidixic acid. A laser flash photolysis study. Photochem Photobiol. 1991;54(5):661–6. doi: 10.1111/j.1751-1097.1991.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 36.Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53(4):549–53. doi: 10.1111/j.1751-1097.1991.tb03669.x. 1991. [DOI] [PubMed] [Google Scholar]
- 37.Pathak MA, Joshi PC. Production of active oxygen species (1O2 and O2-.) by psoralens and ultraviolet radiation (320−400 nm). Biochim Biophys Acta. 1984;798(1):115–26. doi: 10.1016/0304-4165(84)90018-7. [DOI] [PubMed] [Google Scholar]
- 38.Sharman WM, et al. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999;4(11):507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama N, et al. Light-harvesting ionic dendrimer porphyrins as new photosensitizers for photodynamic therapy. Bioconjug Chem. 2003;14(1):58–66. doi: 10.1021/bc025597h. [DOI] [PubMed] [Google Scholar]
- 40.Schuitmaker JJ, et al. Bacteriochlorin a, a new photosensitizer in photodynamic therapy. In vivo results. Invest Ophthalmol Vis Sci. 1990;31(8):1444–50. [PubMed] [Google Scholar]
- 41.Miller H, Miller B. Photodynamic therapy of subretinal neovascularization in the monkey eye. Arch Ophthalmol. 1993;111(6):855–60. doi: 10.1001/archopht.1993.01090060145039. 1993. [DOI] [PubMed] [Google Scholar]
- 42.Mori K, et al. [Potential of photodynamic therapy with a second-generation sensitizer: mono-L-aspartyl chlorin e6]. Nippon Ganka Gakkai Zasshi. 1997;101(2):134–40. [PubMed] [Google Scholar]
- 43.Weng J, Ren J. Luminescent quantum dots: a very attractive and promising tool in biomedicine. Curr Med Chem. 2006;13(8):897–909. doi: 10.2174/092986706776361076. [DOI] [PubMed] [Google Scholar]
- 44.Bakalova R, et al. Quantum dots as photosensitizers? Nat Biotechnol. 2004;22(11):1360–1. doi: 10.1038/nbt1104-1360. [DOI] [PubMed] [Google Scholar]
- 45.Samia AC, et al. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125(51):15736–7. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 46.Borras T. Recent developments in ocular gene therapy. Exp Eye Res. 2003;76(6):643–52. doi: 10.1016/s0014-4835(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 47.Mohan RR, et al. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24(5):537–59. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Chaum E, Hatton MP. Gene therapy for genetic and acquired retinal diseases. Surv Ophthalmol. 2002;47(5):449–69. doi: 10.1016/s0039-6257(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 49. Visudyne. [ www.fda.gov] 2002 December 29, 2004 [cited; U. S. Food and Drug Administration]. Available from: http://www.fda.gov.