Abstract
BCL-2 was the first antideath gene dis-covered, a milestone that effectively launched a new era in cell death research. Since its discovery more than 2 decades ago, multiple members of the human Bcl-2 family of apoptosis-regulating proteins have been identified, including 6 antiapoptotic proteins, 3 structurally similar proapoptotic proteins, and several structurally diverse proapoptotic interacting proteins that operate as upstream agonists or antagonists. Bcl-2–family proteins regulate all major types of cell death, including apoptosis, necrosis, and autophagy. As such, they operate as nodal points at the convergence of multiple pathways with broad relevance to biology and medicine. Bcl-2 derives its name from its original discovery in the context of B-cell lymphomas, where chromosomal translocations commonly activate the BCL-2 protooncogene, endowing B cells with a selective survival advantage that promotes their neoplastic expansion. The concept that defective programmed cell death contributes to malignancy was established by studies of Bcl-2, representing a major step forward in current understanding of tumorigenesis. Experimental therapies targeting Bcl-2 family mRNAs or proteins are currently in clinical testing, raising hopes that a new class of anticancer drugs may be near.
Introduction
Cell death can be either physiologic or pathologic. Although medicine focused on pathologic cell death for centuries, it was the discovery of programmed cell death that breathed new life into the field of cell death research, as well as sparking major advances in multiple areas of physiology and medicine. Physiologic cell death in animal species generally occurs through a mechanism commonly called “apoptosis,” typically involving activation of intracellular proteases known as caspases.1 The proteolytic events mediated by caspases impart characteristic morphologic and ultrastructural changes to dying cells that define the apoptotic phenotype. Among the features of apoptosis are cell shrinkage, blebbing of the plasma membrane without loss of integrity, nuclear fragmentation, and chromatin condensation. In vivo, apoptotic cells are cleared by phagocytosis before they can rupture, maintaining ATP and ion homeostasis even as they are cleared from the body.
It is estimated that the average adult human produces and in parallel eradicates approximately 60 billion cells daily, with new cells formed by cell division and old cells eliminated by apoptosis, thus striking a balance under normal circumstances. This ability to control cell numbers at both the points of entry and exit allows our bodies to more rapidly respond to stress, such as mounting a white blood cell count in the face of acute infection. In this regard, hematopoietic growth factors (eg, granulocyte macrophage–colony-stimulating factor, granulocyte cell-stimulating factor, interleukin-3) typically transduce signals both for expansion and differentiation of hematopoietic progenitors, as well for prolonging survival of leukocytes, thus promoting both new cell production and extending survival of existing cells to achieve rapid increases in leukocyte numbers.
The pathways responsible for adult tissue homeostasis are governed significantly but not exclusively by Bcl-2–family proteins.2 The central pathway involved in daily programmed cell death in most tissues involves mitochondria, energy-producing organelles that play critical roles in both cell life and death.3 Several Bcl-2–family proteins, both antiapoptotic and proapoptotic, have C-terminal transmembrane domains that insert in the outer membrane of mitochondria. Proapoptotic Bcl-2–family proteins, such as Bax and Bak, induce mitochondrial outer membrane permeabilization (MOMP), causing the release of caspase-activating proteins and other cell death mediators, whereas antiapoptotic proteins such as Bcl-2 serve as guardians of the outer membrane and preserve its integrity by opposing Bax and Bak. Other nonmitochondrial pathways for apoptotic cell death also exist, including those governed by tumor necrosis factor-family death receptors, such as Fas—an important regulator of lymphoid homeostasis in vivo. However, even the death receptor pathway (“extrinsic pathway”) converges with the mitochondrial pathway (“intrinsic pathway”) in certain types of cells, through caspase-mediated cleavage and activation of Bid, an endogenous modulator of Bcl-2/Bax–family proteins.4
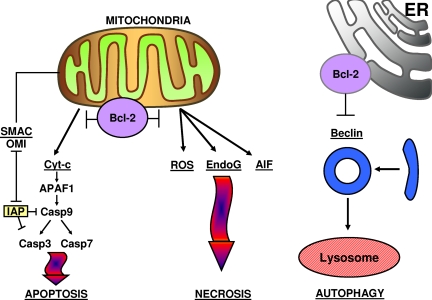
Although mitochondria clearly induce apoptosis by releasing proteins that participate in caspase activation (eg, cytochrome c) and that neutralize endogenous inhibitors of caspases (eg, SMAC, OMI/Htra2), these organelles are also well known mediators of necrotic cell death.5 For example, defects in electron chain transport in respiring mitochondria spew reactive oxygen species into cells, causing lipid peroxidation and membrane damage, which impair normal ion-homeostasis, causing cellular swelling and plasma membrane rupture, as well as rupture of lysosomes and release of hydrolytic enzymes that destroy proteins, nucleic acids, and lipids. Bcl-2–family proteins also modulate these necrotic actions of mitochondria.6 The point of regulation may be linked to the ability of Bcl-2–family proteins to control outer mitochondrial membrane permeability, inasmuch as loss of cytochrome c from mitochondria interrupts electron chain transport between complexes III and IV (although this notion has been challenged).7 In addition, once downstream caspases are activated, they can cleave proteins required for proper function of complex I.8 MOMP also releases several proteins that contribute to nonapoptotic cell death, including DNAse, endonuclease G, and apoptosis-inducing factor, a flavoprotein reported to enter the nucleus and promote genome destruction.9 Alternatively, Bcl-2–family proteins may have other, as yet poorly understood ways of communicating with the mitochondrial inner membrane to affect mitochondrial bioenergetics and control nonapoptotic cell death.10 The mechanistic details notwithstanding, the bottom line is that antiapoptotic proteins such as Bcl-2 protect and proapoptotic proteins such as Bax kill even when caspases are neutralized using broad-spectrum chemical inhibitors, showing that Bcl-2–family proteins control a cell death checkpoint upstream of caspase activation, thus allowing them to govern both apoptotic (caspase-dependent) and nonapoptotic (caspase-independent) cell death.
Antiapoptotic Bcl-2–family proteins are well known for their ability to prolong survival of growth factor–dependent cells when deprived of their obligate growth factors. In fact, the antiapoptotic function of Bcl-2 was first elucidated in gene transfection studies, where interleukin-3–dependent murine hematopoietic cells were shown to cease division but to survive for prolonged periods in the absence of interleukin-3 when Bcl-2 was constitutively overexpressed.11 Growth factor deprivation can also lead to nutrient deprivation, because expression of cell-surface glucose transporters and amino-acid transporters depends on them. Prolonged nutrient deprivation invokes autophagy, an evolutionarily conserved response for catabolizing macromolecules and organelles, thereby generating substrates for ATP production.12,13 Autophagy is initially induced to prolong cell survival, but when taken to extremes, it seems to cause cell death. Bcl-2 and Bcl-XL suppress autophagy by binding the protein Beclin (ATG7),14,15 an essential component of the mammalian autophagy system that marks autophagic vesicles for fusion with lysosomes for digestion and recycling of components.
The antiautophagic function of Bcl-2 has been dissociated from mitochondrial location, and instead appears to be manifested from the endoplasmic reticulum (ER),14 where a considerable proportion of antiapoptotic Bcl-2 and related proteins often resides.16 In this regard, Bcl-2–family proteins have regulatory effects on several proteins and processes in the ER, including those influencing the unfolded protein response (UPR).17 The UPR is an evolutionarily conserved adaptive mechanism that detects accumulation of unfolded proteins in the lumen of the ER, causing induction of chaperones and transporters that strive to restore homeostasis, at least in part, by retrograde transport of unfolded proteins into the cytosol for ubiquitination and proteasome-mediated destruction.18 Given that chaperone-mediated autophagy complements proteasome-dependent destruction of misfolded proteins, representing another major route of disposal of defective proteins,19 it is tempting to speculate that Bcl-2–family proteins have evolved additional functions that serve during times of cell stress to tip the balance toward either survival or death. Indeed, persistent ER stress induces both caspase-dependent and caspase-independent cell death programs, which are modulated by Bcl-2–family proteins.17 Altogether, therefore, Bcl-2–family proteins govern cellular pathways involved in apoptosis, necrosis, and autophagy (Figure 1).
Figure 1.
Bcl-2 suppresses apoptosis, necrosis, and autophagy. ROS indicates reactive oxygen species; Cyt-c, cytochrome-c; EndoG, endonuclease G; AIF, apoptosis-inducing factor; and IAP, inhibitor of apoptosis protein.
Milestones in Bcl-2 research: a brief history
As we commemorate the 50th anniversary of Blood, it seems fitting to take a historical approach to the topic of Bcl-2–family proteins and their roles in hematology. Moreover, a brief historical accounting of some of the milestones in Bcl-2 research serves to illustrate several of the important functions and mechanisms of these proteins. Many of the first-time observations concerning Bcl-2 and related proteins were made in the context of the hematopoietic and lymphoid systems or malignancies originating from these cells. Even with the hematology-centric focus of this review, however, space limitations preclude citing all contributions and all contributors, for which I apologize in advance.
BCL-2 gene activation in hematopoietic malignancies
Cloning of portions of the human BCL-2 gene was first reported in 1985 by Tsujimoto et al,20 who cloned the breakpoints from t(14;18) chromosomal translocations observed in non-Hodgkin lymphomas. In these translocations, the BCL-2 gene becomes fused with the immunoglobulin heavy-chain locus (IgH), bringing the juxtaposed BCL-2 gene under the control of the IgH enhancer and thereby dysregulating BCL-2 gene expression at a transcriptional level. The somatic hypermutation mechanism associated with the IgH gene locus often then peppers the BCL-2 gene with mutations, which may further dysregulate expression and can also lead to point mutations in the coding regions of the Bcl-2 protein,21 some of which have functional significance but are not required for suppressing apoptosis. In 1986, successful cDNA cloning of Bcl-2 transcripts was accomplished by Tsujimoto et al22 and shortly thereafter by Cleary et al,23 allowing the amino-acid sequence of the Bcl-2 protein to be deduced. At the time, the only recognized homology to other proteins was BHRF, an open reading frame in Epstein-Barr virus (EBV),23 an observation that years later would be validated by various studies showing that EBV and several Herpes-family viruses contain functional Bcl-2 homologs.24 In 1992, Hanada et al25 described high levels of BCL-2 gene expression in most chronic lymphocytic leukemias (CLLs), in association with gene hypomethylation. Not until 2005, however, would it be revealed by Cimmino et al26 that BCL-2 gene expression in B-cells is normally repressed by endogenous microRNAs (miRs), and the genes encoding these regulatory, noncoding RNAs (miR15, miR16) become deleted or inactivated by mutations in more than 70% of CLLs. This discovery of loss of miR-mediated repression of BCL-2 represented the first clear elucidation of a miR-dependent mechanism of tumor suppression in humans. In addition to chromosomal translocations as a mechanism for activation of the BCL-2 gene in human malignancies, in 1997, examples of BCL-2 gene amplification were found in non-Hodgkin lymphomas.27
Cytoprotective function of Bcl-2
In 1988, the first demonstration of oncogenic potential of Bcl-2 was made by Reed et al,28 confirming that Bcl-2 is a bona fide protooncogene. Shortly thereafter, the antiapoptotic activity of Bcl-2 was discovered by Vaux et al,11 a milestone in research on Bcl-2 that laid a foundation for the next 2 decades of work. The first Bcl-2 transgenic mice were reported in 1989 by McDonnell et al,29 showing that constitutive overexpression of Bcl-2 in vivo causes polyclonal accumulation of B lymphocytes as a result of delayed programmed cell death, and recapitulating the histologic features of the human follicular lymphomas that commonly bear t(14;18) translocations. In 1990, the first demonstrations that knocking down Bcl-2 expression induces leukemia cell death and inhibits leukemic growth in vivo were made by Reed and colleagues, using antisense methods.30,31 In 1991, bcl-2 gene knockout mice were generated by Sentman et al,32 revealing a requirement for Bcl-2 for postnatal survival of lymphocytes and melanocytes, observations that would later influence selection of indications for testing of Bcl-2–targeted therapies in human clinical trials.
The concept that Bcl-2 controls an evolutionarily conserved pathway for regulating programmed cell death was introduced in 1992 by Vaux et al33 with their demonstration that ectopic expression of human Bcl-2 in mutant worms (Caenorhabditis elegans) could rescue a defect in programmed cell death. Later, in 1994, the defective gene responsible for the excessive cell death observed in these mutant worms (Ced9) was cloned by Hengartner and Horvitz,34 proving that at least some elements of the cellular machinery that controls apoptosis are highly conserved within the animal kingdom. This theme of evolutionary conservation of mechanisms was continued by Kane et al,35 who in 1993 reported that human Bcl-2 could even protect yeast (Saccharomyces cerevisiae) under some circumstances. This work, along with that of Zhong et al,36 also showed some of the early hints that Bcl-2 could suppress both apoptotic and necrotic cell death, focusing on neuronal cell death models.
Bcl-2 confers resistance to chemotherapy
BCL-2 gene rearrangements were associated with poor prognosis in large-cell non-Hodgkin lymphomas in 1989 by Yunis et al,37 an observation subsequently confirmed and extended to immunohistochemical detection of elevated Bcl-2 protein by several groups.38–41 Although implying that Bcl-2 altered the biologic behavior of lymphoma in adverse ways, the experimental link between Bcl-2 and resistance to cytotoxic anticancer drugs was directly made in reports by Miyashita and Reed in 199242 and 1993,43 showing that stable overexpression of Bcl-2 in various lymphoid cell lines conveyed resistance to a broad range of DNA-damaging drugs, antimicrotubule drugs, nucleoside analogs, and glucocorticoids and building on the work of Sentman et al,32 who showed that Bcl-2 could prevent cell death induced by multiple types of cell death stimuli in thymocytes. The findings implied that Bcl-2 operates at a distal point in a conserved cell death pathway used by most anticancer drugs, and revealed a novel form of chemoresistance, distinct from the previously identified mechanisms involving drug efflux, drug metabolism, drug inactivation, and related mechanisms. Shortly thereafter, Campos et al44,45 showed that high levels of Bcl-2 are associated with resistance of patients with acute myeloid leukemia (AML) to chemotherapy and that antisense oligodeoxynucleotides targeting Bcl-2 mRNA improve sensitivity of AML cells to arabinocytosine (AraC) in vitro. Together, these and many other clinical correlative studies strengthened the association between Bcl-2 and chemoresistance in hematolymphoid malignancies, with similar results seen for some solid tumors, such as prostate cancer.46,47 Then, in 1997, the first phase 1 clinical trial of a Bcl-2–targeted therapy was reported by Webb et al,48 a study of an antisense phosphorothioate oligodeoxy-nucleotide that showed promising activity in patients with refractory lymphomas and that raised hopes that neutralizing Bcl-2 could restore apoptosis sensitivity to malignant cells and promote their demise.
Mechanisms of action of Bcl-2–family proteins
Several members of the family contain a stretch of hydrophobic residues at their C terminus that has been variably reported to be important for their functions. In 1987 and 1989, reports by Tsujimoto et al49 and Chen-Levy et al50 groups, respectively, confirmed that Bcl-2 is an integral membrane protein, found in intracellular membranes. Then, in 1990, association of Bcl-2 with mitochondria was discovered by Hockenbery et al51 and the suppressive action of Bcl-2 on mitochondrial reactive oxygen species (ROS) production was elucidated in connection with programmed cell death. In 1994, the first in vitro reconstitution of a Bcl-2–dependent cell death pathway was achieved by Newmeyer et al52 using Xenopus laevis extracts to show that Bcl-2–mediated protection depends on mitochondria. Then, in 1995, Zamzami et al53 showed that the loss of mitochondrial membrane potential was an early event associated with apoptosis, which was suppressible by Bcl-2. Subsequently, in 1996, Liu et al54 and Li et al55 identified cytochrome c as the chief link between mitochondria and apoptosis, binding to and activating the cytoplasmic caspase-activating protein, Apaf1. Bcl-2 was first shown to suppress cytochrome c release from mitochondria in 1997 by Yang et al56 an observation quickly confirmed by many other groups. At about this time, Wolter et al57 and Nechushtan et al58 demonstrated dynamic trafficking of Bcl-2–family proteins between cytosol and mitochondria and elucidated a pathway whereby latent Bax in the cytosol undergoes conformational changes that induce its insertion into mitochondrial membranes, a process suppressed by Bcl-2.57,58
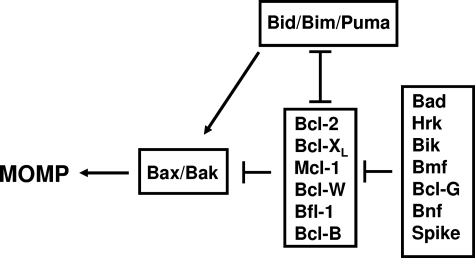
In 1997, the 3-dimensional (3D) structure of Bcl-XL was determined by Muchmore et al59 and Fesik,60 revealing for the first time that several Bcl-2–family members share striking structural similarity to the pore-forming domains of bacterial toxins and sparking a series of subsequent studies that demonstrated an ability of Bcl-XL, Bcl-2, and Bax to form ion channels in synthetic membranes.61–63 It remains a mystery how Bcl-2 and Bax can have essentially the same 3D protein fold64 and yet have diametrically opposed effects on cell death. Probably the best correlate with their opposing activities is oligomerization of Bax in mitochondrial membranes (first demonstrated by Eskes et al65 and Wei et al66 in 2000), which coincides with MOMP. Bcl-2 does not oligomerize in mitochondrial membranes, but rather blocks Bax oligomerization. Wei et al66 showed that BH3-only protein Bid serves as an agonist of Bax oligomerization in 2000. In 2002, Kuwana et al67 reconstituted in synthetic liposomes the Bax pore, showing that Bax can be induced to oligomerize by Bid BH3 peptide, permeabilizing liposomes in vitro. Thus, Bax and structurally related proapoptotic proteins (Bak, Bok) are hypothesized to form proteinaceous or lipidic pores upon oligomerization in mitochondrial membranes, alone or in conjunction with resident mitochondrial proteins, thereby allowing escape of cytochrome c and other proteins from these organelles (reviewed by Reed68). In parallel, in 2001, gene ablation studies in mice by Wei et al69 demonstrated that either Bax or Bak is necessary for MOMP, establishing these pore-forming proapoptotic proteins as the distal elements controlling permeability of mitochondrial membranes and thus the governors of life/death decisions in the mammalian mitochondrial pathway for cell death (Figure 2).
Figure 2.
Functional interactions among the types of mammalian Bcl-2–family proteins at mitochondrial membranes.
In parallel with the early observations concerning effects of Bcl-2–family proteins on mitochondrial membrane permeability, researchers studying Ced-9, the C elegans ortholog of Bcl-2, reported in 1997 that Ced-9 directly binds to and inhibits Ced-4, the caspase-activating Apaf-1 ortholog.70–72 However, this mechanism for inhibition of caspase-activating protein would not find its human analog until 2007, with the discovery by Bruey et al73 that Bcl-2 binds to and suppresses NLR-family member NALP1, an Apaf1-like activator of inflammatory caspases. In 1998, Liang et al74 described interaction of Bcl-2 with Beclin, a protein that later would be recognized as a critical component of the autophagy machinery of mammalian cells, thus linking Bcl-2 to suppression of autophagy.14
Proapoptotic Bcl-2 family members as tumor suppressors
An important link between apoptosis promotion and tumor suppression was made by Miyashita et al75 and Miyashita and Reed76 in 1994 and 1995, respectively, with the discovery that p53 directly binds to and suppresses BAX gene expression, thereby revealing the first proapoptotic target of this important tumor suppressor. Since then, multiple proapoptotic members of the Bcl-2 family, including Bid, Puma, and Noxa, have been shown to be direct targets of p53, with the relative importance of any particular family member dependent on cell lineage and cellular context.77–79 The first examples of loss of function mutations in any proapoptotic Bcl-2–family gene were uncovered by studies of the human BAX gene in colon cancers by Rampino et al80 in 1997 and later observed also for hematopoietic malignancies.81 At about this time, studies of bax−/− mice by Yin et al82 revealed that this proapoptotic gene suppresses tumorigenesis in vivo, firmly establishing Bax as a tumor suppressor. More recently, cytosolic interactions of p53 with pro- and antiapoptotic Bcl-2–family proteins have been observed by Chipuk et al83 and Deng et al,84 respectively, directly modulating the activities of Bax and Bcl-2, and suggesting that p53 regulates the Bcl-2 family at both transcriptional and posttranscriptional levels.
Bcl-2–family protein interaction networks: dimerization and drug discovery
As membership in the Bcl-2 family grew with discovery of additional pro- and antiapoptotic members in the mid-1990s, regions of conserved sequence homology were pinpointed, and given various rubrics, coalescing on the nomenclature proposed by Yin et al85 of Bcl-2 Homology (BH) domains. Today, we recognize up to 4 BH domains. All antiapoptotic members of the human Bcl-2 family contain BH1, BH2, BH3, and BH4. Mutagenesis studies of BH1 and BH2 in antiapoptotic Bcl-2 suggested importance for homo- and heterodimerization with Bax.85 The fundamental motif of proapoptotic proteins required for dimerization with antiapoptotic Bcl-2–family proteins was first identified in 1995 by Chittenden et al,86 later termed BH3 (Bcl-2 Homology domain 3). Subsequently, several proapoptotic proteins were discovered that share sequence homology only in the BH3 domain (“BH3 only” proteins) and that depend upon the BH3 domain for binding Bcl-2 or Bcl-XL, including Bid, Bim, Hrk, Bik, Puma, Noxa, Bcl-GS, Bmf, Bnf, Mule, and possibly Spike (reviewed by Strasser87). These BH3-only proteins uniformly operate as antagonists of antiapoptotic Bcl-2–family proteins in a BH3-dependent manner that correlates with BH3-dependent binding. The BH3-only proteins stand apart from a smaller group of proapoptotic proteins that contain BH1, BH2, and BH3 (sometimes termed “multidomain”), which includes Bax, Bak, and Bok in mammals. A few BH3-only proteins (ie, Bid, Bim, Puma) have dual functions as both antagonists of antiapoptotic Bcl-2 family members and agonists of proapoptotic multidomain proteins Bax and Bak.
Attempts to understand dimerization among Bcl-2 family proteins remained confusing until Muchmore et al59 solved the first 3D structure of a Bcl-2–family member, Bcl-XL, in 1996, and then elucidated the structure of a complex of Bcl-XL with the BH3 peptide from Bak in 1997.88 These seminal studies taught that the BH3 peptide is an amphipathic α-helix of approximately 16 to 20 amino acids that binds a hydrophobic pocket on the surface of antiapoptotic Bcl-2–family proteins, thus revealing the structural basis for antagonism of pro- and antiapoptotic members of the family and setting the stage for subsequent drug discovery strategies based on mimicking BH3 peptides with chemical compounds that bind the same pocket.89 The first chemical inhibitor of Bcl-2 based on this concept was described by Wang et al90 in 2000, who used computational modeling approaches based on the structural data. Since then, a variety of approaches have resulted in successful identification and synthesis of chemical antagonists of antiapoptotic Bcl-2 family proteins, some of which are now in clinical testing (reviewed by Reed and Pellecchia91).
Posttranslational modifications
Many proteins are regulated by posttranslational modifications, and Bcl-2 family proteins are no exception. To date, phosphorylation, proteolysis, ubiquitination, myristoylation, and deamidation are among the documented posttranslational modifications of Bcl-2–family proteins. The first report of phosphorylation of Bcl-2 was made in 1994 by May et al,92 and it was originally reported to be inhibitory, although stimulatory effects have since been described93—an issue that remains unresolved. Phosphorylation of proapoptotic Bcl-2-family proteins can either neutralize (eg, BAD) or potentiate (eg, BIM) their functions. Far more work is needed on biochemical and functional analysis of posttranslational modifications of Bcl-2–family members.
Dual phenotype of Bcl-2
Bcl-2 is best known for its ability to suppress apoptosis, but hints that this protein could also participate in apoptosis induction began to surface as early as 1996.94 In 1997, Cheng et al95 revealed the first mechanism for conversion of Bcl-2 from a protector to a killer, showing that proteolytic removal of N-terminal sequences by caspase-mediated cleavage flips the phenotype of Bcl-2. It is noteworthy that mutations in translocated BCL-2 alleles have been identified in human lymphomas that ablate the aspartic acid residue required for caspase cleavage,21 suggesting that some tumors may evolve strategies for avoiding the “dark side” of Bcl-2. Later, in 2004, Lin et al96 discovered another mechanism for converting Bcl-2 into a killer, showing that the orphan nuclear receptor Nur77 can be induced to translocate from nucleus to cytosol, binding Bcl-2, and inducing a conformational change in Bcl-2 that probably mimics what happens during caspase cleavage, exposing the normally buried BH3 domain of Bcl-2 and causing it to function as a proapoptotic protein. A potentially similar mechanism was identified for Bcl-XL in 2006 by Bivona et al,97 showing that lipid modifications of K-Ras can promote its association with Bcl-XL on mitochondria and induce apoptosis. Thus, depending on the proteins with which Bcl-2 and Bcl-XL interact, their phenotypes can be converted from anti- to proapoptotic, revealing an additional level of complexity to these proteins that has clear therapeutic implications for malignancies that overexpress these Bcl-2-family proteins. Perhaps phenotypic conversion underlies the recent discovery of compounds by Schwartz et al98 that show superior cytotoxic activity in Bcl-2/Bcl-XL–overexpressing cells compared with control cells, as well as providing a potential explanation for why high levels of Bcl-2 expression are sometimes associated with better patient prognosis—first observed in breast cancers in 1994 by Silvestrini et al.99
Unanswered questions
During the 2 decades since the discovery of Bcl-2, we have learned much about the functions of Bcl-2–family proteins. The lessons learned, however, have also taught us that there is much we do not understand, including (1) the structural details of how Bax and Bak permeabilize mitochondrial membranes, (2) the role of Bcl-2–family proteins in ER membranes, where they modulate Ca2+ and influence signal transduction events linked to cell cycle entry and ER stress,17,100 (3) the relevance of Bcl-2–mediated suppression of autophagy on tissue homeostasis and neoplasia, and (4) the many ways that posttranslational modifications (phosphorylation, deamidation, proteolysis) can alter the phenotypes of Bcl-2 family proteins.101
In vivo actions of Bcl-2–family proteins—of mice and men
Although genetic alterations that activate the BCL-2 proto-oncogene or inactivate the BAX tumor suppressor gene in human malignancies have provided important clues about the in vivo roles of Bcl-2–family proteins in the hematopoietic system, the preponderance of what we know has come from investigations of genetically engineered mice. Space limitations allow only a superficial treatment of what we have learned about regulation of programmed cell death in the hematopoietic system from mouse genetics studies, and thus the reader is referred to recent reviews on the topic by Strasser87 and Opferman and Korsmeyer.102 In brief, in terms of basic tissue homeostasis, gene knockout studies have shown that (1) Bcl-2 is required for maintenance of peripheral lymphocyte populations in adulthood, with animals developing lymphopenias at approximately the time they undergo sexual maturation (probably a time when endogenous glucocorticoid levels rise)103; (2) Bax-deficient mice develop lymphocytosis, demonstrating a role for this proapoptotic protein in programmed cell death of mature lymphocytes104; (3) Bcl-X is required for survival of immature thymocytes105; (4) Bid knockout mice are initially hematologically normal but after a long latency period develop chronic myelomonocytic leukemias, suggesting a role for Bid in controlling turnover of some populations of myeloid cells106; (5) proapoptotic gene Bim is required for proper control of granulocyte survival, elimination of autoreactive thymocytes, and programmed cell death of memory B cells and plasma cells (or their progenitors)107–110; (6) antiapoptotic Mcl-1 is required for survival of hematopoietic stem cells, survival of developing T and B lymphocytes, as well as maintenance of mature lymphocyte cell populations111,112; (7) proapoptotic Bak helps to govern the homeostasis of B-cell populations in vivo and bak−/− platelets have extended half-life113; (8) antiapoptotic Bcl-W is expressed in myeloid cells but evidently not essential for survival114; (9) Bad-deficient mice develop diffuse large B-cell lymphoma, suggesting a tumor suppressor role for this proapoptotic protein in mature B cells115; and (10) neutrophils and mast cells from mice deficient in A1a (murine ortholog of Bfl-1) show accelerated apoptosis in culture.116,117 We have also learned from gene knockout studies in mice that defects in programmed cell death promote autoimmunity, presumably by interfering with eradication of autoreactive lymphocytes or their progenitors.
Species-specific differences in some members of the Bcl-2 gene families of humans and mice have made it difficult to extend the in vivo analysis to all members. For example, in humans, the nuclear factor-κB–inducible antiapoptotic gene BFL-1 is represented by a single gene in humans, but consists of 4 highly homologous genes spread around the mouse genome (A1a, A1b, A1c, A1d), making it difficult to perform genetic ablation experiments.118 In addition, in mice, the closest homolog to the human BCL-B (BCL210L) gene is diva/boo, which has entirely different patterns of expression in vivo in humans versus mice, with expression restricted to reproductive tissues in mice but prominent in plasma cell and some populations of B lymphocytes (among other types of cells) in humans.119 Thus, although the hematopoietic systems of diva/boo knockout mice are phenotypically normal,120 we cannot infer that the same would be true in humans lacking the corresponding gene.
Bcl-2–family proteins as central integrators—critical nodes in life's networks
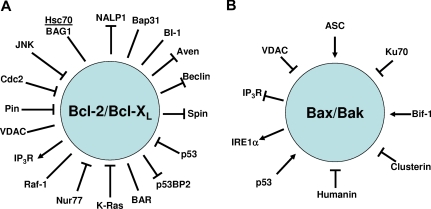
Although much research has focused on homo- and heterodimerization among Bcl-2–family proteins, many if not most of these proteins have other interaction partners that regulate their activity and that link them to a wide variety of cellular pathways, giving the impression that Bcl-2–family proteins operate as critical nodes in complex networks to integrate information and make ultimate life/death decisions. For the more heavily studied members of the family, such as Bcl-2, Bcl-XL, and Bax, the list of potential interacting proteins is almost overwhelming (Figure 3). The structural basis for most of these nonfamilial protein interactions has not been elucidated, and their roles in physiology are not always well understood, but the extensive repertoire of partners suggests that diverse signaling, developmental, and metabolic pathways converge on Bcl-2–family members.
Figure 3.
Nonfamilial interactions with antiapoptotic proteins Bcl-2 or Bcl-XL and with proapoptotic proteins Bax or Bak. (A) The depicted protein interactions have been reported for either Bcl-2 or Bcl-XL. (B) The depicted protein interactions have been reported for Bax or Bak. In many cells, Bax is present in a latent (inactive) conformation in the cytosol,57 in which its C-terminal transmembrane domain is folded onto the protein.64 Activators of Bax induce conformational changes that promote Bax's insertion into membranes of mitochondria, followed by BH3-induced oligomerization in membranes. Several proteins that modulate these steps have been reported.
Future therapeutic opportunities
The fruits of more than 2 decades of research on Bcl-2 and its kin have yielded new strategies for therapeutic applications, some of which have advanced to clinical testing in humans. The first of these is antisense oligonucleotides targeting Bcl-2 mRNA, which have shown promising activity for CLL in randomized phase 3 trials.121 Results for myeloma and AML have not been encouraging, which could be due to the overexpression of antiapoptotic members of the family besides Bcl-2. Multiple chemical antagonists of antiapoptotic Bcl-2–family proteins have been described that bind the same pocket occupied by proapoptotic BH3 domains (reviewed by Reed and Pellecchia91). These compounds have different potencies and variable spectra of activity against the 6 antiapoptotic members. At least 3 such compounds have advanced into human testing to date, with more likely to follow soon. Compounds that appear to convert Bcl-2, Bcl-XL, or their relatives from protectors to killers have been identified, including molecules that directly bind these proteins and others that trigger Nur77 translocation.122 A close analog of a Nur77-modulating compound is in clinical testing currently for cancer treatment. These translational aspects of research on Bcl-2 raise hopes that a new class of anticancer drugs may be forthcoming. Given the prominent role of Bcl-2–family proteins in elimination of autoreactive lymphocytes, these experimental therapeutic agents are likely to also find applications for a variety of autoimmune diseases.
Acknowledgments
I thank M. Hanaii and T. Siegfried for manuscript preparation.
This work was supported by the National Institutes of Health NCI-Drug Discovery Group (CA113318) and a grant on apoptosis and mitochondria (GM60554).
Biography
Dr John Reed is President and CEO of Burnham Institute for Medical Research, a nonprofit research institute with more than 800 employees, an annual operating budget of more than $100 million, and sites of operation in San Diego and Santa Barbara, CA, and in Orlando, FL. Dr Reed's interests in hematology began as an MD/PhD student at the University of Pennsylvania, undertaking studies of lymphocyte proliferation and lymphokines, continuing with work as a postdoctoral fellow at the Wistar Institute on oncogenes involved in lymphoma and leukemia, including the antiapoptotic BCL-2 gene—the first antiapoptotic gene discovered. Since joining Burnham in 1992, Dr Reed's research team has contributed more publications on apoptosis and cell death than has any other laboratory worldwide. According to Dr Reed, “Hematology is a wonderful platform for life-science research, because it provides outstanding models for essentially all major biologic processes, including stem cell biology and cell differentiation, cell division, cell death, cell migration, cellular communication and signaling, and aging.”
Authorship
Contribution: J.C.R. is the sole author.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: John C. Reed, 10901 N Torrey Pines Road, Burnham Institute for Medical Research, La Jolla, CA 92037; e-mail: reedoffice@burnham.org.
References
- 1.Salvesen GS, Abrams JM. Caspase activation—stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- 2.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 4.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Proapoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 6.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci JE, Munoz-Pinedo C, Fitzgerald P, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit (NDUSF1) of Complex I of the electron transport chain. Cell. 2004;117:773–86. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Penninger JM, Kroemer G. Mitochondria, AIF and caspases–rivaling for cell death execution. Nat Cell Biol. 2003;5:97–99. doi: 10.1038/ncb0203-97. [DOI] [PubMed] [Google Scholar]
- 10.Reed JC, Green DR. Remodeling for demolition: Changes in mitochondrial ultrastructure during apoptosis. Mol Cell. 2002;9:1–3. doi: 10.1016/s1097-2765(02)00437-9. [DOI] [PubMed] [Google Scholar]
- 11.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 12.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 14.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit beclin 1- dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 16.Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 17.Xu C, Bailly-Maitre B, Reed JC. Endoplamic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ron D. Cell biology. Stressed cells cope with protein overload. Science. 2006;313:52–53. doi: 10.1126/science.1130469. [DOI] [PubMed] [Google Scholar]
- 19.Kitsis RN, Peng C-F, Cuervo AM. Eat your heart out. Nat Med. 2007;13:539–541. doi: 10.1038/nm0507-539. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto Y, Cossman J, Jaffe E, Croce C. Involvement of the Bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Louie D, Kant J, Reed J. Frequent somatic mutations in translocated BcL2 genes of non-Hodgkin's lymphoma patients. Blood. 1992;79:229–237. [PubMed] [Google Scholar]
- 22.Tsujimoto Y, Croce C. Analysis of the structure, transcripts, and protein products of Bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986;83:5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for Bcl-2 and a hybrid Bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. CELL. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 24.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed J. Bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–1828. [PubMed] [Google Scholar]
- 26.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Nat Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monni O, Joensuu H, Franssila K, Klefstrom J, Alitalo K, Knuutila S. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90:1168–1174. [PubMed] [Google Scholar]
- 28.Reed JC, Cuddy M, Slabiak T, Croce CM, Nowell PC. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988;336:259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- 29.McDonnell TJ, Deane N, Platt FM, et al. Bcl-2-immunoglobulin transgenic mice demonstrate extended B-cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 30.Reed JC, Stein C, Subasinghe C, et al. Antisense-mediated inhibition of BCL2 proto-oncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990;50:6565–6570. [PubMed] [Google Scholar]
- 31.Reed JC, Cuddy M, Haldar S, et al. BCL2-mediated tumorigenicity of a human Tlymphoid cell line: synergy with MYC and inhibition by BCL2 antisense. Proc Natl Acad Sci USA. 1990;87:3660–3664. doi: 10.1073/pnas.87.10.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 33.Vaux D, Weissman I, Kim S. Prevention of programmed cell death in Caenorhabditis elegans by human Bcl-2. Science. 1992;258:1955–1957. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- 34.Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammlian proto-oncogene Bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 35.Kane DJ, Sarafin TA, Auton S, et al. Bcl-2 inhibition of neural cell death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1276. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 36.Zhong LT, Sarafian T, Kane DJ, et al. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yunis JJ, Mayer MG, Arensen MA, Aeppli DP, Oken MM, Frizzera G. Bcl-2 and other genomic alterations in the prognosis of large-cell lymphomas. N Engl J Med. 1989;320:1047–1054. doi: 10.1056/NEJM198904203201605. [DOI] [PubMed] [Google Scholar]
- 38.Hermine O, Haioun C, Lepage E, et al. Prognostic significance of Bcl-2 protein expression in aggressive non-Hodgkin's lymphoma. Blood. 1996;87:265–272. [PubMed] [Google Scholar]
- 39.Tang SC, Visser L, Hepperle B, Hanson J, Poppema S. Clinical significance of bcl-2-MBR gene rearrangement and protein expression in diffuse large-cell non-Hodgkin's lymphoma: an analysis of 83 cases. J Clin Oncol. 1994;12:149–154. doi: 10.1200/JCO.1994.12.1.149. [DOI] [PubMed] [Google Scholar]
- 40.Hill ME, MacLennan KA, Cunningham DC, et al. Prognostic significance of BCL-2 expression and bcl-2 major breakpoint region rearrangement in diffuse large cell non-Hodgkin's lymphoma: a British national lymphoma investigation study. Blood. 1996;88:1046–1051. [PubMed] [Google Scholar]
- 41.Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin's lymphoma. Blood. 1997;90:244–251. [PubMed] [Google Scholar]
- 42.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 43.Miyashita T, Reed JC. Bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992;52:5407–5411. [PubMed] [Google Scholar]
- 44.Campos L, Roualult J-P, Sabido O, et al. High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. [PubMed] [Google Scholar]
- 45.Campos L, Sabido O, Rouault J-P, Guyotat D. Effects of Bcl-2 antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood. 1994;84:595–600. [PubMed] [Google Scholar]
- 46.Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein Bcl-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 47.McDonnell TJ, Troncoso P, Brisbay SM, et al. Expression of the proto-oncogene Bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 48.Webb A, Cunningham D, Cotter F, et al. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet. 1997;349:1137–1141. doi: 10.1016/s0140-6736(96)11103-x. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimoto Y, Ikegaki N, Croce CM. Characterization of the protein product of Bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2:3–7. [PubMed] [Google Scholar]
- 50.Chen-Levy S, Nourse J, Cleary MZ. The Bcl-2 candidate proto-oncogene product is a 24-KD integral membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989;9:701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hockenbery DM, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 52.Newmeyer D, Farschon DM, Reed JC. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 53.Zamzami N, Marchetti P, Castedo M, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 55.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 57.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nechushtan A, Smith C, Hsu YT, Youle R. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR structure of human Bcl-XL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 60.Fesik SW. Insights into programmed cell death through structural biology. Cell. 2000;103:273–282. doi: 10.1016/s0092-8674(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 61.Minn AJ, Velez P, Schendel SL, et al. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 62.Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci U S A. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki M, Youle RJ, Tjandra N. Structure of bax: Coregulation of dimmer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 65.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 67.Kuwana T, Mackey MR, Perkins G, et al. Bid, bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 68.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 69.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chinnaiyan AM, O'Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 71.Spector MS, Desnoyers S, Heoppner DJ, Hengartner MO. Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature. 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 72.Wu D, Wallen H, Inohara N, Nunez G. Interaction and regulation of the c. elegans death protease CED-3 by CED-4 and CED-9. J Biol Chem. 1997;272:21449–21454. doi: 10.1074/jbc.272.34.21449. [DOI] [PubMed] [Google Scholar]
- 73.Bruey JM, Bruey-Sedano N, Luciano F, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 74.Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyashita T, Krajewski S, Krajewska M, et al. Tumor suppressor p53 is a regulator of BCL-2 and BAX in gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 76.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of human Bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 77.Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 78.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 79.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 81.Meijerink JP, Mensink EJ, Wang K, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- 82.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 83.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 84.Deng X, Gao F, Flagg T, Anderson J, May WS. Bcl2's flexible loop domain regulates p53 binding and survival. Mol Cell Biol. 2006;26:4421–4434. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with bax. Nature. 1994;369:321–333. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 86.Chittenden T, Flemington C, Houghton AB, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 88.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 89.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2-family proteins induces regression of solid tumors. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 90.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reed JC, Pellecchia M. Apoptosis-based therapies for hematological malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 92.May WS, Tyler PG, Ito T, Armstrong DK, Qatsha KA, Davidson NE. Interleukin- 3 and bryostatin-1 mediate hyperphosphorylation of Bcl-2 α in association with suppression of apoptosis. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- 93.Itoh T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for antiapoptosis function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 94.Chen J, Flannery JG, LaVail MM, Steinberg RH, Xu J, Simon MI. bcl-2 overexpression reduces apoptotic photoreceptor cell death in three different retinal degenerations. Proc Natl Acad Sci U S A. 1996;93:7042–7047. doi: 10.1073/pnas.93.14.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng E, Clem R, Ravi R, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 96.Lin B, Kolluri SK, Lin F, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor TR3/NGFI-B/Nur77. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 97.Bivona TG, Quatela SE, Bodemann BO, et al. PKC regulates a farnesyl electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz PS, Manion MK, Emerson CB, et al. 2-Methoxy antimycin reveals a unique mechanism for Bcl-x(L) inhibition. Mol Cancer Ther. 2007;6:2073–2080. doi: 10.1158/1535-7163.MCT-06-0767. [DOI] [PubMed] [Google Scholar]
- 99.Silvestrini R, Veneroni S, Daidone MG, et al. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994;86:499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- 100.Demaurex N, Distelhorst C. Apoptosis–the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 101.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 102.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 103.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. CELL. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 104.Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ. Baxdeficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 105.Ma A, Pena JC, Chang B, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci U S A. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zinkel SS, Ong CC, Ferguson DO, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 108.Bouillet P, Purton JF, Godfrey DI, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 109.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 110.Fischer SF, Bouillet P, O'Donnell K, Light A, Tarlinton DM, Strasser A. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 112.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 113.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Print C, Loveland K, Gibson L, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ranger AM, Zha J, Harada H, et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Hatakeyama S. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in mast cell survival after allergic activation. J Exp Med. 2001;194:1561–1569. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reed JC, Doctor KS, Godzik A. The domains of apoptosis: a genomics perspective. Science STKE. 2004;2004:RE9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- 119.Luciano F, Krajewska M, Ortiz-Rubio P, et al. Nur77 converts phenotype of Bcl-B, an anti-apoptotic protein expressed in plasma cells and myeloma. Blood. 2007;109:3849–3855. doi: 10.1182/blood-2006-11-056879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russell HR, Lee Y, Miller HL, Zhao J, McKinnon PJ. Murine ovarian development is not affected by inactivation of the Bcl-2 family member diva. Mol Cell Biol. 2002;22:6866–6870. doi: 10.1128/MCB.22.19.6866-6870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 122.Han YH, Cao X, Lin B, et al. Regulation of Nur77 nuclear export by c-Jun Nterminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]