Abstract
The endothelial cell protein C receptor also exists in soluble form in plasma (sEPCR), resulting from ADAM17 cleavage. Elevated sEPCR levels are observed in subjects carrying the A3 haplotype, which is characterized by a Ser219Gly substitution in the transmembrane domain, rendering the receptor more sensitive to cleavage. Because sEPCR production is not completely blocked by metalloprotease inhibition, we looked for another mechanism. Comparing mRNA expression patterns and levels in A3 and non-A3 cells from 32 human umbilical cord veins, we detected a truncated mRNA in addition to the full-length mRNA. This truncated mRNA was 16 times more abundant in A3 human umbilical vein endothelial cells than in non-A3 human umbilical vein endothelial cells and encoded a protein lacking the transmembrane domain. We stably expressed a recombinant form of this protein (rEPCRisoform) and a protein mimicking the plasma sEPCR (rEPCRsol). Functional studies of the purified recombinant proteins revealed that the rEPCRisoform bound to recombinant protein C with similar affinity than rEPCRsol and that it also inhibited the anticoagulant activity of APC. Trace amounts of the EPCR isoform were found in the plasma of A3 subjects. These results suggest that the sEPCRisoform could contribute to the regulatory effect of sEPCR in plasma.
Introduction
The endothelial cell protein C receptor (EPCR) is a glycoprotein with an important role in the protein C (PC) anticoagulant pathway.1 EPCR is mainly expressed on the surface of large vessels2 and binds PC with high affinity (Kd 30 nM),3 inducing a 20-fold increase in PC activation by thrombin/thrombomodulin complexes in vivo.1 The role of EPCR in regulating blood coagulation has been shown in several studies. The first major finding was obtained in baboons pretreated with anti-EPCR antibodies, which led to a major reduction in activated PC (APC) generation when the animals were infused with thrombin.4 A second showed that EPCR gene disruption led to early embryonic lethality by fibrin deposition in mice.5
EPCR is a 46-kDa type I transmembrane protein homologous to major histocompatibility complex class I /CD1 family proteins.3 This 221-amino acid protein comprises an extracellular region composed of 2 alpha domains (α1 and α2 domains), a 25-amino acid transmembrane domain and a short (3-amino acid) cytoplasmic tail.3,6 The human EPCR gene, PROCR, is located on chromosome 20, spans 8 kilobases, and comprises 4 exons. The first exon contains the 5′-untranslated region and encodes the signal peptide; exons 2 and 3 encode most of the extracellular domain, and exon 4 encodes the transmembrane and intracellular domain and the 3′ untranslated region.7,8
A soluble form of EPCR (sEPCR) is present in normal human plasma.9 sEPCR is a single species of 43 kDa lacking the transmembrane domain and cytoplasmic tail. Like the membrane-associated form, sEPCR binds PC and APC with similar affinity.10 However, its binding to APC inhibits the anticoagulant activity of APC by abrogating its ability to inactivate factor Va, and its binding to PC precludes PC activation by thrombin/thrombomodulin complexes.11,12 It is therefore not surprising that one study13 has reported an odds ratio at 1.9 for increased sEPCR levels and the risk of venous thrombosis.
Approximately 15% to 20% of the general population have plasma sEPCR levels of 200 to 800 ng/mL,13,14 whereas the remainder have levels less than 180 ng/mL. We and others13,15,16 have previously shown that most subjects with elevated sEPCR levels carry the A3 haplotype, one of the 4 most frequent haplotypes of PROCR. This haplotype is characterized by 4 specific nucleotides, 1651G, 3610T, 4216A, and 6936G, the latter leading to Ser 219 substitution by a Gly, in the transmembrane domain of the protein.15 Up to now, sEPCR was thought to be generated exclusively by shedding17 because of the action of thrombin or other sheddases,17 such as the metalloprotease ADAM17.18 It was recently shown that the increased sEPCR levels observed in A3 carriers are the result of the higher sensitivity of the Gly219 receptor isoform compared with the Ser219 isoform.19 Very recently, Pr3 was also shown to be able to cleave EPCR.20
Besides its role in coagulation, EPCR was recently shown to be involved in the inflammation control pathway.21,22 For this reason, and by homology with mechanisms generating soluble forms of other receptors for inflammatory mediators, such as interleukin,23,24 we wondered whether another mechanism, such as mRNA alternative splicing, could contribute to sEPCR production.
We thus studied the mRNA forms present in eukaryote cells and compared the expression pattern according to the cell genotype. In A3-carrying cells, we detected a statistically significant higher amount of a truncated mRNA than in non–A3-carrying cells. This truncated mRNA encoded a protein lacking the transmembrane domain, which was therefore likely to be secreted. We then expressed this protein and showed that it was indeed synthesized and secreted. Finally, we compared its properties with those of sEPCR generated by ADAM17 cleavage.
Methods
Materials
Goat anti–human EPCR and biotinylated anti–human EPCR antibodies were from R&D Systems (Lille, France), HRP-labeled polyclonal antiprotein C antibody was from Dako (Trappes, France), and RNA B was from Bioprobe Systems (Montreuil-sous-bois, France). Oligonucleotides were synthetized by Proligo France (Paris, France). Nhe I and Not I endonucleases and PNGase F were from New England Biolabs (Ozyme, Saint-Quentin en Yvelines, France). The Big Dye Terminator V3.1 Cycle Sequencing Ready Reaction with AmpliTaq DNA polymerase FS kit and the Sybr Green PCR Core Reagent kit were from Applied Biosystems (Courtabeuf, France). Pfu polymerase and the pCI-neo expression vector were from Promega (Charbonnière-les-Bains, France). Trizol reagent, polyacrylamide gels, and cell culture medium and reagents were from Invitrogen (Eragny-sur-Oise, France). PC-deficient plasma and Asserachrom sEPCR kit were from Stago (Asnières, France). Nitrocellulose membranes were from Bio-Rad (Marne-la-Coquette, France). All other reagents were of the highest available grade. HEK293 cells were from ATCC and the EA.hy926 cell line (established by fusion of human umbilical-vein endothelial cells with the permanent cell line A 549) were a kind gift from Dr Cora Edgell (University of North Carolina, Chapel Hill, NC).
Nucleotides
The nucleotides of the EPCR gene PROCR are numbered according to the sequence available under GenBank accession number AF106202.25 The primers are named according to their position on this sequence, the number indicated after the suffix EPCR referring to the first nucleotide at the 5′ end of the primer, Fr indicating a sense primer, and Rv an antisense primer.
Endothelial cells from human umbilical veins
Endothelial cells from 32 different umbilical cord veins (HUVECs) were collected between November 2004 and April 2005.
mRNA isolation
mRNA was isolated from 75-cm2 flasks of cultured HEK293 and EA.hy 926 cells and from hepatocytes using RNA Probe solution, and from HUVECs using Trizol solution, according to the manufacturer's instructions.
Genotyping of HEK 293, EA.hy 926, and HUVECs
The PROCR region encompassing nt 6936 was amplified using EPCR 4993Fr and EPCR 7320Rv (Table 1) and was sequenced using primer EPCR7190Rv after polymerase chain reaction (PCR) purification. The products were analyzed with an ABI Prism 3700 sequencer.
Table 1.
Sequences of oligonucleotides used in the present study
| Name | Sequence (5′ to 3′) | Position in PROCR sequence (AF106202) |
|---|---|---|
| Primers used for cDNA amplification of PROCR genotyping | ||
| EPCR 2347 Fr | CATTGCTGCCGATACTGCTGC | nt 2347 to 2367 |
| EPCR 4993 Fr | CCCAGACACCAACACCACG | nt 4993 to 5010 |
| EPCR 6970 Rv | AAGATGCCTACAGCCACACC | nt 6970 to 6951 |
| EPCR 7320 Rv | GTCTGTCTTTGGAGGATGGGCC | nt 7320 to 7299 |
| EPCR 7190 Rv | CAAGTACTTTGTCCACCTCTCC | nt 7190 to 7169 |
| EPCR 3960 Fr | GAGGCAGAGCAATCCTGACA | nt 3960 to 3980 |
| EPCR 4562 Rv | CATCTCCTCTTCCACTGCCC | nt 4526 to 4507 |
| EPCR 4069 Fr | GGGCCAGGTCTCTAGCAGCC | nt 4069 to 4088 |
| Primers used to clone the EPCR cDNA in the pCI-neo expression vector | ||
| First-round PCR | ||
| EPCR upstream | GGTCCGGAGCCTCAACTTCAG | nt 2315 to 2335 |
| EPCR downstream | ACTTTCCCTTGCCAGCCTCC | nt 7054 to 7035 |
| Nested PCR with oligonucleotides containing the cloning sites | ||
| EPCR Nhe IFr | GACTCACTATAGGGAGACCCAAGCTGgctagcTCAGGATGTTGACAACATTGCTGCCGATAC | |
| EPCR Not IRv | GGTGTTTCAGTTGGGGAGTCTCGAgcggccgcGAGCTGAAACTTTCCCTTGCCAGCCTCCATC | nt 7062 to 7032 |
| Primers used to clone the cDNA of EPCR 1–209 (rEPCRsol) in the pCI-neo expression vector | ||
| pCI-neo upstream 2 | CGAGACAGAGAAGACTCTTGCGTTTC | [in plasmid pCI-neo sequence] |
| EPCRsol1–209NotI | CACAAGGAAAAGGTATTAgcggccgcTTAAGTGTAGGAGCGGCTTGTTTGGCTC | nt 6908 to 6884 |
| Primers used to clone the cDNA corresponding to EPCR isoform (rEPCRisoform) in the pCI-neo expression vector | ||
| 5′ part of the cDNA | ||
| pCI-neo upstream 2 | CGAGACAGAGAAGACTCTTGCGTTTC | [in plasmid pCI-neo sequence] |
| EPCR 6568Rv | CCCGCAGTTCATACCGAGTG | nt 6568 to 6549 |
| 3′ part of the cDNA | ||
| EPCR 4993Fr | CCCAGACACCAACACCACG | nt 4993 to 5012 |
| EPCR 7480 Rv Not I | CACAAGGAAAGGTATTAgcggccgcGGGATTTTACATTTCCACCACTTCTTCGTG | nt 7480 to 7451 |
Restriction site sequences are shown in lowercase letters. The sequences complementary to the EPCR cDNA are in bold letters.
To distinguish between EPCR haplotypes, the PROCR region encompassing nucleotide 4216 was amplified with primers EPCR 3960Fr and EPCR 4526Rv and sequenced as described using EPCR 4069 Fr as the sequencing primer.
Reverse transcription and real-time PCR for mRNA quantification
Reverse transcription (RT) was performed as previously described,26 except that the cDNA was obtained from 1 μg of total mRNA extracted from HUVECs or from HEK 293 and EA.hy926 cells. Quantitative values were obtained from the threshold cycle number (Ct value) essentially as described elsewhere.26
We quantified the 2 PROCR mRNA transcripts (full-length and truncated mRNA) using appropriate primers (Table 2) chosen with the Oligo Express program. To avoid amplification of contaminating gDNA, one of the 2 primers used to amplify the cDNA obtained from the full-length mRNA form overlapped the exon 3/exon 4 junction. The cDNA obtained from the truncated mRNA was amplified using a primer overlapping the newly created junction between exon 3 and the untranslated part of exon 4. Results were normalized using TBP transcripts as controls, and final results were obtained as described by Dupont et al.26
Table 2.
Oligonucleotide primers used for real-time quantitative RT-PCR amplification
| EPCR mRNA forms | Sense primer 5′-3′ | Antisense primer 5′-3′ | PCR product size, bp |
|---|---|---|---|
| Full-length EPCR mRNA | TGGTCACCTTCACCCTGCA | TTTGGCTCCCTTTCGTGTTTT | 132 |
| Truncated EPCR mRNA | TGGTCACCTTCACCCTGCA | TGCCCCAACTCCATTATCTTTC | 140 |
Cloning of EPCR cDNA
Total mRNA was isolated from EA.hy926 cells, and first-strand DNA synthesis and a first round of PCR were performed using the C Therm Polymerase One-Step RT-PCR system (Roche Diagnostics, Basel, Switzerland) with primers indicated in Table 1. The amplified product was purified on agarose gel and used as the template for nested PCR with 2 primers containing Nhe I and Not I restriction sites, respectively (Table 1). After double digestion with NheI/NotI, the amplified fragment containing the EPCR cDNA was ligated into the corresponding sites of a eukaryotic expression vector (pCI-neo). The resulting pCI-neo-EPCR plasmid was either used for stable transfection of HEK293 cells or as a template for site-directed mutagenesis.
Cloning of a recombinant sEPCR
To express a recombinant sEPCR (rEPCRsol) mimicking the plasma sEPCR, we used pCI-neo-EPCR as a template; we deleted, by site-directed mutagenesis, the sequence encoding the EPCR amino acids (210 to 236) predicted to constitute the transmembrane and intracellular domains of EPCR. The cDNA encoding this truncated form of EPCR was obtained by PCR using a primer located in the pCI-neo vector sequence as upstream primer, and a mutagenic primer EPCR1–209NotIhybridizing on the nucleotide sequence corresponding to codons 202 to 209, followed by a TAA codon and a Not I restriction site. The mutated cDNA was inserted into the pCI-neo vector as described in “Cloning of EPCR cDNA.”
Cloning of a recombinant EPCR isoform encoded by the truncated mRNA
The particular form of mRNA encoding the EPCR isoform (rEPCRisoform) was cloned in 2 steps: first, amplification of the 5′ and 3′ parts in 2 separate reactions, and then a final PCR step using the purified products of the first 2 PCR rounds as templates.
The 5′ part of the cDNA was obtained using pCI-neo-Ser EPCR as template and the primers described in Table 1. The 3′ part of the cDNA corresponding to the truncated mRNA required a different approach. We used RT-PCR obtained from mRNA of EA.hy cells (heterozygous for the A3 haplotype) to gel-purify the 3′ truncated mRNA form obtained by PCR with EPCR4993Fr and EPCR7480Rv as amplification primers. The purified product was used as a template to prepare the 3′ part of the truncated mRNA, using EPCR4993Fr and EPCR7480Rv Not Ias amplifying primers. The 2 PCR products were gel-purified and added to a final PCR mixture to obtain the final PCR product using pCI-neo Amont 2 and EPCR7480Rv Not I primers; this product contained the sequence encoding the EPCR isoform and the cloning sites EcoRI/Not I.
The integrity of the wild-type and mutated cDNAs inserted in the vector was checked by sequencing the whole sequence inserted in the expression vector by overlapping PCRs. The amplification products were purified by filtration on Millipore plates and sequenced as previously described, using sets of primers matching the cDNA of interest.
Expression of recombinant rEPCRsol and rEPCRisoform proteins
HEK293 cells were cultured as previously described,27 and subconfluent cells were transfected with 15 μg of plasmid as described by Graham and van der Eb.28 Twenty-four hours after transfection, cells were trypsinized and grown at various initial densities. Drug selection was performed by adding 1 mg/mL G418 2 days after transfection. Drug-resistant clones strongly producing soluble recombinants were identified with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Asserachrom sEPCR) and were mass-produced as previously described.27
Purification of rEPCRsol and rEPCRisoform
Recombinant EPCRsol and rEPCRisoform were purified from 3.2 L of conditioned medium first concentrated by ion-exchange chromatography on QAE A50-Sephadex. Fractions containing the recombinant proteins were identified with a home-made ELISA method described in “Home-made ELISA,” and proteins were then loaded on an immunoaffinity chromatography column containing the antihuman EPCR mAb JRK1496, coupled to Affigel 10 resin, and whose epitope is located in the extracellular domain of EPCR.9 The fractions containing the recombinant proteins were pooled for a final ion-exchange chromatography on a MonoQ column, thus concentrating the recombinant proteins. Protein concentrations were determined by measuring absorbance at 280 nm and using E1%, 1 cm = 10 for rEPCRsol and rEPCRisoform.
N-terminal sequencing of rEPCRsol
The N-terminal part of rEPCRsol was sequenced by Edman degradation in the microsequencing laboratory of the Pasteur Institute (Paris, France) as previously described.29
Binding of recombinant wild-type protein C to rEPCRsol and rEPCRisoform
Binding of recombinant wild-type protein C (rWT PC) to rEPCRsol and rEPCRisoform was evaluated with an ELISA method. Goat anti-EPCR antibody (400 ng) was immobilized on a microtiter plate and saturated with rEPCRsol or rEPCRisoform diluted in Tris-buffered saline (50 mM Tris/HCl 150 mM NaCl pH 7.5) containing 0.2% bovine serum albumin. After one hour of incubation with increasing amounts of rWT PC diluted in Tris-buffered saline containing 1.3 mM Ca2+, 0.6 mM Mg2+ and 0.2% bovine serum albumin, bound rWT PC was detected with peroxidase-conjugated rabbit antihuman-PC. Revelation was performed by adding 50 μL o-phenylene diamine (substrate) and the reaction was stopped by adding 25 μL 3 M H2SO4. Absorbance was measured at 490 nm on a microplate reader (MWGt Discovery HRt Reader; Bio-Tek Instruments, Winooski, VT). The apparent dissociation constants (Kdapp) of rWT PC for rEPCRsol and rEPCRisoform were estimated as previously described.30
Effects of rEPCRsol and rEPCRisof in a clotting assay
The clotting assay was based on that described by Smirnov et al31 and was performed as described by Saller et al,32 with minor modifications.
Concentration and partial purification of human plasma EPCR
A total of 200 mL pooled plasma (collected on trisodium citrate 0.109 M and 10 mM benzamidine) from healthy volunteers heterozygous for the A3 haplotype and from healthy volunteers not carrying the A3 haplotype was diluted with 2 L Tris-HCl 50 mM benzamidine 10 mM pH 9.0 buffer and subjected to QAE-Sephadex chromatography as described in “Purification of rEPCRsol and rEPCRisoform.” Fractions containing the sEPCR were detected with a home-made ELISA, then pooled and loaded on a JRK-1496 immunoaffinity column as described for the recombinant proteins. The sEPCR-containing fractions were lyophilized before SDS-PAGE and Western blot analysis.
Home-made ELISA
A goat anti-EPCR antibody (400 ng) was immobilized on a microtiter plate and allowed to react for one hour with recombinant EPCR forms or plasma EPCR forms contained in QAE- and JRK-1496 elution fractions. EPCR binding was revealed with a biotinylated anti-EPCR antibody, whose binding was detected with streptavidin-HRP diluted 1/1000. Revelation was performed using 50 μL o-phenylene diamine (substrate) as previously described.
Results
Detection of an abnormal mRNA product in cells carrying the PROCR A3 haplotype
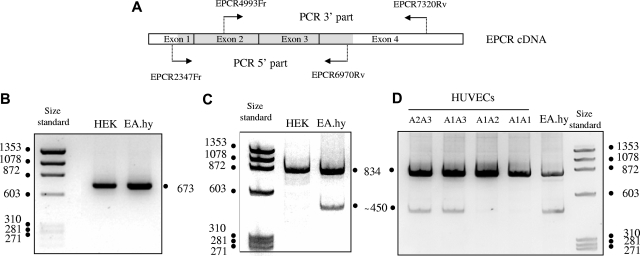
We sought an mRNA abnormality leading to truncation of the C-terminal part of the EPCR protein. After reverse-transcription of mRNAs isolated from HEK293 and EA.hy926 cells, the cDNA 5′ and 3′ parts were amplified as 2 overlapping fragments (see Figure 1A for primer location and Table 1 for primer sequences). Polyacrylamide gel electrophoresis (Figure 1B) identified a single amplification product of the expected size (673 bp) for the 5′ part from both cell types, whereas amplification of the 3′ part of mRNA from EA.hy926 cells gave a shorter product (∼450 bp) in addition to the expected 834-bp product obtained with HEK293 cells and EA.hy926 cells (Figure 1C). This strongly suggested 3′ deletion of some EA.Hy926 mRNA transcripts.
Figure 1.
Polyacrylamide gel electrophoresis of the amplified 5′ parts and 3′ parts of PROCR cDNA derived from HEK293 and EA.hy 926 cells, and of the amplified 3′ part derived from HUVECs from umbilical cords with different PROCR genotypes. (A) Schematic representation of the EPCR cDNA structure and the location of the primers used to amplify the 5′ and 3′ parts. The translated region of the full-length mRNA is in grey. (B) PCR product from HEK 293 EPCR cDNA and EA.hy 926 EPCR cDNA obtained using EPCR2347Fr and EPCR6970Rv as amplification primers. (C) PCR product from HEK 293 EPCR cDNA and EA.hy 926 EPCR cDNA obtained using EPCR4993Fr and EPCR7320Rv as amplification primers. (D) PCR product from HUVECs EPCR cDNA obtained using EPCR4993Fr and EPCR7320Rv as amplification primers.
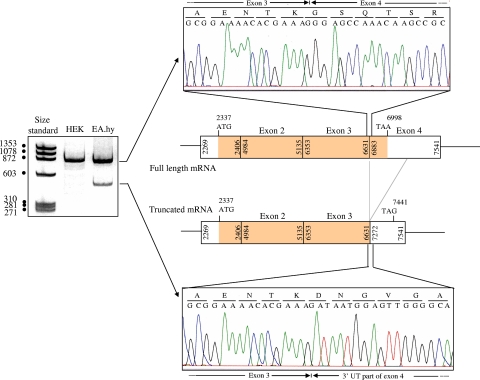
As EA.hy926 cells are heterozygous for the A3 haplotype, we studied a series of mRNAs isolated from HUVECs obtained from 32 cords to determine whether the truncated amplification product was specific for A3 cells. HUVECs were genotyped in terms of their PROCR haplotypes, and PROCR mRNA was isolated and submitted to reverse transcription. PAGE of the 3′ amplification product showed the presence of both the 450-bp amplification product and the 834-bp product only in the 6 HUVECs lines heterozygous for the A3 haplotype. Representative patterns obtained with HUVECs of different genotypes are shown in Figure 1D. The 834-bp and 450-bp products obtained with HUVECs and EA.hy926 cells were gel-purified and used as templates for sequencing. The sequences for the 2 cell types were identical. As expected (Figure 2), the 834-bp product corresponded to amplification of a normal 3′ part of full-length mRNA and exhibited a normal junction between exon 3 and exon 4. Sequencing of the shorter amplification product revealed an mRNA deletion of 390 nucleotide, linking nt 6631 (last nucleotide of exon 3) to nucleotide 7272 within the 3′ untranslated part. This 390-bp-deleted sequence corresponded to the entire coding region of exon 4 (region encoding the transmembrane and intracellular domains) and to a large part of its noncoding sequence.
Figure 2.
Sequences of the 2 amplification products of the 3′ part of EA.hy 926 PROCR cDNA, and location in the mature mRNA. The sequences shown here were obtained using EPCR4993Fr as sequencing primer and are restricted to sequences corresponding to the normal junction (upper electrophoregram) and the unusual junction (lower electrophoregram) of the full-length and truncated mRNAs, respectively. The schema indicates the exonic organization of the full-length and truncated mRNAs. Numbers indicate the first and last nucleotides of the exons of the genomic sequence (according to AF106202 numbering). In the truncated mRNA, exon 3 is linked to nt 7272 of the untranslated region of PROCR exon 4, which leads to a new stop codon at position 7441.
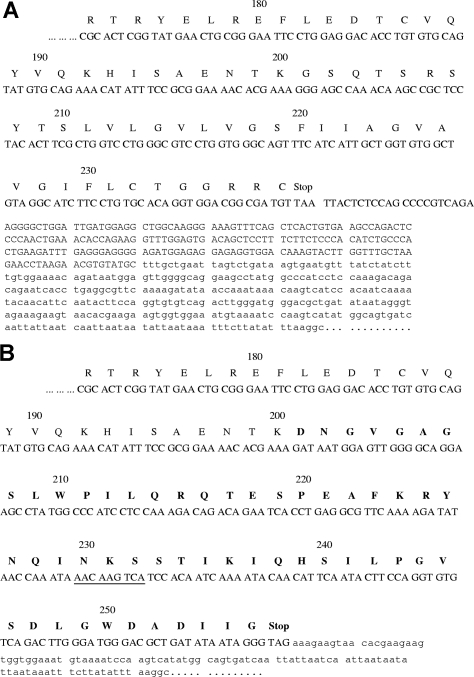
The predicted protein sequence of the C-terminal part of the transcript corresponding to the truncated mRNA is shown in Figure 3B, by comparison with the normal protein (Figure 3A). The TMHMM2.0 program fails to identify putative protein transmembrane helices in the new 56-amino-acid C-terminal part (compared with the membrane EPCR sequence). This suggested direct secretion of this protein, which is referred later as the sEPCRisoform.
Figure 3.
Sequences of the C-terminal parts of transcripts corresponding to the full-length mRNA and the truncated mRNA. Nucleotide sequence and predicted protein sequence of the EPCR isoform (B) compared with those of membrane EPCR (A). Only the C-terminal parts of the proteins are shown. The new exon 3/exon 4 junction generates a C-terminal part with 56 new amino acids (indicated in bold). A potential N-glycosylation site is underlined. The sequence corresponding to the recombinant sEPCR lacking the transmembrane domain prepared here ends at amino acid T 209 (A).
We then used RT-PCR to quantify each EPCR mRNA form (full-length and truncated) in HUVECs. The truncated mRNA represented 3.2% of EPCR mRNA in A3 cells and only 0.2% in non-A3 cells. We also detected the truncated mRNA isoform in hepatocytes (data not shown).
Expression and purification of the protein generated by the truncated mRNA
To study the functions of the EPCR isoform, by comparison with those of the plasma sEPCR derived from ADAM17 cleavage, we expressed both recombinant proteins in eukaryotic cells.
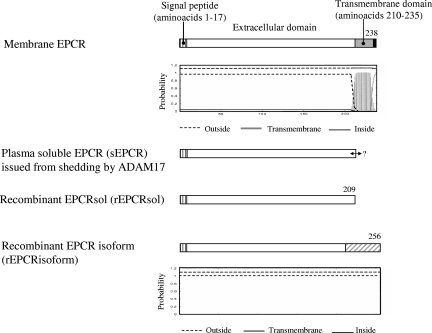
The schematic structures of the recombinant proteins we prepared are shown in Figure 4, by comparison with membrane EPCR and plasma sEPCR. Because the C-terminal amino acid of plasma sEPCR derived from metalloprotease cleavage is unknown, we used the TMHMM program to choose the cDNA region that needed to be deleted to obtain a recombinant EPCR (rEPCR) mimicking the plasma sEPCR. The program predicted a potential transmembrane helix beginning at amino acid 210 (Figure 4). We thus prepared a cDNA encoding EPCR amino acids 1 to 209, using site-directed mutagenesis to delete the sequence encoding amino acids 210 to 238.
Figure 4.
Recombinant proteins prepared in this study and comparison with membrane EPCR and plasma sEPCR resulting from shedding by ADAM17. The amino acid numbering starts at Met + 1. The signal peptide (hatched stretch) that is cut before protein addressing to the membrane (EPCR and sEPCR) or before protein secretion (rEPCRsol and rEPCRisoform) comprises amino acids 1 to 17. The C-terminal amino acid of plasma EPCR (sEPCR) is unknown (↔). The gray stretch denotes the putative transmembrane domain predicted by TMHMM Server, version 2.0 (panel below Membrane EPCR). The hatched gray stretch corresponds to the new C-terminal part of EPCRisoform (relative to membrane EPCR and sEPCR) lacking the transmembrane domain, owing to mRNA truncation.
Preparation of the cDNA encoding the EPCR isoform required a special cloning step to insert the DNA sequence encoding the 56 new C-terminal amino acids of the rEPCRisoform (see “Cloning of a recombinant EPCR isoform encoded by the truncated mRNA” for further details). The final construct encoded the signal peptide, amino acids 18 to 200 of the wild-type sequence, and a new C-terminal part consisting of 56 amino acids.
After stable transfection of HEK293 cells by the constructs, high-producing clones were selected and the rEPCRsol and rEPCRisoform recombinants were purified from conditioned media. Using flow cytometry, we observed that cells transfected with pCI-neoEPCRsol and pCI-neoEPCRisoform expressed the same amount of membrane EPCR as nontransfected cells, indirectly indicating that the 2 constructs were not partially targeted to the membrane (data not shown).
Characterization of the recombinant proteins
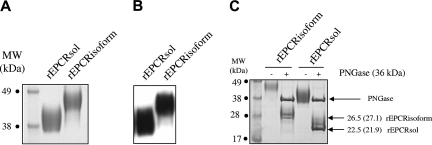
The purified recombinant proteins were analyzed by SDS-PAGE. They migrated as a single band in both reducing and non reducing conditions (Figure 5A; reducing conditions not shown), showing their homogeneity and purity. Both purified proteins were recognized by a polyclonal anti-EPCR antibody on Western blots (Figure 5B).
Figure 5.
SDS-PAGE and Western blot analysis of native forms of rEPCRsol and rEPCRisoform, and SDS-PAGE of deglycosylated rEPCRsol and rEPCRisoform. (A) A total of 2.5 μg rEPCRsol and rEPCRisoform was analyzed by SDS-12% PAGE in reducing conditions. (B) Western blot was performed using 0.5 μg recombinant protein per lane. Recombinant proteins were transferred to nitrocellulose membranes on a semidry apparatus, and proteins were detected with a goat polyclonal biotinylated antibody raised against human EPCR; binding was revealed with HRP-labeled streptavidin. (C) SDS-PAGE of rEPCRsol and rEPCRisoform deglycosylated by PNGase F, and determination of polypeptide chains MW. The theoretical MWs based on the number of amino acids are indicated in parentheses.
N-terminal sequencing of purified rEPCRsol confirmed its homogeneity and showed that the signal peptide was correctly removed by cleavage. Indeed, the N-terminal sequence was SQDA and corresponded to amino acids 18 to 21 of the sequence described by Fukudome and Esmon.3 Importantly, secretion of rEPCRsol demonstrated that the functional signal peptide corresponds to positions 1 to 17 of the EPCR precursor, something that until now was only suspected, on the basis of sequence alignments.
Theoretically, these constructs permit the synthesis of a 192-amino acid polypeptide (mature rEPCRsol without the signal peptide) and a 239-amino acid polypeptide (mature rEPCRisoform without the signal peptide).
The apparent molecular masses (Mrs) of rEPCRisoform and rEPCRsol were 48 kDa and 42 kDa, respectively, as determined by SDS-PAGE. The Mr of rEPCRsol was consistent with that reported in the literature (43 kDa) for plasma sEPCR.9 The Mrs of the 2 recombinants were higher than predicted from the amino acid compositions, at 27.1 kDa (239-amino acid polypeptide corresponding to the mature rEPCRisoform) and 21.9 kDa (molecular weight of the 192-amino acid polypeptide corresponding to mature rEPCRsol). Because rEPCRisoform contains an additional potential N-glycosylation site (at Asn 230) relative to rEPCRsol, we checked that the observed difference in Mrs was not due to glycosylation. As expected, treatment with endoglycosidase-F reduced the molecular masses of both recombinants, to 26.5 and 22.5 kDa for deglycosylated rEPCRisoform and rEPCRsol, respectively, in keeping with the Mrs predicted from the amino acid compositions of the recombinants. For both deglycosylated proteins, we repeatedly observed an additional band over the main deglycosylated form, which appeared to be sharper for rEPCRisoform and remained as a faint band for rEPCRsol. This higher Mr band did not disappear by increasing the amount of PNGase or by increasing the incubation duration. This suggests the presence of a glycosylation chain not accessible for the PNGase F in both constructs.
Binding and inhibitory properties of the recombinant proteins
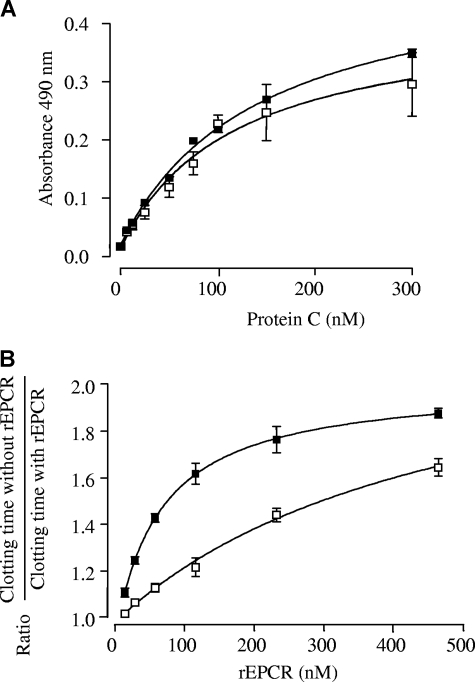
To probe the conformations of the 2 recombinant proteins, we took advantage of a commercial ELISA (Asserachrom sEPCR kit), which uses a monoclonal capture antibody recognizing the EPCR region interacting with APC/PC. The Kdapp of rEPCRsol and rEPCRisoform for this mAb was of the same magnitude (90 and 125 nM, respectively), suggesting that both proteins are able to interact with PC/APC. We then used a solid-phase assay to compare the affinity of rWT PC for the 2 recombinants. The curves of rWT PC binding to immobilized rEPCRsol and rEPCRisoform are shown in the top panel of Figure 6. They yielded Kdapp values of 142 plus or minus 11 nM and 134 plus or minus 30 nM for rEPCRsol and rEPCRisoform, respectively. The same experiment performed with rWT APC yielded Kdapp values of 112 plus or minus 17 nM and 92 plus or minus 10 nM for rEPCRsol and rEPCRisoform, respectively (data not shown).
Figure 6.
Binding of rWT PC to rEPCRsol and rEPCRisoform, and effect of rEPCRsol and rEPCRisoform on the plasma clotting time. The results in each panel are the mean of at least 3 separate experiments. (A) Binding of rWT PC to rEPCRsol (■) and to rEPCRisoform (□) in the presence of CaCl2 and MgCl2. REPCRsol and rEPCRisoform were captured by 400 ng of immobilized polyclonal antibody to EPCR. Various dilutions of rWT PC were added to the wells in the presence of 1.3 mM Ca2+ and 0.6 mM Mg2+, and bound rWT PC was detected with a peroxidase-conjugated anti-PC polyclonal antibody. Solid curves were obtained by nonlinear regression analysis.30 They yielded Kdapp values of 142 nM and 134 nM for rEPCRsol and rEPCRisoform, respectively. The data represent values (mean ± SD) of 4 separate experiments. The standard deviation is not apparent at all points because they are sometimes smaller than the symbols. (B) Increasing concentrations of rEPCRsol (■) and rEPCRisoform (□) were incubated for 2 minutes at 37°C with APC (1.8 nM, final concentration) in the presence of phospholipid vesicles, RVV-X, and PC-deficient plasma. Clotting was initiated by adding 14 mM CaCl2 (final concentration), and the clotting time was recorded by an ST4 analyzer. The APC-inhibiting effect of the 2 recombinant proteins was expressed as the following ratio: Clotting time in the presence of APC alone/Clotting time in the presence of both APC and rEPCRsol or rEPCRisoform. The standard deviations are not visible at all points because they are sometimes smaller than the symbols.
We tested the effect of increasing amounts of rEPCRsol and rEPCRisoform in a one-stage factor Xa clotting assay. Clotting times in the presence of 0 to 1.8 nM rWT APC ranged between 62 and 200 seconds. Results obtained with 1.83 nM rWT APC were expressed as a ratio between the clotting time in the absence of sEPCR and the clotting times in the presence of increasing amounts of sEPCR. As shown in the bottom panel of Figure 6, rEPCRsol and rEPCRisoform both shortened the clotting time in a concentration-dependent manner. Ratios ranged from 0.88 to 1.00 when similar concentrations of rEPCRsol and rEPCRisoform were used in the absence of rWT APC (data not shown). The rEPCRisoform appeared to have a lesser inhibitory effect than rEPCRsol.
Detection of the EPCR isoform in plasma
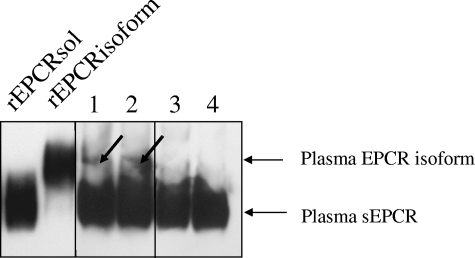
We used ion-exchange chromatography to concentrate the proteins from 200 mL of pooled plasma from A3-carrying subjects and A3 noncarriers. We then purified circulating EPCR on an immunoaffinity column. Because the amounts of sEPCR were very small, we measured the sEPCR concentrations in the elution fractions with a home-made ELISA. The fractions were concentrated by lyophilization before being submitted to Western blot analysis (Figure 7). The migration patterns of the fractions were compared with those of purified rEPCRsol and rEPCRisoform. To obtain sEPCR bands of similar intensity, we loaded protein contained in 2.5 mL of A3-carrying subjects and in 5 mL of A3-noncarrying subjects. sEPCR corresponding to rEPCRsol was present in all fractions. A band migrating at the same level as rEPCRisoform was detected in the 2 fractions of A3-carrying subjects, attesting the presence of sEPCR isoform in the plasma of these subjects.
Figure 7.
Detection of sEPCR in plasma of A3-carrying subjects. Western blot of 2 JRK-1496 column elution fractions, each corresponding to the plasma EPCR forms contained in 2.5 mL of a pool of plasma from A3-carrying subjects (lanes 1 and 2) and of 2 JRK-1496 column elution fractions each corresponding to the plasma EPCR forms contained in 5 mL of pooled plasma from A3-noncarrying subjects (lanes 3 and 4) The 2 lanes on the left were loaded with recombinant rEPCRsol and rEPCRisoform, respectively, used as controls. The membrane were blocked, treated with goat anti-EPCR biotinylated antibody, washed, and then incubated with HRP-conjugated streptavidin. Bands were visualized with enhanced chemiluminescence (Pierce Chemical, Rockford, IL). Vertical lines have been inserted to indicate repositioned gel lanes, and the arrows point to the plasma EPCR isoform.
Discussion
We and others have previously reported that elevated levels of plasma sEPCR are associated with the A3 haplotype of the EPCR gene.13,15,16 This haplotype results from linkage disequilibrium of 14 intragenic polymorphisms, including 4 haplotype-tagging SNPs. The A3 haplotype is characterized by the combination of G/C/A/G at positions 1651, 3610, 4216, and 6936, respectively.15 The latter leads to the replacement of a Ser by a Gly in the transmembrane domain and has been shown to increase EPCR shedding19 by the metalloprotease ADAM17,18 providing an explanation for the elevated sEPCR levels observed in A3 subjects. However, in vitro experiments with cells carrying the A3 haplotype showed that metalloprotease inhibitors failed to completely inhibit sEPCR production, suggesting that another mechanism might contribute to this production.17 Such a double mechanism has already been described for the production of soluble cytokine receptors24,33 (eg, IL6Rα), with simultaneous proteolytic cleavage of the receptor ectodomain and alternative mRNA splicing.23
We studied the mRNA forms in HEK293 and EA.hy926 cells, which we have previously shown to be A1/A2 and A2/A3, respectively. We also studied endothelial cells from 32 human umbilical cord veins, of which 6 carried the A3 haplotype in the heterozygous state. A 390-bp truncated form of EPCR mRNA was clearly detected in all A3 cells, whereas only traces were found in other cells. Interestingly, we also detected the truncated form of EPCR mRNA in hepatocytes. Quantitative real-time PCR revealed that this truncated mRNA was 16 times more abundant in A3 cells than in non-A3 cells, corresponding to 3.2% of total EPCR mRNA in A3 cells in basal conditions. This mRNA level is compatible with the synthesis of a significant amount of protein. An explanation to the increased mRNA splicing associated with the A3 haplotype might be that nucleotide 6936 belongs to an enhancer splicing element, which is more efficient with a G (corresponding to the A3 haplotype) than with an A (A1 and A2 haplotypes). This is currently under investigation.
Sequencing revealed that this truncated mRNA encoded a protein lacking the transmembrane and intracellular domains and possessing a theoretical new C-terminal stretch of 56 amino acids. Compared with the sEPCR form derived from ADAM17 cleavage, this new EPCR soluble form contains approximately 47 additional amino acids compared with plasma sEPCR. We checked that the protein encoded by this truncated mRNA form was synthesized and secreted, by transfecting HEK293 cells with a cDNA encoding “rEPCRisoform”: the protein was secreted into the culture medium, and flow cytometry showed that it was not retained on the cell membrane. This ruled out the formation of a neo-transmembrane domain, which the transmembrane prediction program had failed to identify. The molecular weight difference between the rEPCRisoform and a recombinant protein (rEPCRsol) mimicking the natural sEPCR was clearly shown by SDS-PAGE analysis of the 2 purified recombinant proteins, confirming that rEPCRisoform contains an elongated C-terminal part.
Because PC interaction with EPCR involves the N-terminal part of the receptor (the 2 alpha domains, α1 and α2),34 PC is theoretically able to bind to sEPCRisoform because its N-terminal part is the same as that of sEPCR. This interaction with APC would reduce the capacity of APC to inactivate factor Va. RWT PC bound similarly to rEPCRisoform and to rEPCRsol, with Kdapp values of 134 and 142 nM, respectively, suggesting no major conformational change in the N-terminal part of the protein and no steric hindrance due to the presence of the elongated C-terminal tail of rEPCRisoform. As expected, the affinity of rWT APC for rEPCRsol and rEPCRisoform was similar to the affinity of rWT PC for the 2 recombinant EPCR forms. In addition, in a one-stage FXa assay, rEPCRisoform was also able to inhibit factor Va inactivation by APC, albeit slightly less efficiently than rEPCRsol. Finally, Western blot analysis detected traces of sEPCR isoform in plasma of subjects heterozygous for the A3 haplotype. We could not determine the respective amounts of sEPCRisoform and sEPCR derived from membrane EPCR cleavage because no antibody specific for the C-terminal part of sEPCRisoform is available. Such an antibody is being prepared.
Our results suggest that, in addition to shedding, the soluble form of EPCR is also produced by alternative splicing in A3-carrying cells, pointing to the existence of 2 independent and mutually compatible mechanisms regulating sEPCR release. This situation looks similar to that of other soluble receptors, such as sIL6, sIL2, sIL4, and sTNFα receptors, which originate from different simultaneous mechanisms.24,33
The existence of this new sEPCR isoform may have implications for coagulation and inflammation because EPCR is located at the interface of these 2 pathways. sEPCR has mainly procoagulant properties.10–12 Several studies have shown that increased plasma sEPCR is associated with common disease states and is often taken as a marker of endothelial cell activation.35 Given that the levels of plasma EPCR are apparently governed by 2 separate mechanisms, shedding and alternative splicing, interpreting the basis for these changes in plasma EPCR levels would benefit by determining the relative concentrations of the 2 forms of sEPCR. It remains to be shown how the synthesis of the full-length and truncated mRNA is regulated. We cannot exclude that, in some pathologic situations, one mechanism predominates over the other one; and if alternative splicing is stimulated, this stimulation might be more efficient in A3-carrying subjects. On another hand, the association of the A3 haplotype with deep venous thrombosis is a controversial issue,13,15,16 probably because of the wide range of sEPCR levels (135-800 ng/mL) observed in A3 carriers, with only those with higher levels being at risk for thrombosis. Because the risk associated with the elevated plasma EPCR remained controversial, it would seem quite important to determine the levels of the 2 forms in patients with the A3 haplotype with and without thrombosis. This hypothesis should also be tested in the case of the acute phase of arterial or venous thromboses, which have not been tested so far because patients we tested were explored at distance of the thrombotic episode.15 The latter studies might provide insights into whether the alternatively spliced form of EPCR had unique activities.
EPCR is also involved in inflammation, as APC binding to EPCR triggers signal transduction via PAR-1 cleavage36–38 (explaining the antiapoptotic and anti-inflammatory effects of APC) or via Edg-1 transactivation39,40 (explaining the cytoprotective effects of APC). sEPCR appears to have anti-inflammatory properties, as it is able to bind Mac-141 and thereby to inhibit neutrophils rolling and adhesion. It would be of interest to determine whether sEPCRisoform inhibits neutrophils rolling adhesion as efficiently as the ADAM17 cleaved form after binding to Mac-1.
sEPCR generation through 2 different mechanisms could be a means of regulation: sEPCR derived from ADAM17 cleavage being generated rapidly in response to a stimulus and sEPCRisoform being produced more slowly by alternative splicing and providing a source of sEPCR once all membrane-bound EPCR has been cleaved.
By highlighting the fact that 2 different mechanisms are responsible for sEPCR production, this work lays the basis for further studies attempting to evaluate the role of sEPCR levels in different pathologic situations. It now remains to be determined how the production of EPCRisoform is regulated and to discover whether this alternative splicing is as important for sEPCR function as it is for interleukin receptor/soluble receptor regulatory pathways.
Acknowledgments
The authors thank Gary Ferrell for preparing and providing the JRK1496 anti-EPCR MoAb, Dr Cora-Jean Edgell (University of North Carolina, Chapel Hill, NC) who kindly provided the EA.hy926 cells, and Stago Laboratories for providing the Asserachrom sEPCR kit and the PC-deficient plasma.
This study was supported by a Transatlantique Network for Excellence in Cardiovascular Research grant from Fondation Leducq, Paris, France.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part at the XXIst Congress of the International Society on Thrombosis and Haemostasis (Geneva, 2007) and in abstract form (OW91) in J Thromb Haemost. 2007;5(Suppl 2:CD-ROM/).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.S. and S.G. designed the study, performed molecular biology qualitative and quantitative studies, cloned the full-length EPCR cDNA, expressed, purified, and performed functional studies of the recombinant proteins, analyzed the data, and wrote the manuscript; E.L. prepared the two cDNA to express soluble forms of EPCR; A.L. prepared HUVECs; and M.A. and C.T.E. contributed to manuscript writing and scientific discussions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Gandrille, Inserm U765, UFR des sciences Pharmaceutiques et Biologiques, 4, Avenue de l'Observatoire, Paris 75006, France; e-mail: sophie.gandrille@univ-paris5.fr.
References
- 1.Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006;13:382–385. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- 2.Laszik Z, Mitro A, Taylor FB, Jr, Ferrell G, Esmon CT. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 3.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 4.Taylor FB, Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685–1688. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
- 5.Gu JM, Crawley JT, Ferrell G, et al. Disruption of the endothelial cell protein C receptor gene in mice causes placental thrombosis and early embryonic lethality. J Biol Chem. 2002;277:43335–43343. doi: 10.1074/jbc.M207538200. [DOI] [PubMed] [Google Scholar]
- 6.Villoutreix BO, Blom AM, Dahlbäck B. Structural prediction and analysis of endothelial cell protein C/activated protein C receptor. Protein Eng. 1999;12:833–840. doi: 10.1093/protein/12.10.833. [DOI] [PubMed] [Google Scholar]
- 7.Simmonds RE, Lane DA. Structural and functional implications of the intron/exon organization of the human endothelial cell protein C/activated protein C receptor (EPCR) gene: comparison with the structure of CD1/major histocompatibility complex alpha1 and alpha2 domains. Blood. 1999;94:632–641. [PubMed] [Google Scholar]
- 8.Hayashi T, Nakamura H, Okada A, et al. Organization and chromosomal localization of the human endothelial protein C receptor gene. Gene. 1999;238:367–373. doi: 10.1016/s0378-1119(99)00360-1. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa S, Stearns-Kurosawa DJ, Hidari N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J Clin Invest. 1997;100:411–418. doi: 10.1172/JCI119548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukudome K, Kurosawa S, Stearns-Kurosawa DJ, He X, Rezaie AR, Esmon CT. The endothelial cell protein C receptor: cell surface expression and direct ligand binding by the soluble receptor. J Biol Chem. 1996;271:17491–1748. doi: 10.1074/jbc.271.29.17491. [DOI] [PubMed] [Google Scholar]
- 11.Liaw PC, Neuenschwander PF, Smirnov MD, Esmon CT. Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J Biol Chem. 2000;275:5447–5452. doi: 10.1074/jbc.275.8.5447. [DOI] [PubMed] [Google Scholar]
- 12.Regan LM, Stearns-Kurosawa DJ, Kurosawa S, Mollica J, Fukudome K, Esmon CT. The endothelial cell protein C receptor: inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors. J Biol Chem. 1996;271:17499–17503. doi: 10.1074/jbc.271.29.17499. [DOI] [PubMed] [Google Scholar]
- 13.Uitte de Willige S, Van Marion V, Rosendaal FR, Vos HL, de Visser MC, Bertina RM. Haplotypes of the EPCR gene, plasma sEPCR levels and the risk of deep venous thrombosis. J Thromb Haemost. 2:1305–1310. doi: 10.1046/j.1538-7836.2004.00855.x. 200. [DOI] [PubMed] [Google Scholar]
- 14.Stearns-Kurosawa DJ, Burgin C, Parker D, Comp P, Kurosawa S. Bimodal distribution of soluble endothelial protein C receptor levels in healthy populations. J Thromb Haemost. 2003;1:855–856. doi: 10.1046/j.1538-7836.2003.t01-4-00115.x. [DOI] [PubMed] [Google Scholar]
- 15.Saposnik B, Reny JL, Gaussem P, Emmerich J, Aiach M, Gandrille S. A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis. Blood. 2004;103:1311–1318. doi: 10.1182/blood-2003-07-2520. [DOI] [PubMed] [Google Scholar]
- 16.Medina P, Navarro S, Estelles A, et al. Contribution of polymorphisms in the endothelial protein C receptor gene to soluble endothelial protein C receptor and circulating activated protein C levels, and thrombotic risk. Thromb Haemost. 2004;91:905–911. doi: 10.1160/TH03-10-0657. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Qu D, Esmon NL, Esmon CT. Metalloproteolytic release of endothelial cell protein C receptor. J Biol Chem. 2000;275:6038–6044. doi: 10.1074/jbc.275.8.6038. [DOI] [PubMed] [Google Scholar]
- 18.Qu D, Wang Y, Esmon NL, Esmon CT. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-α converting enzyme/ADAM17. J Thromb Haemost. 2007;5:395–402. doi: 10.1111/j.1538-7836.2007.02347.x. [DOI] [PubMed] [Google Scholar]
- 19.Qu D, Wang Y, Song Y, Esmon NL, Esmon CT. The Ser219->Gly dimorphism of the endothelial protein C receptor contributes to the higher soluble protein levels observed in individuals with the A3 haplotype. J Thromb Haemost. 2006;4:229–235. doi: 10.1111/j.1538-7836.2005.01676.x. [DOI] [PubMed] [Google Scholar]
- 20.Villegas-Mendez A, Montes R, Ambrose LR, Warrens AN, Laffan M, Lane DA. Proteolysis of the endothelial cell protein C receptor by neutrophil proteinase 3. J Thromb Haemost. 2007;5:980–988. doi: 10.1111/j.1538-7836.2007.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmon CT. Coagulation and inflammation. J Endotoxin Res. 2003;9:192–198. doi: 10.1179/096805103125001603. [DOI] [PubMed] [Google Scholar]
- 22.Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med. 2004;32(suppl):S298–S301. doi: 10.1097/01.ccm.0000126128.64614.81. [DOI] [PubMed] [Google Scholar]
- 23.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 24.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 25.European Bioinformatics Institute of the European Molecular Biology Laboratory. [Accessed June 2004]; http://www.ebi.ac.uk/cgi-bin/expasy.
- 26.Dupont A, Fontana P, Bachelot-Loza C, et al. An intronic polymorphism in the PAR-1 gene is associated with platelet receptor density and the response to SFLLRN. Blood. 2003;101:1833–1840. doi: 10.1182/blood-2002-07-2149. [DOI] [PubMed] [Google Scholar]
- 27.Morboeuf O, Borgel D, Aiach M, Kaabache T, Gandrille S, Gaussem M. Expression and characterization of recombinant protein S with the Ser 460 Pro mutation. Thromb Res. 2000;100:81–88. doi: 10.1016/s0049-3848(00)00296-6. [DOI] [PubMed] [Google Scholar]
- 28.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 29.Saposnik B, Borgel D, Aiach M, Gandrille S. Functional properties of the sex-hormone-binding globulin (SHBG)-like domain of the anticoagulant protein S. Eur J Biochem. 2003;270:545–555. doi: 10.1046/j.1432-1033.2003.03423.x. [DOI] [PubMed] [Google Scholar]
- 30.Le Bonniec BF, Guinto ER, Esmon CT. Interaction of thrombin des-ETW with antithrombin III, the kunitz inhibitors, thrombomodulin and protein C. Structural link between the autolysis loop and the Tyr-Pro-Pro-Trp insertion of thrombin. J Biol Chem. 1992;267:19341–19348. [PubMed] [Google Scholar]
- 31.Smirnov MD, Safa O, Esmon NL, Esmon CT. Inhibition of activated protein C anticoagulant activity by prothrombin. Blood. 1999;94:3839–3846. [PubMed] [Google Scholar]
- 32.Saller F, Villoutreix BO, Amelot A, et al. The γ-carboxyglutamic acid domain of anticoagulant protein S is involved in activated protein C cofactor activity, independently of phospholipid binding. Blood. 2005;105:122–130. doi: 10.1182/blood-2004-06-2176. [DOI] [PubMed] [Google Scholar]
- 33.Lainez B, Fernandez-Real JM, Romero X, et al. Identification and characterization of a novel spliced variant that encodes human soluble tumor necrosis factor receptor 2. Int Immunol. 2004;16:169–177. doi: 10.1093/intimm/dxh014. [DOI] [PubMed] [Google Scholar]
- 34.Liaw PC, Mather T, Oganesyan N, Ferrell GL, Esmon CT. Identification of the protein C/activated protein C binding sites on the endothelial cell protein C receptor: implications for a novel mode of ligand recognition by a major histocompatibility complex class 1-type receptor. J Biol Chem. 2001;276:8364–8370. doi: 10.1074/jbc.M010572200. [DOI] [PubMed] [Google Scholar]
- 35.Kurosawa S, Stearns-Kurosawa DJ, Carson CW, D'Angelo A, Della Valle P, Esmon CT. Plasma levels of endothelial cell protein C receptor are elevated in patients with sepsis and systemic lupus erythematosus: lack of correlation with thrombomodulin suggests involvement of different pathological processes. Blood. 1998;91:725–727. [PubMed] [Google Scholar]
- 36.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 37.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signalling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 38.Brueckmann M, Horn S, Land S, et al. Recombinant human activated protein C upregulates cyclooxygenase-2 expression in endothelial cells via binding to endothelial cell protein C receptor and activation of protease-activated receptor-1. Thromb Haemost. 2005;93:743–750. doi: 10.1160/TH04-08-0511. [DOI] [PubMed] [Google Scholar]
- 39.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 40.Feitstritzer C, Schuepbach RA, Mosnier LO, et al. Protective signalling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 41.Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The soluble endothelial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697–4703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]