Abstract
Whether T-cell antigen receptors (TCR) on donor T cells require direct interactions with major histocompatibility complex class I or class II (MHCI/MHCII) molecules on target cells to mediate graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) is a fundamental question in allogeneic stem-cell transplantation (alloSCT). In MHC-mismatched mouse models, these contacts were not required for GVHD. However, this conclusion may not apply to MHC-matched, multiple minor histocompatibility antigen-mismatched alloSCT, the most common type performed clinically. To address this, we used wild-type (wt)→MHCI−/− or wt→MHCII−/− bone marrow chimeras as recipients in GVHD experiments. For GVL experiments, we used MHCI−/− or MHCII−/− chronic-phase CML cells created by expressing the BCR-ABL cDNA in bone marrow from MHCI−/− or MHCII−/− mice. TCR/MHCI contact was obligatory for both CD8-mediated GVHD and GVL. In contrast, CD4 cells induced GVHD in wt→MHCII−/− chimeras, whereas MHCII−/− mCP-CML was GVL-resistant. Donor CD4 cells infiltrated affected skin and bowel in wt→MHCII−/− recipients, indicating that they mediated GVHD by acting locally. Thus, CD4 cells use distinct effector mechanisms in GVHD and GVL: direct cytolytic action is required for GVL but not for GVHD. If these noncytolytic pathways can be inhibited, then GVHD might be ameliorated while preserving GVL.
Introduction
Allogeneic hematopoietic stem-cell transplantation (alloSCT) is a potentially curative therapy for hematologic malignancies, inherited disorders of blood cells including sickle cell anemia, and acquired nonmalignant diseases such as aplastic anemia. Mature donor T cells in allografts play 2 important functions. First, they are pivotal for reconstituting T-cell immunity, particularly in adult patients who have incomplete and delayed generation of progenitor-derived T cells resulting from age-dependent thymic involution and damage to the thymus by conditioning regimens.1–3 Second, they mediate a potent antineoplastic effect called graft-versus-leukemia (GVL).4 Unfortunately, donor T cells can also broadly attack the recipient in a process called graft-versus-host disease (GVHD). Because of GVHD, all patients receive some form of prophylactic immunosuppression. Nonetheless, GVHD and the infectious complications of immunosuppression remain major sources of morbidity and mortality, which prevent the more widespread application of alloSCT. A detailed mechanistic understanding of how T cells mediate GVHD and GVL will be essential for developing strategies for minimizing GVHD and for maximizing GVL and immune reconstitution.
A fundamental question regarding how T cells mediate GVHD is whether cognate interactions between T-cell antigen receptors and major histocompatibility complex (MHC) on nonhematopoietic target tissues (eg, skin, liver, and bowel) are required. Prior work by Teshima et al has suggested that CD4-mediated GVHD, and to a lesser extent CD8-mediated GVHD, do not require such direct interactions.5 In these experiments, GVHD and death were at least in part cytokine-mediated.
However, these conclusions were drawn from rapidly lethal GVHD models in which the donor and recipient were MHC-mismatched. This is in contrast to the majority of human alloSCTs in which donor and recipient MHC are matched or genotypically identical. MHC-mismatched and MHC-matched alloSCTs differ in the identities of the antigens targeted by donor alloreactive T cells. In MHC-mismatched GVHD, T-cell receptors (TCRs) on donor T cells at least in part directly recognize intact recipient MHC.6–8 In contrast, alloreactive T cells in MHC-matched alloSCT recognize minor histocompatibility antigens (miHAs), which are the peptide products of polymorphic genes that distinguish recipients from donors, conventionally presented by MHC molecules.4,9 A consequence of targeting MHC vs miHAs is that the precursor frequency of T cells that recognize MHC is as high as 1% to 10% of T cells, much greater than the estimated 1 in 104 to 1 in 106 T cells that recognize peptide antigens, such as miHAs. Consequently, when T cells are transferred as part of a MHC-disparate alloSCT, a large number synchronously activate and expand, which results in elaboration of cytokines in sufficient quantities to result in death.5,10 In such models, death can occur with few T cells infiltrating target tissues, consistent with the idea that cytokines act at a distance in an endocrine fashion. However, in MHC-matched, multiple miHA-mismatched alloSCTs, because fewer alloreactive T cells are transferred, mice (and humans) typically survive this early burst of cytokines and GVHD is instead primarily manifest by T-cell infiltration of target tissues. Thus, it is possible that in this setting T cells require cognate interactions with target tissues to cause GVHD.
Another implication of the studies by Teshima et al5 was that parenchymal alloantigen itself is not even required for GVHD because in their experiments both donors and recipients were on the B6 background and differed only at loci within the MHC gene complex. The requirement for parenchymal miHA expression has been addressed in MHC-matched murine allogeneic bone marrow transplant (alloBMT) models wherein host→donor bone marrow (BM) chimeras, in which only hematopoietic cells in the recipient are allogeneic, were used as recipients in a second transplant with GVHD-inducing CD4 or CD8 cells.11,12 These chimeras were resistant to both CD4- and CD8-mediated GVHD, clearly demonstrating that allogeneic recipient hematopoietic cells alone are not sufficient for miHA-directed GVHD and that parenchymal tissues must express alloantigen. However, these experiments did not address whether donor T cells must make cognate interactions with recipient parenchyma; rather, they demonstrate that miHA-expressing parenchyma is required.
To determine whether donor T cells require cognate interactions with recipient parenchyma to induce GVHD in MHC-matched alloBMT, we used wild-type (wt) B6→B6 beta-2-microblobulin gene-deficient (β2M−/− and therefore MHCI−) and wt B6→B6 IAb beta chain gene-deficient (IAbβ−/− and therefore MHCII−) BM chimeras as recipients in a second transplant in which GVHD was induced by purified donor CD8 or CD4 cells. We also studied whether cognate interactions are required for CD4- and CD8-mediated GVL in the same strain pairings.
Methods
Mice
Mice were between 7 and 10 weeks of age. C3H.SW (H-2b) mice were originally purchased from The Jackson Laboratories (Bar Harbor, ME) and bred at Yale University. 129S1/SvImJ/Cr (129), C57BL6 (B6), and B6 CD45.1 congenic mice were obtained from the National Cancer Institute (Frederick, MD). IAb beta chain deficient (IAbβ−/−) mice were obtained from Taconic Farms (Germantown, NY). β2M−/− mice were obtained from Jackson ImmunoResearch Laboratories.
Cell purification
CD8 cells were purified from lymph nodes (LN) via negative selection as previously described13 using biotin-conjugated antibodies against CD4 (clone GK1.5; laboratory-prepared), B220 (clone 6B2; laboratory-prepared), CD11c (clone HL3; BD PharMingen, San Diego, CA), and CD11b (clone M1/70; BD PharMingen), followed by streptavidin-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA) and separation on an autoMACS (Miltenyi Biotec). CD8 cells were more than 90% pure with CD4 T cell contamination of less than 0.2% (not shown). CD4 cells were similarly purified by negative selection, except that GK1.5 was omitted and biotin-conjugated anti-CD8 (TIB105; laboratory-prepared) was added to the antibody depletion cocktail. BM T cells were depleted with anti-Thy1.2 magnetic microbeads (Miltenyi Biotec) and the autoMACS.
GVHD transplant protocol
All transplants were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee.
Bone marrow chimeras
To create wt→β2m−/−, wt→wt, or wt→IAbβ−/− chimeras, recipient mice received 2 500-cGy fractions (separated by 3 hours) from a cesium irradiator and were reconstituted with 107 T cell–depleted (TCD) B6 CD45.1 BM. Two to 3 months later, chimeras were reirradiated (2 × 450 cGy) and reconstituted with TCD donor BM (C3H.SW or 129) with purified donor CD4+ or CD8+ T cells. Mice were weighed and scored for GVHD 2 to 3 times per week. Weights from mice that died or were killed because of GVHD morbidity were included in averages for subsequent time points at the last value recorded.
Analysis of dendritic cell engraftment
Spleens and LNs were digested with collagenase as described.13,14 To distinguish residual recipient (CD45.2+/MHCI− or MHCII−), and donor-derived B6 (CD45.1+MHCI+MHCII+) DCs in initial BM chimeras, preparations were stained with antibodies against CD45.1 or CD45.2 (FITC; clones A20 and 104; BD PharMingen), CD11c (APC; clone HL3; BD PharMingen), a cocktail of biotin-conjugated antibodies against Gr-1 (clone RB6-8C5; BD PharMingen), CD19 (BD PharMingen; clone 1D3), Ly76 (TER119; BD PharMingen), and Thy1.2 (clone 30H12; laboratory-prepared), and MHCI (PE; clone 2B-11-5S; BD PharMingen) or MHCII (PE; clone MS/114.15.2, BD PharMingen). Cells were washed and stained with streptavidin-PerCP (BD PharMingen). DCs were identified as being CD11c+/lineagelow/propidium iodide−.
Histologic analysis
Tissues were fixed in 10% phosphate-buffered formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Slides were read by pathologists expert in skin (J.M.) and gastrointestinal disease (D.J.) without knowledge as to experimental group as we have described.15 Images of bowel and liver were obtained with an Olympus BX40 microscope (Olympus America, Lakewood, CO) using a 10× eyepiece and an 20× objective, with a QImaging QColor5 camera (Olympus) and QCapture software (Olympus). Images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). Images of skin were obtained with an Olympus BX40 microscope (Olympus) using a 10× eyepiece, 20× objective, a Scanalytics SPOT RT Slider camera (model 2.3.1; Diagnostic Instruments, Sterling Heights, MI), SPOT soft-ware version 4.06 (Diagnostics Instruments) followed by processing with Adobe Photoshop 7.0.
Immunofluorescent staining
Tissues were fixed in 0.7% formaldehyde overnight followed by dehydration in 30% sucrose and freezing in Tissue-TeK OCT compound (Sakura Finetek, Torrance, CA); 7-μm sections were incubated overnight at 4°C with DAPI and antibodies against CD4 (Alexa647; clone GK1.5; laboratory-prepared) and MHCII (biotin, clone M5/114.15.2; BioLegend, San Diego, CA), followed by incubation with streptavidin-Alexa568 (Invitrogen, Carlsbad, CA). Sections were imaged with an Olympus BX40 microscope using a 10× eyepiece, 40× objective, a Scanalytics SPOT RT Slider camera (model 2.3.1, Diagnostic Instruments) using SPOT software version 4.06 (Diagnostics Instruments). Pictures were reconstituted with Adobe Photoshop 7. CD4 was rendered in green, MHCII in red, and DAPI in blue.
Retrovirus production
MSCV2.2 expressing the human bcr-abl p210 cDNA and a nonsignaling truncated form of the human low affinity nerve growth factor receptor (NGFR), driven by an internal ribosome entry site (Mp210/NGFR), was a gift of Warren Pear. Retroviral supernatants were generated by transfection of Plat-E retrovirus packing cell line16 as described.17–19
Progenitor infections
p210-infected progenitors were generated as previously described.13,17,19 Briefly, B6 mice were injected on day −6 with 5 mg 5-fluorouracil (Pharmacia & Upjohn, Kalamazoo, MI). On day −2, BM cells were harvested and cultured in prestimulation media (Dulbecco modified Eagle medium, 15% fetal bovine serum, interleukin-3 (6 ng/mL), interleukin-6 (10 ng/mL), and stem-cell factor (10 ng/mL; all cytokines from PeproTech; Rocky Hill, NJ). On days −1 and 0, cells underwent “spin infection” with the Mp210/NGFR retrovirus.19
GVL transplant protocol
On day 0, B6 hosts received 2 450-cGy fractions and were reconstituted with 5 × 106 TCD C3H.SW or 129 BM with 7 × 105 wt B6, B6 β2M−/−, or B6 IAbβ−/− BM cells that underwent spin infection, with or without 1.2 × 106 purified wt C3H.SW CD8+ T cells or 4 × 106 129 or C3H.SW CD4+ T cells. For CD8 GVL experiments, all recipients (including those that received wt mCP-CML) were injected with 200 μg intraperitoneally of anti-NK1.1 (PK-136; laboratory-prepared) on days −2, −1, and 7 to prevent NK cell–mediated rejection of β2M−/− mCP-CML. Peripheral blood was analyzed for the presence of NGFR+ cells by flow cytometry. Cause of death was determined by the presence of NGFR+ cells at the bleed before death and by spleen weight at necropsy.
Results
CD8-mediated GVHD
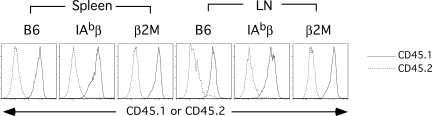
For these experiments, we used the MHC-matched C3H.SW (H-2b)→B6 (H-2b) strain pairing in which purified C3H.SW CD8+ T cells induce GVHD.13,14 To determine whether donor T cell TCR:target MHC I contacts are required for CD8-mediated GVHD across only miHAs, we wanted a recipient mouse with MHCI− parenchyma but with MHCI+ antigen presenting cells (APCs), which are required for GVHD in this model.14 We created such a mouse by making B6 CD45.1→B6 CD45.2 β2M−/− BM chimeras (wt→β2M−/−; Figure 1). We also created control B6 CD45.1→B6 CD45.2 chimeras (wt→wt; Figure 1). Cohorts of mice were killed between days 87 and 90 after alloBMT, and engraftment was analyzed in spleen and LN. Overall, splenocytes were 90% to 95% donor-derived in both sets of chimeras. In particular, dendritic cells (DCs) were well engrafted, with more than 95% of LN and splenic DCs being of donor origin (Figure 2). This confirmed that the wt→β2M−/− chimeras had sufficient wt APCs such that any differences in GVHD between the 2 sets of chimeras would not be attributable to APC composition.
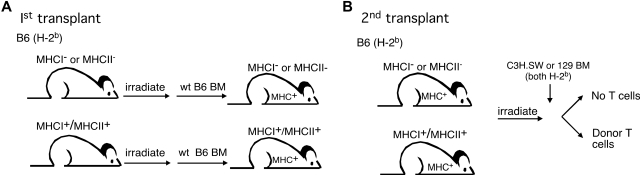
Figure 1.
Experimental design. (A) To create mice with MHCI− or MHCII− parenchyma but with MHC+ hematopoietic cells (including APCs), B6 β2M−/− or IAbβ−/− mice (both CD45.2) were irradiated and reconstituted with TCD wt B6 CD45.1 BM. (B) After 3 months to allow for engraftment of the wt B6 hematopoietic system, these chimeras were used as recipients in a second, GVHD-inducing alloBMT.
Figure 2.
Dendritic cells in B6 CD45.1→B6 β2M−/− or B6 IAbβ−/− are mostly donor in origin. Spleens and LNs were collagenase-treated, and cells were stained with a cocktail of lineage antibodies, anti-CD11c and anti-CD45.1 or CD45.2 (“Analysis of dendritic cell engraftment”). Shown is CD45.1 (——) and CD45.2 (----) staining of lineagelow CD11c+ cells from representative mice (at least 3 mice were analyzed from each group in each experimental repetition).
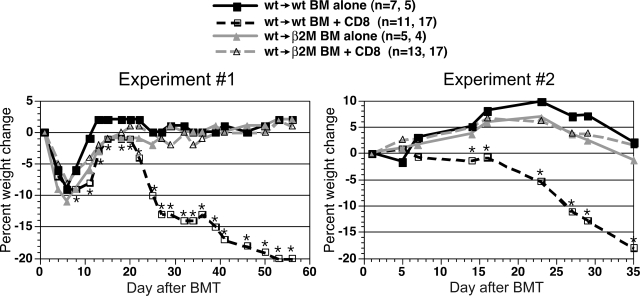
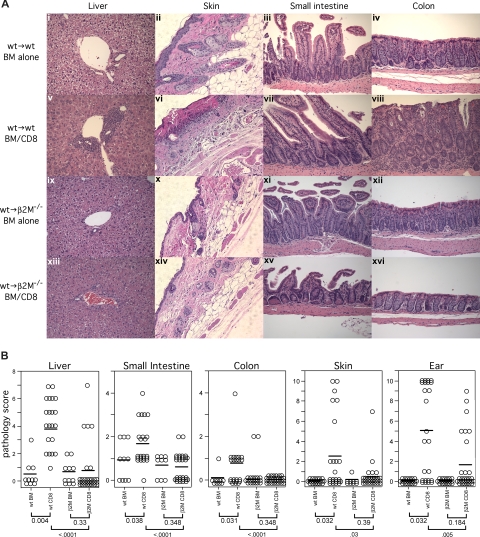
We then used these chimeras as recipients in a second GVHD-inducing alloBMT with C3H.SW donors (Figure 1). In 2 independent experiments, wt→β2M−/− chimeras were nearly completely resistant to CD8-mediated GVHD as measured by weight loss (Figure 3) and histologic analysis of GVHD target organs (Figure 4). We found no evidence of GVHD pathology in skin, liver, small intestine, and colon in wt→β2M−/− recipients of donor CD8 cells. In contrast, wt→wt recipients of donor CD8 cells developed significant GVHD in each organ (Figure 4). The only suggestion of GVHD in wt→β2M−/− CD8 recipients was low-penetrance GVHD of the ear, although, compared with control wt→β2M−/− mice that did not receive CD8 cells, scores did not reach statistical significance (P = .184). Thus, with the possible exception of the ear, donor CD8+ T cells absolutely required cognate interactions with MHCI on parenchyma to cause GVHD.
Figure 3.
Wt→β2M−/− chimeric recipients are resistant to CD8-mediated GVHD. Shown is percentage weight change versus day after alloBMT from 2 independent experiments. Numbers of mice per group in each repetition are listed (separated by commas; *P < .05, CD8 recipients vs recipients of only TCD C3H.SW BM). Weight change was not significantly different at any time point comparing wt→β2M−/− recipients of only C3H.SW TCD BM and TCD BM plus CD8 cells.
Figure 4.
Only wt→wt and not wt→β2M−/− chimeric recipients of C3H.SW CD8 cells develop histologic GVHD. (A) Representative histology (original magnification ×200). (B) Combined histology scores from 2 independent experiments. Each symbol is the score from an individual mouse; horizontal lines represent mean scores. P values are shown below the group labels.
To address the possibility that wt→β2M−/− mice did not develop GVHD because of poor donor CD8 cell engraftment, we enumerated splenic CD8 cells at the time of death (day 35). Hematopoietic cells in wt (CD45.1)→β2M−/− (CD45.2+) chimeras were mostly CD45.1+, which allowed us to distinguish CD45.1− C3H.SW-derived and residual CD45.1+ recipient CD8 cells. However, there were no Thy1.1 or CD45.1 congenic C3H.SW mice available that would have permitted us to use C3H.SW BM and CD8 cells that differed at one of these to allow us to distinguish infused C3H.SW CD8 cells from those derived from donor BM. However, by comparing donor CD8 cell numbers in recipients of only donor BM to recipients of BM and CD8 cells, an estimation of what is derived from infused mature CD8 cells can be made. Moreover, because thymic epithelial cells in wt→β2M−/− recipients do not express MHCI, we anticipated they would have a profound deficiency of BM-derived CD8 cells. As expected, we detected few if any donor-derived CD8 cells in wt→β2M−/− recipients of only C3H.SW BM. In contrast, in a representative GVHD experiment, wt→β2M−/− chimeric recipients of C3H.SW BM and CD8+ T cells had a mean of 1.6 × 106 (SD = 3.3 × 105; n = 15) splenic C3H.SW CD8+ cells, similar to that found in wt→wt recipients of CD8 cells (1.4 × 106; SD = 3.1 × 105; n = 15). These cells were mostly CD44+CD62L−, consistent with having been previously activated or having underwent lymphopenia-induced proliferation, making it highly probable that these were derived from infused mature CD8 cells. Thus, the failure of wt→β2M−/− chimeras to develop GVHD was not the result of poor engraftment of donor CD8+ T cells.
CD4-mediated GVHD
For these experiments, we used the MHC-matched, miHA-disparate 129 (H-2b)→B6 (H-2b) strain pairing. To determine whether donor T cell TCR:target MHCII interactions are required for CD4-mediated GVHD, we generated BM chimeras to create recipient mice in which the parenchyma was MHCII− but the hematopoietic cells, including APCs, were MHCII+. B6 CD45.2 IAbβ−/− and therefore MHCII-deficient mice were irradiated and reconstituted with B6 CD45.1 BM (wt→IAbβ−/−). As controls we also created B6 CD45.1→B6 CD45.2 (wt→wt) chimeras. Cohorts of these chimeric mice were killed between days 90 and 92 after BMT to assess donor B6 CD45.1+ hematopoietic engraftment. Overall, splenocytes were 90% to 92% donor-derived in wt→IAbβ−/− and wt→wt chimeras. In particular, DCs were well engrafted in both sets of chimeras, with > 95% of LN and splenic DCs being of donor origin (Figure 2). This confirmed that the wt→IAbβ−/− chimeras had sufficient wt APCs such that any differences in GVHD seen between the 2 sets of chimeras would not be attributable to APC composition.
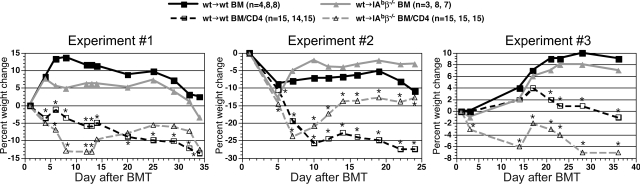
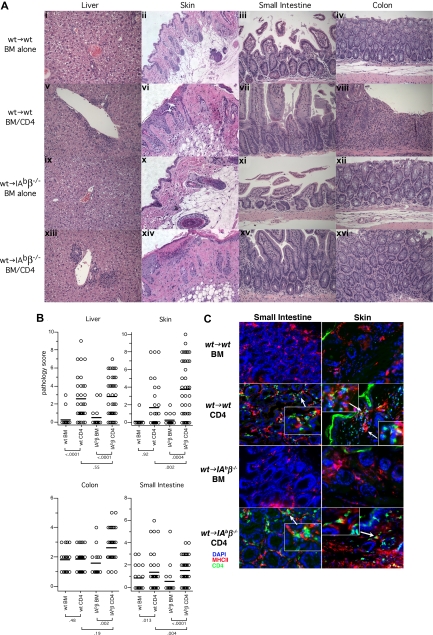
We then used these chimeras as recipients in a second GVHD-inducing alloBMT. In contrast to what we observed with CD8-mediated GVHD, donor CD4+ T cells induced clinical (Figure 5) and histologic GVHD (Figure 6) in wt→IAbβ−/− chimeras that was equivalent to, if not more severe than in, wt→wt chimeras. Compared with wt→IAbβ−/− recipients of only donor BM, wt→IAbβ−/− CD4 recipients had histologic GVHD of the liver, skin, small intestine, and colon. GVHD pathology in wt→IAbβ−/− CD4 recipients was equivalent to that in wt→wt CD4 recipients in the liver and colon and was more severe in the small intestine and skin (Figure 6). Thus, cognate interactions between donor T cells and MHCII on recipient parenchymal tissues were not required for CD4-mediated GVHD across only miHA differences.
Figure 5.
Both wtgwt and wt→IAbβ−/− donor CD4 recipients developed GVHD-induced weight loss. Shown is percentage weight change versus day after BMT from 3 independent experiments. Numbers of mice per group in each repetition are listed, separated by commas (*P < .05, CD4 recipients [either wt→wt or wt→IAbβ−/−] vs the appropriate recipients of only TCD 129 BM).
Figure 6.
Both wt→wt and wt→IAbβ−/− CD4 recipients develop histologic GVHD. (A) Representative histology (original magnification ×200). (B) Combined histology scores from 3 independent experiments. Each symbol is the score from an individual mouse; horizontal lines represent mean scores. P values are shown below the group labels. (C) Immunofluorescent staining of small intestine and skin from both wt→wt and wt→IAbβ−/− recipients of 129 CD4+ T cells. Blue indicates DAPI-stained nuclei; green, CD4+ cells; red, MHCII+ cells. Note infiltrating CD4+ cells in both bowel and skin of CD4 recipients, frequently adjacent to MHCII+ cells.
Grossly similar cellular infiltrates were present in hematoxylin and eosin-stained sections from GVHD target tissues in both wt→wt and wt→IAbβ−/− CD4 recipients (Figure 6), which we presume were pathogenic because they were absent in recipients of only donor BM. To confirm that CD4 cells were a component of the infiltrate, we performed immunofluorescent microscopy on skin and small intestine. We observed clear CD4+ infiltrates in skin and bowel of wt→IAbβ−/− and wt→wt recipients of CD4 cells, not present in BM alone controls (Figure 6C) or in unmanipulated wt mice (not shown). These CD4 cells were frequently adjacent to MHCII+ cells, suggesting that interactions between donor CD4 cells and MHCII+ cells, such as macrophages and tissue DCs, contribute to GVHD pathogenesis (see “Discussion”).
Role of cognate TCR/MHC interactions in GVL
Having established the roles of TCR/MHC interactions in GVHD, we investigated these requirements for T cells mediating GVL. To do so, we used a murine model of chronic-phase chronic myelogenous leukemia (mCP-CML) wherein mCP-CML is induced by the retroviral introduction of the human bcr-abl fusion cDNA into mouse hematopoietic progenitors. mCP-CML phenotypically resembles human CP-CML in that recipients of p210-infected BM cells develop a high white blood cell count and splenomegaly, with hematopoiesis dominated by maturing myeloid cells.13,17,19 An advantage of this model is that we can induce mCP-CML in BM from any strain, including gene-deficient mice. The retroviral construct also expresses a nonsignaling truncated form of the human NGFR,20 which allows transduced cells to be quantitated by flow cytometry.
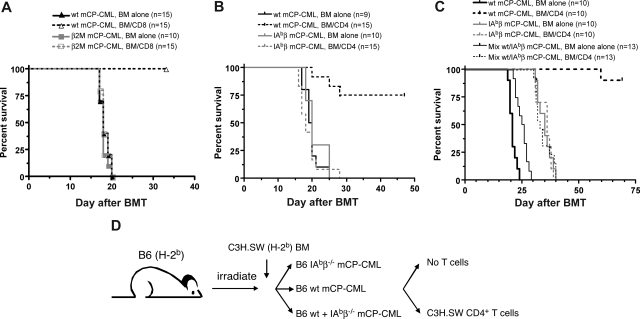
To determine whether cognate T cell/leukemia interactions are required for GVL, we tested whether CD8+ or CD4+ T cells could mediate GVL against mCP-CML created from B6 β2M−/− or B6 IAbβ−/− BM, respectively. To directly compare the roles of cognate interactions in GVL and GVHD, we used the same strain pairings as in the prior GVHD experiments, except in this case recipients were wt B6 mice and not BM chimeras. For CD8-mediated GVL, irradiated B6 mice were reconstituted with TCD C3H.SW BM, either wt B6 or β2M−/− B6 mCP-CML, with or without donor C3H.SW CD8 cells. To prevent NK cell-mediated rejection of β2M−/− mCP-CML, all mice (including recipients of wt mCP-CML) were treated anti-NK1.1 on days −2, −1, and 7 to deplete NK cells.13 As expected, CD8 cells mediated GVL against wt B6 mCP-CML (Figure 7A). However, β2M−/− mCP-CML was completely resistant to CD8-mediated GVL. Thus, donor CD8 cells required cognate interactions with MHCI to mediate GVL.
Figure 7.
β2M−/− and IAbβ−/− mCP-CML are resistant to CD8- and CD4-mediated GVL. (A) B6 mice were irradiated and reconstituted with TCD C3H.SW BM, wt B6 or β2M−/− mCP-CML, with or without C3H.SW donor CD8+ T cells. (B) B6 mice were irradiated and reconstituted with TCD 129 BM, wt B6 or IAbβ−/− mCP-CML, with or without purified 129 CD4+ T cells. (C) To determine whether the GVL resistance of IAbβ−/− mCP-CML is in the effector phase, irradiated B6 mice were reconstituted with TCD C3H.SW BM, wt mCP-CML, or IAbβ−/− mCP-CML or a mix of both wt and IAbβ−/− mCP-CML, with or without purified C3H.SW CD4+ T cells. Note that the only survivors were recipients of wt mCP-CML cells and donor C3H.SW CD4 cells, indicating that IAbβ−/− mCP-CML is GVL-resistant because of a defect in the effector phase. (D) Experimental design for panel C.
For CD4-mediated GVL, irradiated B6 mice were reconstituted with TCD 129 BM, either wt B6 or B6 IAbβ−/− mCP-CML, with or without purified 129 CD4 cells. Donor CD4 cells mediated GVL against wt mCP-CML but not against IAbβ−/− mCP-CML (Figure 7B). Thus, in contrast to CD4-mediated GVHD and consistent with our prior experiments with IAbβ−/− mCP-CML in a different strain pairing,19 CD4-mediated GVL required cognate interactions with MHCII+ mCP-CML cells.
IAbβ−/− mCP-CML could have been GVL-resistant because leukemia cells were important APCs. If so, GVL may have been reduced because of inadequate generation of alloreactive effector CD4 cells rather than because of an inability of effectors to kill MHCII− targets. To address this possibility, we determined whether GVL would be intact against IAbβ−/− mCP-CML if mice also received wt MHCII+ mCP-CML (Figure 7C). We reasoned that, if priming by MHCII+ mCP-CML cells is important, then the presence of wt mCP-CML would “rescue” GVL against IAbβ−/− mCP-CML. For these experiments, we used the C3H.SW→B6 strain pairing. B6 mice were irradiated and reconstituted with C3H.SW TCD BM, B6 wt mCP-CML, or B6 IAbβ−/− mCP-CML, or a 1:1 mix of wt and IAbβ−/− mCP-CML with or without 4 × 106 purified C3H.SW CD4 cells. All mice received 7.5 × 105 BM cells that underwent spin infection (recipients of a mix of wt and IAbβ−/− mCP-CML received 3.75 × 105 cells of each). As expected, donor CD4 cells mediated GVL against wt mCP-CML. In contrast, recipients of IAbβ−/− mCP-CML and CD4+ T cells, with or without additional wt mCP-CML, died from mCP-CML with similar kinetics (P = .96). Survival in these groups was similar to that in recipients of IAbβ−/− mCP-CML and no donor CD4 cells (P = .77 comparing wt + IAbβ−/− mCP-CML and CD4 cells to IAbβ−/− mCP-CML without CD4 cells). Thus, even in the presence of wt mCP-CML, IAbβ−/− mCP-CML was resistant to CD4-mediated GVL. Therefore, the requirement for cognate interactions between TCRs on donor CD4 cells and MHCII on mCP-CML is in the effector and not the priming phase of GVL.
Discussion
In the present work, we define the requirements for cognate T cell contact with parenchymal tissues and leukemia cells in clinically relevant, MHC-matched, multiple miHA-mismatched models of GVHD and GVL. CD8-mediated GVHD (aside from low-penetrance GVHD of ears, which did not make statistical significance) absolutely required direct interactions between TCRs on donor CD8 cells and MHCI on target tissues. We observed neither clinical GVHD nor histologic GVHD in skin, small intestine, colon, and liver of wt→β2M−/− recipients of CD8+ T cells. Thus, in contrast to the situation in MHC-disparate GVHD,5 T cell or T cell–induced cytokine production and cytolytic-targeting of only recipient hematopoietic cells was insufficient for GVHD in a MHC-matched, multiple miHA-mismatched model.
Because alloreactive CD8+ T-cell generation was intact in wt→β2M−/− recipients, given that recipient APCs were MHCI+, initial recruitment of alloreactive activated T cells into potential GVHD target tissues should not have been impaired.21,22 That we saw no evidence of infiltrating lymphocytes in these chimeras highlights the importance of tissue damage in the accumulation of donor CD8+ T cells. Such accumulation could be the result of increased T-cell recruitment from blood, local retention and survival of T cells after entering tissues, and perhaps by local T-cell expansion driven by host antigen-bearing tissue APCs. We previously demonstrated that maximal GVHD in this system requires functional donor APCs which probably promote GVHD by cross-presenting recipient antigen to donor CD8 cells.13 Although this clearly could be occurring in secondary lymphoid tissues, it could also be taking place in GVHD target organs, and parenchymal cells killed by alloreactive T cells could be a source of cross-presented antigen. The topical administration of a Toll-like receptor-7 agonist was recently shown to promote localized GVHD, presumably by enhancing recruitment of alloreactive T cells.23 Our data extend that work by showing that initial T-cell recruitment is not enough and that a critical mass of tissue damage is necessary to establish a sustained GVHD lesion.
We do not know why wt→β2M−/− CD8 recipients may have developed low-penetrance GVHD of the ear. One possibility would be that parenchymal cells acquire sufficient soluble β2M, or even intact MHCI contained in exosomes derived from wt hematopoietic cells, to permit direct cytolytic damage.24–27 Alternatively, local release of inflammatory mediators, including cytokines and perforin/granzymes, by CD8+ T cells reacting with host antigen-bearing MHCI+ donor APCs may injure bystander MHCI− tissues. Either effect could be potentiated by underlying damage from irradiation, which disproportionately affects the ears. Nonetheless, the key conclusion is that overall cognate interactions between TCRs and MHCI are pivotal for histologic GVHD.
Cytolytic mechanisms in CD8-dependent, miHA-disparate, MHC-matched GVHD have been studied using T cells deficient in perforin, FasL, or both.28 Perforin/FasL double-deficient T cells induced GVHD across only miHA differences, albeit with delayed kinetics compared with GVHD induced by wild-type T cells.29 To the extent that the major cytolytic mechanisms were impaired, the GVHD observed could have been the result of activities that do not rely on direct TCR contact with target tissues. Our result that CD8-dependent GVHD requires cognate interactions between donor T cells and parenchyma clarifies that result and suggests that GVHD induced by FasL/perforin double-deficient CD8 cells was likely the result of direct killing of MHCI+ parenchymal cells by other cytolytic effectors, such as tumor necrosis factor-α and tumor necrosis factor-α–related apoptosis-inducing ligand.
In contrast to CD8-mediated GVHD, TCRs on donor CD4 cells did not require cognate interactions with MHCII on parenchymal tissues to mediate GVHD. It was reasonable to propose that CD4-mediated GVHD in MHC-matched transplants might depend on parenchymal interactions between TCR and MHCII as MHCII is expressed by inflamed bowel epithelial cells and keratinocytes, specifically in mice with GVHD.13,30–32 Nonetheless, cognate interactions were not required, although our studies do not exclude that they additionally contribute to GVHD pathology. If anything, GVHD was more severe in wt→IAbβ−/− mice. This increased severity could have been the result of the absence of recipient CD4+CD25+FOXP3+ regulatory T cells which would not have been positively selected as thymic epithelial cells are MHCII− in wt→IAbβ−/− chimeras.33
We observed similar polymorphonuclear infiltrates in GVHD target tissues in either wt→wt or wt→IAbβ−/− CD4 recipients, not present in mice that received only TCD donor BM. Immunofluorescence staining confirmed the presence of infiltrating CD4+ T cells in both wt→wt and wt→IAbβ−/− CD4 recipients, consistent with their having a pathogenic role. We see several nonexclusive mechanisms whereby infiltrating CD4 cells could cause tissue damage without directly contacting nonhematopoietic tissues. After activation in secondary lymphoid organs, donor CD4 cells could migrate into target tissues and elaborate inflammatory mediators without any additional activation. Alternatively or in addition, infiltrating alloreactive CD4 cells could be further activated in target organs by miHA-bearing MHCII+ cells, such as tissue DCs and macrophages, thereby amplifying the GVHD response locally. Our demonstration of CD4 cells adjacent to MHCII+ cells in GVHD target tissues supports this notion. Donor CD4 cells could reciprocally activate tissue MHCII+ cells, and this might need to occur before they activate infiltrating CD4 cells. Consistent with this idea, we previously reported reduced GVHD when donor BM was CD40-deficient.34 CD4 cells could also arm miHA-expressing macrophages to induce tissue damage. This is a compelling mechanism because CD8+ T cells, which could not mediate GVHD indirectly, are not well equipped for this function. A more definitive investigation on a potential role for tissue macrophages will require the use of donors that have constitutive or preferably inducible defects in macrophage effector function.35,36 Further clarification of this mechanism could identify novel therapeutic targets for treating or preventing GVHD that could spare the GVL effect.
By creating MHCI− and MHCII− leukemias, we investigated the requirement for TCR/leukemia target interactions in GVL. CD8-mediated GVL mirrored CD8-mediated GVHD in that it required cognate interactions between donor CD8 cells and leukemic targets. Although this was an anticipated result, a requirement for MHCI expression by target leukemias for CD8-mediated GVL had never been definitively tested. Prior work in an MHCI-mismatched, miHA-matched model suggested that some GVL can occur when donor T cells are syngeneic to and therefore MHCI-matched with the targeted leukemia,37 which implies indirect killing, not observed in our experiments. In contrast, only CD4-mediated GVL, and not CD4-mediated GVHD, required contact with MHCII on targets. mCP-CML cells are uniformly MHCI+; however, because p210 expression does not block myeloid differentiation in human or mCP-CML, mCP-CML cells are heterogeneous, as is MHCII expression (not shown). However, we infer from these data that a key cell, perhaps a leukemia stem cell, is MHCII+ and this is currently being investigated. The requirement for cognate interactions for GVL is not restricted to mCP-CML, but we have also found it to be required for GVL against a murine model of blast crisis CML.38 Thus, alloreactive CD4 cells mediate GVL and GVHD using distinct mechanisms. This was an unexpected result, which has important clinical implications. If the specific pathways engaged by CD4 cells in GVHD and not in GVL are fully elucidated, they could be targeted to mitigate GVHD, possibly without compromising GVL.
Acknowledgments
The authors thank Srividhya Venkatesan and Hung Sheng Tan for expert technical assistance and the Yale University Animal Resources Center for meticulous animal care.
This work was supported by the National Institutes of Health (grant R01-CA96943). W.D.S. is also the recipient of a Clinical Scholar award from the Leukemia and Lymphoma Society.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.M.-M. designed and performed experiments, analyzed results, and wrote the paper; J.L. designed and performed experiments; D.J. and J.M. analyzed histopathology; and W.D.S. designed experiments, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren D. Shlomchik, Section of Medical Oncology, Yale Comprehensive Cancer Center, PO Box 208032, Yale University School of Medicine, New Haven, CT 06520-8032; e-mail: warren.shlomchik@yale.edu.
References
- 1.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 3.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-vs-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 5.Teshima T, Ordemann R, Reddy P, et al. Acute graft-vs-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 6.Aosai F, Ohlen C, Ljunggren HG, et al. Different types of allospecific CTL clones identified by their ability to recognize peptide loading-defective target cells. Eur J Immunol. 1991;21:2767–2774. doi: 10.1002/eji.1830211118. [DOI] [PubMed] [Google Scholar]
- 7.Man S, Salter RD, Engelhard VH. Role of endogenous peptide in human alloreactive cytotoxic T cell responses. Int Immunol. 1992;4:367–375. doi: 10.1093/intimm/4.3.367. [DOI] [PubMed] [Google Scholar]
- 8.Crumpacker DB, Alexander J, Cresswell P, Engelhard VH. Role of endogenous peptides in murine allogenic cytotoxic T cell responses assessed using transfectants of the antigen-processing mutant 174xCEM. T2. J Immunol. 1992;148:3004–3011. [PubMed] [Google Scholar]
- 9.Hambach L, Goulmy E. Immunotherapy of cancer through targeting of minor histocompatibility antigens. Curr Opin Immunol. 2005;17:202–210. doi: 10.1016/j.coi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Hill GR, Krenger W, Ferrara JL. The role of cytokines in acute graft-vs-host disease. Cytokines Cell Mol Ther. 1997;3:257–266. [PubMed] [Google Scholar]
- 11.Korngold R, Sprent J. Features of T cells causing H-2-restricted lethal graft-vs.-host disease across minor histocompatibility barriers. J Exp Med. 1982;155:872–883. doi: 10.1084/jem.155.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SC, Murphy GF, Friedman TM, Korngold R. Importance of minor histocompatibility antigen expression by nonhematopoietic tissues in a CD4+ T cell-mediated graft-vs-host disease model. J Clin Invest. 2003;112:1880–1886. doi: 10.1172/JCI19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 14.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft vs host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-vs-host disease phenotype. J Immunol. 2004;173:5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 16.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 17.Pear WS, Miller JP, Xu L, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 18.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matte CC, Cormier J, Anderson BE, et al. Graft-vs-leukemia in a retrovirally induced murine CML model: mechanisms of T-cell killing. Blood. 2004;103:4353–4361. doi: 10.1182/blood-2003-10-3735. [DOI] [PubMed] [Google Scholar]
- 20.Reddy UR, Venkatakrishnan G, Maul GG, Roy AK, Ross AH. Transient expression of full-length and truncated forms of the human nerve growth factor receptor. Brain Res Mol Brain Res. 1990;8:137–141. doi: 10.1016/0169-328x(90)90058-l. [DOI] [PubMed] [Google Scholar]
- 21.Issekutz TB, Stoltz JM, van der Meide P. Lymphocyte recruitment in delayed-type hypersensitivity: the role of IFN-gamma. J Immunol. 1988;140:2989–2993. [PubMed] [Google Scholar]
- 22.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm [review]. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 23.Chakraverty R, Cote D, Buchli J, et al. An inflammatory checkpoint regulates recruitment of graft-vs-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 25.Emerson SG, Cone RE. I-Kk and H-2Kk antigens are shed as supramolecular particles in association with membrane lipids. J Immunol. 1981;127:482–486. [PubMed] [Google Scholar]
- 26.Emerson SG, Cone RE. Absorption of shed I-Ak and H-2Kk antigens by lymphoid cells. Transplantation. 1982;33:36–40. doi: 10.1097/00007890-198201000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 28.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2:273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]
- 29.Marks L, Altman NH, Podack ER, Levy RB. Donor T cells lacking Fas ligand and perforin retain the capacity to induce severe GvHD in minor histocompatibility antigen mismatched bone-marrow transplantation recipients. Transplantation. 2004;77:804–812. doi: 10.1097/01.tp.0000110416.96307.d5. [DOI] [PubMed] [Google Scholar]
- 30.Giorno R, Choi KL, Katz HR, Claman HN. Monoclonal antibody analysis of skin in chronic murine graft vs host disease produced across minor histocompatibility barriers. Cell Immunol. 1987;106:76–87. doi: 10.1016/0008-8749(87)90151-1. [DOI] [PubMed] [Google Scholar]
- 31.Bland PW, Whiting CV. Induction of MHC class II gene products in rat intestinal epithelium during graft-vs-host disease and effects on the immune function of the epithelium. Immunology. 1992;75:366–371. [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Kurts C, Kontgen F, Holdsworth SR, Tipping PG. Major histocompatibility complex class II expression by intrinsic renal cells is required for crescentic glomerulonephritis. J Exp Med. 1998;188:597–602. doi: 10.1084/jem.188.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-vs-host disease. Blood. 2004;104:1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 34.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-vs-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 35.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 36.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-vs-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 38.Shlomchik WD, Matte-Martone CC, Gilliland DG, Chen LP. Mechanisms of GVL against a murine blast crisis CML [abstract]. Blood. 2006;108:60a–61a. [Google Scholar]