Abstract
In addition to its clinical antimanic effects, lithium also has efficacy in the treatment of depression. However, the mechanism by which lithium exerts its antidepressant effects is unclear. Our objective was to further characterize the effects of peripheral and central administration of lithium in mouse models of antidepressant efficacy as well as to investigate the role of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors in these behaviors. We utilized the mouse forced swim test (FST) and tail suspension test (TST), intracerebroventricular (ICV) lithium administration, AMPA receptor inhibitors, and BS3 crosslinking followed by western blot. Both short- and long-term administration of lithium resulted in robust antidepressant-like effects in the mouse FST and TST. Using ICV administration of lithium, we show that these effects are due to actions of lithium on the brain, rather than to peripheral effects of the drug. Both ICV and rodent chow (0.4% LiCl) administration paradigms resulted in brain lithium concentrations within the human therapeutic range. The effects of lithium to decrease immobility in the FST and TST were blocked by administration of AMPA receptor inhibitors. Additionally, administration of lithium increased the cell surface expression of GluR1 and GluR2 in the mouse hippocampus. Collectively, these data show that lithium exerts centrally mediated antidepressant-like effects in the mouse FST and TST that require AMPA receptor activation. Lithium may exert its antidepressant effects in humans through AMPA receptors, thus further supporting a role of targeting AMPA receptors as a therapeutic approach for the treatment of depression.
Keywords: alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA), mood stabilizer, antidepressant, lithium, forced swim test (FST), tail suspension test (TST), glutamate, bipolar disorder
Depression is a severe neuropsychiatric disorder that manifests both as a component of bipolar disorder and unipolar depressive disorder. Bipolar disorder affects at least 1% of the world’s population when a strict definition (DSM IV bipolar I disorder) is applied. Major depression is more common than bipolar disorder in the overall population (~ 15% lifetime risk as defined by DSM IV) (Kessler et al., 2005; Sullivan et al., 2000). The recognition of the significant morbidity and mortality (suicide rate of 15%) associated with these severe mood disorders (Evans et al., 2005; Jamison, 2000; Kupfer, 2005), as well as the growing appreciation that a significant percentage of patients either do not respond fully to existing treatments or are intolerant of undesirable side effects, has made the challenge of discovering improved therapeutic agents increasingly important (Zarate et al., 2006).
The results of a number of double-blind studies have confirmed the efficacy of lithium for both the acute treatment of mania, and mania prophylaxis (Schou, 2001). Findings from a number of studies, including meta-analyses, have led to the opinion that lithium is useful for the acute and the prophylactic treatment of depression in some patient populations (Bauer and Mitchner, 2004; Mendels et al., 1972; Souza and Goodwin, 1991). Furthermore, lithium has clear utility as an adjunct antidepressant in treatment-refractory patients (de Montigny et al., 1983; Heninger et al., 1983). Overall, in placebo-controlled trials, lithium has been found useful as an adjunct medication for approximately 45% of patients (Bauer et al., 2003). However, it is unclear how the clinically relevant antidepressant actions of lithium are exerted. Although a number of targets, both direct and indirect, have been suggested as potentially interacting with lithium to produce its therapeutic effects, it is currently unknown which of these targets holds primary responsibility in improving the symptoms of patients with depression or bipolar disorder. Thus, lithium has a clinical profile as an antidepressant medication, but understanding the therapeutic mechanism of lithium action has been a major challenge for psychiatric neuroscience, and lack of knowledge regarding how lithium exerts its therapeutic effects has likely hindered the development of novel antidepressant medications. To narrow the effects of lithium that are most likely related to the therapeutic actions of the drug, we have begun studying the neurobiology underlying the effects of lithium in established behavioral models.
Among the many biochemical effects of lithium, evidence has suggested actions on the glutamateric system (Antonelli et al., 2000; Dixon and Hokin, 1998; Dixon et al., 1994; Du et al., 2004; Hokin et al., 1996; Marcus et al., 1986). A substantial body of data suggests that the AMPA receptor subclass of glutamate receptors may be particularly interesting targets for the treatment of mood disorders (Alt et al., 2006; Li et al., 2001; Li et al., 2003; Martinez-Turrillas et al., 2002; Svenningsson et al., 2002). Lithium has been reported to have diverse effects on AMPA receptors including modulation of receptor levels in rats (Du et al., 2004; Du et al., 2006; Gray et al., 2003) and, at high concentrations or when replacing extracellular sodium with lithium, direct effects on AMPA-mediated currents in model systems such as Xenopus oocytes or larval lamprey spinal cord neurones (Karkanias and Papke, 1999a, b; Nanou and El Manira, 2007). Glutamate is the most prevalent excitatory neurotransmitter in the mammalian brain. AMPA receptors are prevalent throughout the central nervous system, found at highest concentrations in regions of the brain involved in mood regulation such as the prefrontal cortex and hippocampus, and thus likely involved in the regulation of mood (Bleakman et al., 2007; Monaghan et al., 1984). However, limited data exist to suggest that the behavioral effects of lithium in rodent models of mood disorders may be linked to any actions of lithium on AMPA receptor function. Multiple groups have recently shown that long-term administration of lithium in rodent chow results in robust antidepressant-like effects in the FST (Bersudsky et al., 2007; Cryns et al., 2006; Gould et al., 2007a; O’Brien et al., 2004; Shaldubina et al., 2006). The tail suspension test, another measure of antidepressant efficacy, was established in 1985 by Steru and colleagues (Steru et al., 1985), but limited evidence exists for the effects of lithium on this behavioral model. It is important to discern the effect of lithium in additional behavioral tests beyond the FST, in order to allow the use of multiple tests to confirm any putative antidepressant-like mechanism of lithium, as well as to use an alternative test when using background mouse strains that are historically unresponsive to antidepressants in the FST (Lucki et al., 2001; Petit-Demouliere et al., 2005). Despite the face value similarities of behavioral endpoints, the spectrum of drugs that are active in the FST and TST are not identical, suggesting possible fundamental differences in the neurochemistry involved in the behavioral output (Bai et al., 2001; Lucki et al., 2001; Petit-Demouliere et al., 2005; Ripoll et al., 2003).
Our aims in the current study include the investigation of the effects of both short-and long-term actions of lithium in the FST and TST. We additionally investigated the relationship between the antidepressant-like effects of lithium in these models and AMPA receptor blockade and cell surface expression.
Methods
Animals
C57BL/6J mice (The Jackson Laboratories; Bar Harbor, Maine) were transported to our laboratory and experimentation started no less than one week later, to allow for appropriate acclimatization time. Mice were housed 4 per cage in an animal room with constant temperature (22 ± 1°C) and a 12 hour light/dark cycle (lights on/off at 6:00 A.M/P.M.), with free access to food and water. All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee, and were conducted in full accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources (U.S.), 1996). Mice were 8–10 weeks of age at the time of testing. Experiments were performed between 9 A.M. and 12 P.M. during the light phase of the light/dark cycle. Mice were used for a single experiment only, with the exception of open field test, in which mice studied at day 8 in the TST were tested at day 17 in the open field test, and a cohort of mice used for ICV lithium administration, where mice were tested in the open field on day 7 and the FST on day 9.
Drugs
Mice were randomly assigned to treatment groups. Lithium-containing chow (lithium chloride, 4.0 g/kg) was identical to control chow (sodium chloride, 4.0 g/kg) with the exception of the added chloride salt. To help prevent ion imbalances due to administration of lithium, both lithium- and control-treated mice were provided with a second water bottle containing 0.9% saline. Cages for both lithium- and control-treated mice were changed every 2 days because of polyuria in the lithium group. NBQX (Tocris) was administered i.p. after being dissolved in 50% DMSO/50% saline. GYKI 52466 (Sigma) was dissolved in 30% DMSO/70% saline and administered i.p. For each drug treatment control mice received the respective vehicle alone.
ICV Lithium Administration
In order to determine the specific behavioral effects of lithium in the brain, intracerebroventricular (ICV) administration of a lithium chloride solution was accomplished by implanting in each mouse an Alzet™ osmotically regulated micro-pump (model 1200) attached to an Alzet™ brain infusion kit. Pumps were implanted subcutaneously into mice under anesthesia (1.5% isoflurane), while the cannula of the brain infusion kit was inserted into the right lateral ventricle (Bregma -1.00mm, 0.25mm). The micro-pumps contained LiCl at a concentration of 1328 mM in an artificial CSF vehicle or vehicle alone. Pumps are active immediately following implantation, and continue delivering solution at 0.25 μl/hour over 14 days. Mice were single-housed after the surgical procedure until sacrifice.
Open Field Test
For the open field test, mice were placed in the center of a Plexiglas open field (35 × 35 cm) and their behavior was recorded by the Ethovision videotracking system (Noldus; Leesburg, VA). The Ethovision system follows the center of the body of the mouse and records its position according to the location of this point.
Tail Suspension Test
Minor variations were required to allow us to reduce the tendency of C57BL/6J mice to climb their tails (Mayorga and Lucki, 2001). A 15 cm length of tape 1.9 cm in width (TimeMed Labeling Systems, Inc, Burr Ridge, Illinois) was positioned with approximately 2 mm of tail protruding. This short distance of the tape from the end of the tail and long length of tape utilized prevented the mice from being able to balance themselves in preparation for any climbing behavior. Mice were suspended by their tails for 6 minutes during video taped sessions. Utilizing this procedure in pilot experiments we observed that 13% of mice climbed their tails, which is substantially less than the 70% observed by Mayorga and Lucki as well as our group in preliminary experiments using the standard technique (Mayorga and Lucki, 2001). Any mice that did climb their tails were removed from the experimental analysis. Mobility was defined as movement of the hind legs. A blind observer (KCO) scored the videotapes for the full 6 minutes.
Forced Swim Test
Mice were placed in a cylinder of water between 23 and 25°C. Mice were videotaped during a 6-minute session, which was later analyzed for activity during the final 4 minutes. Mobility was defined as any movement beyond what is necessary to maintain the head above water. At the end of the trial session, mice were taken out of the water, dried with a paper towel and placed back in their home cages. A blind observer (KCO) scored the videotapes.
Lithium Concentration Assay
To determine serum lithium levels (mmol/l), serum was isolated from whole blood by centrifugation. Whole brain lithium levels (mmol/kg wet weight) were determined following polytron homogenization of the entire brain (Kinematica AG Model PCU 11, Littau, Switzerland) in 3 volumes of 0.5 N trichloroacetic acid (Hamburger-Bar et al, 1986)(Gould et al., 2007b). Following centrifugation, lithium assays were performed on the supernatant with a digital flame photometer (Cole-Palmer Model 2655-00, Chicago, Illinois).
Surface receptor cross-linking with BS3 to distinguish surface and intracellular AMPARs in vivo
BS3 does not penetrate cell membranes and therefore selectively cross-links cell surface receptors, forming high molecular weight aggregates, while not modifying intracellular receptors (Boudreau and Wolf, 2005; Hall and Soderling, 1997). Surface and intracellular receptor pools can thereafter be distinguished based on molecular weight using SDS-PAGE and Western blotting. Our protocol was similar to Boudreau and Wolf (2005), with some minor variations. After treatment with lithium or control chow for 1.5 days, mice were decapitated. Brains were rapidly removed followed by dissection of frontal cortex and hippocampal tissue on wet ice. Bilateral pieces of prefrontal cortex or hippocampal tissue from each mouse were then chopped into 400μm slices using a McIllwain tissue chopper (The Vibratome Company, O’Fallon, MO). Slices were placed in 6-well plates containing ice-cold artificial CSF with 2 mM BS3 (Pierce, Rockford, IL). Incubation with gentle agitation proceeded for 30 min on ice. The cross-linking reaction was terminated by quenching with 100 mM glycine (10 min, 4°C). The slices were then pelleted by brief centrifugation, and the supernatant discarded. Pellets were resuspended in ice-cold lysis buffer (10 mM Tris-HCL, 15 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-Glycerophosphate) with phosphotase inhibitors cocktail I and II (1:100) and protease inhibitor cocktail (1:100) (Sigma, St. Louis, MO). Samples were aliquoted and stored at −80°C for future analysis. Protein concentrations were determined by using the Bradford protein assay (Bio-Rad, Hercules, CA (Bradford, 1976)). Equal amounts of proteins were subjected to 6% SDS-PAGE gels and separated by electrophoresis (Invitrogen, Carlsbad, CA), transferred to 0.45 micron pore-size PVDF membranes (Millipore) and immuno-blotted with anti-GluR1 (Chemicon AB1504, 1:500) and anti-GluR2 (Zymed 32-0300, 1:1000) antibodies. The secondary antibody used was horseradish peroxidase (HRP)-conjugated anti-mouse (7076) or anti-rabbit (7074) antibody (Cell Signaling, Danvers, MA). AMPA receptors are tetramers, which are composed of various combinations of GluR1, GluR2, GluR3, and GluR4 subunits. Among all possible combinations, GluR1/2, GluR2/3, or GluR1/3 are the common forms with a size range of 430–450kD. Non-crosslinked GluR1 and GluR2 are each 100kD in size. Immunoreactive bands were visualized by enhanced chemoluminescence (ECL+) (Amersham, Piscataway, NJ) and exposed to Kodak Biomax or Biolight film (New Haven, CT). The ECL signal intensities were quantified using a Kodak Image system (New Haven, CT).
Statistical analysis
Statistical analysis was performed by SPSS 11 for Macintosh and Graphpad Prism Version 4. Statistical analysis utilized either Student’s t-test, one-way ANOVA, or two-way ANOVA. A Newman-Keuls post hoc test was utilized to compare significant ANOVA results. All statistics were un-paired with the exception of the western blot studies, which used paired t-tests to take into account microenvironment changes between gels. Data are reported as mean +/− SE. Any point greater than 2 SD from a group mean was considered an outlier, and was not considered in the analysis. All statistics were 2-tailed. A p value less than 0.05 was considered significant.
Results
Experiment 1: Determination of brain and serum lithium levels
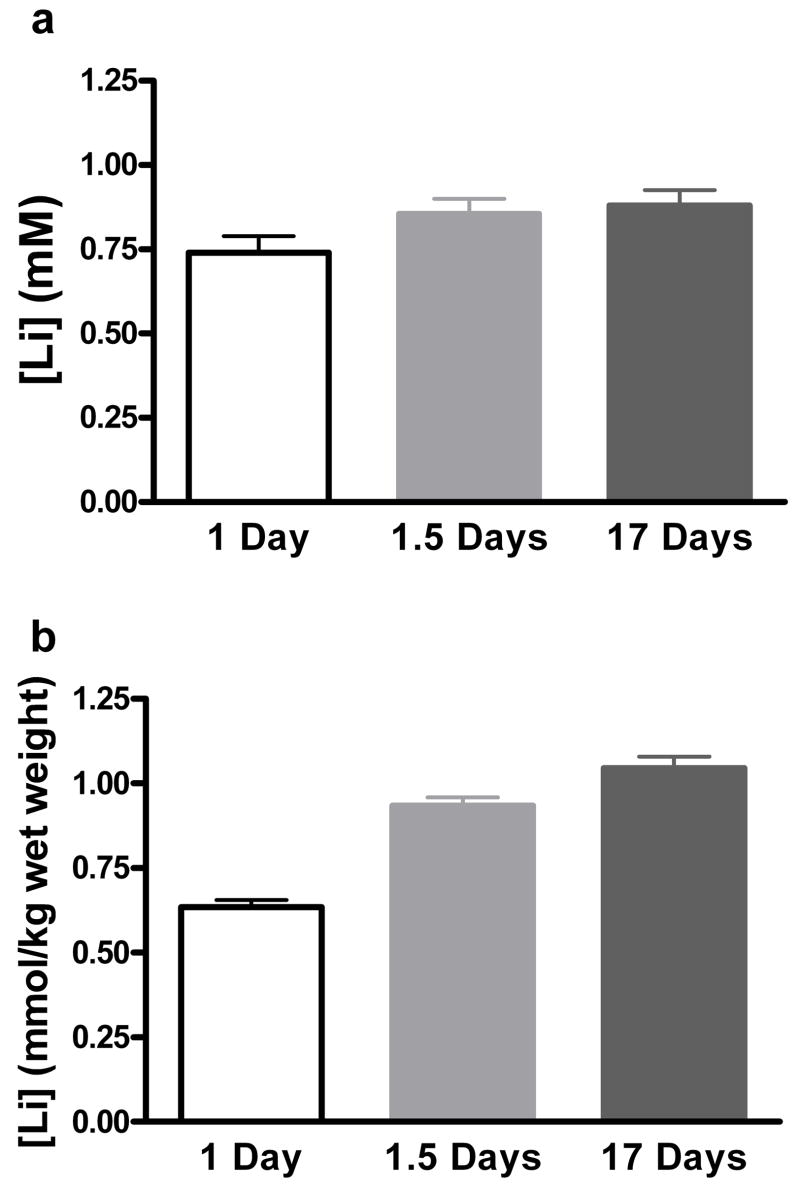
Prior to examining a time course for behavioral effects of lithium in the FST and TST, we were interested in establishing a short-term lithium administration paradigm that would result in brain lithium levels similar to what was observed following long-term treatment. We first examined lithium levels following long-term (17 days) treatment. The serum levels were 0.88 ± 0.05mM and brain levels were 1.05 ± 0.03 mmol/kg. To establish the minimum amount of feeding to reach high brain levels, serum and brain lithium levels following 1 night of chow (over 24 hours), and 2 consecutive nights of chow (over ~1.5 days) were studied. These two administration times were designed to take into account the fact that mice consume most of their chow during the dark cycle. Figure 1 shows that serum levels at all 3 time points were not significantly different, and were all within the human therapeutic range of 0.6 to 1.2 mM (F(2, 23) = 2.63, p = 0.096). However, brain lithium levels (Figure 1b) were significantly different among groups: F(2, 23) = 62.53, p < 0.0001. This is consistent with the finding from numerous groups that establishment of steady-state brain lithium levels in the rodent brain lags behind serum lithium levels (Ghoshdastidar et al., 1989; Gould et al., 2007b; Morrison et al., 1971). Bersudsky and colleagues recently reported that the effect of lithium in the FST after long-term treatment requires serum levels above 0.8 mM (Bersudsky et al., 2007). Following long-term administration of lithium to rodents, whole brain lithium concentrations and serum lithium concentrations are generally equal (Ghoshdastidar et al., 1989; Gould et al., 2007b), suggesting that brain lithium levels above 0.8 mM would be required to elicit antidepressant-like effects in the FST. Brain levels for 1 day and 1.5 days were 0.64 ± 0.02 and 0.94 ± 0.02 respectively. Based upon these findings we reasoned that if short-term lithium also had antidepressant-like actions in the FST, then 1.5 days of administration should allow for sufficient brain lithium levels to exert these effects. Thus, we proceeded with 1.5 days as our earliest time point to study the effects of lithium in the FST and TST.
Figure 1. Brain and serum concentrations of lithium following administration of LiCl in rodent chow.
Serum lithium concentration following 1 day, 1.5 days, and 17 days of lithium administration (a). Brain lithium concentration following 1 day, 1.5 days, and 17 days of lithium administration (b). N = 8 mice per group.
Experiment 2: Time course for antidepressant-like effects of lithium in the FST and TST
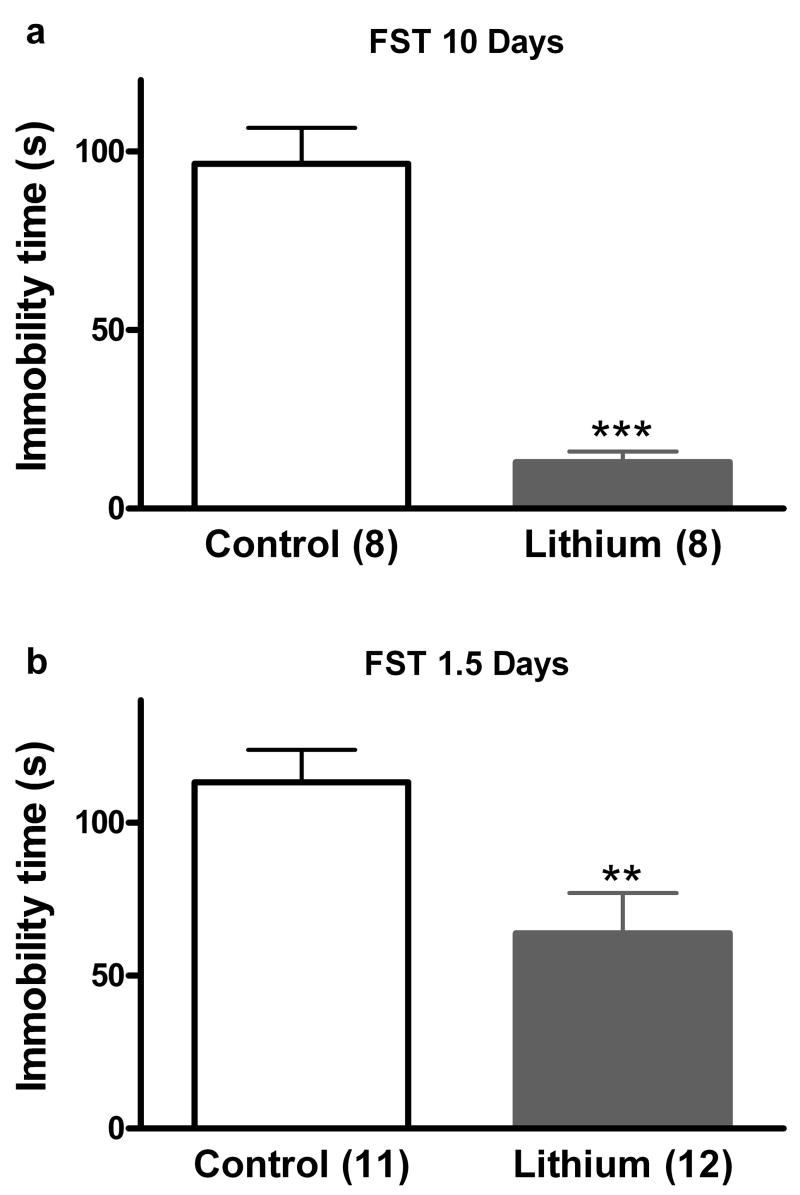
Consistent with results reported previously for time points between 10 and 35 days (Bersudsky et al., 2007; Cryns et al., 2006; Gould et al., 2007a; O’Brien et al., 2004; Shaldubina et al., 2006), in our experiments 10 days of lithium administration resulted in decreased immobility time in the mouse FST (t(14) = 7.98, p < 0.001; Figure 2a). We have previously reported that i.p. administration of LiCl 30 minutes prior to the FST did not result in antidepressant-like effects (Gould et al., 2007a). However, this administration paradigm results in whole brain lithium levels lower than those necessary to result in antidepressant-like effects in the FST (Bersudsky et al., 2007; Gould et al., 2007a). Administration of lithium over 1.5 days via rodent chow resulted in a significant decrease in immobility time, t(21) = 2.89, p < 0.01 (Figure 2b).
Figure 2. Short- and long-term administration of LiCl in rodent chow results in antidepressant-like effects in the FST.
Lithium exerts antidepressant-like effects in the mouse FST following 10 days (a) or 1.5 days (b) of treatment. The number of mice per group is indicated in each individual graph. ** p < 0.01; *** p <0.001
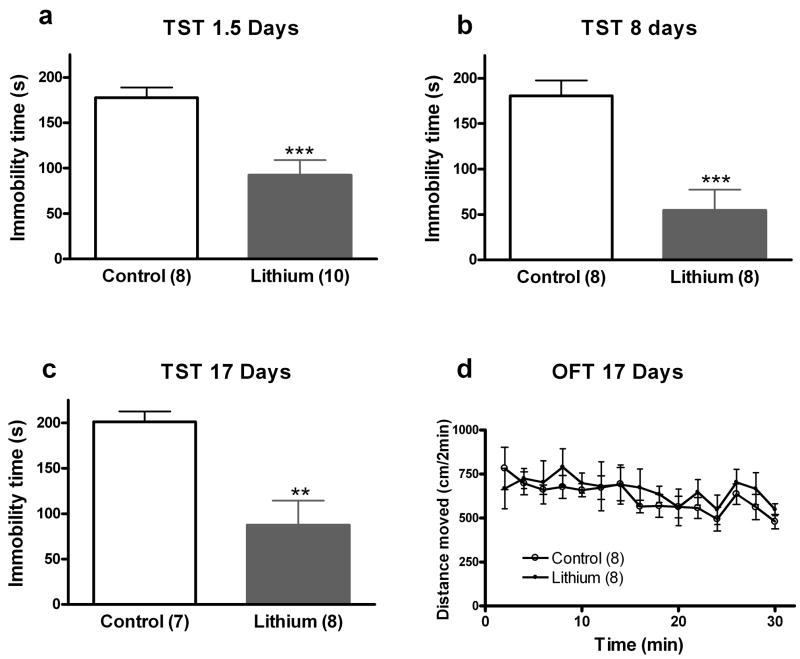
Next we were interested in determining whether lithium administration had antidepressant-like effects in another validated mouse model of antidepressant efficacy, the TST. As detailed in the Methods and Materials section, minor variations were made in the TST procedure that allowed us to reduce the tendency of C57BL/6J mice to climb their tails. We tested mice following administration of lithium chow for 1.5, 8, or 17 days (Figure 3a, b, c). Administration of lithium resulted in decreased immobility time at each time point (1.5 days: t(16) = 4.01, p < 0.001; 8 days: t(14) = 4.38, p < 0.001; 17 days: t(13) = 3.70, p < 0.01). Because stimulants are also capable of decreasing immobility in the FST and TST, we determined whether the reduced immobility was caused by general hyperactivity induced by lithium; as such, we tested mice treated for 17 days with lithium in a small open field. Repeated measure ANOVA revealed that over a 30 minute period there was no significant effect of lithium to alter open field activity (F(1, 196) = 0.35, p = NS), a significant effect of time (F(14, 196) = 2.51, p < 0.001), and no interaction between the two (F(14, 196) = 0.42, p = NS), suggesting that effects of lithium in the FST and TST were not due to a non-specific activating effect of the drug (Figure 3d).
Figure 3. Short- and long-term administration of LiCl in rodent chow results in antidepressant-like effects in the TST.
Lithium exerts antidepressant-like effects in the mouse TST following 1.5 days (a), 8 days (b), or 17 days of treatment (c). There was no change in open field activity following 17 days of lithium treatment (d). The number of mice per group is indicated in each individual graph. ** p < 0.01; *** p <0.001
Experiment 3: Effects of ICV administration of lithium on the mouse FST and TST
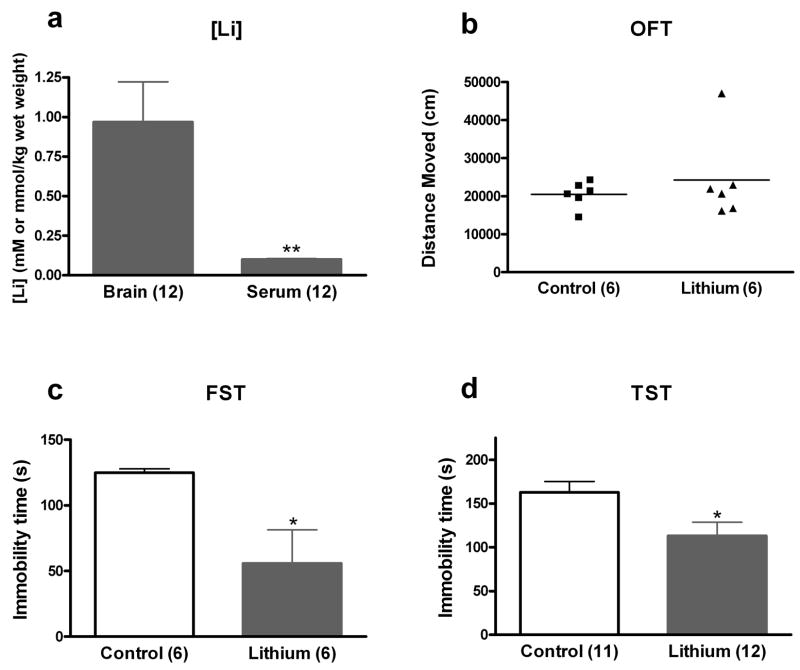
Lithium has a number of direct and indirect cellular targets, both within the central nervous system and in the periphery. Administering lithium via rodent chow does not allow us to discern whether the effects of lithium in the FST and TST are due to its effects on the brain or elsewhere in the body. To determine if the behavioral effects of lithium administration in the FST and TST were due to actions of lithium on the brain, we administered lithium via an Alzet™ micro-osmotic pump attached to a brain infusion kit. Our goal was to administer lithium chloride at human therapeutic lithium levels in the brain with negligible levels in the periphery. Pilot dose experiments revealed that a concentration of 1328mM LiCl in artificial CSF consistently resulted in mean brain lithium levels around 1 mM, with negligible levels in the serum. This was confirmed following behavioral testing in a cohort (n = 12) of mice: brain concentrations were measured as 0.97 ± 0.25 mmol/kg wet weight, and serum concentrations as 0.10 ± 0.004 mM (Figure 4a).
Figure 4. Intracerebroventricular administration of LiCl results in antidepressant-like effects in the mouse FST and TST.
Mice receiving ICV LiCl or vehicle were tested in the open field test, FST, or TST. Following completion of the behavioral experiments a subset of mice were sacrificed to evaluate brain and serum lithium levels. Our paradigm resulted in brain lithium levels within the human therapeutic concentration range and low levels in the serum (a). This paradigm resulted in no significant change in open field activity (b) but a significant antidepressant-like effect in the FST (c) and TST (d). The number of mice per group is indicated in each individual graph. * p <0.05; ** p < 0.01
The first cohort of mice was tested for 60 minutes in an open field, 7 days post-surgery. The open field test showed that lithium-treated animals did not differ from vehicle-treated mice in total distance moved, t(10) = 0.78, p = NS (Figure 4b). Nine days after surgery, mice from the same cohort were tested in the FST, the statistical analysis of which revealed a significant decrease in immobility time in lithium-treated mice, t(10) = 2.69, p < 0.05 (Figure 4c). A separate cohort of mice was analyzed in the TST 9 days following surgery. Mice treated with lithium spent significantly less time immobile in the TST as compared to control animals treated with CSF only, t(21) = 2.45, p < 0.05 (Figure 4d). These results support the hypothesis that the effects of lithium to decrease immobility in the FST and TST are due to actions of lithium on the brain, rather than to peripheral effects of the drug.
Experiment 4: Effects of AMPA receptor blockade on the antidepressant-like effects of lithium
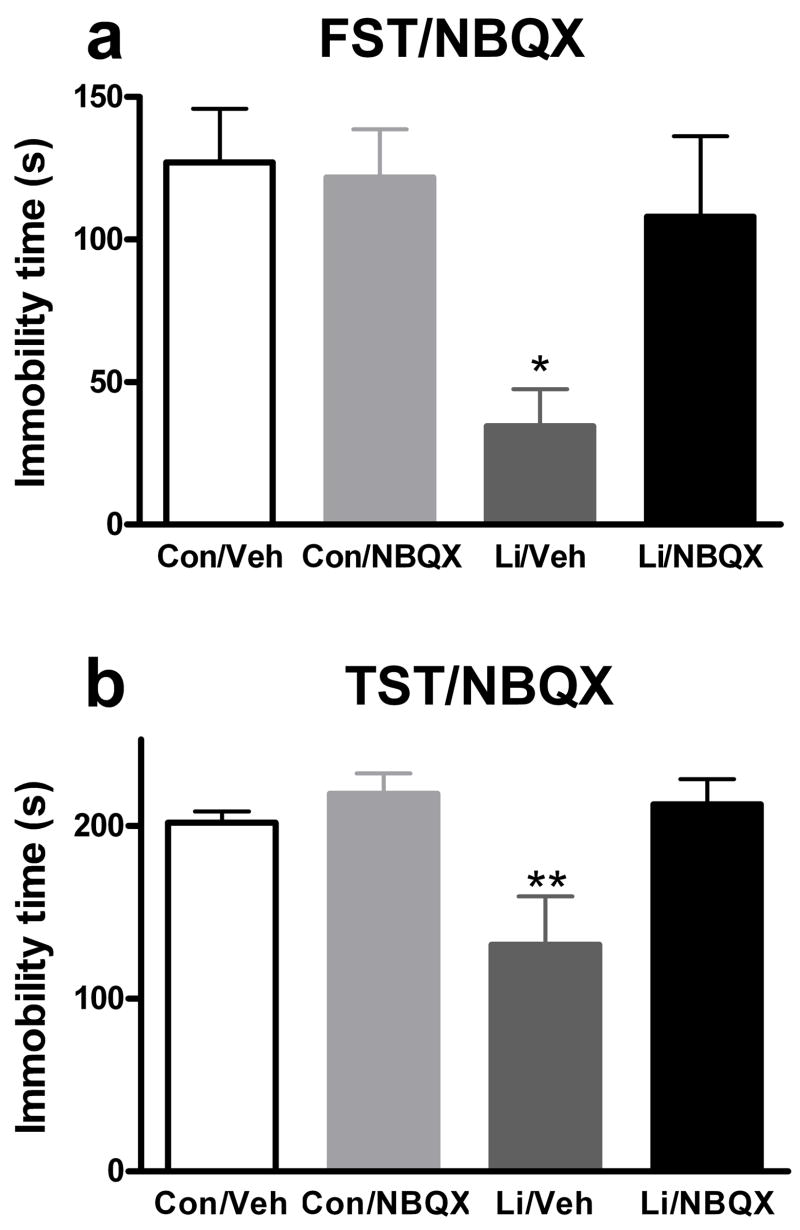
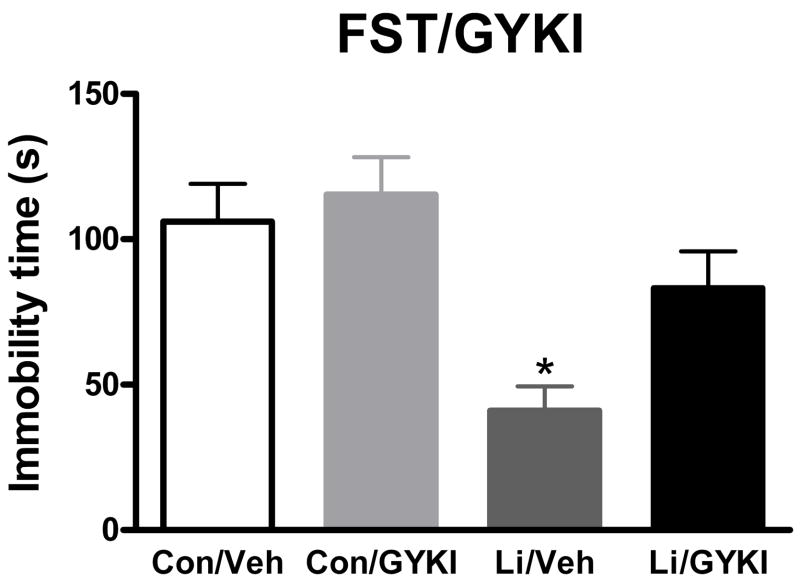
Increasing evidence implicates the glutamatergic system, and in particular the AMPA receptor subclass of glutamate receptors, in the mechanism of action of antidepressant drugs (Alt et al., 2006; Li et al., 2001; Li et al., 2003; Martinez-Turrillas et al., 2002; Svenningsson et al., 2002). Lithium, in particular, has been implicated in modulating the glutamatergic system and AMPA receptors (Antonelli et al., 2000; Dixon and Hokin, 1998; Dixon et al., 1994; Du et al., 2004; Hokin et al., 1996; Marcus et al., 1986). Given the clinical profile of lithium, we hypothesized that regulation of AMPA receptor activation may be implicated in the antidepressant-like effects of lithium in the FST and TST. To determine whether activation of AMPA receptors is required for the antidepressant-like effects of lithium in the FST and TST, we administered lithium or control chow to mice for 1.5 days followed by administration of NBQX (a competitive AMPA receptor antagonist) or vehicle 30 minutes prior to testing in these behavioral tasks. We chose a dose of 10 mg/kg because preliminary studies suggested non-specific effects of higher doses to decrease baseline locomotion (data not shown). A dose in this range has been shown to decrease AMPA-induced seizures in mice (Chapman et al., 1991). NBQX attenuated the antidepressant-like effects of lithium in the FST (Figure 5a). Two-way ANOVA revealed a significant effect of lithium (F(1, 20) = 7.05, p < 0.05), no significant effect of NBQX (F(1, 20) = 2.91), and a trend for an interaction between the two (F(1, 20) = 3.86, p = 0.064). Post hoc analysis revealed a significant effect of lithium alone compared to the each of the other 3 groups (p < 0.05) and no other significant differences. Similarly, the antidepressant-like effects of 1.5 days of lithium administration in the TST were attenuated by NBQX (Figure 5b). Two-way ANOVA revealed a significant effect of lithium (F(1, 27) = 4.76, p < 0.05), a significant effect of NBQX (F(1, 27) = 7.79, p < 0.05), and a trend for an interaction between the two (F(1, 27) = 4.76, p = 0.076). Post hoc analysis revealed a significant effect of lithium alone compared to each of the other 3 groups (p < 0.01), and no other significant differences. Importantly, for both the FST and TST there was no significant effect of NBQX alone to change immobility time compared to vehicle treated mice. To rule out the possibility that the effect of NBQX was non-specific, or unrelated to its AMPA receptor antagonism, we administered the non-competitive AMPA receptor antagonist, GYKI 52466, prior to the FST. We chose a dose of 5 mg/kg because preliminary studies suggested non-specific effects of higher doses to decrease baseline locomotion (data not shown). A dose in this range has been reported to decrease AMPA-induced seizures in mice (Chapman et al., 1991). Similar to the results seen with NBQX, GYKI 52466 attenuated the effects of lithium in the FST (Figure 6). Two-way ANOVA revealed a significant effect of lithium (F(1, 44) = 16.65, p < 0.001), a significant effect of GYKI 52466 (F(1, 44) = 4.66, p < 0.05), and no significant interaction between the two (F(1, 44) = 1.91). Post hoc analysis revealed a significant effect of lithium alone compared to control (p < 0.01), GYKI 52466 alone (p < 0.001), or lithium and GYKI 52466 (p < 0.05) and no other significant differences. Importantly, there was no significant effect of GYKI 52466 alone to change immobility time compared to vehicle treated mice.
Figure 5. Administration of the AMPA receptor inhibitor, NBQX, attenuates the effects of lithium in the mouse FST and TST.
Mice were treated with lithium or control chow for 1.5 days. NBQX or vehicle was administered 30 minutes prior to behavioral testing. NBQX, at a dose of 10 mg/kg, attenuated the antidepressant-like effects of lithium in the FST (a) and TST (b). N = 6 to 8 mice per group. * p <0.05; ** p < 0.01, posthoc test versus all other groups
Figure 6. Administration of the AMPA receptor inhibitor GYKI 52466, attenuates the effects of lithium in the mouse FST.
Mice were treated with lithium or control chow for 1.5 days. GYKI 52466 or vehicle was administered 30 minutes prior to behavioral testing. GYKI 52466, at a dose of 5 mg/kg, attenuated the antidepressant-like effects of lithium in the TST. N = 12 mice per group. * p <0.05, posthoc test versus all other groups
Experiment 5: Effects of lithium administration on membrane levels of GluR1 and GluR2
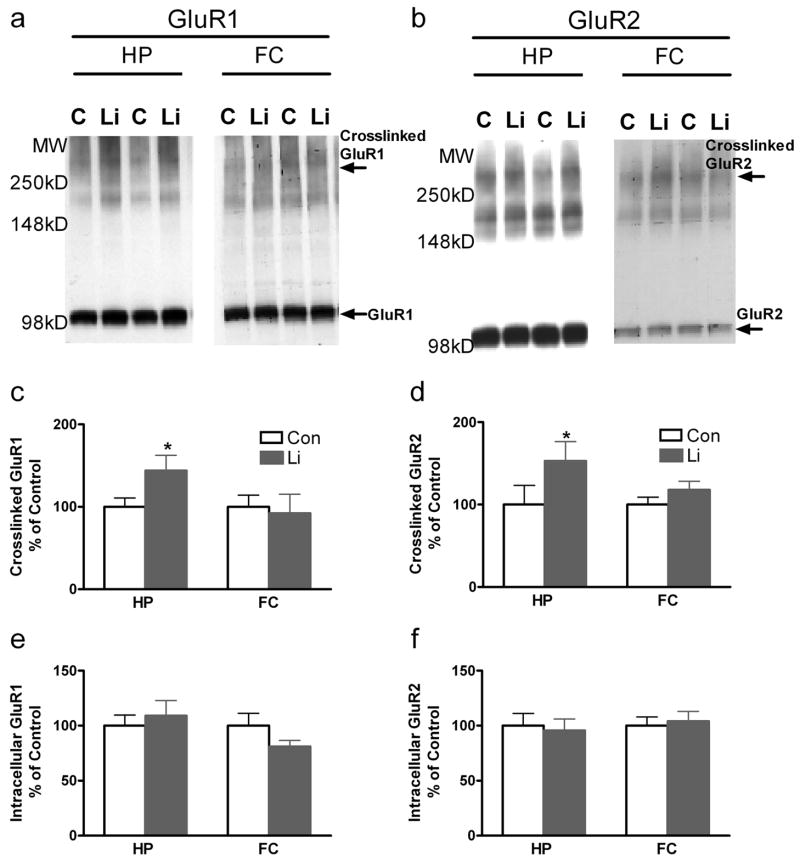
To determine whether the AMPA-dependent behavioral changes we observed were associated with changes in levels of membrane-associated AMPA receptors, we used the BS3 cross-linking method followed by western blot quantification of surface GluR1 and GluR2 tetramers, as well as intracellular levels of these receptors. Since the cross linking reagent BS3 does not cross the cell membrane it reacts solely with surface proteins, including glutamate receptor tetramers (Boudreau and Wolf, 2005; Hall and Soderling, 1997). Western blot can then distinguish cross-linked (surface) and non-cross-linked (intracellular) receptors (Figure 7a, b). Lithium administration for 1.5 days significantly enhanced surface cross-linked GluR1 and GluR2 in hippocampus, but not in frontal cortex (p < 0.05) (Figure 7c, d). There was no significant change in non-cross-linked (intracellular) levels of either GluR1 or GluR2 (Figure 7e, f).
Figure 7. Lithium treatment significantly increases surface GluR1/GluR2 in the hippocampus, but not frontal cortex in vivo.
Mice were treated with lithium or control chow for 1.5 days. Following treatment, mice were decapitated and brains were rapidly removed. Hippocampal (HP) and frontal cortex (FC) tissues were isolated and the BS3 cross-linking procedure was performed to identify surface GluR1/GluR2 tetramers. Western blot analyses were performed with anti-GluR1 and anti-GluR2 antibodies. Representative images for cross-linked and intracellular GluR1 and GluR2 by western blot analysis (a, b). Densitometry analysis: Lithium significantly enhanced surface cross-linked GluR1 and GluR2 in hippocampus, but not in prefrontal cortex vivo. N=6 mice per group (b-e). * p <0.05
Discussion
We show that lithium, following both short- and long-term administration to mice, exerts robust antidepressant-like effects in the TST as well as previously documented long-term effects in the FST. These effects are also observed with direct administration of lithium to the ventricular system, indicating that the effects of lithium in these behavioral models are most likely not due to any peripheral effects of the drug. The antidepressant-like effects of lithium in these models are blocked by administration of AMPA receptor inhibitors, indicating that activation of AMPA receptors is required for the antidepressant-like effects of lithium. Further, administration of lithium increases the cell surface expression of GluR1 and GluR2 in the hippocampus, suggesting that lithium-induced increases in surface AMPA receptor levels may underlie these behavioral effects of lithium and the requirement for AMPA activation.
We hypothesize that although lithium is generally most efficacious clinically as an adjunct antidepressant, rather than as monotherapy, its actions when used alone in mouse models of antidepressant efficacy are nevertheless related to its therapeutic effects in the treatment of depression. Due to lithium’s narrow dose range in humans it may not be possible to administer lithium at doses high enough to elicit strong effects as a monotherapy. Though our results were observed in the human therapeutic range, responsivity in humans and mice may not be precisely identical. Previous studies have addressed adjunctive effects of lithium in both the TST and FST. In the FST lithium has been shown to augment the antidepressant-like effects of a number of different classes of antidepressants, including SSRIs, MAOIs, tricyclic, and atypical antidepressants (Bourin et al., 1996; Hascoet et al., 1994; Nixon et al., 1994). One study has reported adjunctive effects of lithium to various classes of antidepressant medications in the TST (Redrobe and Bourin, 1997). An antidepressant-like effect of lithium alone in the FST has been reported following chronic i.p. administration to rats (Eroglu and Hizal, 1987), acute i.p. administration to mice (Hascoet et al., 1994), and in mice following long-term administration in the chow (Bersudsky et al., 2007; Cryns et al., 2006; Gould et al., 2007a; O’Brien et al., 2004; Shaldubina et al., 2006). To the best of our knowledge, our report is the first to show that lithium by itself has antidepressant-like effects in the TST. Additional studies will clearly be necessary to discern how lithium interacts with classical antidepressants in the FST and TST models, the role of AMPA receptors in these interactions, and where any diverse mechanisms of action converge.
Biochemically diverse effects of lithium on the glutamateric system have been identified by a number of groups (Antonelli et al., 2000; Dixon and Hokin, 1998; Dixon et al., 1994; Du et al., 2004; Du et al., 2006; Gray et al., 2003; Hokin et al., 1996; Marcus et al., 1986). Specifically, these effects include evidence suggesting a decrease in glutamate reuptake, decrease in glutamate release, and/or modulation of receptor levels. Direct effects of lithium to modify AMPA receptor inward and outward currents in a subtype specific manner have also been noted (Karkanias and Papke, 1999a, b; Nanou and El Manira, 2007). However, these direct effects on receptors have been obtained at lithium concentrations well above the therapeutic range observed in humans and may be most relevant to in vitro studies of receptor biophysics (Karkanias and Papke, 1999a, b; Nanou and El Manira, 2007). Overall, there are likely complex, brain region specific effects of lithium on AMPA activation and receptor regulation that will need to be considered in future studies. For example, long-term treatment of rats with lithium decreases the levels of GluR1 found in hippocampal synaptosomes, findings that may be species or time-frame specific (Du et al., 2004). To the best of our knowledge, our study is the first to relate lithium’s actions on AMPA receptors to the behavioral effects of lithium. Clinically, as monotherapy, lithium is generally considered to be a more robust antimanic than antidepressant medication. In the present manuscript we do not address the role of AMPA receptors in mediating the effects of lithium in models of manic-like behavior. Available rodent models for the antimanic effects of lithium include attenuation of amphetamine-induced hyperlocomotion and attenuation of aggression (O’Donnell and Gould, 2007), which should be evaluated for the relevance of AMPA receptors.
Modulation of the AMPA receptor subclass of glutamate receptors has been linked to the mechanism of action of antidepressant medications as well as a target for future medications (Alt et al., 2006; Du et al., 2006; Li et al., 2001; Li et al., 2003; Martinez-Turrillas et al., 2002; Svenningsson et al., 2002). It has recently been reported by us that the antidepressant-like effects of ketamine in the FST, as well as the NMDA antagonist MK801 and NR2B antagonist, Ro25-6981, are prevented by preadministration of NBQX (Maeng et al., 2007). Though it is not clear precisely how NMDA receptor antagonism results in AMPA receptor activation, we hypothesize that it may be through increased release of glutamate following NMDA receptor blockade. This hypothesis is supported by the finding that ketamine increases the release of glutamate, a process that might be mediated by presynaptic NMDA autoreceptors and/or interneurons (Lorrain et al., 2003; Moghaddam et al., 1997; Razoux et al., 2007). It has also been reported that the antidepressant-like effects of imipramine in the FST are not affected by antagonism of AMPA receptors (Li et al., 2001; Maeng et al., 2007). However, while traditional antidepressants such as imipramine may not act directly on AMPA receptors, indirect effects cannot be excluded. For example, Svenningson and colleagues (2002) reported that administration of fluoxetine to mice increased phosphorylation of the AMPA receptor subunit GluR1 at Ser-831 and Ser-845. Antidepressants have also been reported to decrease GluR3 expression both in fontal cortex and hippocampus of rats as well as altering the editing levels of either the flip or flop form of GluR3, AMPA-mediated release of monamines, AMPA receptor protein interactions, and the release of glutamate (Barbon et al., 2006; Bonanno et al., 2005; Martinez-Turrillas et al., 2007; Pittaluga et al., 2007). Similar to our findings with lithium, it has been reported that monamine-acting antidepressants increase membrane levels of AMPA receptors (Martinez-Turrillas et al., 2005; Martinez-Turrillas et al., 2002). Electroconvulsive shock, an animal model of electroconvulsive therapy, was shown to increase the levels of GluR1 mRNA in the rat hippocampus (Naylor et al., 1996). It is unclear what the mechanism is by which modulation of AMPA receptors may be involved in the mechanism of antidepressant action, or to what extent AMPA activation is required for therapeutic action in humans (Bleakman et al., 2007). One hypothesis suggests that an increase in expression of neurotrophic factors such as brain derived neurotrophic factor (BDNF) and a resultant increase in neuroplasticity and protection of neurons from neurotoxic insults may be relevant. Similar to antidepressants, AMPA receptor potentiators have been shown increase the production of BDNF and cell proliferation in the hippocampus (Bai et al., 2003; Lauterborn et al., 2000; Legutko et al., 2001; Mackowiak et al., 2002). Another possibility is modulation of amine neurotransmission. For example, AMPA has been shown to enhance the release of noradrenaline and serotonin from rat synaptosomes (Pittaluga et al., 1997; Pittaluga et al., 2007; Wang et al., 1992).
Several classes of compounds can allosterically modulate AMPA receptors. These compounds (referred to as AMPA receptor-positive modulators or AMPA receptor potentiators, ARPs) do not activate AMPA receptors themselves, but slow the rate of receptor desensitization and/or deactivation in the presence of an agonist (e.g., glutamate and AMPA). ARPs have been reported to have antidepressant effects in animal models of antidepressant efficacy including the FST, TST, and a reduction in antidepressant-sensitive submissive behaviors (Alt et al., 2006; Bai et al., 2001; Knapp et al., 2002; Li et al., 2001). Similar to lithium, ARPs have been shown to have a robust augmentative effect on the actions of antidepressants in the FST (Li et al., 2003). These AMPA receptor modulators that are in clinical development increases the likelihood that the results reported in the present manuscript will influence translational studies for the treatment of patients with mood disorders (Black, 2005). Our results suggest that lithium may exert its antidepressant-like effects in the FST and TST by increasing synaptic levels of GluR1 and GluR2. However, additional effects of lithium on glutamate release or reuptake, or postsynaptic effects on glutamate receptors, downstream signaling, or direct effects on the receptor cannot be excluded. Recent data have suggested that lithium exerts some of its behavioral actions through inhibition of GSK-3 (Beaulieu et al., 2004; Gould et al., 2004; Kaidanovich-Beilin et al., 2004; O’Brien et al., 2004). Future studies will examine the link between GSK-3 inhibition and regulation of AMPA receptors. These and other recent insights into the actions of lithium on validated animal models of depression make it likely that relevant mechanisms can soon be determined and thereafter used to develop and test novel therapeutics for the treatment of depression and bipolar disorder.
Acknowledgments
The Intramural Research Program of the National Institute of Mental Heath and the National Association on Schizophrenia and Depression (Young Investigator Award to TDG) supported this research. The authors have no conflicts of interest, financial or otherwise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–1288. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Antonelli T, Ferioli V, Lo Gallo G, Tomasini MC, Fernandez M, O’Connor WT, Glennon JC, Tanganelli S, Ferraro L. Differential effects of acute and short-term lithium administration on dialysate glutamate and GABA levels in the frontal cortex of the conscious rat. Synapse. 2000;38:355–362. doi: 10.1002/1098-2396(20001201)38:3<355::AID-SYN15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bai F, Bergeron M, Nelson DL. Chronic AMPA receptor potentiator ( LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44:1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Bauer M, Forsthoff A, Baethge C, Adli M, Berghofer A, Dopfmer S, Bschor T. Lithium augmentation therapy in refractory depression-update 2002. Eur Arch Psychiatry Clin Neurosci. 2003;253:132–139. doi: 10.1007/s00406-003-0430-9. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Mitchner L. What is a “mood stabilizer”? An evidence-based response. Am J Psychiatry. 2004;161:3–18. doi: 10.1176/appi.ajp.161.1.3. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky Y, Shaldubina A, Belmaker RH. Lithium’s effect in forced-swim test is blood level dependent but not dependent on weight loss. Behav Pharmacol. 2007;18:77–80. doi: 10.1097/FBP.0b013e32801416ed. [DOI] [PubMed] [Google Scholar]
- Black MD. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology (Berl) 2005;179:154–163. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Witkin JM. AMPA receptors in the therapeutic management of depression. CNS Neurol Disord Drug Targets. 2007;6:117–126. doi: 10.2174/187152707780363258. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, Raiteri M, Racagni G, Popoli M. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, Colombel MC, Redrobe JP, Baker GB. Differential effects of clonidine, lithium and quinine in the forced swimming test in mice for antidepressants: possible roles of serotoninergic systems. Eur Neuropsychopharmacol. 1996;6:231–236. doi: 10.1016/0924-977x(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Smith SE, Meldrum BS. The anticonvulsant effect of the non-NMDA antagonists, NBQX and GYKI 52466, in mice. Epilepsy Res. 1991;9:92–96. doi: 10.1016/0920-1211(91)90018-b. [DOI] [PubMed] [Google Scholar]
- Cryns K, Shamir A, Shapiro J, Daneels G, Goris I, Van Craenendonck H, Straetemans R, Belmaker RH, Agam G, Moechars D, Steckler T. Lack of Lithium-Like Behavioral and Molecular Effects in IMPA2 Knockout Mice. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301154. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Cournoyer G, Morissette R, Langlois R, Caille G. Lithium carbonate addition in tricyclic antidepressant-resistant unipolar depression. Correlations with the neurobiologic actions of tricyclic antidepressant drugs and lithium ion on the serotonin system. Arch Gen Psychiatry. 1983;40:1327–1334. doi: 10.1001/archpsyc.1983.01790110069012. [DOI] [PubMed] [Google Scholar]
- Dixon JF, Hokin LE. Lithium acutely inhibits and chronically up-regulates and stabilizes glutamate uptake by presynaptic nerve endings in mouse cerebral cortex. Proc Natl Acad Sci U S A. 1998;95:8363–8368. doi: 10.1073/pnas.95.14.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JF, Los GV, Hokin LE. Lithium stimulates glutamate “release” and inositol 1,4,5-trisphosphate accumulation via activation of the N-methyl-D-aspartate receptor in monkey and mouse cerebral cortex slices. Proc Natl Acad Sci U S A. 1994;91:8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The Anticonvulsants Lamotrigine, Riluzole, and Valproate Differentially Regulate AMPA Receptor Membrane Localization: Relationship to Clinical Effects in Mood Disorders. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Eroglu L, Hizal A. Antidepressant action of lithium in behavioral despair test. Pol J Pharmacol Pharm. 1987;39:667–673. [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ghoshdastidar D, Dutta RN, Poddar MK. In vivo distribution of lithium in plasma and brain. Indian J Exp Biol. 1989;27:950–954. [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004:1–4. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. beta-Catenin Overexpression in the Mouse Brain Phenocopies Lithium-Sensitive Behaviors. Neuropsychopharmacology. 2007a doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007b;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gray NA, Du J, Falke CS, Yuan P, Manji HK. Lithium regulates total and synaptic expression of the AMPA glutamate receptor GluR2 in vitro and in vivo. Ann N Y Acad Sci. 2003;1003:402–404. doi: 10.1196/annals.1300.036. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience. 1997;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Khimake S. Additive effect of lithium and clonidine with 5-HT1A agonists in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:381–396. doi: 10.1016/0278-5846(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS, Sternberg DE. Lithium carbonate augmentation of antidepressant treatment. An effective prescription for treatment-refractory depression. Arch Gen Psychiatry. 1983;40:1335–1342. doi: 10.1001/archpsyc.1983.01790110077013. [DOI] [PubMed] [Google Scholar]
- Hokin LE, Dixon JF, Los GV. A novel action of lithium: stimulation of glutamate release and inositol 1,4,5 trisphosphate accumulation via activation of the N-methyl D-aspartate receptor in monkey and mouse cerebral cortex slices. Adv Enzyme Regul. 1996;36:229–244. doi: 10.1016/0065-2571(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C: 1996. [DOI] [PubMed] [Google Scholar]
- Jamison KR. Suicide and bipolar disorder. J Clin Psychiatry. 2000;61(Suppl 9):47–51. [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Karkanias NB, Papke RL. Lithium modulates desensitization of the glutamate receptor subtype gluR3 in Xenopus oocytes. Neurosci Lett. 1999a;277:153–156. doi: 10.1016/s0304-3940(99)00878-2. [DOI] [PubMed] [Google Scholar]
- Karkanias NB, Papke RL. Subtype-specific effects of lithium on glutamate receptor function. J Neurophysiol. 1999b;81:1506–1512. doi: 10.1152/jn.1999.81.4.1506. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, Crites G, Malatynska E. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in bipolar disorder. Jama. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legutko B, Li X, Skolnick P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology. 2001;40:1019–1027. doi: 10.1016/s0028-3908(01)00006-5. [DOI] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator ( LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of alpha-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Marcus SR, Nadiger HA, Chandrakala MV, Rao TI, Sadasivudu B. Acute and short-term effects of lithium on glutamate metabolism in rat brain. Biochem Pharmacol. 1986;35:365–369. doi: 10.1016/0006-2952(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Del Rio J, Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Del Rio J, Frechilla D. Neuronal proteins involved in synaptic targeting of AMPA receptors in rat hippocampus by antidepressant drugs. Biochem Biophys Res Commun. 2007;353:750–755. doi: 10.1016/j.bbrc.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Frechilla D, Del Rio J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- Mendels J, Secunda SK, Dyson WL. A controlled study of the antidepressant effects of lithium carbonate. Arch Gen Psychiatry. 1972;26:154–157. doi: 10.1001/archpsyc.1972.01750200058012. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Yao D, Cotman CW. Distribution of [3H]AMPA binding sites in rat brain as determined by quantitative autoradiography. Brain Res. 1984;324:160–164. doi: 10.1016/0006-8993(84)90636-x. [DOI] [PubMed] [Google Scholar]
- Morrison JM, Jr, Pritchard HD, Braude MC, D’Aguanno W. Plasma and brain lithium levels after lithium carbonate and lithium chloride administration by different routes in rats. Proc Soc Exp Biol Med. 1971;137:889–892. doi: 10.3181/00379727-137-35687. [DOI] [PubMed] [Google Scholar]
- Nanou E, El Manira A. A postsynaptic negative feedback mediated by coupling between AMPA receptors and Na+-activated K+ channels in spinal cord neurones. Eur J Neurosci. 2007;25:445–450. doi: 10.1111/j.1460-9568.2006.05287.x. [DOI] [PubMed] [Google Scholar]
- Naylor P, Stewart CA, Wright SR, Pearson RC, Reid IC. Repeated ECS induces GluR1 mRNA but not NMDAR1A-G mRNA in the rat hippocampus. Brain Res Mol Brain Res. 1996;35:349–353. doi: 10.1016/0169-328x(95)00264-s. [DOI] [PubMed] [Google Scholar]
- Nixon MK, Hascoet M, Bourin M, Colombel MC. Additive effects of lithium and antidepressants in the forced swimming test: further evidence for involvement of the serotoninergic system. Psychopharmacology (Berl) 1994;115:59–64. doi: 10.1007/BF02244752. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007 doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Bonfanti A, Raiteri M. Differential desensitization of ionotropic non-NMDA receptors having distinct neuronal location and function. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:29–38. doi: 10.1007/pl00005025. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Raiteri L, Longordo F, Luccini E, Barbiero VS, Racagni G, Popoli M, Raiteri M. Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacology. 2007;53:27–36. doi: 10.1016/j.neuropharm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Lena I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Effects of pretreatment with clonidine, lithium and quinine on the activities of antidepressant drugs in the mouse tail suspension test. Fundam Clin Pharmacol. 1997;11:381–386. doi: 10.1111/j.1472-8206.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Schou M. Lithium treatment at 52. J Affect Disord. 2001;67:21–32. doi: 10.1016/s0165-0327(01)00380-9. [DOI] [PubMed] [Google Scholar]
- Shaldubina A, Johanson RA, O’Brien WT, Buccafusca R, Agam G, Belmaker RH, Klein PS, Bersudsky Y, Berry GT. SMIT1 haploinsufficiency causes brain inositol deficiency without affecting lithium-sensitive behavior. Mol Genet Metab. 2006;88:384–388. doi: 10.1016/j.ymgme.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Souza FG, Goodwin GM. Lithium treatment and prophylaxis in unipolar depression: a meta- analysis. Br J Psychiatry. 1991;158:666–675. doi: 10.1192/bjp.158.5.666. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci U S A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Andrews H, Thukral V. Presynaptic glutamate receptors regulate noradrenaline release from isolated nerve terminals. J Neurochem. 1992;58:204–211. doi: 10.1111/j.1471-4159.1992.tb09297.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]