Abstract
TRPP2 (transient receptor potential polycystin-2) channels function in a range of cells where they are localized to specific subcellular regions including the endoplasmic reticulum (ER) and primary cilium. In humans, TRPP2/PC-2 mutations severely compromise kidney function and give rise to autosomal dominant polycystic kidney disease (ADPKD). The Caenorhabditis elegans TRPP2 homolog, PKD-2, is restricted to the somatodendritic (cell body and dendrite) and ciliary compartment of male specific sensory neurons. Within these neurons PKD-2 function is required for sensation. To understand the mechanisms regulating TRPP2 subcellular distribution and activity, we performed in vivo structure-function-localization studies using C. elegans as a model system. Our data demonstrate that somatodendritic and ciliary targeting requires the transmembrane (TM) region of PKD-2and that the PKD-2 cytosolic termini regulate subcellular distribution and function. Within neuronal cell bodies, PKD-2 colocalizes with the OSM-9 TRP vanilloid (TRPV) channel, suggesting that these TRPP and TRPV channels may function in a common process. When human TRPP2/PC-2 is heterologously expressed in transgenic C. elegans animals, PC-2 does not visibly localize to cilia but does partially rescue pkd-2 null mutant defects, suggesting that human PC-2 and PKD-2 are functional homologs.
Keywords: autosomal dominant polycystic kidney disease (ADPKD), C. elegans, trafficking, TRPP2, cilia, behavior, PKD-2, polycystin
INTRODUCTION
The transient receptor potential (TRP) channel superfamily is classified into six groups of ion channels that share a common structure. Defects in TRP receptor function cause a variety of diseases [1], including autosomal dominant polycystic kidney disease (ADPKD [2]). Many TRP receptors are multifunctional, specializing in either a sensory or developmental capacity to act as intrinsic environmental sensors. Typically TRP channels form cation-permeable tetramers that respond to extracellular stimulation [3]. TRP channel function is dependent upon complex assembly, subcellular localization, and downstream signaling [4].
The human TRP polycystin-2 protein (TRPP2/PC-2) was identified based upon its involvement in ADPKD [5]. In the kidney, TRPP2/PC-2 acts as a mechanosensor to regulate kidney homeostasis in response to urine flow [6]. TRPP2/PC-2 also regulates a variety of other biological processes including the establishment of polarity, cell proliferation, and apoptosis [7]. TRPP2/PC-2 functions as a nonselective calcium permeable ion channel that, depending upon the cellular environment, is targeted to a variety of subcellular locations including the plasma membrane, primary cilia, endoplasmic reticulum (ER), centrosome, and mitotic spindles [7, 8]. TRPP2 contains a variety of signaling and protein interaction motifs including a calcium binding EF hand and ER retention signal in the cytoplasmic C-terminus, and a large and unique extracellular loop between the first two transmembrane (TM) domains. The cytosolic N-terminus contains a glycogen synthase kinase 3 (GSK3) phosphorylation site required for plasma membrane localization [9] and a ciliary targeting motif [10]. TRPP2/PC-2 colocalizes with polycystin-1 (PC-1) [11] to form a functional heteromeric complex, and this interaction is mediated by their carboxy termini coiled-coil regions [12]. Moreover, the presence of partner proteins like PC-1 affects the subcellular distribution of TRPP2/PC-2: co-assembly of these two proteins is required for TRPP2/PC-2 channel function at the membrane in some cell types [11]. In addition to PC-1, other adaptor proteins regulate the distribution of the TRPP2/PC-2 channel including GSK3 [9], PIGEA-14 [13] and phosphofurin acidic cluster sorting (PACS) proteins [14]. This dynamic subcellular distribution may allow TRPP2/PC-2 to perform multiple roles required for cellular homeostasis. Understanding how TRPP channels are targeted to and selectively distributed within the cell is critical to understanding their physiological and pathological roles.
Research in simple experimental organisms has revealed numerous unique features about the TRP superfamily. TRP channels were first identified in photoreceptors of Drosophila melanogaster [15]. In Caenorhabditis elegans it was first demonstrated that the LOV-1 (PC-1) and PKD-2 (PC-2) polycystins localize to cilia of male specific sensory neurons where they regulate male mating behavior [16]. In the polycystin neurons (four CEM head neurons, 16 RnB ray and one HOB hook tail neuron) C. elegans PKD-2 localizes to the somatodendritic region and cilium [17, 18]. Within the somatodendritic region PKD-2::GFP labeled vesicles are actively transported from the cell body along the dendrite to the ciliary base [17]. Similar to human PC-2, PKD-2 colocalizes with ER markers in the cell body [17]. The ‘ciliary base’ corresponds to the distal most region of the dendrite and the transition zone [17, 19]. The transition zone is presumably the site where dendritic vesicles dock and membrane proteins are inserted into the ciliary membrane [20]. The ‘cilium proper’ extends from the transition zone to end of the cilium and is composed of a microtubule axoneme and the overlying membrane. PKD-2::GFP localizes to both the ciliary base and cilium proper, and is enriched in the former region [17, 21]. Previously we proposed that a cell body checkpoint restricts PKD-2 to the dendritic compartment, and that a ciliary checkpoint regulates the abundance of PKD-2 in the cilium [17]. PKD-2 levels in the ciliary membrane are regulated by a complex scheme of retrograde transport, degradation, and recycling [21, 22].
To further understand PKD-2 trafficking, we took a molecular approach to identify regions within PKD-2 that are required for localization and function. We considered three main regions of PKD-2: the cytoplasmic N-terminus (amino acids 1–73), the transmembrane (TM) region (aa 74–541), and the cytoplasmic C-terminus (aa 542–716). The N-terminus contains two recognizable motifs: an N-myristoylation sequence (aa 48–53) and a casein kinase II (CK2) phosphorylation site (aa 58–61 [22]). The TM region is the most related to the TRPP2 family and includes 6 hydrophobic regions corresponding to the TM domains and two polycystin motifs in the first extracellular loop. The C-terminus contains multiple CK2 sites that regulate TRPP2 and PKD-2 localization in mammalian cells and transgenic worms, respectively [14, 22, 23]. Our results indicate that distinct regions of PKD-2 mediate trafficking and function and that PKD-2 and human TRPP2/PC-2 are orthologs.
MATERIALS AND METHODS
GFP expression constructs
C-terminal GFP-tagged full length PKD-2 was described in [17] and used as a template for PKD-2 deletion constructs. Truncated pkd-2 cDNA clones were generated by PCR, subcloned into pCR-II TOPO (Invitrogen), and sequenced. These deletion cassettes were cloned in frame into the appropriate vectors containing an upstream Ppkd-2 promoter and downstream GFP [17]. The following constructs were generated: ΔN::GFP (pKK102 and pKK111, encoding amino acids (aa) 67–716 or 64–716), ΔC::GFP (pKK96, aa 1–541), ΔER::GFP (pKK98, aa 1–711), and ΔNΔC::GFP (pKK104, aa 64–541). pXD5 [24] provided a full length FLAG-tagged human polycystin-2 (TRPP2/PC-2) that was subcloned into an expression vector containing the Ppkd-2 promoter (pKK59). A full-length human TRPP2/PC-2 cDNA (minus the stop codon) was PCR amplified from pXD5, subcloned into pCR-II TOPO (Invitrogen), and sequenced. PC-2 was subcloned in frame into a Ppkd-2::GFP vector to generate PC-2::GFP (pKK91, aa 1–968). A YFP tagged PC-2 was similarly generated using the Ppkd-2::venusYFP vector (PC-2::YFP, pKK135). PC-2ΔC::GFP (pKK109, aa 1–693) was constructed by amplifying the amino and TM regions of PC-2 and subcloning this fragment into Ppkd-2::gfp. Portions of the C. elegans PKD-2 and human PC-2 proteins were generated by PCR amplifying the N- terminal, C-terminal, and TM regions of TRPP2/PC-2 or PKD-2 and subcloning fragments into Ppkd-2::GFP. HsNCeTMHsC::GFP is the full length PC-2/PKD-2 chimera containing the N-terminus of PC-2 (aa 1–210), TM region of PKD-2 (aa 64–546) and the PC-2 C-terminus (aa 691–968, pKK151). For construct details please see Supplementary Table 2. Further cloning details available upon request.
C. elegans maintenance and transgenic animals
Nematodes were raised using standard conditions [25]. Strains used for injections and behavioral characterization were: “WT”: CB1490, him-5(e1490)V and PS2172, pha-1(e2123ts)III; him-5(e1490)V, and “mutant”: PT9, pkd-2(sy606)IV; him-5(e1490)V, and PT22, pha-1(e2123ts)III; pkd-2(sy606)IV; him-5(e1490)V.
Transgenic worms were generated using standard microinjection technology. A mixture of deletion construct (50–60 ng/μl) and a marker DNA (pBX1 ~60 ng/μl [26] or Punc122:GFP, ~60 ng/μl [27] was injected into pha-1(e2123ts)III; pkd-2(sy606) IV; him-5(e1490) V or pkd-2(sy606) IV; him-5(e1490) V hermaphrodites, respectively. F2 progeny that stably expressed the marker DNA were maintained as transgenic lines. Multiple (2–5) transgenic lines were characterized for each analysis. For strain details please see Supplementary Table 2.
Epifluorescent or confocal microscopic analysis of transgenic worms
L4 males and hermaphrodites were isolated and placed overnight at 15°C to obtain a synchronized sample. Within twenty-four hours of isolation young adult male worms were mounted on agarose pads set on microscope slides and anesthetized using 5 mM Levamisole or 50 mM NaN3. Fluorescence analysis was performed on a Zeiss Axioskop II using a 63× (NA 1.4) oil objective. Confocal images were collected using a 63× (NA 1.4) objective on a BioRad MRC 1024 laser-scanning microscope (Lasersharp2000™ software). Optical sections were collected between 0.5–1 μm and projected as Z-series that were stored as TIFF files and manipulated using Adobe PhotoShop™. The subcellular distribution of PKD-2::GFP was assessed in the CEM head neurons of staged transgenic males using identical fluorescence microscopy settings. The subcellular distribution patterns of wild-type or mutant PKD-2 proteins were evaluated in three ways. First, PKD-2 distribution was examined in neuronal cell bodies. Wild-type PKD-2::GFP localizes in a reticular pattern in the cell body [17, 18]. ‘Minimal’ distribution was noted when it was not possible to clearly define the cell body or distinguish between the cytoplasm and nucleus; aggregates were counted and scored as having a random or polar location. Second, dendritic localization was examined. PKD-2::GFP is typically not visible in the distal dendrite of transgenic neurons. Increased dendritic localization was scored as ‘abnormal.’ Finally, PKD-2 distribution at the ciliary base and/or cilium proper was determined: it was noted when any construct was visible in the cilium proper. Phenotypes observed in the CEM neurons were also observed in the RnB ray neurons of the tail.
Mating Behavior Analysis
Multiple observation trials of male mating behavior were performed blindly using experimental and control animals in each trial. L4 transgenic males were isolated and maintained overnight at 15°C. For characterization of transgenic strains, GFP or pBX expressing males were selected 12–18 hours prior to behavioral testing or analyzed for GFP expression after mating analysis. Mating behavior assays involved placing 1–6 virgin adult males on a newly seeded plate containing 10–12 unc-31(e169) adult hermaphrodites. The mating behavior of individual males was observed and response behavior was scored using the following criterion: a male who successfully responded to hermaphrodite contact within four minutes was scored as response positive (a male who failed to respond was negative). Responsiveness reflects the percentage of response positive males divided by the total number of males scored. Pair-wise comparisons were made using Mann-Whitney nonparametric and two-sided t-tests.
RESULTS
The PKD-2 transmembrane domain is sufficient for somatodendritic and ciliary targeting
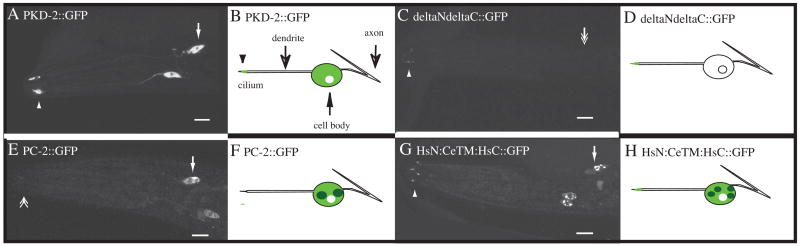
In pkd-2(sy606) null animals, transgenic expression of PKD-2::GFP fully rescues male mating behavior defects [17, 18] and thus represents an appropriate measure of protein localization and function. To determine structure-function-localization relationships, the cytoplasmic tails and portions of the TM region of PKD-2 were deleted (Supplementary Figure 1). Wild-type PKD-2::GFP localizes to a reticular network in the cell body, the proximal dendrite, and cilium (Figure 1A, B). Deletion of the PKD-2 N-terminus (ΔN::GFP contains amino acids 64–716) or the C-terminus (ΔC::GFP aa 1–541) does not affect targeting to the somatodendritic or ciliary region (Supplementary Table 1). However, deletion of both cytosolic domains (ΔNΔC::GFP aa 64–541) results in stable expression only in the ciliary regions (Figure 1C, D, Supplementary Table 1). While it is possible that these deletion proteins are not stable, we consider it a significant observation that all deletion constructs are still targeted to the ciliary subcompartment. These data confirm that the TM region is sufficient for PKD-2 localization and that the PKD-2 cytoplasmic tails do not direct PKD-2 subciliary distribution (Supplementary Table 1)
Figure 1. Subcellular distribution of GFP-tagged PKD-2/PC-2 constructs.
A, B) Confocal micrograph of an adult male expressing GFP-tagged full length PKD-2 (PKD-2::GFP) in the CEM head neurons. Localization is perinuclear within the cell body (arrow), restricted to the proximal dendrite and in cilia (arrowhead). B) Schematic of panel A.
C, D) Deletion of PKD-2 cytosolic tails affects subcellular distribution. There is little observable cell body localization of ΔNΔC::GFP (double arrow) yet ciliary targeting is normal (arrowhead).
E, F) GFP-tagged TRPP2 (PC-2::GFP) is not targeted to cilia (double arrowhead) but is present in perinuclear aggregates in the cell body (arrow). G, H) GFP-tagged chimera containing the cytoplasmic tails of TRPP2 fused with the TM region of PKD-2 (HsN:CeTM:HsC::GFP) is targeted to the cell body (arrow) and cilia (arrowhead). Small perinuclear aggregates are visible in the cell body. Scale bars 10 μm.
Human TRPP2/PC-2 is structurally related to C. elegans PKD-2 (26% identity overall; N-terminus: 8%; TM region: 43%; C-terminus: 17%, Supplementary Figure 2), and localizes to the primary cilia [28], ER [29, 30], plasma membrane [31–33], centrosome [34] and mitotic spindles [35] of renal epithelial cells. To test whether PC-2 is targeted to the somatodendritic and ciliary regions in C. elegans, we used the pkd-2 promoter to drive expression of fluorescent protein (FP)-tagged PC-2 in transgenic nematodes and evaluated the localization of PC-2::GFP or PC-2::YFP (aa 1–968). PC-2::GFP is visible only in the cell body, but not in dendrites or cilia (Figure 1E, F). These results indicate that PC-2 lacks elements necessary for somatodendritic and ciliary targeting in C. elegans sensory neurons. A chimeric protein containing the cytosolic regions of PC-2 and the C. elegans PKD-2 TM domain (HsNCeTMHsC::GFP encodes PC-2 N-terminal aa 1–210, PKD-2 TM region: aa 64–546, PC-2 C-terminus: aa 691–968) is properly transported to the somatodendritic and ciliary regions (Figure 1G, H). These data confirm that the TM region is necessary and sufficient for PKD-2 targeting and that the PC-2 cytoplasmic tails do not direct PKD-2 ciliary targeting.
PKD-2 cytoplasmic tails regulate cell body retention
If the TM region targets PKD-2 to the somatodendritic and ciliary regions, what is the function of the cytoplasmic tails? While ΔNΔC:GFP localizes to cilia, little fusion protein is detected in neuronal cell bodies (Figure 1C, D; 100% of n=203 CEM neurons exhibited ‘minimal’ cell body localization, Supplementary Table 1). In contrast, PKD-2::GFP and ΔN::GFP exhibit normal expression levels in cell bodies (n=310 and 196 CEM neurons scored respectively). Deletion of the entire C-terminus (aa 543–716) reduces cell body distribution (ΔC::GFP, 6% of neurons scored exhibited ‘minimal’ cell body localization (n=178)) and deletion of a predicted ER retention sequence at the C-terminal end of PKD-2 (aa 711–716, DKKEE) also reduces cell body localization (ΔER::GFP (9%, n=174)). We conclude that retention of PKD-2 in the cell body requires both cytoplasmic tails.
The cytoplasmic C-terminus of human PC-2 also regulates cell body distribution. PC-2::GFP forms cytoplasmic aggregates in the cell body of transgenic neurons (Figure 1E, F). Deletion of the C-terminus (PC-2ΔC::GFP (aa 1–693)), which removes CK2 phosphorylation and PACS binding sites of PC-2, eliminates aggregate formation (Supplemental Table 1). The chimeric protein containing the C. elegans PKD-2 TM domain and the cytosolic regions of PC-2 (HsNCeTMHsC::GFP) is properly targeted to the somatodendritic and ciliary regions yet aggregates form in neuronal cell bodies (Figure 1G, H). No cytoplasmic aggregates are formed when a chimeric protein that contains the human PC-2 N-terminus and the C. elegans TM domain (HsNCeTM::GFP) is expressed in vivo (data not shown). These data suggest that the PC-2 cytoplasmic tails do not direct PKD-2 ciliary targeting but do regulate protein abundance and distribution within the cell body.
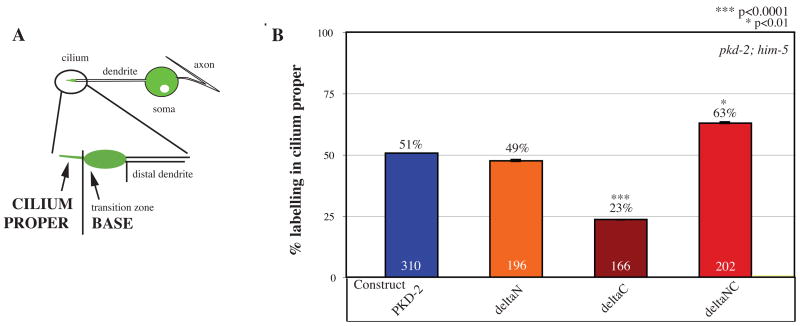
PKD-2 C-terminus regulates subciliary distribution
Do PKD-2 C-tails also regulate protein distribution within the cilium? A polycystin sensory cilium has two morphological sub-compartments (Figure 2A): the cilium proper is comprised of the microtubule axoneme and ciliary membrane and the ciliary base includes the distal dendrite and the transition zone [17, 19]. Full-length PKD-2::GFP is detected in both the cilium proper and base of one half (51% in pkd-2; him-5 mutants) of CEM neurons [17, 18]. PKD-2::GFP is visible only in the base of the remaining 49% of CEM neurons (n=310 total scored). The percentage of neurons expressing ΔN::GFP in both the cilium proper and base is equivalent to PKD-2::GFP (49%, n=196) indicating that the PKD-2 N-terminus is not necessary for ciliary distribution. However, deletion of the C-terminus or just the ER retention motif, significantly reduces distribution in the cilium proper (ΔC::GFP, 23% n=166, p<0.0001 vs. PKD-2::GFP; ΔER::GFP 30% n=168, p<0.0001 vs. PKD-2::GFP, not shown). In contrast, deletion of both cytosolic tails increases distribution in the cilium proper (Figure 2B, ΔNΔC::GFP 63% n=231, p=0.0256 vs. PKD-2::GFP) suggesting that both tails anchor PKD-2 at the ciliary base.
Figure 2. PKD-2 cytosolic tails regulate protein distribution in the cilium proper.
A) Schematic describing ciliary organization and normal PKD-2::GFP distribution in worm polycystin neurons. The cilium proper is located at the distal end of the dendrite beyond the more proximal base and transition zone.
B) Deletion of the C-terminus of PKD-2 reduces targeting of GFP-tagged fusion proteins to the cilium proper. 51% of transgenic pkd-2(sy606); him-5(e1490) (n=310 CEM neurons scored) worms expressing PKD-2::GFP in CEM neurons demonstrate visible labeling of the cilium proper (remaining neurons show localization in the base region, ST1). Deletion of the N-terminus (ΔN::GFP, 49% n=196) does not affect subciliary distribution. Removal of the C-terminus significantly reduces the targeting of deletion constructs to the cilium proper (ΔC::GFP, 23% n=166, p<0.0001 vs PKD-2::GFP). Deletion of both cytosolic termini increases the number of neurons with visible GFP labeling of the cilium proper (ΔNΔC::GFP, 63% n=202, p=0.0178 vs PKD-2::GFP).
PKD-2 cytoplasmic tails regulate sensory function
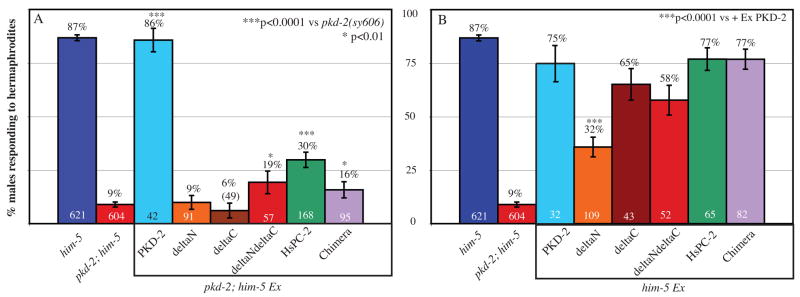
The human TRPP2/PC-2 C-terminus interacts with a variety of proteins, contains an ER localization sequence, and is deleted in several disease causing mutants [11]. To study TRPP2 structure-function relationships, GFP-tagged PKD-2 deletion constructs and domains were expressed in pkd-2 mutants and the response behavior of transgenic males was evaluated (Figure 3A). Male mating behavior is a powerful in vivo assay for PKD-2 function. pkd-2 null mutants are severely defective in two substeps of mating behavior: response to mate contact and location of the mate’s vulva, which requires male tail neurons [18, 36]. The CEM head neurons are required for chemotaxis to sex pheromones produced by Caenorhabditis remanei females [37]. pkd-2 mutant males are also defective in this chemotactic sexual behavior [37]. We therefore consider male mating behavior to be an accurate measure of PKD-2 transgene function. Transgenic expression of PKD-2::GFP rescues the response defect of pkd-2 mutant defects. Expression of an N- or C-terminal PKD-2 deletion construct (ΔN::GFP or C::GFP) fails to rescue pkd-2 response defects, indicating that both the N- and C-termini are required for function. Deletion of an ER retention motif significantly interferes with but does not eliminate mating response (Supplementary Table 2). The mating behavior of transgenic animals expressing ΔNΔC::GFP was evaluated to determine if the TM region retains any unregulated function. Expression of ΔNΔC::GFP partially rescues mating response but not to wild-type levels (Figure 3A). In summary, the cytosolic domains regulate PKD-2 activity.
Figure 3. PKD-2 cytosolic domains regulate protein function in vivo.
A) Transgenic expression of certain deletion and chimeric constructs significantly restores mutant male mating response behavior. Wild-type males (him-5(e1490)V) respond to hermaphrodite contact 87% (n=621) of the time while pkd-2 mutant (pkd-2 (sy606)IV; him-5(e1490)V) adult males respond 9% (n=604) of the time. Transgenic pkd-2 mutants expressing PKD-2::GFP exhibit normal mating response behavior (86% n=42, p<0.0001 vs. pkd-2 mutant). Expression of the ΔN::GFP (9% n=91), or ΔC::GFP (6% n=49) constructs fails to rescue male mating response relative to their controls. Expression of the ΔNΔC::GFP construct significantly rescues male response behavior (19% n=57, p=0.0102 vs. pkd-2 mutant). TRPP2/PC-2 significantly rescues male mating response in transgenic mutant worms (PC-2::GFP, 30% n=168, p<0.0001 vs. pkd-2 mutant). Transgenic expression of a chimeric TRPP2/PKD-2 protein (HsNCeTMHsC::GFP, 16% n=95, p=0.0320 vs. pkd-2 mutant) also weakly but significantly restores response in transgenic mutant worms.
B) Transgenic expression of some deletion constructs interferes with the function of endogenous protein. Wild-type worms expressing PKD-2::GFP respond to hermaphrodites normally (75% (32)), as do transgenic worms expressing ΔC::GFP (65% n=43),ΔNΔC::GFP (58%, n=52), PC-2::GFP (77%, n=65), and the chimera (77%, n=82). Transgenic expression of ΔN::GFP (32%, n=109, p<0.0001 vs. pkd-2 mutant) significantly disrupts the response behavior of wild-type worms.
PKD-2 N-terminus inhibits C-terminal function
TRP channel function requires heteromeric or homomeric interactions and complex formation with adaptor and signaling proteins [3]. If the cytosolic tails of PKD-2 regulate this process, then deletion constructs may interfere with the function of endogenous channels. To test this hypothesis we expressed various constructs in the wild-type background (Figure 3B). PKD-2::GFP has no inhibitory effect while ΔN::GFP significantly interferes with mating behavior of wild-type males. 75% of wild-type males expressing full-length GFP-tagged PKD-2 (n=32) respond to hermaphrodite contact whereas ΔN::GFP transgenic males respond only 32% of the time (n=109). The ΔN::GFP dominant negative phenotype is suppressed by deletion of the C-terminus. Expression of ΔNΔC::GFP does not significantly interfere with wild-type mating (58% n=52) but does minimally rescue the pkd-2 response defect (Figure 3A: 19% n=57). Thus, while the TM region is capable of forming a functional multimeric complex that is targeted to the cilia, this complex does not interfere with endogenous channel function.
The OSM-9 TRPV channel is restricted to the cell body ER of polycystin neurons
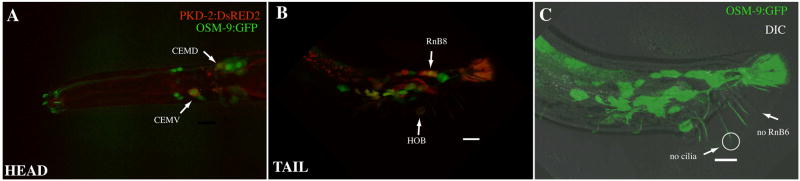
Mammalian TRPP2 interacts with a TRPC (canonical) channel [38]. To test if other TRP channels are coexpressed with pkd-2, we evaluated the expression patterns of several TRP superfamily channel members. The TRPV1 channel OSM-9 is the sole TRPV gene expressed in the polycystin neurons (data not shown). In non sex-specific neurons, OSM-9 is targeted to the cilia only when co-expressed with another TRPV family member [39]. Consistent with this predicted requirement for another TRPV channel in ciliary targeting, OSM-9::GFP is localized to the cell body, but not cilia, of the polycystin neurons (CEM, HOB, RnB, where n = 1–9 except R6B, Figure 4). osm-9 mutant males exhibit normal response and vulva location behavior (data not shown), indicating that OSM-9 does not play a direct role in polycystin-mediated sensory behaviors. Bargmann and colleagues have proposed that non-cilial OSM-9 functions in sensory adaptation [39]. Whether OSM-9 plays a similar role in polycystin neurons is not known.
Figure 4. osm-9 and pkd-2 are coexpressed in polycystin neurons.
A) In the adult male head, OSM-9::GFP (green) is expressed in the cell body and cilia of the amphid, OLQ, and IL1 sensory neurons that are shared with the hermaphrodite [48]. In the CEMs, OSM-9::GFP is restricted to cell bodies (arrows) and not observed in cilia. PKD-2::DsRed2 is targeted to cilia.
B) In the male tail, the ray RnB and hook HOB sensory neuron cell bodies co-express PKD-2::DsRed2 and OSM-9::GFP (arrows).
C) OSM-9::GFP localization is restricted to the cell body of the RnB and HOB sensory neurons, and not observed in cilia (circle and arrow). Like pkd-2, OSM-9 GFP is not expressed in the R6B sensory neuron.
Human PC-2 and C. elegans PKD-2 are orthologs
Unlike PKD-2, TRPP2/PC-2 is not targeted to cilia but is restricted to the soma of C. elegans polycystin neurons (Figure 1). Regardless of this difference in subcellular distribution, TRPP2/PC-2 and PKD-2 are structurally similar and may share conserved function (Supplementary Figure 2). PC-2::GFP partially rescues pkd-2 defects (30% response, n=168, p<0.0001 vs. PKD-2::GFP, Figure 3A). Heterologous expression of a wild-type (unlabelled) PC-2 also complements pkd-2 null mutant defect (Supplemental Table 2). Deletion of the PC-2 cytoplasmic tail (PC-2ΔC::GFP) completely eliminates function (12%, n=85), emphasizing the importance of the C-terminus. Our data supports the hypothesis that TRPP2/PC-2 and PKD-2 are orthologs.
DISCUSSION
TRPP2 is localized to the ER, plasma membrane, and ciliary membrane of renal epithelial cells [8]. The subcellular distribution of TRPP2 depends upon the cell type and differentiation state and is regulated by endogenous elements and interactions with adaptor proteins. In the ER [29], TRPP2 acts as an intracellular calcium channel [30]. TRPP2 also localizes to the plasma membrane where it functions as a Ca2+ permeable, nonselective cation channel [11, 32, 40]. In non-dividing kidney epithelial cells, TRPP2 is targeted to the to the ciliary membrane where it may be anchored to the axoneme for function as a flow sensor [6, 28, 41]. Like TRPP2, C. elegans PKD-2 is targeted to the ER and cilia. In C. elegans sensory neurons, PKD-2 regulates the ability of adult males worms to respond to mating cues that likely involve both chemosensory and mechanosensory components [16, 18, 37]. In spite of diverse physiological roles in the vertebrate kidney and nematode sensory neurons, trafficking of both TRPP2 and PKD-2 involves anterograde movement from the ER to the ciliary membrane as well as retrograde transport back to and retention in the ER/Golgi compartments.
ClustalW analysis showed that C. elegans PKD-2 is closely related to human PC-2/TRPP2 and polycystin-2-like proteins 1 and 2 (PKD2L1/polycystin-L/TRPP3 and PKD2L2/TRPP5) as well as Drosophila and Chlamydomonas PKD-2 [3, 42–44]. TRPP2 and PKD-2 are structurally similar (Supplemental Figure 2). A variety of known targeting elements exist within the cytoplasmic regions of the TRPP2 protein sequence. Some of these motifs are important for anterograde transport from the ER and targeting to the plasma membrane or cilium, others are required for ER retention. Several are unique to TRPP2 and others are conserved within PKD-2. The N-terminal 72 amino acids of TRPP2 are necessary and sufficient to regulate ciliary targeting in mammalian cell culture [10]. This sequence is not conserved in PKD-2. However, there are myristoylation sites in the N-terminus of both PKD-2 (aa 48–53) and TRPP2 (aa 37–42); these may be involved in promoting an association with the plasma membrane. Myristoylation sites are enriched in ciliary and flagellar membrane proteins [45]. Phosphorylation of an N-terminal serine (S76) by glycogen synthase kinase 3(GSK3) is important for ER retention of TRPP2 [9]. It is not known if this mechanism is employed in C. elegans.
CK2 phosphorylation regulates PKD-2 ciliary and TRPP2 plasma membrane abundance in C. elegans and mammalian cells, respectively [14, 22]. The C-terminus of TRPP2 contains elements that regulate ER localization: deletion of the C-terminus results in a dramatic translocation to the plasma membrane [11, 23, 29]. PKD-2 does not possess this motif but instead contains a classic ER retention motif at the end of the carboxy terminus (Supplemental Figure 2). Here we show that this ER localization motif is essential for full PKD-2 activity (Supplemental Table 2). A unique cluster of amino acids in the C-terminus of TRPP2 is recognized by the phosphofurin acidic cluster sorting proteins PACS-1 and -2 [14]. The interaction of the C-terminus of TRPP2 with PACS-1 and -2 is required for the retrieval of TRPP2 to the ER and Golgi and is dependent upon CK2 phosphorylation at serine 812 [14]. Intracellular trafficking of the PACS proteins depends upon the AP-1 adaptor protein. While there is no acidic cluster in the PKD-2 C-terminus, the C. elegans homolog of the mu subunit of AP-1 (unc-101) is required to retain PKD-2 in intracellular vesicles [17]. We conclude that TRPP2 and PKD-2 likely share some trafficking and functional elements, but also possess divergent properties.
We previously showed that a GFP-tagged PKD-2 fragment containing the N-terminus, first TM domain and a portion of the extracellular domain including the first polycystin motif (PKD-2::GFP2) localizes to cilia of transgenic nematodes [16]. Likewise, the amino terminus of TRPP2 is sufficient for ciliary targeting in mammalian cells [46]. Here, we show that the TM region (including 6 hydrophobic domains and the extracellular loop typical of all TRPP2 proteins) is sufficient for somatodendritic and ciliary targeting and that the N- and C-termini are not necessary for PKD-2 localization in vivo. Combined, these data indicate that there are multiple ciliary localization signals in these TRP polycystin channels.
Other members of the TRP family are known to retain localization and function despite the absence of cytosolic tails. Mammalian TRPV4 rescues the osmolarity and mechanosensory defects of an osm-9 mutant and is localized to the somatodendritic and ciliary compartments in a subset of ciliated sensory neurons [47]. Deletion of the cytosolic tails of TRPV4 does not affect the somatodendritic or ciliary targeting of the TRPV4 [47]. Like the PKD-2 ΔNΔC construct, the TRPV4 ΔNΔC deletion construct retains some function [47].
Male worms lacking pkd-2 will not respond to contact with a potential mate. If pkd-2 mutant worms containing human PC-2 expressed under the control of the C. elegans pkd-2 promoter, they will respond to contact with a mate at a significantly higher level. These results indicate that the sensory function of polycystin-2 has been conserved in evolution, and we therefore propose that TRPP2 and PKD-2 are orthologs. In transgenic nematodes, TRPP2 is not visibly targeted to cilia but does partially complement the sensory defects of pkd-2 mutant males. One possibility is that TRPP2 functions in the cell body ER in worm sensory neurons and requires its partner PC-1 for ciliary targeting and function. In some mammalian cell lines, PC-1 is a prerequisite for TRPP2 protein translocation to the plasma membrane [11]. In C. elegans, the PC-1 homolog LOV-1 is required for efficient translocation to the ciliary membrane [17]. It is likely that common and species-specific molecular mechanisms regulate TRPP2 protein trafficking. Understanding how a TRP polycystin channel is transported and functions in its native environment will contribute to a better understanding of ADPKD pathophysiology.
Supplementary Material
Supplementary Figure 1: Schematic representation of the GFP-tagged PKD-2 and TRPP2/PC-2 deletion constructs used in this study. C. elegans PKD-2 constructs are shown in black, human TRPP2/PC-2 sequences are blue. Identified protein domains are indicated in diagram of full length proteins.
Supplementary Figure 2: Alignment of C. elegans PKD-2 and human TRPP2/PC-2 protein sequences. Conserved residues are indicated in purple. Characterized domains/motifs are indicated in the sequences as shared or unique. PKD-2 shares 26% overall identity with TRPP2/PC-2, with similarity varying significantly between regions (EMBOSS Align, http://www.ebi.ac.uk/emboss/align/). There are substantial differences between the PKD-2 and TRPP2/PC-2 cytoplasmic N- and C- termini. The N-terminus of PKD-2 is significantly shorter than PC-2 (73 aa vs. 223) sharing only 8% identity between the two. The C-termini of TRPP2/PC-2 and PKD-2 share 17% identity and contain a coiled-coil domain and an EF hand [5, 16, 49]. Contained within the TM region of TRPP2/PC-2 and PKD-2 are six hydrophobic TM domains, two polycystin domains in the first extracellular loop, and the “p-loop” between TM5 and TM6 that is characteristic of TRP ion channels. The TM regions of TRPP2/PC-2 and PKD-2 are 43% identical.
Supplementary Table 1: Subcellular and subciliary localization phenotypes of deletion constructs.
Supplementary Table 2: Table indicating nature of deletion construct (construct name, encoded amino acids), wild-type and pkd-2 mutant C. elegans strains made with these constructs and mating response behavior of transgenic worms.
Acknowledgments
We thank members of the Barr laboratory for insight and discussion, especially Young-Kyung Bae and Dr. Jamie Lyman-Gingerich for reading the manuscript. Doug Braun, Joe Wolfe, David Saber, Julie Karl and Chad Hall provided technical support. Y.-K. Bae generated the confocal image used in Figure 4. We thank Dr. Judith Kimble for use of her confocal microscope. Drs. Feng Qian and Gregory Germino kindly provided the TRPP2 cDNA. This research was supported by the PKD Foundation (to K.M.K and M.M.B.) and NIH (DK059418 to M.M.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 2.Kottgen M. TRPP2 and autosomal dominant polycystic kidney disease. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbadis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 4.Ambudkar IS. Trafficking of TRP channels: determinants of channel function. Handb Exp Pharmacol. 2007:541–557. doi: 10.1007/978-3-540-34891-7_32. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 6.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 7.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal. 2007;19:444–453. doi: 10.1016/j.cellsig.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch. 2005;451:286–293. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 9.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet. 2006;15:1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 11.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 12.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidaka S, Konecke V, Osten L, Witzgall R. PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem. 2004;279:35009–35016. doi: 10.1074/jbc.M314206200. [DOI] [PubMed] [Google Scholar]
- 14.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. Embo J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 16.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 17.Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 18.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 19.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Wittekind SG, Barr MM. STAM and Hrs Down-Regulate Ciliary TRP Receptors. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Bae YK, Knobel KM, Barr MM. Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol Biol Cell. 2006;17:2200–2211. doi: 10.1091/mbc.E05-10-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- 24.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 25.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res. 1994;22:1762–1763. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyabayashi T, Palfreyman MT, Sluder AE, Slack F, Sengupta P. Expression and function of members of a divergent nuclear receptor family in Caenorhabditis elegans. Dev Biol. 1999;215:314–331. doi: 10.1006/dbio.1999.9470. [DOI] [PubMed] [Google Scholar]
- 28.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 30.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 31.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol. 2003;23:2600–2607. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet. 2002;11:59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- 34.Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, Peters DJ, Doxsey S. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol. 2004;166:637–643. doi: 10.1083/jcb.200405023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J Biol Chem. 2004;279:29728–29739. doi: 10.1074/jbc.M400544200. [DOI] [PubMed] [Google Scholar]
- 36.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 37.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci U S A. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci U S A. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 42.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas flagella. J Cell Biol. 2007 doi: 10.1083/jcb.200704069. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaletta T, Van der Craen M, Van Geel A, Dewulf N, Bogaert T, Branden M, King KV, Buechner M, Barstead R, Hyink D, Li HP, Geng L, Burrow C, Wilson P. Towards understanding the polycystins. Nephron Exp Nephrol. 2003;93:e9–17. doi: 10.1159/000066650. [DOI] [PubMed] [Google Scholar]
- 44.Venglarik CJ, Gao Z, Lu X. Evolutionary conservation of Drosophila polycystin-2 as a calcium-activated cation channel. J Am Soc Nephrol. 2004;15:1168–1177. doi: 10.1097/01.asn.0000125616.42669.51. [DOI] [PubMed] [Google Scholar]
- 45.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Montalbetti N, Wu Y, Ramos A, Raychowdhury MK, Chen XZ, Cantiello HF. Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J Biol Chem. 2006;281:37566–37575. doi: 10.1074/jbc.M603643200. [DOI] [PubMed] [Google Scholar]
- 47.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 49.Koulen P, Duncan RS, Liu J, Cohen NE, Yannazzo JA, McClung N, Lockhart CL, Branden M, Buechner M. Polycystin-2 accelerates Ca2+ release from intracellular stores in Caenorhabditis elegans. Cell Calcium. 2005;37:593–601. doi: 10.1016/j.ceca.2005.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Schematic representation of the GFP-tagged PKD-2 and TRPP2/PC-2 deletion constructs used in this study. C. elegans PKD-2 constructs are shown in black, human TRPP2/PC-2 sequences are blue. Identified protein domains are indicated in diagram of full length proteins.
Supplementary Figure 2: Alignment of C. elegans PKD-2 and human TRPP2/PC-2 protein sequences. Conserved residues are indicated in purple. Characterized domains/motifs are indicated in the sequences as shared or unique. PKD-2 shares 26% overall identity with TRPP2/PC-2, with similarity varying significantly between regions (EMBOSS Align, http://www.ebi.ac.uk/emboss/align/). There are substantial differences between the PKD-2 and TRPP2/PC-2 cytoplasmic N- and C- termini. The N-terminus of PKD-2 is significantly shorter than PC-2 (73 aa vs. 223) sharing only 8% identity between the two. The C-termini of TRPP2/PC-2 and PKD-2 share 17% identity and contain a coiled-coil domain and an EF hand [5, 16, 49]. Contained within the TM region of TRPP2/PC-2 and PKD-2 are six hydrophobic TM domains, two polycystin domains in the first extracellular loop, and the “p-loop” between TM5 and TM6 that is characteristic of TRP ion channels. The TM regions of TRPP2/PC-2 and PKD-2 are 43% identical.
Supplementary Table 1: Subcellular and subciliary localization phenotypes of deletion constructs.
Supplementary Table 2: Table indicating nature of deletion construct (construct name, encoded amino acids), wild-type and pkd-2 mutant C. elegans strains made with these constructs and mating response behavior of transgenic worms.