Abstract
Inactivation of the cyclin-dependent kinase (CDK) inhibitor p21Waf1/Cip1 (CDKN1; hereafter p21) has previously been implicated in the induction of numerical centrosome alterations. It is unclear, however, whether p21 deficiency deregulates the centrosome duplication cycle itself or causes an accumulation of centrosomes due to cell division failure and/or polyploidization. Using a novel marker for maternal centrioles, Cep170, we show here that knock-down of p21 protein expression in murine myeloblasts can stimulate excessive centriole numbers in the presence of only one or two mature centrioles. These results indicate that p21 deficiency can trigger a bona fide overduplication of centrioles and that aberrant centrosome numbers cannot solely be explained by polyploidization as suggested by previous studies. Our findings underscore that impaired p21 expression may function as a driving force for chromosomal instability and highlight the importance of markers for maternal centrioles such as Cep170 to elucidate the pathogenesis of numerical centriole aberrations in tumor cells.
Keywords: p21, centrosome, CDK2, genomic instability, cancer
Aberrant centrosome numbers are detected in virtually all cancers where they can increase the risk for cell division errors and chromosomal instability1–3. Centrosomes function as major microtubule organizing centers during interphase and mitosis in most animal and human cells4. Each centrosome consists of two centrioles, short barrel-shaped microtubule cylinders, embedded in a pericentriolar protein matrix. In order to ensure bipolar mitotic spindle formation, the single centrosome of a cell duplicates precisely once prior to mitosis5. During this process, the two pre-existing centrioles disengage followed by a de novo synthesis of a single daughter centriole adjacent to each of the older centrioles during S phase6. The restriction to a single round of centriole duplication per cell cycle and the formation of only one daughter per maternal centriole prevents multipolar mitotic spindles and their potentially deleterious consequences. Aberrant centrosome numbers in tumor cells can in principle arise through two different mechanisms: a genuine overduplication i.e., excessive formation of daughter centrioles, or, centriole accumulation after failed mitosis and/or polyploidization1, 7. This distinction is critical since a persistent failure of a cell to complete mitosis and to produce viable progeny does not propagate genomic instability in a tumor. Conversely, cells that acquire supernumerary centrioles through overduplication during S phase are likely to have an increased propensity to generate abnormal mitotic spindles in a subsequent mitosis1, 7.
Among the first molecules that have been implicated in the control of centrosome numbers was the CDK inhibitor p218, 9. p21 mainly inhibits cyclin/CDK2 complexes thereby arresting the cell cycle and also binds to PCNA to block DNA synthesis10, 11. p21 has not only antiproliferative activities but is also involved in the assembly of D-cyclin-containing CDK complexes12. p21 is a transcriptional target of p53 and an important mediator of a stress-induced cell cycle arrest13. Mutations of p21 are rare but genetic and/or epigenetic alterations of p21 regulators including p53 or MYC are common findings in malignant tumors.
In one of the first studies related to the role of p21 in centrosome homeostasis, cells deficient of p21 and exposed to ionizing radiation were found to accumulate abnormal numbers of centrosomes following abortive mitoses14. In keeping with these findings, depletion of p21 in human hematopoietic cells was found to cause abnormal centrosome numbers together with a deformed nuclear architecture and polyploidy15. Additional support for a role of p21 in centrosome duplication came from studies showing that p21 overexpression can inhibit centrosome overduplication associated with a prolonged treatment of cells with hydroxyurea16. Similar results were obtained when the related CDK inhibitor p27Kip1 was overexpressed17, 18. Conversely, cells lacking p21 were prone to centrosome amplification following a prolonged S phase arrest19. Inhibitory effects of Cip/Kip type CDK inhibitors on repeated centrosome reproduction were reported in frog embryos18 and Xenopus egg extracts20. Furthermore, the human papillomavirus type 16 E7 (HPV-16 E7) oncoprotein, which inactivates p21 21, 22 and several other key regulators of the G1/S cell cycle checkpoint, was found to stimulate centrosome overduplication23. Cells deficient of p53 frequently show abnormal centrosome numbers24 and the failure to upregulate p21 following genotoxic stress in such cells may contribute importantly to this phenotype25.
Based on these results, no straightforward explanation for the development of abnormal centrosome numbers in p21-deficient cells exists and it is possible that these alterations develop as a secondary phenomenon of cell division failure and/or polyploidization14, 15. This notion is further supported by findings implicating p21 in the prevention of polyploidization26–28.
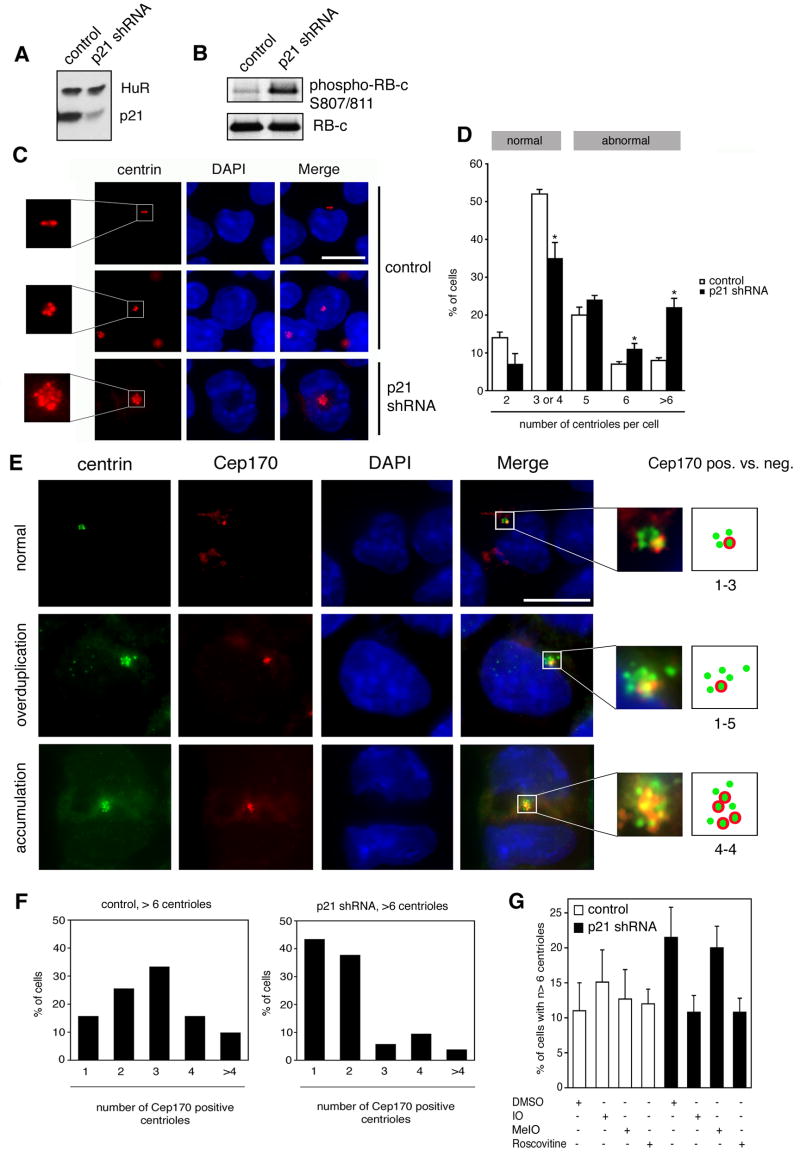
To determine whether deficiency of p21 can cause a genuine centrosome overduplication, we engineered the murine myeloblast cell line 32Dcl3 (32D) to stably express a hairpin siRNA construct targeting murine p2129. A significant reduction of p21 protein expression was detected (Fig. 1A) that was associated with enhanced in vitro CDK2 kinase activity towards a C-terminal fragment of the retinoblastoma protein (pRB) that contained serine residues 807 and 811 (Fig. 1B).
Figure 1.
(A) Immunoblot analysis of 32D murine myeloblasts for p21 expression using a polyclonal antibody (C-19; Santa Cruz) following stable expression of p21 shRNA or control shRNA constructs as previously described29. Immunoblot for HuR is shown to demonstrate loading of equal amounts of protein.
(B) In vitro CDK2 kinase assay of control or p21-depleted 32D cells using a recombinant C-terminal fragment of pRB (RB-c; Upstate) as a substrate followed by immunoblot analysis using a phospho-specific pRB antibody (S807/811; Cell Signaling) as previously described32. CDK2 complexes were isolated by immunoprecipitation with an anti-CDK2 polyclonal antibody (M-2; Santa Cruz).
(C) Immunofluorescence analysis of control or p21-depleted 32D cells for centrin (antibody kindly provided by Jeffrey L. Salisbury, Mayo Clinic, Rochester, MN, USA) as described previously41. Examples of cells with unduplicated centrioles (top panels), duplicated centrioles (middle panels) or supernumerary centrioles (bottom panels) are shown. Nuclei stained with DAPI. Scale bar indiates 10 μm.
(D) Quantification of the percentage of cells with normal centriole numbers (up to four) or abnormal centriole numbers (five, six or more than six) in control (open bars) or p21-depleted cells (black bars). Each bar represents mean and standard error of at least three different experiments with a minimum of 50 cells counted per experiment. Statistical significance was calculated using Student’s t test for independent samples (indicated by asterisks).
(E) Co-immunofluorescence analysis of p21-depleted 32D cells for centrin and Cep17031. Briefly, cells were stained for centrin as previously described41 followed by an anti-Cep170 antibody (kindly provided by Erich A. Nigg, Max Planck Institute for Biochemistry, Martinsried, Germany) and a Rhodamine Red-conjugated anti-rabbit secondary antibody (Jackson Immunoresearch). All antibody incubations following the first primary antibody were for 2 h at 37°C. Examples of cells with normal centriole duplication (one Cep170-positive and three Cep170-negative centrioles; top panels), centriole overduplication (one Cep170-positive and five Cep170-negative centrioles; middle panels) or centriole accumulation (four Cep170-positive and four Cep170-negative centrioles; bottom panels) are shown (see also drawings). Nuclei stained with DAPI. Scale bar indicates 10 μm.
(F) Quantification of the percentage of control (left panel) or p21-depleted (right panel) cells with more than six centrioles and the indicated numbers of Cep170-positive centrioles. Results of a representative experiment with 50 cells counted per cell population are shown.
(G) Quantification of control (open bars) or p21-depleted (black bars) 32D cells with more than six centrioles following 24 h treatment with either 0.1% DMSO used as solvent control or 1 μM indirubin-3′-oxime (IO), 1 μM 1-methyl-indirubin-3′-oxime (MeIO) or 1 μM roscovitine (all reagents kindly provided by Laurent Meijer, CNRS, Roscoff, France). Each bar represents mean and standard error of at least three different experiments with a minimum of 50 cells counted per experiment.
Immunofluorescence analysis of p21-deficient and control cells for centrin30 was performed to visualize individual centrioles. Cells deficient of p21 frequently showed excessive numbers of centrioles (Fig. 1C). Quantification of centriole numbers on a cell-per-cell basis revealed a decrease of cells with normally duplicated centrioles (three or four per cell) from 52% in controls to 35% in p21-deficient populations (p≤0.05; Fig. 1D). At the same time, an increase of cells with overduplicated centrioles (more than four per cell) was detected. Cells containing five (24%), six (11%) or more than six (22%) centrioles were increased in p21-deficient cells when compared to controls (20%, 7%, and 8%, respectively; Fig. 1D). The increase of the proportion of cells with six or more than six centrioles in p21-depleted populations in comparison to controls was found to be statistically significant (p≤0.05 and p≤0.005, respectively).
To analyze whether aberrant centriole numbers were generated through overduplication or accumulation, a double-immunofluorescence analysis for centrin and Cep170 (centrosomal protein of ~170 kD) was performed (Fig. 1E). Cep170 has recently been identified as a novel marker for mature centrioles and can hence be used to distinguish between newly synthesized centrioles (Cep170-negative) and maternal centrioles that have undergone maturation (Cep170-positive)31. Cells usually contain one Cep170-positive maternal centriole (Fig. 1E, top panels) until late G2 phase when the second maternal centriole becomes positive (not shown). A genuine centriole overduplication is characterized by the presence of a single mature centriole and more than three immature daughter centrioles 31. In p21-deficient 32D cells, staining for Cep170 enabled us to distinguish between overduplication of centrioles in the presence of a single Cep170-positive maternal centriole and centriole accumulation leading to multiple Cep170-positive centrioles. The example for centriole overduplication shown in Fig. 1E (middle panels) highlights a cell with a single Cep170-positive mature centriole and five Cep170-negative centrioles whereas centriole accumulation is illustrated by a cell displaying four Cep170-positive centrioles in the presence of the same number of Cep170-negative centrioles (Fig. 1E, bottom panels).
In order to quantify the staining results, we analyzed cells containing more than six centrioles since the difference in the frequency of such cells was most significant between control and p21-deficient populations (p≤0.005; Fig. 1D). Quantification of the Cep170 staining revealed that 58.8% of control cells with more than six centrioles contained three, four or more than four Cep170-positive centrioles (Fig. 1F, left panel). These results suggest cytokinesis failure and/or polyploidization in a high number of control cells. Centriole overduplication, however, is also detectable in control populations as indicated by cells with more than six centrioles and only one or two Cep170-positive centrioles.
In cells deficient of p21, the steady state level of cells with centriole overduplication had significantly shifted and the majority of cells containing more than six centrioles showed only one or two Cep170-positive maternal centrioles (81.1%; Fig. 1F, right panel). It is noteworthy that the proportion of cells with more than six centrioles and only one Cep170-positive centriole was increased from 15.7% in controls to 43.4% in p21-deficient cells indicating that loss of p21 promotes a genuine centriole overduplication. The presence of cells with three, four or more than four Cep170-positive centrioles in p21-deficient cells furthermore suggests that cytokinesis failure and/or polyploidization contribute to the overall number of cells with more than six centrioles but to a much lesser extent than in controls.
Given that centriole duplication requires CDK2 and that p21-depleted cells showed an increased in vitro CDK2 kinase activity (Fig. 1B), we next tested whether chemical CDK inhibitors can abrogate centriole overduplication32. The two CDK inhibitors indirubin-3′-oxime (IO) and roscovitine33, 34 were found to reduce the proportion of p21-deficient cells with excessive centriole numbers to the level detected in control cells (Fig. 1G). In contrast, when we treated cells with 1-methyl-indirubin-3′-oxime (MeIO), no effect on the proportion of cells with centriole overduplication was detected. MeIO is an analogue of IO that lacks the ability to donate a hydrogen bond to the peptidyl carbonyl oxygen of CDK2’s amino acid residue glutamine 81 and thus cannot bind the ATP binding pocket and inhibit its kinase activity33, 35–37. These results are in line with previous findings showing an abrogation of centriole overduplication in the absence of CDK2 activity32, 38.
Several studies have suggested that abnormal centrosome numbers in p21-deficient cells arise from centrosome accumulation14, 15. Here, we provide evidence that p21-deficiency can trigger a bona fide centriole overduplication. Our results do not preclude that cell division failure and/or polyploidization followed by centrosome accumulation contribute to aberrant centrosome numbers in cells with reduced p21 expression. Nonetheless, the fact that p21-deficiency represents an oncogenic stimulus that can provoke centriole overduplication may help to explain previous findings reporting that p21-deficient cells become aneuploid39. A recent report has shown that loss of the CDK inhibitor p16INK4A can promote chromosomal instability through the induction of supernumerary centrosomes40. Despite probable differences in the underlying mechanisms that lead to numerical centrosome abnormalities, these findings, together with results presented here, lend support to the notion that impaired function of CDK inhibitors can trigger centrosome-mediated chromosomal instability. Centriole overduplication results from disruption of the centriole duplication cycle itself and our results suggest that p21 helps to prevent centriole overduplication, most likely by regulating CDK2 activity16–20, 25. Further studies are warranted to explore the precise mechanism through which disruption of the p21/cyclin/CDK2/pRB axis leads to centriole overduplication.
Acknowledgments
We are grateful to Erich A. Nigg (Max Planck Institute for Biochemistry, Martinsried, Germany), Giulia Guarguaglini (University of Rome “La Sapienza”, Rome, Italy), Jeffrey L. Salisbury (Mayo Clinic, Rochester, MN, USA) and Laurent Meijer (CNRS, Roscoff, France) for sharing important reagents. We would like to thank Giulia Guarguaglini for critical reading of the manuscript and helpful comments. Supported by NIH/NCI grant R01 CA112598 and the Susan G. Komen Breast Cancer Foundation.
Abbreviations
- CDK2
cyclin-dependent kinase 2
- DAPI
4′,6′-diamidino-2-phenylindole
- HPV-16
human papillomavirus type 16
- pRB
retinoblastoma protein
- shRNA
short hairpin RNA
References
- 1.Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev Cancer. 2002;2:1–11. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 2.Brinkley BR. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 2001;11:18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- 3.Salisbury JL, Whitehead CM, Lingle WL, Barrett SL. Centrosomes and cancer. Biol Cell. 1999;91:451–60. [PubMed] [Google Scholar]
- 4.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 5.Sluder G. Centrosome duplication and its regulation in the higher animal cell. In: Nigg EA, editor. Centrosomes in Development and Disease. Weinheim, Germany: Wiley-VCH; 2004. pp. 167–89. [Google Scholar]
- 6.Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006 doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 7.Duensing S. A tentative classification of centrosome abnormalities in cancer. Cell Biol Int. 2005;29:352–9. doi: 10.1016/j.cellbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 9.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 10.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–8. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 12.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–62. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 13.Di Leonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–51. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 14.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 15.Mantel C, Braun SE, Reid S, Henegariu O, Liu L, Hangoc G, Broxmeyer HE. p21cip1/waf-1 deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood. 1999;93:1390–8. [PubMed] [Google Scholar]
- 16.Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–32. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- 17.Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2- cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 18.Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci U S A. 1999;96:2817–22. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarapore P, Horn HF, Tokuyama Y, Fukasawa K. Direct regulation of the centrosome duplication cycle by the p53- p21Waf1/Cip1 pathway. Oncogene. 2001;20:3173–84. doi: 10.1038/sj.onc.1204424. [DOI] [PubMed] [Google Scholar]
- 20.Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–4. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 21.Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–11. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11:2090–100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–7. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–7. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 25.D’Assoro AB, Busby R, Suino K, Delva E, Almodovar-Mercado GJ, Johnson H, Folk C, Farrugia DJ, Vasile V, Stivala F, Salisbury JL. Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene. 2004;23:4068–75. doi: 10.1038/sj.onc.1207568. [DOI] [PubMed] [Google Scholar]
- 26.Stewart ZA, Leach SD, Pietenpol JA. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–15. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 28.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–6. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 29.Ghanem L, Steinman RA. p21Waf1 inhibits granulocytic differentiation of 32Dcl3 cells. Leuk Res. 2006;30:1285–92. doi: 10.1016/j.leukres.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 31.Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The Forkhead-associated Domain Protein Cep170 Interacts with Polo-like Kinase 1 and Serves as a Marker for Mature Centrioles. Mol Biol Cell. 2005;16:1095–107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duensing S, Duensing A, Lee DC, Edwards KM, Piboonniyom S, Manuel E, Skaltsounis L, Meijer L, Munger K. Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene. 2004;23:8206–15. doi: 10.1038/sj.onc.1208012. [DOI] [PubMed] [Google Scholar]
- 33.Hoessel R, Leclerc S, Endicott JA, Nobel MEM, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L. Indirubin, the active constituent of a chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–7. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 34.Meijer L, Leclerc S, Leost M. Properties and potential applications of chemical inhibitors of cyclin-dependent kinases. Pharmacol Ther. 1999:82. doi: 10.1016/s0163-7258(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 35.Davies TG, Tunnah P, Meijer L, Marko D, Eisenbrand G, Endicott JA, Nobel MEM. Inhibitor binding to active and inactive CDK2. The crystal structure of a CDK2-cyclin A/indirubin-5′-sulphonate. Structure. 2001;9:389–97. doi: 10.1016/s0969-2126(01)00598-6. [DOI] [PubMed] [Google Scholar]
- 36.Polychronopoulos P, Magiatis P, Skaltsounis L, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase -3 and cyclin-dependent kinases. J Med Chem. 2004;47:935–46. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 37.Meijer L, Skaltsounis L, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CD, Brivanlou A, Dajani R, Tarricone A, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3 selective inhibitors derived from Tyrian purple indirubins. Chem & Biol. 2003;10:1255–66. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–9. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen KC, Heng H, Wang Y, Lu S, Liu G, Deng CX, Brooks SC, Wang YA. ATM and p21 cooperate to suppress aneuploidy and subsequent tumor development. Cancer Res. 2005;65:8747–53. doi: 10.1158/0008-5472.CAN-05-1471. [DOI] [PubMed] [Google Scholar]
- 40.McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD. p16(INK4a) Prevents Centrosome Dysfunction and Genomic Instability in Primary Cells. PLoS Biol. 2006;4:e51. doi: 10.1371/journal.pbio.0040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duensing A, Liu Y, Spardy N, Bartoli K, Tseng M, Kwon JA, Teng X, Duensing S. RNA polymerase II transcription is required for human papillomavirus type 16 E7- and hydroxyurea-induced centriole overduplication. Oncogene. 2006 doi: 10.1038/sj.onc.1209782. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]