Abstract
In vitro, small Hsps (heat-shock proteins) have been shown to have chaperone function capable of keeping unfolded proteins in a form competent for Hsp70-dependent refolding. However, this has never been confirmed in living mammalian cells. In the present study, we show that Hsp27 (HspB1) translocates into the nucleus upon heat shock, where it forms granules that co-localize with IGCs (interchromatin granule clusters). Although heat-induced changes in the oligomerization status of Hsp27 correlate with its phosphorylation and nuclear translocation, Hsp27 phosphorylation alone is not sufficient for effective nuclear translocation of HspB1. Using firefly luciferase as a heat-sensitive reporter protein, we demonstrate that HspB1 expression in HspB1-deficient fibroblasts enhances protein refolding after heat shock. The positive effect of HspB1 on refolding is completely diminished by overexpression of Bag-1 (Bcl-2-associated athanogene), the negative regulator of Hsp70, consistent with the idea of HspB1 being the substrate holder for Hsp70. Although HspB1 and luciferase both accumulate in nuclear granules after heat shock, our results suggest that this is not related to the refolding activity of HspB1. Rather, granular accumulation may reflect a situation of failed refolding where the substrate is stored for subsequent degradation. Consistently, we found 20S proteasomes concentrated in nuclear granules of HspB1 after heat shock. We conclude that HspB1 contributes to an increased chaperone capacity of cells by binding unfolded proteins that are hereby kept competent for refolding by Hsp70 or that are sorted to nuclear granules if such refolding fails.
Keywords: chaperone activity, heat-shock protein 27 (Hsp27), interchromatin granule cluster (IGC), intranuclear distribution, phosphorylation
Abbreviations: Bag-1, Bcl-2-associated athanogene; CMV, cytomegalovirus; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; EGFP, enhanced green fluorescent protein; FBS, foetal bovine serum; Hsp, heat-shock protein; HspB1, gene name for Hsp25 (murine) or Hsp27 (human); IEF, isoelectrofocusing; IGC, interchromatin granule cluster; MAPK, mitogen-activated protein kinase; NLS, nuclear localization sequence; SAPK, stress-activated protein kinase; TEA, triethanolamine; WT, wild-type
INTRODUCTION
The human small Hsp (heat-shock protein) 27 or HspB1 (also referred to as Hsp25 in rodents) confers resistance to a variety of stresses, especially heat shock [1]. The mechanism by which HspB1 protects cells has only been partially elucidated, but clearly the members of the small Hsp family are functionally implicated in the stabilization and/or restoration of the cytoskeletal elements in stressed cells [2,3]. Besides that, in vitro data have indicated that small Hsps can act as ATP-independent molecular chaperones by binding to partially unfolded proteins, preventing their aggregation [4]. HspB1 needs to be dissociated from large oligomers into small oligomers (presumably dimers) [5] and these dimers are thought to bind the unfolded proteins. The client protein cannot be refolded by HspB1 alone, but can be presented to the ATP-dependent Hsp70 chaperone machinery for subsequent refolding [6]. In cells, the oligomeric size of HspB1 seems to be regulated by stress-dependent post-translational modifications. The most prominent modification is early stress-induced phosphorylation of HspB1 [5,7] and HspB1 is known as a terminal substrate in the MAPK (mitogen-activated protein kinase) cascade [8]. Herein, serine residues Ser15 and Ser86 in rodent HspB1 or Ser15, Ser78 and Ser82 in human HspB1 were identified as the sites of phosphorylation. Phosphorylation promotes dissociation of the large HspB1 oligomers to the smaller ones and eventually to tetramers and dimers [9,10].
Direct proof for chaperone activity of HspB1 in living mammalian cells is lacking. Clearly, HspB1 can bind to the cytoskeletal elements, which seems associated with the stabilization or restoration of cellular F-actin after stress [2,3], which may depend on its chaperone-like activities. In addition, overexpressed HspB1 exhibits accelerated recovery from heat stress-induced nuclear protein aggregates [11]. The latter data, however, do not directly provide proof for the improved chaperone activity in vivo or provide insight as to the identity or possible fate (e.g. refolding, degradation) of putative HspB1-bound substrates.
The finding of an effect on recovery from nuclear protein aggregation [11] suggests a role for HspB1 in the cell nucleus. Indeed, in parallel with the well-known stress-induced nuclear translocation of components of the Hsp70 machine [12,13], previous reports describe that a fraction of HspB1 also enters the cell nucleus [3,7,14–17]. Heat or ATP-depletion stress causes detergent insolubility of HspB1 and formation of large (∼106 kDa) HspB1-containing structures inside the nucleus [7,18] visible as granules [3,14,16] that also contain heat-denatured proteins [3]. Neither the mechanism of (sub)nuclear redistribution nor the nuclear function(s) of HspB1 has been elucidated. It has been suggested that phosphorylation might induce the nuclear translocation [15] possibly via effects on the oligomeric size. On the other hand, however, indirect evidence has suggested that HspB1 present in intranuclear granules is dephosphorylated [3,16]. Thus the role of phosphorylation of HspB1 in intranuclear sorting is yet unclear.
For Hsp70, nuclear translocation has been clearly demonstrated to promote resolubilization of heat-induced protein aggregates by reactivation of thermally denatured enzymes in the nuclei of recovering cells [11,19]. More specifically, Hsp70 and Hsp40 accumulate into the nucleoli of heat-shocked cells [12,13], which is associated with the refolding activity of the Hsp70 machine [20]. In contrast, the non-nucleolar granules in which HspB1 accumulates after cellular stress seem not to be associated with refolding [20], but do contain heat-unfolded proteins [3].
In the present study, we provide a comprehensive analysis of the stress-induced HspB1 redistributions in relation to its presumed cellular chaperone functions in vivo. It is demonstrated, for the first time, that HspB1 can prevent the irreversible denaturation/aggregation of heat-unfolded, non-cytoskeletal proteins in living mammalian cells. This activity, however, is not restricted to the nuclear compartment, but is also seen in the cytoplasm. The chaperone activity and nuclear translocation are influenced by phosphorylation, but HspB1 phosphorylation status is insufficient for both events. The appearance in nuclear granules, identified to be IGCs (interchromatin granule clusters), is also associated with HspB1 phosphorylation, but does not seem to be linked to refolding of the HspB1-bound substrates. Rather, indirect evidence suggests that denatured substrate that accumulates with HspB1 in nuclear granules is degraded by the proteasome.
EXPERIMENTAL
Plasmids
pN-luc-EGFP (where EGFP is enhanced green fluorescent protein) expressing NLS (nuclear localization sequence)-tagged luciferase fused to EGFP has been described previously [20]. pCyt-luc-EGFP is a derivative of pN-luc-EGFP lacking the NLS, which results in a luciferase distributed to both the cytoplasm and partially to the nucleus. Plasmids encoding the full-length gene of human Hsp27 and its phosphorylation mutants were created by subcloning the EcoRI–XbaI coding fragment from corresponding templates [pBC KS(+)27 WT/3A/3D/3G, a gift from Dr L. A. Weber, Department of Biology, University of Nevada, Reno, NV, U.S.A.] into the pCMV5 (where CMV is cytomegalovirus) mammalian expression vector to obtain pCMV-hum27 [WT (wild-type)], pCMV-hum27 (3A), pCMV-hum27 (3D) and pCMV-hum27 (3G). These constructs have been verified by sequencing and were used for overexpression of wild-type human HspB1 (WT), phosphorylation mutants HspB1 S15A/S78A/S82A (3A), HspB1 S15D/S78D/S82D (3D), and HspB1 S15G/S78G/S82G (3G) in mammalian cells [21]. pCMV-70 and pCMV-Bag-1 (Bcl-2-associated athanogene) were used to express Hsp70 and Bag-1 [22].
Cell lines and transfections
The rat cardiac fibroblasts Rat-1 and murine fibroblasts L929 were cultured at 37 °C in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% FBS (foetal bovine serum) in the presence of 5% CO2 in air. For transfections, cells were plated on to 6-well plates or 35-mm tissue dishes at 1×105 cells/well. A day later the cells were incubated for 2–5 h in Optimem (Gibco) containing pre-made complexes of DNA and a delivery reagent (GenePorter, Gene Therapy Systems). After incubation the mixture was replaced with DMEM containing 20% (for Rat-1 cells) or 10% (for L929 cells) FBS. After 18–24 h cells were either fixed, immunostained and analysed by fluorescence microscopy or further subcultured for biochemical assays. We use terms Hsp27 and Hsp25 to indicate the human or rodent orthologues respectively.
Immunofluorescence
Cells were plated on to glass coverslips in a 24-well plate at a density of 8×104 cells/well and were grown for 1–2 days. When the cells reached a sub-confluent monolayer stage they were fixed with 4% formaldehyde [prepared in PBS with 0.1% (v/v) Triton X-100] for 5–10 min and processed for immunostaining. To visualize rat Hsp25 and human Hsp27, appropriate rabbit and mouse antibodies from StressGen were used (catalogue numbers SPA801 and SPA800 respectively). Secondary antibodies were Cy3-conjugated goat anti-rabbit and FITC-labelled goat anti-mouse (Jackson Immunoresearch). Localization of luciferase was visualized by its fluorescent EGFP tag without additional immunolabelling. DNA was co-stained with 0.5 μg/ml DAPI (4′,6-diamidino-2-phenylindole).
To reveal 20S proteasome localization, the PW8155 rabbit polyclonal antibody (Affinity Research) was used. For double staining of 20S proteasomes along with Hsp25, cells were first incubated with an anti-20S antibody, washed with PBS-T [PBS containing 0.05% (v/v) Tween 20], and subsequently incubated with secondary Cy3-labelled Fab fragments of goat anti-rabbit antibody (Jackson Immunoresearch). After this incubation and an additional washing step an anti-Hsp25 antibody (SPA801) was applied followed by incubation with a goat anti-rabbit FITC-labelled antibody (Jackson Immunoresearch). The absence of cross-staining was verified by appropriate controls omitting primary antibodies at different steps of staining. Reference cells were stained with either of the antibodies alone. Alternatively, cells transiently expressing human Hsp27 were used for a conventional double-labelling procedure with primary rabbit anti-20S PW8155 and mouse anti-Hsp27 SPA800 antibodies and the corresponding secondary antibodies. This approach produced very similar staining patterns to the method described above thus confirming its reliability. In addition, cells were transiently transfected with a GFP-tagged proteasomal subunit LMP-2 [23] (a gift from Dr J. Neefjes, NKI, Amsterdam, The Netherlands) and subsequently immunostained for endogenous Hsp25.
Electron microscopy
To reveal intranuclear compartments, cells were briefly (45 s) permeabilized in TEA (triethanolamine)/Mg buffer [20 mM TEA, 1% (v/v) Triton X-100 and 4 mM MgCl2] before fixation [24]. After lysis, cells were fixed with 3.7% paraformaldehyde in TEA/Mg buffer for 20 min, blocked sequentially with 20 mM glycine and 1% BSA in the same buffer and immunolabelled with a primary anti-Hsp25 antibody (StressGen, SPA801) and a secondary goat anti-rabbit 5 nm gold-labelled antibody diluted in TEA/Mg buffer. To enlarge the gold particles the technique of silver enhancement was applied as described previously [25,26]. Immunolabelled samples were routinely dehydrated and embedded in Epon 812. A 2% uranyl/acetate solution in 70% ethanol was applied for 1 h in the course of the embedding procedure. Embedded specimens were sliced into ultrathin sections and mounted on formvar-coated copper grids. Specimens were analysed and photographed using a transmission electron microscope (HU-11; Hitachi).
Luciferase in vivo refolding assay
Luciferase inactivation and refolding was measured as described previously with slight modifications. At day 1 after transfection with pN-luc-EGFP (nuclear luciferase) or pCyt-luc-EGFP (cytosolic luciferase) cells were plated on to 24-well plates in quadruplicates and allowed to grow for 24 h. Before heat shock (30 min at 43 °C), the growth medium (DMEM+10% FCS) was refreshed with medium containing 20 mM Mops (pH 7.0). Immediately after heat shock, an equal volume (0.5 ml/well) of growth medium containing a double concentration of cycloheximide (40 μg/ml) was added to block de novo protein synthesis. Cells were lysed and scraped prior to, and 0–180 min after, heat shock in BLUC [25 mM Tris/H3PO4 (pH 7.8), 10 mM MgCl2, 1% (v/v) Triton X-100, 15% glycerol and 1 mM EDTA]. Luciferase activity in the samples was measured for 10 s (integrated) after injecting the substrate buffer (BLUC, 1.25 mM ATP and 0.087 mg/ml D-luciferin) in a Berthhold Lumat 9501.
IEF (isoelectrofocusing)
Separation of Hsp25 isoforms was performed essentially as described previously [3]. Briefly, cells were lysed with IEF buffer [9 M urea, 1% Nonidet P40, 2% 2-mercaptoethanol and protease and phosphatase inhibitor cocktails (Roche)] and run in 7% acrylamide gel containing 4% ampholytes (pH range 5–7) in the IEF apparatus (LKB). The focusing gels were stripped from underlying gel bonds and processed for Western blot analysis.
Size pore-exclusion gel electrophoresis
To estimate the oligomeric size of HspB1 complexes, the native size pore-exclusion pore electrophoresis was performed as described earlier [27] with minor modifications. Rat-1 cells were lysed in PELE sample buffer [20 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 0.5% (v/v) Triton X-100, 10% glycerol, 10 mM NaF, 1 mM dithiothreitol and anti-protease cocktail] before and after heat shock, vortexed for 30 s, and then centrifuged for 10 min at maximum speed in a desktop centrifuge (Eppendorff). Aliquots of the supernatants were applied on a discontinuous 2–18% acrylamide gradient gel. Samples in the gels were run in large electrophoresis chambers at a constant 120 V, usually for 18 h in a cold box. The electrode buffer consisted of 90 mM Tris/HCl, (pH 8.4), 80 mM boric acid and 2 mM EDTA. Separated protein complexes in gels were soaked in SDS-containing Tris/glycine buffer [25 mM Tris (pH 8.3), 192 mM glycine and 0.25% SDS] and then electroblotted on to nitrocellulose in Tris/glycine buffer without SDS.
Western blotting
Transfected cells, growing on 35-mm tissue culture dishes, were washed with PBS and harvested on ice using a cell scraper into BLUC buffer (100 μl/dish) supplemented with anti-protease cocktail (Complete™, Roche). The samples were mixed 1:1 with 2×PAGE sample buffer (100 mM Tris, 4% SDS, 10% 2-mercaptoethanol, 20% glycerol and 0.1 mg/ml Bromophenol Blue), sonicated at 50 W for 5 s and boiled for 5 min. Prepared samples were run in 10% polyacrylamide gels and blotted on to nitrocellulose membranes. After blocking with 5% (w/v) non-fat milk, membranes were probed with a primary rabbit anti-Hsp27 antibody (StressGen, SPA803). Antibody binding was detected using a peroxidase-conjugated secondary antibody (Pierce) and an ECL® (enhanced chemiluminescence) kit (Amersham Pharmacia).
RESULTS
Stress-inducible nuclear redistribution of HspB1
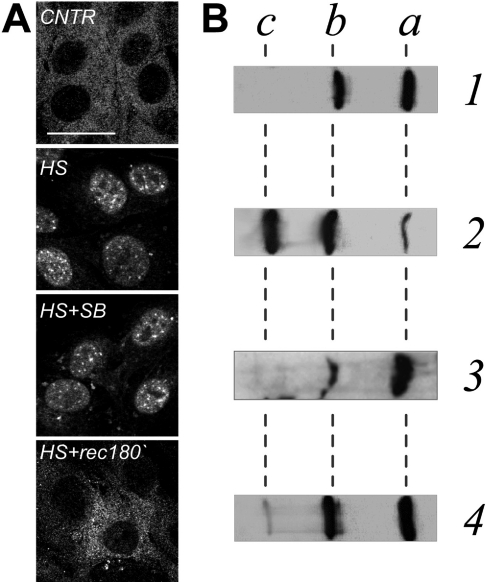
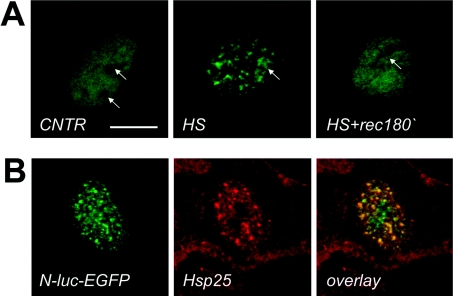
First, the intracellular localization of the endogenous rodent HspB1 (here referred to as Hsp25) was evaluated in Rat-1 cardiac fibroblasts since these cells express sufficient amounts of this protein. Under normal conditions Hsp25 was diffusely distributed throughout the cytoplasm of Rat-1 cells, with minimal nuclear staining (Figure 1A, CNTR). After a mild heat shock (43 °C for 30 min), a significant proportion of Hsp25 accumulated in the nucleus and partially reorganized into nuclear granules (Figure 1A, HS). Nuclear accumulation of Hsp25 and its subnuclear patterns was totally reversible and after 3 h of recovery most of the protein was back in the cytoplasm (Figure 1A, HS+rec180′).
Figure 1. Distribution and phosphorylation patterns of endogenous HspB1 (Hsp25) in Rat-1 fibroblasts before, during and after heat shock.
Localization of Hsp25 in cells (A) or phosphorylation status of Hsp25 in cell lysates (B) at normal conditions (CNTR, lane 1), after heat shock at 43 °C for 30 min without (HS, lane 2) or with (HS+SB, lane 3) the kinase inhibitor SB202190, or after heat shock followed by a recovery for 180 min at 37 °C (HS+rec180′, lane 4). (A) Confocal images of cells immunostained with the antibody against rodent Hsp25. Bar=30 μm. (B) Cells were lysed and run on 7% acrylamide IEF gels and processed for Western blot analysis using the antibody against rodent Hsp25. Positions of non-phosphorylated (a), monophosphorylated (b), and diphosphorylated (c) isoforms are indicated.
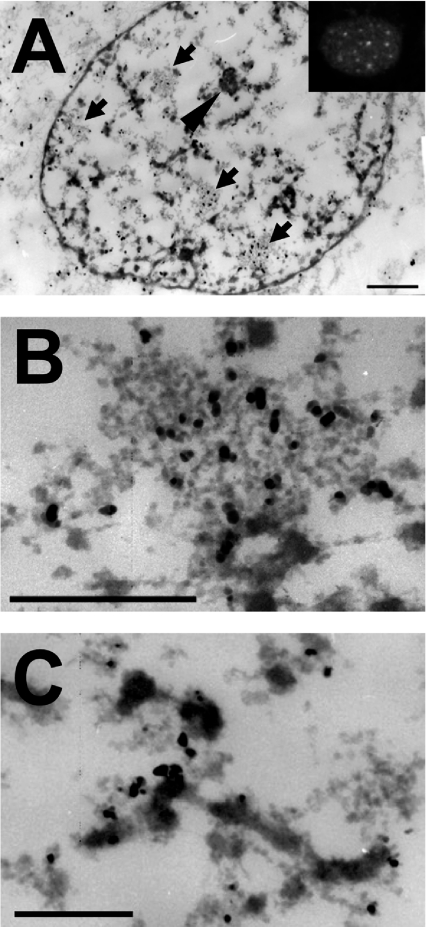
To precisely identify the different nuclear compartments in which Hsp25 resides after heat shock, we employed immunoelectron microscopy. A special protocol was used for this purpose. Prior to fixation, the cells were briefly lysed in TEA buffer containing Mg2+. This treatment liberates the nuclei from the soluble (nucleosolic) components and condenses chromatin [28]. Other architectural features such as nucleolus, IGCs and perichromatin material remain intact (for a review, see [29]) and become more visible [30,31]. At 30 min after heat shock, Hsp25 was detected throughout the nucleus as well as in IGCs of various sizes (Figures 2A and 2B) clearly without labelling inside of condensed chromatin blocks. Interestingly, IGCs also contain splicing factors (e.g. SC35) and are identified at the light microscopy level as nuclear speckles [32]. Consistent with this finding, others [14,33] have revealed that small Hsps co-localize with the SC35 in nuclear speckles (see also below; Figure 6). However, unlike αB-crystallin that localizes to nuclear speckles under non-stress conditions [33], we never found Hsp25 present in nuclear patterns in control cells. Our electron microscopy data also revealed that Hsp25 was decorating perichromatin fibres (Figure 2C) corresponding to a detergent-insoluble diffuse pattern seen by immunofluorescent staining (Figure 2A, inset).
Figure 2. Ultrastructural immunolocalization of Hsp25 in the nuclei of heat-shocked Rat-1 fibroblasts.
To identify the intranuclear localization of rodent Hsp25 after heating at the ultrastructural level, heated (30 min at 43 °C) Rat-1 cells were briefly lysed in TEA buffer containing Mg2+ prior to standard fixation in paraformaldehyde. After immunolabelling with a primary anti-Hsp25 antibody and a secondary goat anti-rabbit 5 nm gold-labelled antibody, ultrathin sections of the cells were processed for transmission electron microscopy. (A) Entire cell nucleus of a cell heat shocked for 30 min. Arrows indicate IGCs and the arrowhead indicates the nucleolus. The inset represents a typical nucleus of a similarly treated cell, stained for immunofluorescence. Bar=1 μm. (B) Enlarged image of an IGC clearly enriched with the Hsp27 label. Bar=1 μm. (C) Enlarged fragment of a perichromatin fibre, containing the label along its periphery. Bar=1 μm.
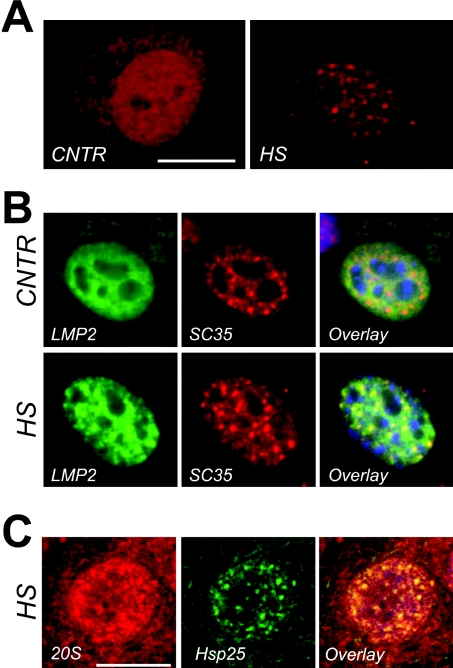
Figure 6. Association between nuclear granules of Hsp25, nuclear speckles and the 20S proteasomal machine.
(A) Confocal images of cell nuclei decorated with anti-20S proteasomal antibodies in the nuclei of cells before (CNTR) and 60 min after a 30 min heat shock at 43 °C (HS). Bar=10 μm. (B) Multicolour channel images of nuclei, expressing GFP-labelled 20S core subunit LMP2 before (panel labelled CNTR) and after a 30-min heat shock at 43 °C (panel labelled HS). Each horizontal series represent the same nucleus in which epigenetical LMP2–GFP (LMP: green) and endogenous SC35 (SC35: red) are revealed and overlaid (Overlay: co-localization is evident by the presence of yellow colour). 20S proteasomes (LMP) become concentrated in nuclear speckles (SC35) after heat shock. Bar=5 μm. (C) Confocal images of heat-shock cells immunostained with the anti-Hsp25 antibody (Hsp25: green) and the anti-20S proteasome antibody (20S: red). Co-localization is evident in the overlay image (Overlay: yellow). On the overlaid images nuclear outline is indicated with blue (DAPI staining). Bar=10 μm.
Stress-inducible HspB1 phosphorylation and its nuclear redistribution
Heat-shock-inducible redistribution of Hsp25 in Rat-1 cells was paralleled by changes in its phosphorylation status. Under non-stress conditions, Hsp25 was only partly phosphorylated (Figure 1B, lane 1) correlating with its cytoplasmic localization (Figure 1A, CNTR). Hsp25 promptly became hyperphosphorylated during heat shock (Figure 1B, lane 2) coinciding with nuclear translocation. The peak of heat-shock-inducible phosphorylation coincided with nuclear accumulation of Hsp25 (Figure 1A, HS). When the phosphorylation status of Hsp25 was back to control levels after longer recovery periods (Figure 1B, lane 4) all Hsp25 had returned to the cytoplasm (Figure 1A, HS+rec180′). To test whether phosphorylation is required for nuclear translocation of Hsp25, cells were pre-treated with SB202190, an inhibitor of stress-induced Hsp25 phosphorylation [3]. Under such conditions Hsp25 was indeed not hyperphosphorylated after heating (Figure 1B, lane 3), but yet was still able to enter the nucleus and form nuclear granules (Figure 1A, HS+SB). So, although phosphorylation of Hsp25 positively correlates with the changes in the subcellular distribution, it is not a prerequisite for nuclear translocation under heat-shock conditions.
Phosphorylation mutants of HspB1 exhibit different properties for nuclear entry
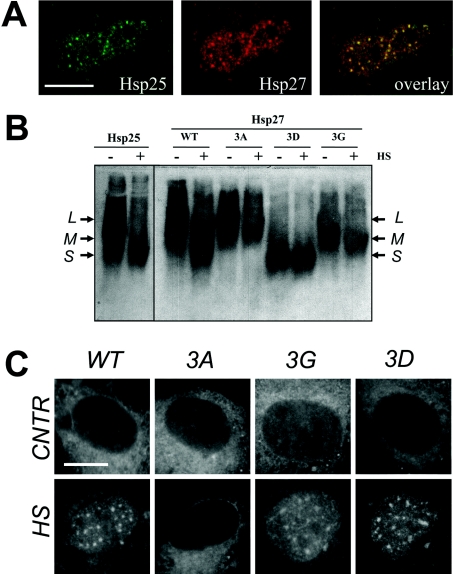
To elucidate further the role of phosphorylation in the nuclear translocation of Hsp25, we transiently expressed human WT HspB1 (further referred to as Hsp27) and three of its phosphorylation mutants in Rat-1 cells. First, in situ double-labelling using species-specific antibodies was used to test whether the ectopically expressed human Hsp27 is localized in the nuclei of heat-shocked cells in the same manner as the endogenous rodent Hsp25. After heat shock, a fraction of the human Hsp27 was found in the cell nucleus concentrated into nuclear granules (Figure 3A), demonstrating that this protein behaves exactly like the endogenous Hsp25 after heat stress.
Figure 3. Intracellular distribution and oligomeric states of endogenous Hsp25 and ectopically expressed WT and mutants of human Hsp27 in Rat-1 fibroblasts.
(A) Rat-1 cells were transiently transfected with a plasmid encoding human Hsp27, heat shocked and processed for immunofluorescence analysis using confocal microscopy. Endogenous rat Hsp25 (green) and ectopically expressed human Hsp27 (red) and the merged image show their excellent co-localization (overlay: yellow). Bar=10 μm. (B) Rat-1 cells were transiently transfected with plasmids encoding WT human Hsp27 or its mutants Hsp27-3A (3A), Hsp27-3G (3G), and Hsp27-3D (3D). The oligomeric size distribution of the endogenous rodent Hsp25 and ectopically expressed Hsp27 variants was analysed in cells before (−) and after (+) heat shock at 43 °C for 30 min (HS). Cell lysates were run by native non-reducing pore size-exclusion electrophoresis, blotted on to nitrocellulose membrane and probed with species-specific anti-Hsp antibodies, recognizing either Hsp25 or Hsp27. Arrows show three major intermediates in oligomers corresponding to their large (L), medium (M) and small (S) size. For details on the various Hsp27 phosphorylation mutants see the text. (C) Rat-1 cells were transiently transfected with plasmids encoding WT human Hsp27 or its mutants Hsp27-3A (3A), Hsp27-3G (3G) and Hsp27-3D (3D) and their intracellular localization was analysed using confocal microscopy as described in (A), before (CNTR) and after heat shock (HS). Bar=10 μm.
Next, Hsp27 phosphorylation mutants were used in which all three known phosphorylatable serine residues at positions 15, 78 and 82 were mutated either to alanine residues (Hsp27-3A, a non-phosphorylatable mutant) or aspartic acid residues (Hsp27-3D, a pseudophosphorylated mutant). Since phosphorylation also dramatically changes the oligomerization state of Hsp27, we also used another non-phosphorylatable mutant, Hsp27-3G, obtained with appropriate substitutions for glycine residues, which results in impaired oligomerization ability. All mutants formed oligomers of different sizes that varied from mutant to mutant, but did not change in response to heat shock (Figure 3B). In contrast, WT proteins (ectopically expressed Hsp27 and endogenous Hsp25) did change their oligomers to smaller sizes following heat shock (Figure 3B).
Surprisingly, under normal growth conditions all mutants, regardless of their phosphorylation or oligomerization status, mostly remained in the cytoplasm (Figure 3C, CNTR). However, heat shock resulted in nuclear accumulation of the smaller oligomers of the Hsp27-3D and Hsp27-3G mutants, whereas the larger multimeric Hsp27-3A did not relocalize into the nucleus (Figure 3C, HS). Since the two non-phosphorylatable mutants (Hsp27-3G and Hsp27-3A) behaved differently in terms of relocalization, nuclear translocation of Hsp27 seems not to depend on phosphorylation. Rather, nuclear entry was associated with reduced oligomeric size. However, since none of the Hsp27 mutants showed nuclear staining under normal growth conditions, oligomeric size alone appeared to be insufficient to trigger nuclear translocation of Hsp27. It must thus be concluded that additional heat-induced events along with the de-oligomerization are required for nuclear accumulation of HspB1.
Overexpression of HspB1 is associated with enhanced refolding of a heat-inactivated reporter protein
To investigate the biological significance of the Hsp27 redistribution after heat shock and whether this may be related to the chaperone function that small Hsps seem to have in cell-free systems [4], the heat-sensitive, nuclear targeted luciferase reporter (N-luc-EGFP) was expressed in Rat-1 cells. The luciferase domain of this chimaeric protein is highly thermosensitive whereas EGFP is thermoresistant. Tracing the signal from EGFP allows us to reveal the localization of N-luc-EGFP in the nucleus regardless of its luciferase activity [20]. Under normal conditions N-luc-EGFP was localized diffusely throughout the nucleosol (Figure 4A, CNTR). Heat shock at 43 °C for 30 min resulted in insolubilization (results not shown) and redistribution of N-luc-EGFP to the nucleoli and some small foci throughout the nucleus (Figure 4A, HS), in a similar manner to previous observations made in other cell lines [3,20]. Interestingly, there was always a fraction of N-luc-EGFP that was dispersed diffusely throughout the nucleus (Figure 4A, HS), despite the finding that N-luc-EGFP completely lost Triton X-100 solubility and became enzymatically inactive upon heat shock (results not shown). Upon recovery (Figure 4A, HS+rec180′), a partial restoration of the N-luc-EGFP staining towards a more diffuse, control-like pattern was observed. The heat-shock-induced N-luc-EGFP re-allocation patterns in and out of the nuclear foci closely resembled those of HspB1 (Figures 1 and 2). Indeed, confocal analysis revealed that non-nucleolar N-luc-EGFP distribution after heat shock substantially overlapped with that of the endogenous Hsp25 (Figure 4B). These results suggest that HspB1 may associate with some heat-unfolded nuclear proteins. However, the subnuclear compartment of such an interaction seems not to be exclusively restricted to the granules only, since fractions of both luciferase and Hsp25 were also found outside the granules in a diffuse pattern. The biological significance of these interactions needs further investigation.
Figure 4. Localization of the nuclear stress-sensitive luciferase reporter before and after heating Rat-1 cells.
(A) Rat-1 cells were transiently transfected with plasmids encoding EGFP-tagged nuclear luciferase (N-luc-EGFP) and either left unheated (CNTR), or heated for 30 min at 43 °C (HS) or heated and allowed to recover for 180 min at 37 °C (HS+rec180′) before processing to confocal microscopy using EGFP as a tracer. Arrows indicate the position of the nucleoli. Note the similarity in kinetic behaviour after heat shock between nuclear luciferase and Hsp25 (Figure 1). Bar=10 μm. (B) Rat-1 cells transiently expressing N-luc-EGFP were heat shocked before processing to confocal microscopy using EGFP as a tracer for luciferase localization (green) or anti-Hsp25 immunostaining (red). The merged image clearly demonstrates an excellent co-localization (overlay: yellow). DNA was counterstained with DAPI in blue.
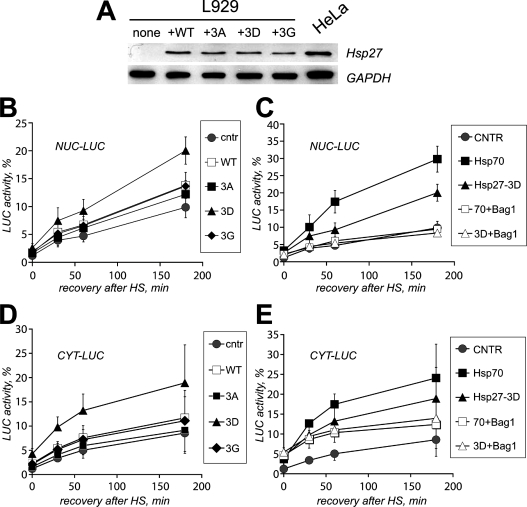
To test for a possible role of Hsp27 in the post-stress fate of denatured proteins, we examined whether HspB1 overexpression had an effect on the renaturation kinetics of N-luc-EGFP and whether this may be associated with the presence of HspB1 in nuclear granules or not. However, when WT human Hsp27 was overexpressed in Rat-1 cells, it had only a minor effect on luciferase reactivation rates (results not shown). Since Rat-1 cells already constitutively express rather high levels of endogenous Hsp25, we reasoned that these levels might already be optimal for refolding. Therefore we turned to the murine fibroblasts L929, which are deprived of constitutive Hsp25 expression. Human Hsp27 was overexpressed in L929 cells and its in situ behaviour after heat shock at 43 °C for 30 min resembled that of the endogenous Hsp25 seen in Rat-1 cells, i.e. it was nuclear and partially present in granules. Also, the various phosphorylation mutants behaved similarly in L929 and Rat-1 cells: after heat shock, Hsp27-3A showed no nuclear entry whereas the Hsp27-3D and Hsp27-3G mutants accumulated in nuclei and nuclear granules (results not shown). The expression of WT Hsp27 in L929 cells (Figure 5A) resulted in a clear enhancement of refolding of heat-denatured luciferase as compared with control cells transfected with an empty vector (Figures 5A and 5B). Equal expression of the non-phosphorylatable Hsp27-3A, incapable of entering the nucleus upon heat shock, had no significant effects on refolding of N-luc-EGFP (Figure 5B). Interestingly, expression of Hsp27-3D to similar levels as WT Hsp27 (Figure 5A) resulted in the largest, 2-fold enhancement of N-luc-EGFP refolding. Finally, Hsp27-3G expression enhanced refolding to similar levels as WT Hsp27 (Figure 5B), which could be explained by relatively poor phosphorylation of human Hsp27 in rodent cells (A. L. Bryantsev, unpublished work). These results show for the first time that Hsp27 contributes to the chaperone activity in living mammalian cells and link this activity to the smallest (phosphorylated) oligomers of HspB1 (see also Figure 3B).
Figure 5. Hsp27 overexpression enhances refolding of heat-inactivated luciferase reporter in L929 cells.
L929 cells lacking endogenous Hsp25 were transiently transfected with plasmids encoding EGFP-tagged nuclear luciferase (A–C) or cytosolic luciferase (D, E) and co-transfected with an empty plasmid (CNTR) or with plasmids encoding WT human Hsp27 or its mutants Hsp27-3A (3A), Hsp27-3G (3G), or Hsp27-3D (3D). (A) Expression levels of WT Hsp27 and its mutants in L929 upon transient transfection as detected by Western blotting (upper lanes). Transfection efficiencies were equal for all plasmids used. Extracts of HeLa cells, naturally expressing moderate levels of human Hsp27, are provided as a positive control. Immunostaining for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to demonstrate equal loading between lanes (bottom panel). (B) L929 cells expressing nuclear luciferase were heated (30 min at 43 °C) and cells were lysed 0–180 min after heating. CHX (cycloheximide, 20 μg/ml) was added just prior to heating to prevent new luciferase synthesis. Recovery of luciferase activity is indicated for L929 cells co-transfected with an empty vector (●) or co-transfected with WT human Hsp27 (□), Hsp27-3A (■), Hsp27-3G (♦), and Hsp27-3D (▲). Activity was calculated as a percentage of the initial luciferase activity before heat shock. (C) Same as (B), but here recovery of nuclear luciferase activity is depicted for cells co-transfected with an empty vector (●), Hsp27-3D alone (▲), Hsp27-3D and Bag-1 (Δ), Hsp70 alone (■) and Hsp70 and Bag-1 (□). (D) Same as (B), now showing recovery of cytosolic luciferase activity (CYT-LUC). (E) Same as (C), now showing recovery of cytosolic luciferase activity (CYT-LUC).
Using cell-free experiments, it has been demonstrated that HspB1 alone can prevent irreversible aggregation of proteins but cannot enable their refolding unless the Hsp70 machine is added. To test whether the effects of HspB1 on in vivo refolding are also dependent on the action of Hsp70, we used co-expression of the Hsp70 modulator Bag-1. We previously showed that overexpression of Bag-1 inhibits Hsp70-dependent refolding in hamster O23 cells [22]. The same was found to be true in L929 cells (Figure 5C). Next, Bag-1 was co-expressed with Hsp27-3D and this completely negated the effect of the small Hsp on refolding (Figure 5C). A similar effect was seen for the WT Hsp27 (results not shown). These results clearly indicate that Hsp27-mediated effects on refolding were completely dependent on the activity of the Hsp70 machine.
Since large fractions of HspB1 remain cytoplasmic during and after heat shock, we next tested whether the observed chaperone-like function was confined to the cell nucleus only. Using a cytosolic luciferase, we found that this was not the case. The overexpression of, especially, Hsp27-3D enhanced refolding of heat-damaged cytosolic luciferase (Figure 5D); the WT and Hsp27-3G had some effects on refolding of heat-denatured cytosolic luciferase, whereas Hsp27-3A had no effect at all (Figure 5D). Again, the action of Hsp27 depended on a functional Hsp70 machine as shown in Figure 5(E) for Hsp27-3D. Similar results were obtained for the WT and Hsp27-3G (results not shown). So, the best chaperone activity of Hsp27-3D seems not to be necessarily linked to its localization in the nucleus and nuclear granules after heat shock but rather is linked to its presence in both compartments in a (pseudo)phosphorylated form.
Nuclear granules of HspB1 co-localize with components of the proteasomal machine
Although we demonstrated that denatured luciferase does partially co-localize to nuclear granules (Figure 4; [3]), we have previously shown that, for more severe heat shock conditions than used here, granules with luciferase were not associated with refolding [20]. Since the effects of Hsp27 on luciferase refolding were also not exclusively linked to its presence in nuclear granules, we wondered what else could be the functional significance of the presence of HspB1 in nuclear granules. Previously, Boelens group has found that the small Hsp αB-crystallin can bind to FBX4 [33], an F-box protein implicated in protein degradation [34], and can recruit this protein to nuclear speckles [33]. Moreover, a direct association between αB-crystallin and one of the proteasomal subunits has also been observed [35]. To test whether HspB1 in nuclear granules may be related to degradation of heat-denatured proteins, we probed heat-treated cells with a specific antibody to the 20S proteasomal complex. Confocal analysis revealed the presence of 20S proteasomal complexes diffusely throughout the nucleus of unheated cells (Figure 6A, CNTR). Heat shock promoted the partial redistribution of these proteasomal complexes into granular structures (Figure 6A, HS). This re-localization was also seen when using transient transfection with the GFP-tagged proteasomal subunit LMP-2 [23] (Figure 6B). These results furthermore revealed that LMP-2 co-localized with the splicing factor SC-35 consistent with these structures being IGCs (Figure 2) or nuclear speckles [32]. Clearly, nuclear speckles are also present under control conditions whereas the proteasomal subunit LMP-2 or 20S complexes only co-associate with these speckles after heat shock (Figures 6A and 6B). Also, only after heat shock, Hsp25 is co-localized in these nuclear speckles with the 20S complexes (Figure 6C) or with LMP-2 (results not shown).
DISCUSSION
The stress-induced nuclear accumulation of several Hsps, including HspB1, suggests the need for their activity in the cell nucleus. Whereas Hsp70/40 translocation to the nucleus has been clearly demonstrated to function in enhanced protein refolding [20,36], the role of nuclear translocation of HspB1 had remained unclear. In the present study, we show that in two different cell lines (Rat-1 and L929) HspB1 is translocated into the nucleus after heat shock. Although the nuclear translocation does not absolutely require HspB1 phosphorylation, the intracellular distribution after heating is associated with the phosphorylation status of HspB1 that is correlated with a reduction in its oligomeric size. Moreover, we show for the first time that ectopically expressed human HspB1 (Hsp27) shows a similar translocation upon heat shock as the endogenously expressed rodent HspB1 (Hsp25) and results in the enhanced capacity of the transfected cells to refold heat-denatured nuclear proteins. Also, overexpression of Hsp27 enhanced refolding in the cytoplasm. This enhanced refolding in both the cytoplasm and the nucleus was dependent on a functional Hsp70 machine. Interestingly, enhanced refolding in the nucleus was not related to the association with nuclear granules as such. Rather, the co-localization of the 20S proteasome with the nuclear granules and the findings that inhibitors of proteasomal degradation reduced the reversibility of Hsp27 in granules (A. L. Bryantsev and H. H. Kampinga, unpublished work) suggest an association of Hsp27 in these granules with degradation of those protein substrates that could not be refolded by the Hsp70 machine.
Nuclear entry and speckle localization of HspB1
HspB1 is known to undergo phosphorylation-sensitive changes in its oligomerization state [9,10]. We now show that these correlate with the presence or absence of HspB1 in the nucleus (see above) since the ectopically expressed human non-phosphorylatable Hsp27-3A mutant does not translocate into the nucleus upon heating, whereas the pseudophosphorylated mutant Hsp27-3D does. In some cell lines, HspB1 was found to be present in nuclei of non-heated cells in a phosphorylation-dependent manner [15,37]. Although we can only speculate why, we (the present study, [3]) and others [14] did not find nuclear HspB1 in non-heated cells, it is interesting to note that the stress-activated kinases p38 MAPK and SAPK (stress-activated protein kinase)/JNK (c-Jun N-terminal kinase) were already active in non-heated hippocampal progenitor cells used in the work by Geum et al. [15]. Maybe the viral infection used in the latter study to deliver Hsp27 or the sensitivity of some cell types to the 37 °C incubation temperature was sufficient to activate the phosphorylation and consequential oligomeric changes needed to drive HspB1 into the nucleus.
As no apparent NLSs are found in mammalian HspB1, the most likely model for nuclear translocation could be that upon phosphorylation, HspB1 oligomers become reduced in size, partially into dimers of 54 kDa, that are small enough to enter passively through the nuclear pore complex. Such an idea is consistent with findings that EGFP-tagged WT HspB1 (114 kDa in size if reduced to dimers) was not detected in the cell nucleus after heating (results not shown and [38]). Although stress-induced phosphorylation may drive nuclear translocation of WT HspB1, the oligomeric size seems to be the most critical prerequisite for nuclear entry rather than phosphorylation. This is supported by the notions that (i) inhibition of phosphorylation did not prohibit nuclear entry and that (ii) the smaller oligomers of the non-phosphorylatable Hsp27-3G mutant also entered the nucleus after heating. Even more so, irrespective of size and/or phosphorylation, additional stress-inducible events seem to be required for nuclear accumulation of HspB1 since none of the smaller oligomeric mutants accumulated in the nucleus under non-stress situations. Perhaps heat stress-inducible but phosphorylation-independent reductions in the HspB1 oligomerization may play a role. However, we see no changes in the size distribution of the Hsp-3G or Hsp-3D mutant after heat shock and yet they do accumulate in the nucleus upon heating. If entry occurs via passive diffusion, one could envision that nuclear retention of HspB1 due to binding to unfolded nuclear substrates is responsible for the accumulation of HspB1 in the nucleus after heat shock. Alternatively, HspB1 nuclear export could be inhibited under heat-shock conditions.
Secondary to the nuclear translocation, we found that HspB1 accumulated in nuclear granules that were identified as interchromatin granules, or nuclear speckles. In line with the results on αB-crystallin [39], it was found that endogenous and transfected HspB1 accumulated in nuclear speckles after heat shock. However, HspB1 behaves differently from its family member αB-crystallin that is already found in nuclear speckles of unheated cells [14,39], whereas no evidence for accumulation in nuclear speckles of HspB1 is apparent under non-stress conditions (the present study and [14]).
Chaperone activity of HspB1: Hsp70-dependent refolding or proteasomal degradation
Our current results present the first evidence that overexpression of HspB1 indeed can enhance the capacity of living mammalian cells to refold heat-denatured, non-cytoskeletal proteins in both the cytoplasm and the nucleus. By inhibiting the activity of the Hsp70 machine with Bag-1 [40], we could demonstrate that this HspB1 positive effect on refolding is lost, consistent with the models based on cell-free data [4,6] that HspB1 can keep unfolded substrates in a form competent for refolding by the Hsp70 machine.
Regarding the role of phosphorylation and oligomeric size, in vitro models have suggested that small HspB1 oligomers (as small as dimers) appear to be the active, substrate-binding species [6,41,42]. Although Hsp70 (or the DnaK system in Escherichia coli) appears to be able to refold small, soluble HspB1–substrate complexes, this system alone cannot efficiently refold substrates from large, insoluble HspB1–substrate aggregates [43]. This is all consistent with our in vivo findings where the pseudophosphorylated Hsp27-3D (smallest oligomers) shows the best chaperone activity both in the nucleus and cytosol. Because Hsp27 size distributions were measured in total lysates, we can only speculate about the oligomeric size of Hsp27 complexes in the nucleus or nuclear speckles. However, our results could be consistent with the following model. As long as these smaller oligomeric HspB1–substrate complexes are not accumulating into the larger oligomeric insoluble complexes, such as the nuclear granules, in which luciferase is indeed insoluble [20], Hsp70 can take over the HspB1-bound substrates. If the latter fails and/or, with time after heat shock, HspB1 accumulates in the nuclear granules with unfolded luciferase still associated to it [3]. In the present study, Hsp70 can no longer act on the damaged reporter as evident from our previous findings [20]. Yet, the reversibility of the speckle-associated Hsp27 and luciferase suggests that there is subsequent processing. It is known that the AAA+ proteins ClpB (E. coli) and Hsp104 (yeast) can rescue large insoluble HspB1-protein aggregates [43,44]. No functional equivalents for ClbB/Hsp104 in mammals have yet been described. Therefore we would like to speculate that in the case where HspB1-bound substrates have accumulated into large aggregates in nuclear granules (formed at sites of regular subnuclear domains, nuclear speckles), they are substrates for degradation by the proteasome. Several (indirect) lines of evidence support this hypothesis. First, nuclear speckles (with accumulated HspB1 and luciferase) stain positively for the 20S proteasomal complex (the present study). Secondly, accumulation of heat-denatured luciferase in speckles is no longer reversible after more severe heat treatments [20] that inhibit activity of the proteasome. Thirdly, HspB1 overexpression in various cell types has been shown to enhance the degradation of ubiquitinated proteins by the proteasome [45]. Fourthly, HspB1 binds to ubiquitin [33,46] and αB-crystallin associates with FBX4, an adapter protein for a ubiquitin ligase and a proteasomal subunit [33] also localizes in SC35-positive nuclear speckles [33]. Fifthly, recent data have clearly shown that nuclear speckles are sites for proteasomal activity [47]. Finally, nuclear αB-crystallin can also enhance refolding of heat-denatured luciferase (results not shown), but this protein that is associated with nuclear speckles before heat shock is released from the speckles during and is present in the nucleosol upon recovery after heating during the time period of refolding (den Engelsman and Boelens, personal communication). However, although the results strongly suggest that HspB1 accumulation in granules is not related to refolding, we realize that none of these results are conclusive evidence for a role of HspB1 and nuclear speckles in the degradation of stress-induced unfolded proteins. Since heat-denatured luciferase is a poor substrate for degradation by the proteasome [22], this could not be tested directly. A possible alternative explanation could be that nuclear granules of HspB1 are the nuclear equivalents of the cytosolic aggresomes [48] in which damaged proteins accumulate after failure of the protein quality control system to refold or degrade them. Irrespective of their function, nuclear speckles (or nuclear granules of HspB1) seem to be ideal sites for storage of unfolded or non-essential proteins as being interchromosomal domains that are relatively less crowded regions outside the expressed chromosomal territories: storage in nuclear speckles will thus not interfere with normal chromatin functioning [49,50].
In summary, we propose that phosphorylation-dependent de-oligomerization of HspB1 provides the cells with a rapid increase in the chaperone capacity to bind unfolded proteins that are hereby kept competent for refolding by Hsp70 or that are sorted to nuclear granules if such refolding fails.
Acknowledgments
This study was financially supported by The International Association for the Promotion of Co-operation with Scientists from the New Independent States (NIS) of the Former Soviet Union (INTAS) (grant 01-0409). We thank Dr W. Boelens for critically reading our manuscript. Also, we thank Dr Lee Weber and Dr J. Neefjes for providing us with the plasmids encoding the (mutant) Hsp27 and LMP2-GFT respectively.
References
- 1.Landry J., Chretien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J. Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J. Biol. Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 3.Bryantsev A. L., Loktionova S. A., Ilyinskaya O. P., Tararak E. M., Kampinga H. H., Kabakov A. E. Distribution, phosphorylation, and activities of Hsp25 in heat-stressed H9c2 myoblasts: a functional link to cytoprotection. Cell Stress. Chaperones. 2002;7:146–155. doi: 10.1379/1466-1268(2002)007<0146:dpaaoh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 5.Ehrnsperger M., Buchner J., Gaestel M. Structure and function of small heat-shock proteins. In: Fink A., Goto Y., editors. Molecular chaperones in the life cycle of proteins. New York: Marcel Dekker, Inc.; 1997. pp. 533–575. [Google Scholar]
- 6.Haslbeck M., Miess A., Stromer T., Walter S., Buchner J. Disassembling protein aggregates in the yeast cytosol. The cooperation of Hsp26 with Ssa1 and Hsp104. J. Biol. Chem. 2005;280:23861–23868. doi: 10.1074/jbc.M502697200. [DOI] [PubMed] [Google Scholar]
- 7.Arrigo A. P., Landry J. Expression and function of the low-molecular-weight heat shock proteins. In: Morimoto R. I., Tissieres A., Georgopoulos C., editors. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 335–373. [Google Scholar]
- 8.Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 9.Kato K., Hasegawa K., Goto S., Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J. Biol. Chem. 1994;269:11274–11278. [PubMed] [Google Scholar]
- 10.Lambert H., Charette S. J., Bernier A. F., Guimond A., Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 11.Kampinga H. H., Brunsting J. F., Stege G. J., Konings A. W., Landry J. Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem. Biophys. Res. Commun. 1994;204:1170–1177. doi: 10.1006/bbrc.1994.2586. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka K., Nakamura H., Sato C. Intracellular distribution of 73000 and 72000 dalton heat shock proteins in HeLa cells. Int. J. Hyperthermia. 1986;2:267–275. doi: 10.3109/02656738609016485. [DOI] [PubMed] [Google Scholar]
- 13.Welch W. J., Mizzen L. A. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J. Cell Biol. 1988;106:1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari A. S., Sridhar R. K., Rangaraj N., Parnaik V. K., Mohan R. C. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for αB-crystallin and Hsp25. Exp. Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Geum D., Son G. H., Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J. Biol. Chem. 2002;277:19913–19921. doi: 10.1074/jbc.M104396200. [DOI] [PubMed] [Google Scholar]
- 16.Loktionova S. A., Ilyinskaya O. P., Gabai V. L., Kabakov A. E. Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human Hsp27 in endothelial cells. FEBS Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- 17.van de Klundert F. A., Gijsen M. L., van den IJssel P. R., Snoeckx L. H., de Jong W. W. αB-crystallin and hsp25 in neonatal cardiac cells: differences in cellular localization under stress conditions. Eur. J. Cell Biol. 1998;75:38–45. doi: 10.1016/s0171-9335(98)80044-7. [DOI] [PubMed] [Google Scholar]
- 18.Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stege G. J., Li G. C., Li L., Kampinga H. H., Konings A. W. On the role of hsp72 in heat-induced intranuclear protein aggregation. Int. J. Hyperthermia. 1994;10:659–674. doi: 10.3109/02656739409022446. [DOI] [PubMed] [Google Scholar]
- 20.Nollen E. A., Salomons F. A., Brunsting J. F., Want J. J., Sibon O. C., Kampinga H. H. Dynamic changes in the localization of thermally unfolded nuclear proteins associated with chaperone-dependent protection. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12038–12043. doi: 10.1073/pnas.201112398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landry J., Lambert H., Zhou M., Lavoie J. N., Hickey E., Weber L. A., Anderson C. W. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 22.Nollen E. A., Brunsting J. F., Song J., Kampinga H. H., Morimoto R. I. Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol. Cell. Biol. 2000;20:1083–1088. doi: 10.1128/mcb.20.3.1083-1088.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reits E. A., Benham A. M., Plougastel B., Neefjes J., Trowsdale J. Dynamics of proteasome distribution in living cells. EMBO J. 1997;16:6087–6094. doi: 10.1093/emboj/16.20.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poliakov V. I., Kir'ianov G. I., Manamsh'ian T. A., Fais D., Chentsov I. [Ultrastructure of the DNP fibrils and of the interchromatin granules in isolated nuclei of the rat liver] Tsitologiia. 1979;21:514–519. [PubMed] [Google Scholar]
- 25.Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 26.Li G., Sudlow G., Belmont A. S. Interphase cell cycle dynamics of a late-replicating, heterochromatic homogeneously staining region: precise choreography of condensation/decondensation and nuclear positioning. J. Cell Biol. 1998;140:975–989. doi: 10.1083/jcb.140.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson L., Borg H., Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972;20:199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- 28.Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J. Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dundr M., Misteli T. Functional architecture in the cell nucleus. Biochem. J. 2001;356:297–310. doi: 10.1042/0264-6021:3560297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiryanov G. I., Smirnova T. A., Polyakov V. Y. Nucleomeric organization of chromatin. Eur. J. Biochem. 1982;124:331–338. doi: 10.1111/j.1432-1033.1982.tb06596.x. [DOI] [PubMed] [Google Scholar]
- 31.Prusov A. N., Polyakov V. Y., Zatsepina O. V., Chentsov Y., Fais D. Rosette-like structures from nuclei with condensed (chromomeric) chromatin but not from nuclei with diffuse (nucleomeric or nucleosomic) chromatin. Cell Biol. Int. Rep. 1983;7:849–858. doi: 10.1016/0309-1651(83)90189-3. [DOI] [PubMed] [Google Scholar]
- 32.Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991;10:3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Engelsman J., Bennink E. J., Doerwald L., Onnekink C., Wunderink L., Andley U. P., Kato K., de Jong W. W., Boelens W. C. Mimicking phosphorylation of the small heat-shock protein αB-crystallin recruits the F-box protein FBX4 to nuclear SC35 speckles. Eur. J. Biochem. 2004;271:4195–4203. doi: 10.1111/j.1432-1033.2004.04359.x. [DOI] [PubMed] [Google Scholar]
- 34.Craig K. L., Tyers M. The F-box: a new motif for ubiquitin-dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 1999;72:299–328. doi: 10.1016/s0079-6107(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 35.Boelens W. C., Croes Y., de Jong W. W. Interaction between αB-crystallin and the human 20S proteasomal subunit C8/α7. Biochim. Biophys. Acta. 2001;1544:311–319. doi: 10.1016/s0167-4838(00)00243-0. [DOI] [PubMed] [Google Scholar]
- 36.Michels A. A., Kanon B., Konings A. W., Ohtsuka K., Bensaude O., Kampinga H. H. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- 37.van den Ijssel P. R., Wheelock R., Prescott A., Russell P., Quinlan R. A. Nuclear speckle localisation of the small heat shock protein αB-crystallin and its inhibition by the R120G cardiomyopathy-linked mutation. Exp. Cell Res. 2003;287:249–261. doi: 10.1016/s0014-4827(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 38.Borrelli M. J., Bernock L. J., Landry J., Spitz D. R., Weber L. A., Hickey E., Freeman M. L., Corry P. M. Stress protection by a fluorescent Hsp27 chimera that is independent of nuclear translocation or multimeric dissociation. Cell Stress. Chaperones. 2002;7:281–296. doi: 10.1379/1466-1268(2002)007<0281:spbafh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Engelsman J., Gerrits D., de Jong W. W., Robbins J., Kato K., Boelens W. C. Nuclear import of αB-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J. Biol. Chem. 2005;280:37139–37148. doi: 10.1074/jbc.M504106200. [DOI] [PubMed] [Google Scholar]
- 40.Takayama S., Bimston D. N., Matsuzawa S., Freeman B. C., Aime-Sempe C., Xie Z., Morimoto R. I., Reed J. C. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haslbeck M., Walke S., Stromer T., Ehrnsperger M., White H. E., Chen S., Saibil H. R., Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Montfort R. L., Basha E., Friedrich K. L., Slingsby C., Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 43.Mogk A., Schlieker C., Friedrich K. L., Schonfeld H. J., Vierling E., Bukau B. Refolding of substrates bound to small Hsps relies on a disaggregation reaction mediated most efficiently by ClpB/DnaK. J. Biol. Chem. 2003;278:31033–31042. doi: 10.1074/jbc.M303587200. [DOI] [PubMed] [Google Scholar]
- 44.Lindquist S., Patino M. M., Chernoff Y. O., Kowal A. S., Singer M. A., Liebman S. W., Lee K. H., Blake T. The role of Hsp104 in stress tolerance and [PSI+] propagation in Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 1995;60:451–460. doi: 10.1101/sqb.1995.060.01.050. [DOI] [PubMed] [Google Scholar]
- 45.Parcellier A., Schmitt E., Gurbuxani S., Seigneurin-Berny D., Pance A., Chantome A., Plenchette S., Khochbin S., Solary E., Garrido C. HSP27 is a ubiquitin-binding protein involved in IκBα proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Engelsman J., Keijsers V., de Jong W. W., Boelens W. C. The small heat-shock protein αB-crystallin promotes FBX4-dependent ubiquitination. J. Biol. Chem. 2003;278:4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- 47.Rockel T. D., Stuhlmann D., von Mikecz A. Proteasomes degrade proteins in focal subdomains of the human cell nucleus. J. Cell Sci. 2005;118:5231–5242. doi: 10.1242/jcs.02642. [DOI] [PubMed] [Google Scholar]
- 48.Kopito R. R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 49.Bridger J. M., Herrmann H., Munkel C., Lichter P. Identification of an interchromosomal compartment by polymerization of nuclear-targeted vimentin. J. Cell Sci. 1998;111:1241–1253. doi: 10.1242/jcs.111.9.1241. [DOI] [PubMed] [Google Scholar]
- 50.Richter K., Reichenzeller M., Gorisch S. M., Schmidt U., Scheuermann M. O., Herrmann H., Lichter P. Characterization of a nuclear compartment shared by nuclear bodies applying ectopic protein expression and correlative light and electron microscopy. Exp. Cell Res. 2005;303:128–137. doi: 10.1016/j.yexcr.2004.09.022. [DOI] [PubMed] [Google Scholar]