Abstract
FXR (farnesoid X receptor), a nuclear receptor activated by BAs (bile acids), is a key factor in the regulation of BA, lipid and carbohydrate metabolism. The recent development of synthetic FXR agonists and knockout mouse models has accelerated the discovery of FXR target genes. In the present study, we identify human fetuin-B as a novel FXR target gene. Treatment with FXR agonists increased fetuin-B expression in human primary hepatocytes and in the human hepatoma HepG2 cell line. In contrast, fetuin-B expression was not responsive to FXR agonist treatment in murine primary hepatocytes. Fetuin-B induction by FXR agonist was abolished upon FXR knockdown by siRNA (small interfering RNA). In addition to the previously described P1 promoter, we show that the human fetuin-B gene is also transcribed from an alternative promoter, termed P2. Transcription via the P2 promoter was induced by FXR agonist treatment, whereas P1 promoter activity was not sensitive to FXR agonist treatment. Two putative FXR-response elements [IR-1 (inverted repeat-1)] were identified in the region –1.6 kb upstream of the predicted P2 transcriptional start site. Both motifs bound FXR–RXR (retinoid X receptor) complexes in vitro and were activated by FXR in transient transfection reporter assays. Mutations in the IR-1 sites abolished FXR–RXR binding and activation. Taken together, these results identify human fetuin-B as a new FXR target gene in human hepatocytes.
Keywords: alternative promoter, cancer, cystatin superfamily, fetuin-B, farnesoid X receptor, retinoid X receptor
Abbreviations: AHSG, α2-Heremans and Schmid glycoprotein; Apo, apolipoprotein; BA, bile acid; BCP, basic calcium phosphate; CDCA, chenodeoxycholic acid; DMEM, Dulbecco's modified Eagle's medium; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift assay; FCS, foetal calf serum; FXR, farnesoid X receptor; FXRE, FXR-binding element; hFXR, human FXR; RXR, retinoid X receptor; hRXR, human RXR; I-BABP, intestinal BA-binding protein; IR-1, inverted repeat-1; PLTP, phospholipid transfer protein; PPARα, peroxisome-proliferator-activated receptor α; QPCR, quantitative PCR; RT, reverse transcriptase; SHP, small heterodimer partner; siRNA, small interfering RNA; TBE, Tris/borate/EDTA; wt, wild-type
INTRODUCTION
The FXR (farnesoid X receptor; NR1H4) is a member of the nuclear receptor family that is expressed at highest levels in the liver, kidney, intestine and the adrenal gland [1,2]. FXR is activated by BAs (bile acids), such as CDCA (chenodeoxycholic acid) [3], or by synthetic FXR agonists, such as GW4064 [4]. In most cases, FXR binds to IR-1 (inverted repeat-1) elements on DNA as a heterodimer with the RXR (retinoid X receptor). FXR regulates the expression of a variety of genes related to BA, cholesterol, triacylglycerol and carbohydrate metabolism. For example, FXR negatively regulates BA synthesis by decreasing the expression of cholesterol-7α-hydroxylase (Cyp7A1), the rate-limiting enzyme in the pathway of BA synthesis [5,6] through the induction of another nuclear receptor, SHP (small heterodimer partner). Enterohepatic circulation of BAs is also controlled by FXR, which modulates the expression of genes such as the I-BABP (intestinal BA-binding protein), BSEP (bile salt export pump) and NTCP (Na+-taurocholate co-transporting polypeptide) [7–9]. FXR is also an important factor in the control of cholesterol metabolism through the inhibition of ApoAI (apolipoprotein AI) expression and through the induction of PLTP (phospholipid transfer protein) expression [10,11]. Similarly, the expression of factors involved in the control of plasma triacylglycerol levels, such as ApoCII (apolipoprotein CII), ApoCIII and PPARα (peroxisome-proliferator-activated receptor α), is under the control of FXR [12–14]. As a consequence, FXR-deficient mice show increased liver and serum triacylglycerol levels [15]. It has also been postulated that the lipogenic response is negatively regulated by FXR [16,18]. Several recent reports have demonstrated that FXR activity is essential to maintain normal glucose homoeostasis [19–21]. The role of FXR in the negative regulation of both hepatic glucose production and lipogenesis has been postulated [17,18]. Finally, fxr gene ablation in mice results in an impaired insulin-sensitivity [19–21]. These reports suggest that FXR is a critical factor involved in lipid and carbohydrate homoeostasis and a potential clinical target to treat the metabolic syndrome.
Fetuin-A and fetuin-B are members of the fetuin family. Human fetuin-B (382 amino acids) shares 22% sequence similarity with fetuin-A. Also known as AHSG (α2-Heremans and Schmid glycoprotein), fetuin-A is a major globular protein in foetal serum and is a member of the cystatin superfamily of cysteine protease inhibitors. Fetuin-A is the only known protein inhibitor of ectopic calcification that is systemic [22]. Owing to a high affinity for hydroxyapatite, fetuin-A accumulates in the skeleton, where it inhibits the formation and precipitation of the apatite precursor mineral, BCP (basic calcium phosphate) [23]. In addition, fetuin-A has been implicated in several processes related to osteogenesis, bone resorption and inhibition of vascular calcification [22,24]. In addition, it has been reported that fetuin-A acts as a negative regulator of insulin receptor tyrosine kinase activity [25]. Fetuin-B is a paralogue of fetuin-A and functional analysis has revealed that fetuin-B is able to act as a calcium phosphate precipitation inhibitor albeit with lower affinity [26,27]. Since the fetuin-B concentration in human serum tends to be low, a major quantitatively systemic contribution to calcium phosphate precipitate removal is rather unlikely. Other authors have reported that fetuin-B expression is up-regulated in certain primary skin tumours and in a subset of human cancer cell lines [28]. Indeed, overexpression of fetuin-B in skin squamous carcinoma cells led to suppression of tumour growth in nude mice [28].
Based on a number of recent reports suggesting a role for FXR in certain cancers [29–31], in the present study we decided to search for novel FXR target genes in hepatocytes using a pharmacological approach and microarray technology. Our results identified the human fetuin-B gene as an FXR agonist-regulated gene. Subsequently, we investigated the molecular mechanisms of fetuin-B regulation by FXR.
MATERIALS AND METHODS
Culture and treatment of HepG2 cells and human primary hepatocytes
Human hepatoblastoma HepG2 cells (A.T.C.C.) were seeded on 6-well plates (5×105 cells/well) and grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FCS (foetal calf serum), streptomycin/penicillin, sodium pyruvate, glutamine and non-essential amino acids (Invitrogen, Cergy-Pontoise, France) at 37 °C in a humidified 5% CO2 atmosphere. Human primary hepatocytes were purchased from Biopredic International (Rennes, France) and grown in the culture medium that was provided by the supplier at 37 °C in a humidified 5% CO2 atmosphere.
To induce FXR target gene expression, HepG2 cells and human primary hepatocytes were treated for 24 h with CDCA (75 μM) (Sigma, Saint Quentin en Yvelines, France) or with the synthetic FXR agonist GW4064 (5 μM) in DMEM supplemented with 1% FCS. siRNA (small interfering RNA)-mediated FXR knockdown was performed as described previously by Sirvent et al. [32,33]. GW4064 was synthesized at GENFIT (Loos, France) as described by Maloney et al. [4].
Culture and treatment of mouse primary hepatocytes
Mouse primary hepatocytes were isolated as described previously [18]. Before the addition of collagenase (0.0025%) (Sigma), livers were perfused with Hanks balanced salt solution (Sigma) at a rate of 5 ml/min via the portal vein. Cell viability was assessed by the Trypan Blue exclusion test and it was always higher than 60%. Hepatocytes were cultured as monolayers in serum-free DMEM (Invitrogen) supplemented with 0.1% BSA, 50 nM dexamethasone, 1% glutamine, 1% gentamicin, 25 mM glucose and 2% ULTROSER (Biosepra) at 37 °C in a humidified 5% CO2 atmosphere. FXR target gene expression was studied in mouse hepatocytes after 24 h of treatment with either CDCA (50 μM) or GW4064 (5 μM).
RNA extraction, PCR analysis and DNA microarray analysis
Total RNA was isolated using the TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized by the reverse transcription reaction from 1 μg of total RNA, 200 ng of random hexamer primers and 200 units of MMLV (Moloney-murine-leukaemia virus) RT (reverse transcriptase) (Invitrogen). PCR analysis was performed using the following reaction buffer: 0.125 mM dNTP, 1.25 mM MgCl2, 1 unit of GoldStar Taq polymerase (Eurogentec, Seraing, Belgium) and 100 nM specific primers. Standard PCR conditions were denaturation at 95 °C for 5 min, followed by 40 cycles of 1 min at 95 °C, 1 min at 55 °C and 1 min at 72 °C. DNA microarray analysis was performed as described previously [32,33].
Real-time QPCR (quantitative PCR) analysis
Real-time QPCR analysis was performed using the Brilliant SYBR Green QPCR master mix (Stratagene) and specific primers according to the manufacturer's instructions. QPCR reactions were run on MX4000 or MX3000 apparatus (Stratagene). Reaction conditions were denaturation at 95 °C for 10 min, followed by 40 cycles of 30 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C. Fetuin-B mRNA levels were normalized to 28S mRNA. Statistical significance was assessed using a Student's t test.
PCR primers
Human fetuin-A Fwd: 5′-ACAACGGCTCCAATTTTCAG-3′; human fetuin-A Rev: 5′-TACACTTGGCTGCCTCTGTG-3′; human fetuin-B P1 Fwd: 5′-AGTTGTAACAAAACCGCTCAA-3′; human fetuin-B P1 Rev: 5′-TCTGTGGAGAACAAGGCCCAG-3′; human fetuin-B P2 Fwd: 5′-AACCCTGAAGGTGTGACTGG-3′; human fetuin-B P2 Rev: 5′-GCACAGGACTAGGATGCAGA-3′; human fetuin-B ex7 Fwd: 5′-TGTGCATCTGGACCTAACCA-3′; human fetuin-B ex7 Rev: 5′-GCTTTTTCTTTGGGGAAAGG-3′; human fetuin-B ex5 Rev: 5′-GTCGGAGGACTGAAGTGAACA-3′; mouse fetuin-B Fwd: 5′-CCCCATTGACTTGTCAAACC-3′; mouse fetuin-B Rev: 5′-ACACCCACTGGTTCATAGCC-3′; 28S Fwd: 5′-AAACTCTGGTGGAGGTCCGT-3′; 28S Rev: 5′-CTTACCAAAAGTGGCCCACTA-3′; cyclophilin Fwd: 5′-GCATACGGGTCCTGGCATCTTGTCC-3′; cyclophilin Rev: 5′-ATGGTGATCTTCTTGCTGGTCTTGC-3′.
Plasmid cloning and site-directed mutagenesis
A human fetuin-B promoter sequence (fragment between –1978 and +263 with respect to the transcriptional start site) was amplified from human genomic DNA by PCR with the following primers: (5′-TGTGGTACCGTTTAAATAACTTGTATTGAG-3′) and (5′-TGTACGCGTCACAGTTGTGCTGGTTTATGA-3′). The resulting PCR product was cloned into the pGL3 basic reporter vector (Promega, Madison, WI, U.S.A.) using KpnI and MluI to yield the reporter plasmid phFetuin-B-pGL3. The sequence of the FXR-response elements was mutated with the QuikChange® site-directed mutagenesis kit (Stratagene). The oligonucleotides 5′-TAAATCAGTTCCCAGAATCATTGTTTTCTGATGTAAGCCCACCAG-3′ and 5′-GTAAGCCCACCAGAAATCAGGGATTTTTGCGTTAGCAGAAGCTGG-3′ were used to introduce mutations into the FXRE1(−1592) (where FXRE1 is FXR-binding element 1) and FXRE2(−1559) sites respectively.
Reporter plasmids that contain three copies of each FXR-response element were produced by the ligation of a synthetic double-stranded DNA cassette into the TK-pGL3 vector cut with SmaI.
The following oligonucleotides were used to assemble 3xFXRRE cassettes. FXRE1(1592)wt (where wt is wild-type) oligonucleotide sense (5′-TTCCCAGGGTCATTGTCCTCTGATGTTCCCAGGGTCATTGTCCTCTGATGTTCCCAGGGTCATTGTCCTCTGATG-3′) and oligonucleotide antisense (5′-CATCAGAGGACAATGACCCTGGGAACATCAGAGGACAATGACCCTGGGAACATCAGAGGACAATGACCCTGGGAA-3′); FXRE1(–1592)mt oligonucleotide sense (5′-TTCCCAGAATCATTGTTTTCTGATGTTCCCAGAATCATTGTTTTCTGATGTTCCCAGAATCATTGTTTTCTGATG-3′) and oligonucleotide antisense (5′-CATCAGAAAACAATGATTCTGGGAACATCAGAAAACATGATTCTGGGAACATCAGAAAACAATGATTCTGGGAA-3′); FXRE2(–1559)wt oligonucleotide sense (5′-CACCAGAGGTCAGGGACCTTTGCGTCACCAGAGGTCAGGGACCTTTGCGTCACCAGAGGTCAGGGACCTTTGCGT-3′) and oligonucleotide antisense (5′-ACGCAAAGGTCCCTGACCTCTGGTGACGCAAAGGTCCCTGACCTCTGGTGACGCAAAGGTCCCTGACCTCTGGTG-3′); FXRE2(–1559)mt oligonucleotide sense (5′-CACCAGAAATCAGGGATTTTTGCGTCACAGAAATCAGGGATTTTTGCGTCACCAGAAATCAGGGATTTTTGCGT-3′) and oligonucleotide antisense (5′-ACGCAAAAATCCCTGATTTCTGGTGACGCAAAAATCCCTGATTTCTGGTGACGCAAAAATCCCTGATTTCTGGTG-3′).
All constructs were verified by restriction enzyme digestion and sequence analysis.
Cell culture and transfection assays
Reporter assays were performed in HepG2 cells. The standard transfection procedure with the cationic lipid RPR 120535B has been described in detail elsewhere [34]. Briefly, reporter plasmids (100 ng) were co-transfected with both pcDNA3 hFXR (human FXR) (30 ng) and pSG5 hRXRα (human RXRα) (30 ng). pSG5 (Stratagene, La Jolla, CA, U.S.A.) and pcDNA3 (Invitrogen, Leek, The Netherlands) empty vectors were used as negative controls. All samples were complemented with pBSK+ plasmid (Stratagene) to bring the total amount of DNA to 500 ng. Following 3 h of incubation, transfection medium was replaced with a medium containing 1% (v/v) FCS with or without CDCA. Luciferase activity was measured 36 h later using a TR717 luminometer (Applied Biosystems). Transfection efficiency was monitored by a co-transfection with 10 ng of a CMV (cytomegalovirus)-driven β-galactosidase expression plasmid. Transfection experiments were performed at least three times in triplicate.
EMSAs (electrophoretic mobility-shift assays)
hFXR and hRXR were produced in vitro using the TNT Quick Coupled Transcription/Translation System (Promega). Double-stranded probes were produced by annealing sense and antisense oligonucleotides (5 μg each) in the following buffer: 50 mM Tris/HCl (pH 8.0), 50 mM KCl, 5 mM MgCl2 and 1 mM DTT (dithiothreitol). Oligonucleotides were denatured at 95 °C for 5 min, then the temperature was reduced to 65 °C for 10 min and finally the mix was incubated at room temperature (20 °C) for 16 h. The probe was subsequently labelled with [γ-32P]ATP using T4-polynucleotide kinase. FXR and/or RXR protein preparations (2 μl) were incubated for 15 min at room temperature in a total volume of 20 μl with 1 μg of poly(dI-dC)·poly(dI-dC) and 1 μg of herring sperm DNA in binding buffer [10 mM Hepes, pH 7.6, 2.5 mM MgCl2, 10% (v/v) glycerol, 2.5 mg/ml BSA, 50 mM NaCl and 0.5 mM DTT] before the radiolabelled probe (1 ng) was added. Binding reactions were further incubated for 15 min and resolved by 6% non-denaturing PAGE in 0.25×TBE (Tris/borate/EDTA) (1×TBE=45 mM Tris/borate and 1 mM EDTA) buffer at room temperature. Excess of the unlabelled probe was included in the binding reaction, just before adding the labelled probe, to perform competition assays.
Oligonucleotides used in the present study were: FXRE1wt sense: 5′-GTTCCCAGGGTCATTGTCCTCTGAT-3′; FXRE1wt antisense: 5′-ATCAGAGGACAATGACCCTGGGAAC-3′; FXRE1mt sense: 5′-GTTCCCAGAATCATTGTTTTCTGAT-3′; FXRE1mt antisense: 5′-ATCAGAAAACAATGATTCTGGGAAC-3′; FXRE2wt sense: 5′-ACCAGAGGTCAGGGACCTTTGCGTT-3′; FXRE2wt antisense: 5′-AACGCAAAGGTCCCTGACCTCTGGT-3′; FXRE2mt sense: 5′-ACCAGAAATCAGGGATTTTTGCGTT-3′; FXRE2mt antisense: 5′-AACGCAAAAATCCCTGATTTCTGGT; I-BABP IR-1 sense: 5′-GATCTCAAGAGGTCATTGACCTT TTTG-3′; I-BABP IR-1 antisense: 5′-CAAAAAGGTCAATGACCTCTTGAGATC-3′.
An FXR-specific antibody SC-1204 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) was added to the binding reaction (2 μl per tube) before addition of the probe, to perform supershift assays.
RESULTS
FXR agonists induce fetuin-B expression in human but not in murine primary hepatocytes
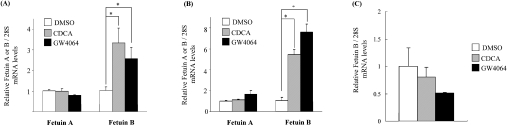
To identify new FXR target genes, we have performed DNA microarray analysis using human primary hepatocytes treated with 5 μM GW4064 for 24 h. The results revealed that fetuin-A (1.6-fold) and fetuin-B (4.5-fold) were induced by FXR agonists (results not shown). To confirm these results, fetuin-A and fetuin-B regulation by FXR was studied in a human hepatoma cell line HepG2 (Figure 1A) and in primary human hepatocytes (Figure 1B). Gene expression analysis by real-time QPCR revealed that fetuin-B but not fetuin-A mRNA level was significantly increased in cells treated with either the natural (CDCA) or synthetic (GW4064) FXR agonist. Surprisingly, fetuin-B expression in mouse primary hepatocytes was completely insensitive to FXR agonist treatment (Figure 1C). These results suggest that the effect of FXR agonists on fetuin-B expression is species-specific.
Figure 1. Fetuin-B expression is induced by FXR agonists in human but not in mouse hepatocytes.
HepG2 cells (A), human primary hepatocytes (B) or mouse primary hepatocytes (C) were treated with either 50 μM CDCA or 5 μM GW4064 for 24 h. Total RNA was extracted and human fetuin-A, human fetuin-B and mouse fetuin-B mRNA levels were measured by real-time QPCR and normalized to 28S mRNA levels. Results are expressed as the fold change over the DMSO; results are means±S.E.M. (n=3). Statistical significance was assessed using a Student's t test. *P<0.05 compared with the control.
Induction of fetuin-B expression by CDCA and GW4064 requires FXR expression
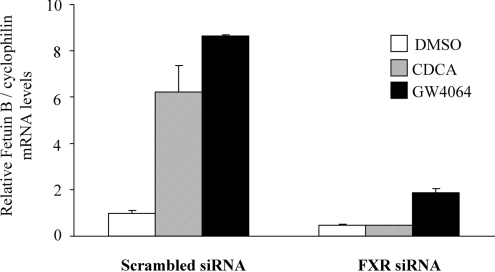
Gene silencing by siRNA was used to determine whether the regulation of fetuin-B expression by FXR agonists observed in human liver cells was dependent on FXR expression. HepG2 cells were therefore transfected with siRNA complexes (anti-FXR and/or negative control) followed by FXR ligand treatment. Basal fetuin-B expression level was reduced by approx. 49% in FXR-siRNA-treated cells as compared with the negative control (Figure 2, white columns). Moreover, CDCA- and GW4064-dependent increase in fetuin-B expression was strongly attenuated in FXR-siRNA-treated cells (Figure 2, grey and black columns). As a control, FXR agonist induction of SHP expression was abolished by FXR siRNA and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression was not affected by either FXR siRNA or the negative control siRNA in the same experiment [32,33].
Figure 2. The induction of fetuin-B expression by CDCA and/or GW4064 is FXR-dependent.
HepG2 cells were transfected with either siRNA targeting FXR or negative control siRNA. At 12 h post siRNA transfection, cells were treated with either 75 μM CDCA or 1 μM GW4064 for the next 24 h. Human fetuin-B mRNA levels were then measured by real-time QPCR and normalized to cyclophilin mRNA levels. Results are expressed as a relative expression compared with DMSO in the control group; results are means±S.E.M. (n=3).
Induction of fetuin-B through FXR occurs via the P2 but not the P1 promoter
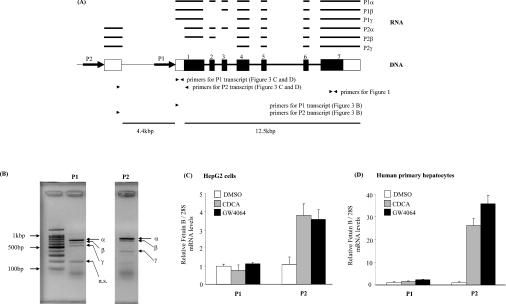
A previous report [28] has identified a fetuin-B promoter (Figure 3A, P1) and three transcripts, which arise by differential splicing of 5′-terminal exons. We were unable to identify any putative FXR-binding sites in the vicinity of this fetuin-B promoter. In addition, a 1.1 kb reporter construct (sequence –1000 to +102 bp) derived from the published promoter sequence was not regulated by FXR overexpression and agonist treatment (results not shown). We therefore suspected that the fetuin-B gene has other, alternative, promoters. A database search identified one expressed sequence tag (DA449793), which mapped both to the 5′-terminal fetuin-B exons and to a genomic sequence that is located 6 kb upstream of the published exon 1. Subsequent RT–PCR analysis confirmed the existence of transcripts that originate at an alternative promoter (Figures 3A and 3B). Detailed analysis with either P1- or P2-specific primers revealed that each promoter produced three different transcripts that differ in size (Figure 3B). Sequencing of P1 promoter transcripts identified the fetuin-B P1α, P1β and P1γ isoforms, which have been previously described by Hsu et al. [28]. Three novel fetuin-B forms, called P2α, β and γ, were identified by sequencing the PCR fragments obtained with the P2-specific primer (Figure 3A). The fetuin-B P2α transcript contains the untranslated exon that follows the P2 promoter, the coding part of exon 1 and exons 2–7. Fetuin-B P2β is missing exon 3. Finally, exons 4–7 are directly spliced to the P2-specific 5′-untranslated exon in the fetuin-B P2γ transcript.
Figure 3. FXR-dependent increase in fetuin-B expression is mediated through the P2 promoter.
(A) Three human fetuin-B transcripts, which originate from the P1 promoter, have previously been described by Hsu et al. [28]. Fetuin-B P1α contains exons 1–7. Fetuin-B P1β is missing exon 2. Fetuin-B P1γ is missing exons 2 and 3. Fetuin-B P2 α, β and γ isoforms arise from the alternative P2 promoter. Fetuin-B P2α contains the untranslated exon P2, the translated part (black box) of exon 1 and exons 2–7. Fetuin-B P2β is missing exon 3. Fetuin-B P2γ is missing exons 1–3. Solid boxes and empty boxes indicate translated and untranslated regions respectively. Arrows indicate P1 and P2 promoters. Arrowheads indicate PCR primers. (B) The differential splicing pattern of the fetuin-B gene was validated by PCR analysis with P1- or P2-specific primers in human primary hepatocytes. PCR products were analysed by gel electrophoresis on 1.5% agarose with TBE and verified by sequencing. Selective, promoter-dependent fetuin-B regulation by FXR in both HepG2 cells (C) and in human primary hepatocytes (D). Relative expression level of human fetuin-B transcripts, which originate either at the P1 or at the P2 promoter, was measured by real-time QPCR and normalized to 28S mRNA. Results are expressed as the fold change compared with DMSO in each cell type; results are means±S.E.M. (n=3).
Real-time QPCR assays, specific for either P1-derived or P2-derived transcripts, were developed to determine which of the two fetuin-B promoters was responsive to FXR. As shown in Figure 3(C), the level of P2-specific transcripts was increased over 3-fold by both CDCA and GW4064 in HepG2 cells, whereas P1-specific transcripts did not respond. Similarly, FXR agonist treatment increased the abundance of P2-specific transcripts 20-fold in human primary hepatocytes (Figure 3D). The level of P1-specific transcripts was not affected by FXR agonist treatment in human primary hepatocytes.
Functional FXR-response elements are present in the human fetuin-B P2 promoter
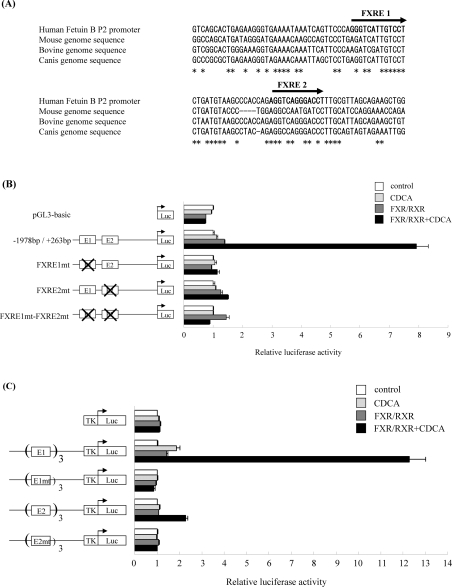
The P2 promoter flanking sequence, obtained from NCBI (National Center for Biotechnology Information) human genome resources (build 36.2 at http://www.ncbi.nlm.nih.gov/genome/guide/human/), was analysed by VISTA tools (http://genome.lbl.gov/vista/index.shtml) for putative FXR-binding sites. Two IR-1 motifs GGGTCATTGTCCT (−1592 to −1577 bp) and AGGTCAGGGACCT (−1559 to −1546 bp) were identified as putative FXR-binding sites. Genomic sequence alignment with fetuin-B orthologues from different species (Figure 4A) showed that only one half-site was conserved in each FXRE motif in mice. G into A conversion at the third position of an IR-1 half-site is usually detrimental to receptor binding.
Figure 4. Functional FXR-response elements are present within the fetuin-B P2 promoter.
(A) Human fetuin-B P2 promoter sequence was aligned with genomic sequences from different species. Two putative FXR-binding sites (FXRE1 and FXRE2) were identified in the human sequence within 1.6 kbp upstream of the predicted transcriptional start site. Boldface letters and arrows indicate putative IR-1 sites. (Mouse chromosome 16: position 19829761 to 19829857 bp; bovine chromosome 1: position 48748 to 48848 bp; Canis chromosome 34: position 6763848 to 6763947 bp.). HepG2 cells were transfected (B, C) with the reporter construct in the presence of either empty vector (control) or FXR and RXR expression vectors. Cells were treated with 50 μM CDCA for 36 h. RLU (relative light unit) signal was normalized to the β-galactosidase activity. FXR agonist-mediated induction is expressed as the fold change over DMSO. (B) Human fetuin-B P2 promoter (−1978 bp/+263 bp) was cloned into the pGL3-basic reporter vector. Mutations were introduced in the FXRE1 or FXRE2 elements (E1mt, E2mt) as described in the Materials and methods section. Values are means±S.E.M. (n=3). (C) HepG2 cells were transfected with reporter constructs containing three copies of either the FXRE1 or FXRE2 element. TK-pGL3 plasmid was used as a control. Mutations were introduced in the FXRE1 or FXRE2 elements (E1mt, E2mt) as described in the Materials and methods section. Luc, luciferase.
The human fetuin-B P2 promoter (positions: –1978 bp/+263 bp) was tested in a transient transfection reporter assay in HepG2 cells. As shown in Figure 4(B), CDCA treatment increased the transcriptional activity of the reporter plasmid (8-fold over control) in the presence of co-transfected FXR and RXR expression plasmids. The control plasmid (pGL3-basic) was not affected. Mutations introduced in each IR-1 motif compromised this induction. These results demonstrate that both FXRE1 and FXRE2 motifs are important for the activation of the fetuin-B P2 promoter by FXR.
Next, both IR-1 motifs were tested in the context of a heterogeneous promoter to check whether those motifs are sufficient to direct FXR-mediated regulation. HepG2 cells were transfected with reporter constructs which carried three copies of either the FXRE1 or FXRE2 motif in the TKpGL3 vector. As shown in Figure 4(C), CDCA-activated FXR–RXR strongly induced the FXRE1 motif (12-fold increase over the control), whereas the FXRE2 motif was only activated 2.5-fold under the same conditions. This difference in the response strength to FXR might probably be attributed to the presence of a guanine at the last position in the second half-site in FXRE2. This position is usually occupied by an adenine in nuclear-receptor-binding motifs. Mutations introduced in either FXRE1 and FXRE2 motifs completely abolished FXR–RXR-mediated regulation (Figure 4C), which confirm that these motifs are functional FXREs mediating FXR-specific regulation of the human fetuin-B promoter.
FXRE1 and FXRE2 motifs identified in the human fetuin-B P2 promoter bind FXR–RXR complexes in an EMSA
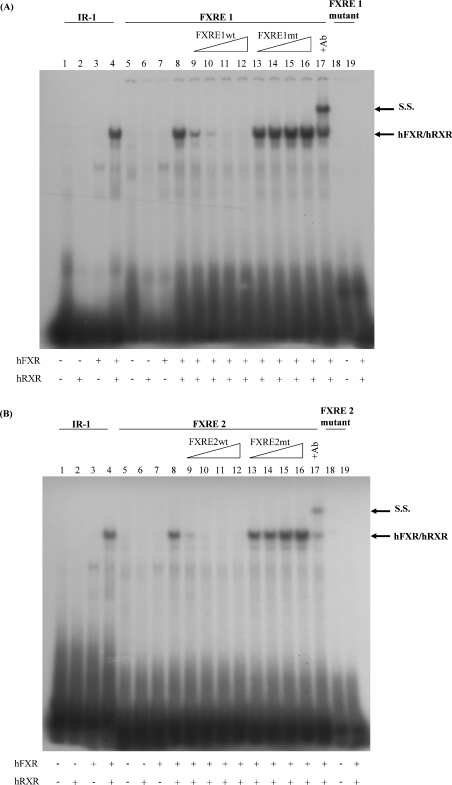
EMSAs were performed to test direct binding of the FXR–RXR complexes to the FXRE1 and FXRE2 motifs. A complex formed by the in vitro translated hFXR–hRXR but not by FXR alone, was able to bind both FXRE1 (Figure 5A, lanes 8–17) and FXRE2 (Figure 5B, lanes 8–17) motifs. FXR–RXR binding was easily displaced with a wt (Figures 5A and 5B, lanes 9–12) but not with a mutant (Figures 5A and 5B, lanes 13–16) unlabelled probe. The identity of the FXR–RXR complex was confirmed by supershift analysis (Figures 5A and 5B, lane 17) using a specific anti-FXR antibody. These results demonstrate that the FXR–RXR heterodimer is able to bind specifically to both FXRE motifs identified in the human fetuin-B promoter P2.
Figure 5. The FXR–RXR complex binds in vitro to the human fetuin-B FXRE1 and FXRE2 motifs.
EMSAs were performed using end-labelled fetuin-B FXREs (wt probe, lanes 5–17; mutant probe, lanes 18–19). An IR-1 element, previously identified in the I-BABP promoter (lanes 1–4), was used as a positive control. Unprogrammed reticulocyte lysate (lanes 1, 5 and 18), hRXR (lanes 2 and 6), hFXR (lanes 3 and 7), hRXR and hFXR (lanes 4, 8–17 and 19). Competition was performed by adding 10-fold (lanes 9 and 13), 50-fold (lanes 10 and 14), 100-fold (lanes 11 and 15), or 200-fold (lanes 12 and 16) excess of unlabelled, wt (lines 9–12) or mutated probe (lines 13–16). Anti-FXR antibody (2 μl per tube) was added to the binding reaction before addition of the probe (lane 17) to obtain the supershift. Ab, antibody; S.S., supershift.
DISCUSSION
Fetuins are abundant foetal serum α-globulins, which belong to the cystatin family of cysteine protease inhibitors. The vast majority of physiological data available to date come from studies on fetuin-A (mouse) or on its human orthologue AHSG, which acts as a systemic inhibitor of ectopic calcification [22,23]. In the clinic, AHSG concentrations in serum were significantly lower in patients on haemodialysis than in healthy controls [35]. Low concentrations of the glycoprotein were associated with raised amounts of CRP (C-reactive protein) and with enhanced cardiovascular and all-cause mortality [35]. In addition, sera from patients on long-term dialysis with low AHSG concentrations showed impaired ex vivo capacity to inhibit calcium phosphate precipitation. Reconstitution of sera with purified AHSG returned this impairment to normal. Similarly, low serum concentrations of AHSG in peritoneal dialysis patients are associated with valvular calcification, higher cardiovascular mortality and development of MIAC (malnutrition, inflammation, atherosclerosis/calcification) syndrome [36].
Fetuin-A is also implicated in a variety of other metabolic regulations, through the inhibition of the TGF-β (transforming growth factor-β) pathway in relation to bone growth and remodelling [24] and acting as a negative regulator of insulin receptor tyrosine kinase activity [24,25]. High levels of fetuin-A in subjects with coronary artery disease were associated with the metabolic syndrome and with a pro-atherogenic lipid profile in the Heart and Soul Study [37].
Fetuin-A and fetuin-B share overlapping but not identical tissue distribution patterns and functions. At the RNA level, the highest expression of both fetuin-A and fetuin-B was reported in the liver, followed by kidney and tongue [27]. Fetuin-B but not fetuin-A mRNA has been detected in mouse ovaries. Interestingly, unlike fetuin-A, the fetuin-B concentration in serum is higher in females [27]. On a structural basis, fetuin-B is clearly homologous with fetuin-A, as illustrated by the shared arrangement in three domains, including two cystatin-like domains D1 and D2 [26]. Mutational analysis showed that in the case of fetuin-A, the BCP activity resides in the N-terminal cystatin-like domain D1 [38]. Functional experiments have shown that fetuin-B is indeed capable of inhibiting BCP, although with lower efficiency [27]. In addition, both fetuin-B expression in human hepatocytes and its protein level in the serum are low as compared with fetuin-A. Therefore fetuin-B can potentially function as a BCP inhibitor in vivo, but it is unlikely to make a major systemic contribution.
In the present study, we show that in human primary hepatocytes the expression of the gene coding for fetuin-B is directly up-regulated by agonist-activated FXR through binding sites which are located in the alternative P2 promoter. Three novel fetuin-B transcripts, which originate at the P2 promoter and arise by differential splicing of their 5′-terminal exons, were identified in the present study. Interestingly, the untranslated exon P2 is directly connected to exon 4 in the fetuin-B P2γ transcript. This situation implies that the translation of the P2γ transcript is initiated at a different site. Indeed, a putative alternative start codon exists in exon 4 at the position 21 bp downstream from splicing site in exon 4. This putative, P2γ-derived protein should only contain the cystatin-like domain D2. The abundance and the functional importance of this new isoform remain to be explored.
Interestingly, fetuin-B expression in primary mouse hepatocytes was not affected by FXR agonist treatment. Genomic sequence alignment of fetuin-B promoters from different species revealed that neither the first FXRE nor the second FXRE is conserved between mouse and human. In addition, those putative FXRE elements in the mouse promoter are positioned relatively far (3.9 kb) from the nearest putative transcriptional start site. Finally, basal fetuin-B concentration in serum is 31 times higher in mouse than in humans [27]. Therefore we postulate that the mouse fetuin-B gene is regulated by different mechanisms than its human orthologue.
Recently, reports have shown that FXR regulates apoptosis and metastasis in breast cancer cells [29,30]. FXR agonists induce apoptosis in human breast carcinoma cells and this effect is diminished by knockdown of FXR [29]. On the other hand, the transfection of fetuin-B expression plasmids suppresses the growth of the carcinoma cell line, B9 cells [28]. These results suggest that the FXR–fetuin-B pathway might regulate proliferation and apoptosis in cancer cells.
In summary, we have demonstrated that FXR agonist treatment results in the activation of fetuin-B gene expression in human hepatocytes. This effect is selectively mediated through the P2 promoter and is species-specific. Since the functional importance of fetuin-B protein has not been firmly established, it is difficult to speculate on the metabolic consequences of FXR-mediated fetuin-B regulation.
Acknowledgments
This work was supported by EU (European Union) Grant Hepadip 018734 and Agence National de la Recherche Grant A05056GS to B. S. and Région Nord Pas-de-Calais/FEDER.
References
- 1.Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 2.Seol W., Choi H. S., Moore D. D. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 4.Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 5.Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 7.Grober J., Zaghini I., Fujii H., Jones S. A., Kliewer S. A., Willson T. M., Ono T., Besnard P. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 8.Ananthanarayanan M., Balasubramanian N., Makishima M., Mangelsdorf D. J., Suchy F. J. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 2000;276:8857–8865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 9.Denson L. A., Sturm E., Echevarria W., Zimmerman T. L., Makishima M., Mangelsdorf D. J., Karpen S. J. The orphan nuclear receptor, SHP, mediates bile acid-induced inhibition of the rat bile acid transporter, NTCP. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 10.Claudel T., Sturm E., Duez H., Torra I. P., Sirvent A., Kosykh V., Fruchart J. C., Dallongeville J., Hum D. W., Kuipers F., Staels B. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urizar N. L., Dowhan D. H., Moore D. D. The farnesoid X-activated receptor mediates bile acid activation of phospholipid transfer protein gene expression. J. Biol. Chem. 2000;275:39313–39317. doi: 10.1074/jbc.M007998200. [DOI] [PubMed] [Google Scholar]
- 12.Kast H. R., Nguyen C. M., Sinal C. J., Jones S. A., Laffitte B. A., Reue K., Gonzalez F. J., Willson T. M., Edwards P. A. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 13.Claudel T., Inoue Y., Barbier O., Duran-Sandoval D., Kosykh V., Fruchart J., Fruchart J. C., Gonzalez F. J., Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 14.Pineda, Torra I., Claudel T., Duval C., Kosykh V., Fruchart J. C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 15.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamagata K., Daitoku H., Shimamoto Y., Matsuzaki H., Hirota K., Ishida J., Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J. Biol. Chem. 2004;279:23158–23165. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 18.Duran-Sandoval D., Cariou B., Percevault F., Hennuyer N., Grefhorst A., van Dijk T. H., Gonzalez F. J., Fruchart J. C., Kuipers F., Staels B. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting–refeeding transition. J. Biol. Chem. 2005;280:29971–29979. doi: 10.1074/jbc.M501931200. [DOI] [PubMed] [Google Scholar]
- 19.Ma K., Saha P. K., Chan L., Moore D. D. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J. C., Gonzalez F. J, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 22.Schafer C., Heiss A., Schwarz A., Westenfeld R., Ketteler M., Floege J., Muller-Esterl W., Schinke T., Jahnen-Dechent W. The serum protein α2-Heremans–Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schinke T., Amendt C., Trindl A., Poschke O., Muller-Esterl W., Jahnen-Dechent W. The serum protein α2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J. Biol. Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 24.Szweras M., Liu D., Partridge E. A., Pawling J., Sukhu B., Clokie C., Jahnen-Dechent W., Tenenbaum H. C., Swallow C. J., Grynpas M. D., Dennis J. W. α2-HS glycoprotein/fetuin, a transforming growth factor-β/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 25.Mathews S. T., Singh G. P., Ranalletta M., Cintron V. J., Qiang X., Goustin A. S., Jen K. L., Charron M. J., Jahnen-Dechent W., Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 26.Olivier E., Soury E., Ruminy P., Husson A., Parmentier F., Daveau M., Salier J. P. Fetuin-B, a second member of the fetuin family in mammals. Biochem. J. 2000;350:589–597. [PMC free article] [PubMed] [Google Scholar]
- 27.Denecke B., Graber S., Schafer C., Heiss A., Woltje M., Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003;376:135–145. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu S. J., Nagase H., Balmain A. Identification of Fetuin-B as a member of a cystatin-like gene family on mouse chromosome 16 with tumor suppressor activity. Genome. 2004;47:931–946. doi: 10.1139/g04-043. [DOI] [PubMed] [Google Scholar]
- 29.Swales K. E., Korbonits M., Carpenter R., Walsh D. T., Warner T. D., Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 30.Silva J., Dasgupta S., Wang G., Krishnamurthy K., Ritter E., Bieberich E. Lipids isolated from bone induce the migration of human breast cancer cells. J. Lipid Res. 2006;47:724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Kim I., Morimura K., Shah Y., Yang Q., Ward J. M., Gonzalez F. J. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;228:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirvent A., Claudel T., Martin G., Brozek J., Kosykh V., Darteil R., Hum D. W., Fruchart J. C., Staels B. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Sirvent A., Verhoeven A. J., Jansen H., Kosykh V., Darteil R. J., Hum D. W., Fruchart J. C., Staels B. Farnesoid X receptor represses hepatic lipase gene expression. J. Lipid Res. 2004;45:2110–2115. doi: 10.1194/jlr.M400221-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Raspé E., Madsen L., Lefebvre A., Leitersdorf I., Gelman L., Peinado-Onsurbe J., Dallongeville J., Fruchart J., Berge R., Staels B. Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid (TTA) via PPARα activation. J. Lipid Res. 1999;40:2099–2110. [PubMed] [Google Scholar]
- 35.Ketteler M., Bongartz P., Westenfeld R., Wildberger J. E., Mahnken A. H., Bohm R., Metzger T., Wanner C., Jahnen-Dechent W., Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;36:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang A. Y., Woo J., Lam C. W., Wang M., Chan I. H., Gao P., Lui S. F., Li P. K., Sanderson J. E. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2005;20:1676–1685. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 37.Ix J. H., Shlipak M. G., Brandenburg V. M., Ali S., Ketteler M., Whooley M. A. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heiss A., DuChesne A., Denecke B., Grotzinger J., Yamamoto K., Renne T., Jahnen-Dechent W. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]