Abstract
The SLTM [SAF (scaffold attachment factor)-like transcription modulator] protein contains a SAF-box DNA-binding motif and an RNA-binding domain, and shares an overall identity of 34% with SAFB1 {scaffold attachment factor-B1; also known as SAF-B (scaffold attachment factor B), HET [heat-shock protein 27 ERE (oestrogen response element) and TATA-box-binding protein] or HAP (heterogeneous nuclear ribonucleoprotein A1-interacting protein)}. Here, we show that SLTM is localized to the cell nucleus, but excluded from nucleoli, and to a large extent it co-localizes with SAFB1. In the nucleus, SLTM has a punctate distribution and it does not co-localize with SR (serine/arginine) proteins. Overexpression of SAFB1 has been shown to exert a number of inhibitory effects, including suppression of oestrogen signalling. Although SLTM also suppressed the ability of oestrogen to activate a reporter gene in MCF-7 breast-cancer cells, inhibition of a constitutively active β-galactosidase gene suggested that this was primarily the consequence of a generalized inhibitory effect on transcription. Measurement of RNA synthesis, which showed a particularly marked inhibition of [3H]uridine incorporation into mRNA, supported this conclusion. In addition, analysis of cell-cycle parameters, chromatin condensation and cytochrome c release showed that SLTM induced apoptosis in a range of cultured cell lines. Thus the inhibitory effects of SLTM on gene expression appear to result from generalized down-regulation of mRNA synthesis and initiation of apoptosis consequent upon overexpressing the protein. While indicating a crucial role for SLTM in cellular function, these results also emphasize the need for caution when interpreting phenotypic changes associated with manipulation of protein expression levels.
Keywords: apoptosis, nucleus, oestrogen, scaffold attachment factor (SAF-box), transcription
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; E2,17β-oestradiol; ERE, oestrogen response element; EYFP, enhanced yellow fluorescent protein; HA, haemagglutinin; HET, heat-shock protein 27 ERE and TATA-box-binding protein; MOI, multiplicity of infection; pol II, polymerase II; poly(A)+, polyadenylated; RRM, RNA recognition motif; SAF, scaffold attachment factor; SC-35, splicing factor SC-35; SERM, selective oestrogen receptor antagonist; SFC, splicing factor compartment; SLTM, SAF-like transcription modulator; S/MAR, scaffold/matrix attachment region; STA, staurosporine; TRITC, tetramethylrhodamine isothiocyanate
INTRODUCTION
SAFB1 {scaffold attachment factor-B1; also known as SAF-B (scaffold attachment factor B), HET [heat-shock protein 27 ERE (oestrogen response element) and TATA-box-binding protein] or HAP [hnRNP (heterogeneous nuclear ribonucleoprotein) A1-interacting protein]} was originally isolated on the basis of its ability to bind the S/MARs (scaffold/matrix attachment regions) of genomic DNA [1]. Such sequences are present throughout eukaryotic genomes and are thought to play a role in organizing chromatin structure and regulating gene expression [2]. SAFB1 contains a number of domains that may be responsible for functions such as tethering components of the nuclear matrix to S/MARs. These include an N-terminal SAF-box DNA binding motif [3,4], an RNA-binding domain [5] and a C-terminal sequence rich in arginine and glutamic acid residues that has been shown to interact with components of the transcriptional and translational machinery [6,7].
A protein that subsequently proved to be identical with SAF-B was identified independently and named HET, owing to its ability to bind and modulate transcription of the heat-shock protein 27 promoter [8]. Subsequent studies showed that overexpression of this protein suppressed the transcriptional activity of the oestrogen receptor, augmenting the antagonistic effects of the SERM (selective oestrogen receptor antagonist) tamoxifen in breast-cancer cells [9]. This inhibitory effect appears to be explained, in part, by recruitment of the N-CoR co-repressor [10]. Overexpression of SAFB1 is also associated with reduced rates of cell proliferation and multinuclearity [11]. Conversely, deletion of SAFB1 in mouse embryonic fibroblasts leads to spontaneous immortalization and lack of contact inhibition [12]. Loss of heterozygosity at chromosome 19p13.2-3, the site at which HET has been localized in the human genome, has been observed in breast-cancer cells, suggesting that it may act as a tumour suppressor gene [13]. A third protein which proved to be identical with SAFB1 was isolated independently by Weighardt et al. [14]. This protein, initially designated ‘HAP’, was detected using a two-hybrid screen to identify proteins interacting with heterogenous nuclear ribonuclear protein A1. Interestingly, the subnuclear distribution of HAP was found to be markedly altered in response to heat shock. More recently, a closely related protein, SAFB2, was isolated, and SAFB1 was proposed as an alternative name for SAFB/HET/HAP [15].
A novel gene that we described (GenBank® Accession Number AF462146) also encodes a protein containing a SAF-box DNA-binding motif and an RNA-binding domain [16]. This gene was initially named ‘Met’, but has now been re-named SLTM (SAF-like transcription modulator), in agreement with the Mouse Genomic Nomenclature Committee (http://www.informatics.jax.org/mgihome/nomen/index.shtml#mgnc) recommendations. Overall, the sequence of this gene was found to have 34% sequence identity at the predicted amino acid level with SAF-B1. Here, we show that SLTM co-localizes with SAFB1 in the nucleus and also inhibits an oestrogen reporter gene. Rather than mediating a specific inhibitory effect on oestrogen action, however, SLTM is shown to exert a generalized inhibitory effect on gene expression which is associated with induction of apoptosis in a wide range of cell lines.
EXPERIMENTAL
Plasmids and adenoviral vector
Plasmids expressing full length HA (haemagglutinin)-tagged SLTM cDNA (pSLTM-HA), HA-tagged SAFB1 (pHA-SAFB1) and EYFP (enhanced yellow fluorescent protein)-tagged SLTM (pEYFP-SLTM) were kindly provided by Dr Shane Colley (Henry Wellcome Laboratories for Integrative Neuroscience and Endocrinology, University of Bristol, Bristol, U.K.). To construct an adenoviral vector, SLTM was cloned into the plasmid pXcX.CMV (Microbix Biosystems Inc). Recombinant AdSLTM-HA virus was generated by homologous recombination in HEK-293 (human embryonic kidney-293) cells grown to high titre, then purified by double caesium chloride density-gradient centrifugation as described in [17]. Viral titre, determined by plaque assay, was 2×109 plaque-forming units/ml. As a control, adenovirus lacking a transgene (Ad0) was used.
Cell culture and transfection
Cell lines were obtained from the European Collection of Cell Cultures (ECACC), and maintained in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% (v/v) fetal-calf serum (Gibco BRL), 2 mM glutamine and penicillin/streptomycin. Cells were transfected using FuGENE™ 6 transfection reagent (Roche).
Confocal microscopy
Cells were washed with PBS and fixed in 2% (w/v) paraformaldehyde for 20 min at room temperature (19–21 °C) before permeabilization with 0.1% Triton X in PBS for 5 min. Non-specific protein binding was then blocked with 1% (w/v) BSA in PBS for 20 min before incubation with anti-HA mouse monoclonal antibody (1:5000 dilution; Sigma) or mouse anti-(splicing factor SC-35) primary antibody (1:1000 dilution; Sigma) at 4 °C overnight. After washing in 1% BSA/PBS, anti-mouse antibody linked to Cy3 (Jackson ImmunoResearch Laboratories) was added over 1.5 h at room temperature in the dark. Coverslips were then mounted on glass microscope slides using DAKO® Fluorescent Mounting Medium (DAKO Corp.). Confocal images were obtained using a Leica TCS SP scanning laser microscope equipped with a Kr/Ar laser using a 63× oil-immersion objective. EYFP was detected using 510-nm-excitation and a 530-nm-emission filter set. Cy3 was visualized using 568 nm excitation and 590 nm emission filters. Digital images were acquired using Leica TCSNT confocal software.
Reporter assays
MCF-7 cells were seeded in 48-well plates at 3×104 cells/well. The following day, cells were transiently transfected with either 250 ng of pERE-tk-luc reporter plasmid [18] or 60 ng of pSV-β-galactosidase control vector (Promega)/well, plus pSLTM-HA or pHA-SAFB1 plasmids using FuGENE™ 6 transfection reagent. Where necessary, pCR3.1 plasmid was used to maintain equal quantities of DNA for transfection. Following overnight incubation, cells were washed and cultured in serum-free medium (Phenol Red-free DMEM/F-12 mix (Gibco BRL) containing 0.1 mg/ml BSA and 10 μg/ml apo-transferrin) for a further 6 h. Cells were subsequently exposed to 10−8 M E2 (17β-oestradiol) for 18 h prior to cell lysis. Reporter gene activity was measured using the Luciferase Assay System (Promega) in a Microtitre Plate Luminometer (Dynex). β-Galactosidase activity was measured following the addition of Chlorophenol Red–β-D-galactopyranoside substrate (Roche). Assays were performed in triplicate and the results presented are representative of at least three independent experiments.
RNA synthesis
To measure [3H]uridine incorporation into total RNA, HeLa cells (3×104) were seeded into 96-well plates. Cells were either transfected with 0.1 μg of empty plasmid or pSLTM-HA and incubated for a further 48 h or treated with STA (staurosporine, a kinase inhibitor derived from Streptomyces which has a well-characterized ability to induce apoptosis) for 3 h. [3H]Uridine (1 μCi/well) was then added 1 h before the cells were lysed and transferred to Printed Filtermats (Wallac) for the determination of radioactivity. To measure [3H]uridine incorporation into mRNA, HeLa cells (1.5×105) were seeded into 60-mm-diameter dishes before being treated with STA as described above or transfected with either 2 μg of pcDNA3 (empty plasmid) or pSLTM-HA. After 48 h, cells were exposed to 20 μCi of [3H]uridine/dish for 1 h before being collected, lysed and RNA extracted using the RNeasy Mini Kit. Poly(A)+ (polyadenylated) RNA was then isolated using the PolyATract kit (Promega) and radioactivity measured using a Wallac 1409 DSA liquid-scintillation counter.
Cell-cycle analysis
For cell-cycle analysis, cells (3×105) were seeded into each well of a six-well plate and incubated overnight. The cells were co-transfected with pEYFP-C1 and either pcDNA3.1 (empty plasmid), or pSLTM-HA (1 μg of each plasmid). The cells were then incubated overnight before serum starvation in serum-free medium for 24 h. The cells were collected, fixed in 70% (v/v) ethanol for 45 min at 4 °C, stained with propidium iodide by incubation at 4 °C for 1 h, and the cell-cycle profile analysed by flow cytometry. The method of fixation in this instance was modified to a shorter incubation period, since EYFP is known to leak out of permeabilized cells.
Cytochemical detection of apoptosis
Induction of apoptosis in HeLa cells transfected with plasmid expressing SLTM was assessed by Hoechst staining. HeLa cells were seeded into 60-mm-diameter dishes and transfected with 1 μg of pEYFP-C1 or pEYFP-SLTM. The cells were then incubated overnight before being transferred to serum-free medium for 24 h. Next cells were fixed and stained with Hoechst and assessed by fluorescent microscopy. In HeLa cells transduced with adenovirus expressing SLTM, nuclear morphology was assessed by fixing cells with 4% (w/v) paraformaldehyde and nuclear staining for 15 min at room temperature with 100 ng/ml DAPI (4′,6-diamidino-2-phenylindole). For release of mitochondrial cytochrome c, cells grown on coverslips were washed once with PBS, fixed for 5 min in ice-cold methanol and immediately rehydrated in PBS. Primary antibodies [rabbit anti-(cytochrome c) from Becton Dickinson and mouse anti-HA from Sigma] were diluted to working concentrations in PBS containing 1% (w/v) BSA. After incubation overnight at 4 °C, coverslips were washed three times with PBS. Secondary antibodies [FITC-conjugated anti-mouse IgG goat antibody and TRITC (tetramethylrhodamine isothiocyanate)-conjugated anti-rabbit IgG swine antibody (both from Sigma)] were diluted to the recommended final concentration in PBS containing 2% BSA. After 1 h at room temperature, coverslips were washed three times in PBS before being mounted in Vectastain ABC (Vector Laboratories). Microscopy was performed with a Leica TCS confocal laser apparatus.
RESULTS
Cellular localization of SLTM protein
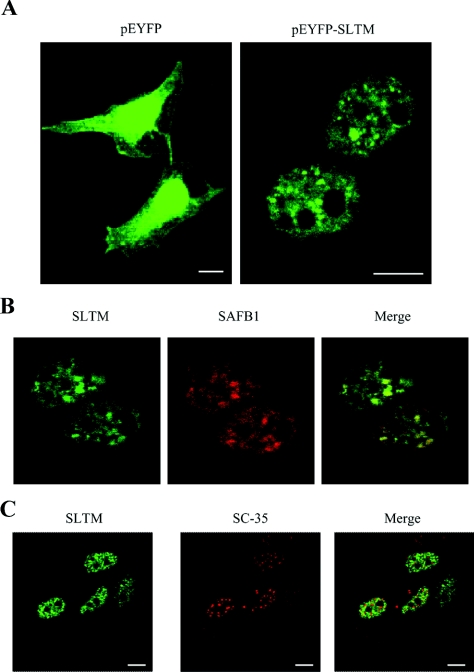
Previous studies have shown that SAFB1 is expressed with a punctate distribution in the cell nucleus [6,14]. To investigate the subcellular distribution of SLTM, confocal microscopy was used to examine HeLa cells transfected with plasmids expressing EYFP or the EYFP–SLTM chimaera. In cells transfected with pEYFP-C1 alone, EYFP staining was evenly distributed throughout the cells, whereas cells transfected with pEYFP-SLTM showed a punctate nuclear localization pattern excluded from nucleoli (Figure 1A). A similar distribution pattern was observed in MCF-7 cells (results not shown). In order to determine whether the distribution of SLTM coincided with that of SAFB1, HeLa cells were co-transfected with pEYFP-SLTM and pHA-SAFB1. After 48 h, the cells were fixed, permeabilized and stained for the SAFB1 protein using an anti-HA mouse monoclonal antibody. As shown in Figure 1(B), SLTM co-localizes with SAFB1, but co-localization is not complete, suggesting that the functions of the two proteins may not be identical.
Figure 1. Cellular localization of SLTM expression.
(A) HeLa cells were grown on coverslips and transfected with either pEYFP-C1 or pEYFP-SLTM, fixed, then viewed under a confocal microscope. (B) Co-localization of EYFP–SLTM with HA–SAFB1. HeLa cells were transfected with pEYFP-SLTM and pHA-SAFB1. The cells were fixed and stained with anti-HA antibody before viewing under the confocal microscope. The Figure shows the distribution of SLTM, SAFB1 and an overlay of both images. (C) SLTM does not co-localize with SFCs. Distribution of SLTM compared with that of SC-35, an SR protein. HeLa cells were grown on coverslips and transfected with pEYFP–SLTM, fixed, permeabilized and stained for SC-35. The white horizontal bars represent 5 μm.
Splicing factor compartments (SFCs) are well-characterized nuclear structures that also display a speckled pattern of distribution. To determine whether SLTM is associated with SFCs, distribution of the SC-35 detected by anti-SC-35 immunostaining was compared with pEYFP-SLTM fluorescence. As shown in Figure 1(C), the nuclear location of SLTM is clearly different from that of SFCs. Initial studies showed that a partial SAFB1 sequence co-localized with SC-35 [6], but a subsequent study showed that speckles associated with intact SAFB1, like those formed by SLTM, are distinct from SC-35 speckles [14].
Transcriptional activity of the oestrogen receptor in cells overexpressing SLTM
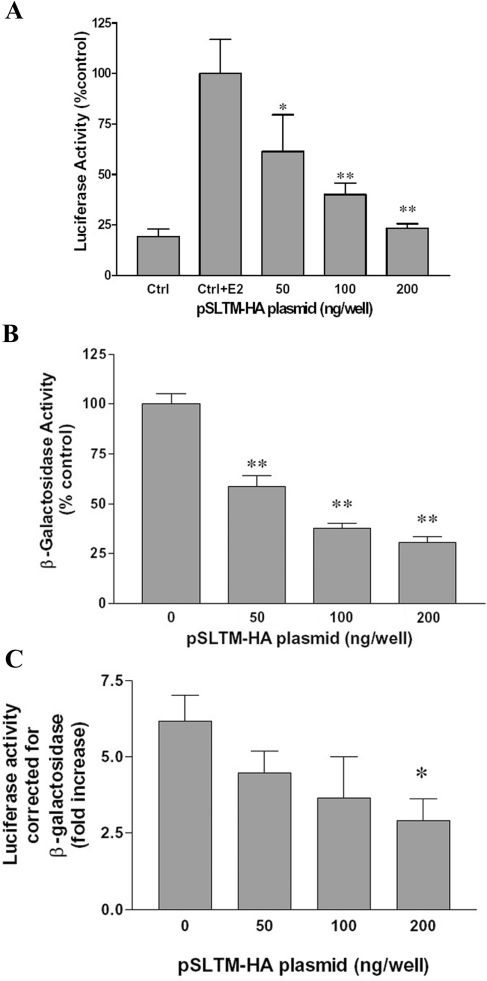
In the light of previous data showing that SAFB1 suppresses oestrogen-induced transcription [9], we decided to test the possibility that SLTM might exert a similar effect. MCF-7 cells were co-transfected with an ERE–luciferase reporter construct, pERE-tk-luc, along with plasmid expressing SLTM. Co-transfected cells were subsequently exposed to oestrogen or vehicle and the activation of reporter genes compared. Luciferase reporter expression was induced fourfold in MCF-7 cells exposed to oestrogen compared with untreated controls (Figure 2A). Co-transfection with increasing amounts of SLTM resulted in reduced luciferase activity, the oestrogen effect being completely reversed when the highest amount (200 ng) of plasmid was used.
Figure 2. Oestrogen receptor transcriptional activity in cells overexpressing SLTM.
(A) MCF-7 cells were transfected with pERE-tk-Luc and various amounts of pSLTM-HA, with pcDNA3.1 used to bring total DNA transfected up to 300 ng. Control cells were transfected with 300 ng of pcDNA3.1 only. At 24 h after infection, cells were serum-starved for 6 h before treatment with 10−8 M E2 for 18 h. This experiment was repeated on five separate occasions, and the results (mean±S.D.) of a typical experiment are shown. Control cells were treated with and without E2 and all cells transfected with SLTM were treated with E2. (B) Effect of SLTM on the transcriptional activity of a constitutively active β-galactosidase reporter. MCF-7 cells were co-transfected with pSV-β-galactosidase (60 ng) and either 300 ng of pcDNA3.1 (control) or various amounts of pSLTM-HA plus pcDNA3.1 up to a total of 300 ng, then serum-starved for 24 h before assaying for β-galactosidase activity (in each case the activity of the control cells was taken as 100%). The Figure shows the result (mean±S.D.) from a typical experiment that was performed five times. (C) MCF-7 cells were transfected with pERE-tk-Luc and various amounts of pSLTM-HA, together with pSV-β-galactosidase as described above. Luciferase activity is expressed as the fold increase in the presence of E2 compared with controls after correction for β-galactosidase activity. The mean results for three separate experiments (±S.D.), each performed in triplicate, are shown. *P<0.05, **P<0.01, compared with control (Student's t test) from results obtained in the absence of SLTM transfection.
Constitutively active β-galactosidase vectors are widely used to control for transfection efficiency. Surprisingly, we found that SLTM also caused a profound inhibition of β-galactosidase activity when MCF-7 cells were co-transfected with SLTM expression plasmid (Figure 2B). Nevertheless, inhibition of β-galactosidase was less than that of luciferase, so that correction of luciferase results for β-galactosidase showed an apparent inhibition of the ability of oestrogen to activate reporter by SLTM (Figure 2C).
Overexpression of SLTM results in a generalized inhibition of transcription
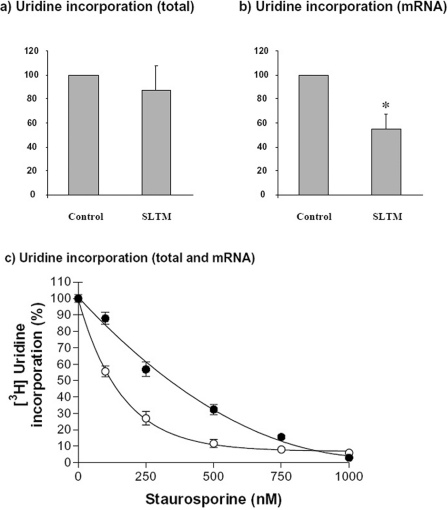
Given the nuclear localization of SLTM, together with evidence from previous studies showing that SAFB1 interacts with RNA pol II (polymerase II) [6], we postulated that SLTM might produce a generalized inhibition of mRNA transcription. Therefore, we tested the effect of SLTM on [3H]uridine incorporation into RNA. These experiments were performed in HeLa cells using a transfection protocol which resulted in transfection of 50–60% of cells (results not shown). There was very little effect on incorporation into total RNA (representing predominantly ribosomal RNA), consistent with the exclusion of SLTM from nucleoli (Figure 3a). When incorporation into poly(A)+ RNA (representing mRNA) was measured, however, there was a strong inhibition to 47±14% (P<0.005) of control values (Figure 3b). When account is taken of transfection efficiency, this result suggests a near total inhibition of mRNA synthesis in cells overexpressing SLTM. Preliminary results suggested that these inhibitory effects might indicate induction of apoptosis by over expression of SLTM. For comparison, we therefore repeated these experiments after exposing cells for 3 h to different concentrations of STA. As shown in Figure 3(c), incorporation of uridine into mRNA was again more severely inhibited than total cellular incorporation of uridine. At an STA concentration of 100 nM, the effect was similar to that produced by expression of SLTM, with total incorporation of [3H]uridine being decreased to 87.9±3.6% and incorporation into mRNA reduced to 55.6±3.3%. These results led us to investigate in greater detail the possibility that the inhibition of RNA synthesis caused by SLTM was associated with induction of apoptosis.
Figure 3. Inhibition of transcription in cells overexpressing SLTM.
(a) Total RNA synthesis. HeLa cells were transfected with 0.1 μg of either empty plasmid or pSLTM-HA and incubated for 48 h. The cells were then exposed to [3H]uridine (1 μCi/well) for 1 h before being lysed, harvested and the radioactivity measured. (b) mRNA synthesis. HeLa cells were treated as described above and poly(A)+ RNA isolated before measurement of radioactivity. (c) For comparison, inhibition of [3H]uridine incorporation was measured in HeLa cells exposed to different concentrations of STA. ●, Total cellular incorporation; ○, incorporation into mRNA. Values represent the means±S.D. for three separate experiments. *P<0.005; (Student's t test).
Overexpression of SLTM induces apoptosis in cultured cells
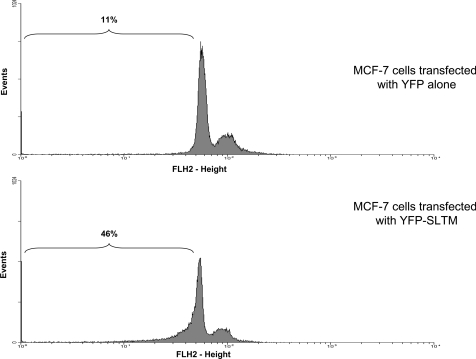
Plasmids encoding HA-tagged SLTM cDNA were used to assess whether the protein altered the cell-cycle profile of MCF-7 cells. Transfection efficiencies of MCF-7 cells are relatively low. Therefore, to allow identification of transfected cells, a plasmid encoding EYFP (pEYFP-C1; Clontech) was co-transfected with plasmid expressing SLTM. Using a gate that only accepted fluorescent cells, the cell-cycle profile of the selected cell population was then analysed. A typical experiment in which MCF-7 cells were transfected with SLTM (Figure 4) showed a clear increase in the pre-G1 population. To ascertain whether the effect of SLTM on the cell cycle was limited to MCF-7 cells, we also investigated its effect on MC3T3 cells (a mouse calvarial fibroblast cell line), Ros (rat osteosarcoma), HepG2 (human hepatocarcinoma), HeLa (human cervical adenocarcinoma) and SF (human fibroblast, primary culture) cell lines. For these experiments, 3×105 cells from each line, seeded in six-well plates, were transfected with 1 μg of pEYFP-C1 or pEYFP-SLTM as described above. Table 1 shows that SLTM induces significant apoptosis in all of these cell lines, albeit to different extents.
Figure 4. Effect of EYFP–SLTM on the cell-cycle profile of MCF-7 cells.
MCF-7 cells were transfected with pEYFP-C1 (1 μg) (upper panel) or pEYFP-SLTM (1 μg) (lower panel), then serum-starved for 24 h before collection and staining with propidium iodide for cell-cycle analysis. The experiment was performed four times, and a typical experiment is shown.
Table 1. Percentage of pre-G1 cells after transfection of various cell lines with EYFP or EYFP-SLTM.
| Percentage of cell population in pre-G1 phase [mean±S.D. (n)] | ||
|---|---|---|
| Cell line | EYFP-transfected | EYFP-SLTM-transfected |
| MCF-7 | 10±5 (15) | 37±10 (16) |
| HeLa | 17±3 (11) | 33±5 (12) |
| HepG2 | 5±3 (16) | 24±15 (16) |
| MC3T3 | 6±3 (12) | 21±7 (12) |
| Ros | 9±4 (12) | 18±6 (12) |
| SF | 4±2 (15) | 17±2 (14) |
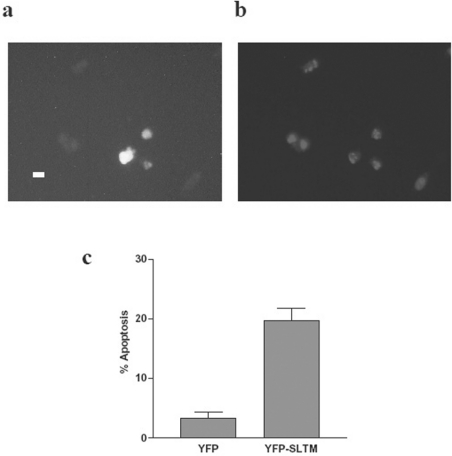
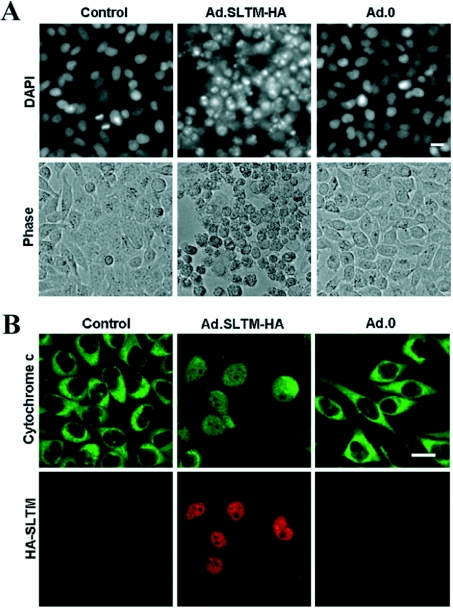
As additional confirmation of the ability of SLTM to induce apoptosis, HeLa cells (2×105) were seeded into 60-mm-diameter dishes, incubated overnight and then transfected with either 1 μg of pEYFP-C1 or pEYFP-SLTM. After incubation for a further 24 h, cells were serum-starved for 24 h before being fixed and stained with Hoechst. Fluorescent microscopy was performed, and 300 fluorescent cells per dish were counted. As shown in Figures 5(a) and 5(b), pEYFP-SLTM-transfected cells correspond to cells with the classical features of apoptosis, shown by Hoechst staining. Cell counts of the pEYFP- and pEYFP-SLTM-transfected cells (Figure 5c) show SLTM increases the apoptotic index from 3±1% (mean±S.D.) to 20±2% (P<0.001), results consistent with those obtained by cell-cycle analysis. In order to provide further confirmation of an apoptotic process, and also to avoid possible ‘artefactual’ results caused by transfection, we carried out additional studies using an adenoviral vector expressing SLTM. As with Hoechst staining of HeLa cells transfected with plasmid expressing SLTM, nuclear condensation and fragmentation were clearly demonstrated by DAPI staining of cells transduced by adenovirus expressing SLTM, but not by the Ad0 empty virus (Figure 6A). Furthermore, extensive release of cytochrome c was apparent in cells transduced with virus expressing SLTM, but not in non-transduced cells (Figure 6B).
Figure 5. Induction of apoptosis in HeLa cells by SLTM, assessed by Hoechst staining.
HeLa cells were seeded into 60-mm-diameter dishes and transfected with 1 μg of pEYFP-C1 or pEYFP-SLTM. The cells were incubated overnight and then transferred to serum-free medium for 24 h. Next, cells were fixed and stained with Hoechst and assessed by fluorescent microscopy. (a) Fluorescence of cells transfected with pEYFP-SLTM; (b) Hoechst staining of the same field as that shown in (a) (the scale bar represents 15 μm); and (c) change in apoptotic index of HeLa cells after expression of SLTM, assessed by cell counting. This experiment was performed in triplicate (results are means±S.D.) and repeated three times. The results shown are those obtained in a typical experiment.
Figure 6. Induction of apoptosis in cells transduced with adenovirus expressing SLTM.
HeLa cells were transduced with AdSLTM-HA [MOI (multiplicity of infection) 50] or Ad.0 (both at MOI 50). (A) DAPI staining. At 48 h after transduction, cells were stained with DAPI. Bright-field images are also shown. (B) Confocal fluorescent micrographs of cells stained with rabbit anti-(cytochrome c) or anti-HA mAb antibody. FITC-conjugated anti-rabbit secondary antibody (green) and a TRITC-conjugated anti-mouse secondary donkey antibody (red) were used to visualize cytochrome c and SLTM–HA respectively. White scale bars represents 50 μm.
DISCUSSION
In the paper we describe some properties of the protein encoded by the SLTM gene. The proteins most closely related to SLTM appear to be SAFB1 [1] and SAFB2 [15], which share similar structures to SLTM, including a SAF Box, an RNA binding domain and a region rich in glutamine and arginine residues. SAFB1 is a nuclear protein, whereas SAFB2 is also found in the cytoplasm [15]. The compartmentalized architecture of the nucleus is increasingly recognized, with nuclear factors which may be highly mobile or localized to distinct compartments. Both SAFB1 [6,14] and SLTM have a punctate, nuclear distribution that is excluded from nucleoli, and to a large extent the two proteins co-localize (Figure 1b), suggesting that they share similar functions. SFCs are well-characterized nuclear structures that are thought to represent storage sites for splicing factors [19]. Because there is evidence that SAFB1 functions as a splicing factor [6], and given the similarity between SLTM and SAFB1, we were interested to determine whether or not SLTM co-localizes with SFCs. Although the speckled distribution pattern of SLTM resembles that of SFCs, we found no co-localization of SC-35 (a splicing factor localized to SFCs) and SLTM (Figure 1C). Initially, it was thought that SAFB1 co-localized with SC-35 [6], but these studies were performed with a C-terminal fragment of SAFB1 and a subsequent study with the complete protein showed that the distribution of SAFB1, like SLTM, is quite distinct from that of SC-35 speckles [14]. It will be important to determine whether SAFB1 and SLTM identify novel nuclear compartments, or whether they correspond to previously described nuclear structures such as paraspeckles, Cajal bodies or promyelocytic leukaemia bodies [20,21].
Further clues to the precise function of SAF proteins might be provided by protein–protein interactions. SAFB1 has been reported to interact with a surprisingly wide range of proteins. These include SR (serine/arginine) [6] and hnRNP splicing proteins [7,14], the oestrogen receptor [9] and several other nuclear receptors, including the peroxisome proliferator-activated receptor, the farnesoid X receptor, the ROR (retinoid-related orphan receptor), the vitamin D receptor, steroidogenic factor 1 and liver receptor homologue 1 [22]. In addition, SAFB1 has also been reported to interact with RNA pol II [6], P2P-R (proliferation potential protein-related) [23], SRPK1 (SR-rich protein-specific kinase 1) [24], ZO-2 (tight junction protein 2) [25], CHD1 (chromodomain helicase DNA binding protein 1) [26] and oncoprotein c-Jun [22]. Evidence for specific transcriptional regulatory functions of SAFB1 has been accumulated by Oesterreich et al. [8]. Thus SAFB1 was found to exert a negative regulatory effect on the hsp27 promoter [8], whereas both SAFB1 and SAFB2 were found to inhibit expression of oestrogen reporter genes [9,15]. In addition, glutathione transferase pull-down experiments were used to show that the oestrogen receptor interacts with both SAFB1 [9] and SAFB2 [15]. More recently, this group has shown that recruitment of a co-repressor, N-CoR, is in part responsible for the inhibitory effect of SAFB1 on transcription by the oestrogen receptor [10]. The presence of RRMs (RNA recognition motifs) in SLTM is also compatible with studies showing that RNA [27] and RRM proteins [28] can regulate transcriptional activity of the oestrogen receptor. For these reasons, we were interested to determine whether or not SLTM would exert a similar effect on oestrogen signalling. Unexpectedly, results with reporter genes led us to conclude that the inhibitory effects of SLTM on oestrogen signalling are primarily a reflection of its ability, when overexpressed, to produce a generalized inhibition of transcription. Thus, although oestrogen reporter results corrected for transfection efficiency by measurement of β-galactosidase suggested that oestrogen signalling was inhibited by SLTM (Figure 2C), this is likely to result primarily from the shorter half-life of luciferase as compared with β-galactosidase. Several previous reports have cautioned against the potential for misinterpretation of reporter assays corrected by use of internal standards [29–31], and conditions that affect transcription and/or translation clearly require particular care. As shown in Figures 3(a) and 3(b), overexpression of SLTM in HeLa cells has little effect on incorporation of radioactive uridine into total RNA, but incorporation into poly(A)+ RNA is strongly inhibited. Because efficiency of transfection for these cells is about 50%, it would appear that mRNA synthesis is almost completely inhibited. Similar results were obtained when cells were exposed to STA, suggesting that the decreased transcription associated with overexpression of SLTM might be linked to activation of an apoptotic pathway. This possibility was confirmed by Hoechst staining of HeLa cells transfected with plasmid expressing SLTM, and further confirmed by DAPI staining together with cytochrome c release in cells transduced with adenovirus expressing SLTM. We have also found similar effects when SAFB1 is overexpressed (results not shown).
Apoptosis may be induced as a response to blockage of transcription via several possible mechanisms, including induction of a p53 response, a relative decrease in anti-apoptotic factors and aberrant accumulation of proteins in the nucleus [32]. Although the function of SAF family proteins remains poorly understood, the presence of DNA and RNA binding domains, as well as a C-terminus capable of interacting with multiple transcription factors, suggests that a precise balance in the stoichiometry of SLTM relative to other components of the transcriptional apparatus is critical. The apoptotic response to over expression of SLTM that we observed in every cell type tested (Table 1) could reflect such a requirement for accurate regulation of SLTM expression. Further studies will be required, however, to confirm that apoptosis resulting from overexpression of SLTM is the direct result of decreased transcription, rather than diminished transcription being a consequence of an apoptotic process that is activated by some other mechanism.
In conclusion, SLTM is a nuclear protein with a distribution similar to that of SAFB1 (but unlike that of SAFB2, which is also found in the cytoplasm [15]). The SLTM protein contains a putative SAF-box and RNA binding domains and, overall, displays significant homology with SAFB1 and SAFB2, which have both been reported to inhibit oestrogen signalling [15]. Rather than regulating expression of specific genes, however, changes in the expression level of SLTM have profound effects on mRNA transcription in general and eventually lead to apoptosis. Although overexpression is a widely used technique to identify protein function, these results serve as a reminder that disturbing the balance in levels of protein expression may give misleading results.
Acknowledgments
This work was supported by grants from the Medical Research Council and the Wellcome Trust. C. W. C. was supported by the Government of Singapore. We thank Dr Mark Jepson and Mr Alan Leard (Cell Imaging Facility, Department of Biochemistry, University of Bristol, Bristol, U.K.) for generous assistance with confocal microscopy, and Dr Niall Kerr (Laboratories for Integrative Neurosciences and Endocrinology, University of Bristol, Bristol, U.K.) for numerous technical discussions. The pERE-tk-luc reporter plasmid was kindly provided by Professor Malcolm Parker (Institute of Reproductive and Developmental Biology, Imperial College, London, U.K.).
References
- 1.Renz A., Fackelmayer F. O. Purification and molecular cloning of the scaffold attachment factor B (SAF-B), a novel human nuclear protein that specifically binds to S/MAR-DNA. Nucleic Acids Res. 1996;24:843–849. doi: 10.1093/nar/24.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulikas T. Chromatin domains and prediction of MAR sequences. Int. Rev. Cytol. 1995;162A:279–388. doi: 10.1016/s0074-7696(08)61234-6. [DOI] [PubMed] [Google Scholar]
- 3.Aravind L., Koonin E. V. SAP – a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 4.Kipp M., Gohring F., Ostendorp T., van Drunen C. M., van Driel R., Przybylski M., Fackelmayer F. O. SAF-Box, a conserved protein domain that specifically recognizes scaffold attachment region DNA. Mol. Cell Biol. 2000;20:7480–7489. doi: 10.1128/mcb.20.20.7480-7489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birney E., Kumar S., Krainer A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayler O., Stratling W., Bourquin J. P., Stagljar I., Lindemann L., Jasper H., Hartmann A. M., Fackelmayer F. O., Ullrich A., Stamm S. SAF-B protein couples transcription and pre-mRNA splicing to SAR/MAR elements. Nucleic Acids Res. 1998;26:3542–3549. doi: 10.1093/nar/26.15.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arao Y., Kuriyama R., Kayama F., Kato S. A nuclear matrix-associated factor, SAF-B, interacts with specific isoforms of AUF1/hnRNP D. Arch. Biochem. Biophys. 2000;380:228–236. doi: 10.1006/abbi.2000.1938. [DOI] [PubMed] [Google Scholar]
- 8.Oesterreich S., Lee A. V., Sullivan T. M., Samuel S. K., Davie J. R., Fuqua S. A. Novel nuclear matrix protein HET binds to and influences activity of the HSP27 promoter in human breast cancer cells. J. Cell Biochem. 1997;67:275–286. [PubMed] [Google Scholar]
- 9.Oesterreich S., Zhang Q., Hopp T., Fuqua S. A., Michaelis M., Zhao H. H., Davie J. R., Osborne C. K., Lee A. V. Tamoxifen-bound estrogen receptor (ER) strongly interacts with the nuclear matrix protein HET/SAF-B, a novel inhibitor of ER-mediated transactivation. Mol. Endocrinol. 2000;14:369–381. doi: 10.1210/mend.14.3.0432. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S., Meyer R., Kang K., Kent O. C., Wong J., Oesterreich S. Scaffold attachment factor SAFB1 suppresses ERα-mediated transcription in part via interaction with N-CoR. Mol. Endocrinol. 2006;20:311–220. doi: 10.1210/me.2005-0100. [DOI] [PubMed] [Google Scholar]
- 11.Townson S. M., Sullivan T., Zhang Q., Clark G. M., Osborne C. K., Lee A. V., Oesterreich S. HET/SAF-B overexpression causes growth arrest and multinuclearity and is associated with aneuploidy in human breast cancer. Clin. Cancer Res. 2000;6:3788–3796. [PubMed] [Google Scholar]
- 12.Dobrzycka K. M., Kang K., Jiang S., Meyer R., Rao P. H., Lee A. V., Oesterreich S. Disruption of scaffold attachment factor B1 Leads to TBX2 up-regulation. Lack of p19ARF induction, lack of senescence, and cell immortalization. Cancer Res. 2006;66:7859–7863. doi: 10.1158/0008-5472.CAN-06-1381. [DOI] [PubMed] [Google Scholar]
- 13.Oesterreich S., Allredl D. C., Mohsin S. K., Zhang Q., Wong H., Lee A. V., Osborne C. K., O'Connell P. High rates of loss of heterozygosity on chromosome 19p13 in human breast cancer. Br. J. Cancer. 2001;84:493–498. doi: 10.1054/bjoc.2000.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weighardt F., Cobianchi F., Cartegni L., Chiodi I., Villa A., Riva S., Biamonti G. A novel hnRNP protein (HAP/SAF-B) enters a subset of hnRNP complexes and relocates in nuclear granules in response to heat shock. J. Cell Sci. 1999;112:1465–1476. doi: 10.1242/jcs.112.10.1465. [DOI] [PubMed] [Google Scholar]
- 15.Townson S. M., Dobrzycka K. M., Lee A. V., Air M., Deng W., Kang K., Jiang S., Kioka N., Michaelis K., Oesterreich S. SAFB2, a new scaffold attachment factor homolog and estrogen receptor corepressor. J. Biol. Chem. 2003;278:20059–20068. doi: 10.1074/jbc.M212988200. [DOI] [PubMed] [Google Scholar]
- 16.Colley S. M., Flynn A., Norman M., Wynick D., Tobias J. H. MET, a novel stimulator of estrogen-induced transcription isolated from mouse bone marrow. J. Bone Miner. Res. 2002;17:S153. [Google Scholar]
- 17.Graham F. L., Prevec L. Methods for construction of adenovirus vectors. Mol. Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 18.White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135:175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- 19.Lamond A. I., Spector D. L. Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 20.Spector D. L. The dynamics of chromosome organization and gene regulation. Annu. Rev. Biochem. 2003;72:573–608. doi: 10.1146/annurev.biochem.72.121801.161724. [DOI] [PubMed] [Google Scholar]
- 21.Fox A. H., Lam Y. W., Leung A. K., Lyon C. E., Andersen J., Mann M., Lamond A. I. Paraspeckles: a novel nuclear domain. Curr. Biol. 2002;12:13–25. doi: 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 22.Debril M. B., Dubuquoy L., Feige J. N., Wahli W., Desvergne B., Auwerx J., Gelman L. Scaffold attachment factor B1 directly interacts with nuclear receptors in living cells and represses transcriptional activity. J. Mol. Endocrinol. 2005;35:503–517. doi: 10.1677/jme.1.01856. [DOI] [PubMed] [Google Scholar]
- 23.Scott R. E., Giannakouros T., Gao S., Peidis P. Functional potential of P2P-R: a role in the cell cycle and cell differentiation related to its interactions with proteins that bind to matrix associated regions of DNA? J. Cell Biochem. 2003;90:6–12. doi: 10.1002/jcb.10618. [DOI] [PubMed] [Google Scholar]
- 24.Nikolakaki E., Kohen R., Hartmann A. M., Stamm S., Georgatsou E., Giannakouros T. Cloning and characterization of an alternatively spliced form of SR protein kinase 1 that interacts specifically with scaffold attachment factor-B. J. Biol. Chem. 2001;276:40175–40182. doi: 10.1074/jbc.M104755200. [DOI] [PubMed] [Google Scholar]
- 25.Traweger A., Fuchs R., Krizbai I. A., Weiger T. M., Bauer H. C., Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J. Biol. Chem. 2003;278:2692–2700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 26.Tai H. H., Geisterfer M., Bell J. C., Moniwa M., Davie J. R., Boucher L., McBurney M. W. CHD1 associates with NCoR and histone deacetylase as well as with RNA splicing proteins. Biochem. Biophys. Res. Commun. 2003;308:170–176. doi: 10.1016/s0006-291x(03)01354-8. [DOI] [PubMed] [Google Scholar]
- 27.Lanz R. B., Razani B., Goldberg A. D., O'Malley B. W. Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA) Proc. Natl. Acad. Sci. U.S.A. 2002;99:16081–16086. doi: 10.1073/pnas.192571399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris J. D., Fan D., Sherk A., McDonnell D. P. A negative coregulator for the human ER. Mol. Endocrinol. 2002;16:459–468. doi: 10.1210/mend.16.3.0787. [DOI] [PubMed] [Google Scholar]
- 29.Huszar T., Mucsi I., Terebessy T., Masszi A., Adamko S., Jeney C., Rosivall L. The use of a second reporter plasmid as an internal standard to normalize luciferase activity in transient transfection experiments may lead to a systematic error. J. Biotechnol. 2001;88:251–258. doi: 10.1016/s0168-1656(01)00277-2. [DOI] [PubMed] [Google Scholar]
- 30.Adam G. I., Miller S. J., Ulleras E., Franklin G. C. Cell-type-specific modulation of PDGF-B regulatory elements via viral enhancer competition: a caveat for the use of reference plasmids in transient transfection assays. Gene. 1996;178:25–29. doi: 10.1016/0378-1119(96)00318-6. [DOI] [PubMed] [Google Scholar]
- 31.Deroo B. J., Archer T. K. Proteasome inhibitors reduce luciferase and β-galactosidase activity in tissue culture cells. J. Biol. Chem. 2002;277:20120–20123. doi: 10.1074/jbc.C200173200. [DOI] [PubMed] [Google Scholar]
- 32.Ljungman M., Lane D. P. Transcription – guarding the genome by sensing DNA damage. Nat. Rev. Cancer. 2004;4:727–737. doi: 10.1038/nrc1435. [DOI] [PubMed] [Google Scholar]