Abstract
The effects of PAR2-activating PAR2-activating peptides, SLIGRL (SL)-NH2, and trans-cinnamoyl-LIGRLO (tc)-NH2 were compared with the action of trypsin, thrombin, and the PAR1 selective-activating peptide: Ala-parafluoroPhe-Arg-cyclohexylAla-Citrulline-Tyr (Cit)-NH2 for stimulating intestinal ion transport. These agonists were added to the serosa of stripped rat jejunum segments mounted in Ussing chambers, and short circuit current (Isc) was used to monitor active ion transport. The relative potencies of these agonists also were evaluated in two bioassays specific for the activation of rat PAR2: a cloned rat PAR2 cell calcium-signaling assay (PAR2-KNRK cells) and an aorta ring relaxation (AR) assay. In the Isc assay, all agonists, except thrombin, induced an Isc increase. The SL-NH2-induced Isc changes were blocked by indomethacin but not by tetrodotoxin. The relative potencies of the agonists in the Isc assay (trypsin≫SL-NH2>tc-NH2>Cit-NH2) were strikingly different from their relative potencies in the cloned PAR2-KNRK cell calcium assay (trypsin≫>tc-NH2 ≅ SL-NH2≫>Cit-NH2) and in the AR assay (trypsin≫>tc-NH2 ≅ SL-NH2). Furthermore, all agonists were maximally active in the PAR2-KNRK cell and AR assays at concentrations that were one (PAR2 -activating peptides) or two (trypsin) orders of magnitude lower than those required to activate intestinal transport. Based on the distinct potency profile for these agonists and the considerable differences in the concentration ranges required to induce an Isc effect in the intestinal assay compared with the PAR2-KNRK and AR assays, we conclude that a proteinase-activated receptor, pharmacologically distinct from PAR2 and PAR1, is present in rat jejunum and regulates intestinal transport via a prostanoid-mediated mechanism.
The PAR2, like the receptor for thrombin [proteinase-activated receptor-1 (PAR1)], belongs to the large superfamily of G protein- coupled receptors (1–4). The unique mechanism whereby the proteinases thrombin (for PAR1) and trypsin (for PAR2) activate the PARs involves the proteolytic cleavage and unmasking of an N-terminal receptor sequence, that in turn acts as an anchored receptor-stimulating ligand (2, 5). Remarkably, short synthetic peptides based on the proteolytically revealed receptor sequences [PAR-activating peptides (APs)] can, in isolation, activate either PAR1 (e.g., the peptide SFLLR-NH2) or PAR2 [e.g., SLIGRL (SL)-NH2]. Studies of the structure–activity relationships for the activation of PAR1 and PAR2 by PAR-APs derived from either PAR1 or PAR2 have revealed that the PAR2AP SL-NH2 can selectively activate PAR2 and not PAR1; but the PAR1-derived peptide SFLLR-NH2 can activate both PAR1 and PAR2 (3, 6, 7). Thus, we have begun to design PAR1APs that are selective for PAR1 and that do not activate PAR2 (and vice versa) (7). Such selective synthetic PAR-APs have become useful probes to assess the biological consequence of the activation of either PAR1 or PAR2 in vivo, and thus mimicking the actions of the proteinases, thrombin and trypsin.
Northern blot analysis has revealed the presence of PAR2 in a variety of tissues, both in rodents and humans, with particularly prominent expression observed in the gastrointestinal tract (3, 8). Because PAR2 was discovered fortuitously in the course of the screening of a mouse-genomic library for an unrelated receptor (3), the precise physiological role that PAR2 plays is open to question. Functional studies with PAR2APs have pointed to a role for PAR2 in regulating the contractility of vascular and gastric smooth muscle (9–12). Recent work using immunofluorescence and confocal microscopy has localized PAR2 in intestinal enterocytes, neuronal elements, and myocytes and also has demonstrated that both trypsin and the PAR2AP SL-NH2, when applied to the luminal side of rat jejunal tissue, can cause the secretion of prostaglandin E2 (PGE2) (13, 14). We were interested in the potential functional effects of activating PAR2 on the serosal rather than the mucosal side of intestinal tissue, and we wondered whether PAR2APs or trypsin, when applied to the serosal surface of jejunal strips, might modulate intestinal transport. To answer this question, we designed two new PAR-APs, one selective for PAR1: Ala-parafluoroPhe-Arg-cyclohexylAla-Citrulline-Tyr- (Cit)-NH2 and a second selective for PAR2: trans-cinnamoyl-LIGRLO (tc)-NH2. We used these two receptor-selective PAR-APs, along with the originally described PAR2AP, SL-NH2, to evaluate their structure–activity relationship for the regulation of active ion transport [by measuring short circuit current (Isc)], when applied to the serosal side of jejunal strips. Our data show not only that the PAR-APs can modulate intestinal transport by acting at the serosal surface, but the structure–activity relationships for this action of the PAR-APs point to the existence of a receptor, distinct from PAR1 and PAR2, that is responsible for regulating intestinal function.
METHODS
Animals.
Male, Hooded Lister rats (150–200 g) were obtained from the Department of Biological Sciences at the University of Calgary (Calgary, Alberta, Canada) and were used for all tissue bioassay experiments. Animals had free access to food and water and were housed under constant temperature (22°C) and photoperiod (12-hr light–dark cycle). All experimental procedures were approved by the Animal Care Committee of the University of Calgary and were performed in accordance with the guidelines established by the Canadian Council on Animal Care.
Ussing Chamber Assay.
Rats were killed by cervical dislocation, and immediately, segments of jejunum 10-cm distal to the ligament of Treitz were removed and flushed with ice-cold Krebs solution. The segments were stripped of external muscle by blunt dissection and were mounted between halves of standard Ussing chambers. Once mounted, the tissues were bathed with Krebs buffer (37°C; pH 7.4). Krebs buffer contained (mM): NaCl (115), KH2 PO4 (2.0), MgCl2 (2.4), NaHCO3 (25.0), KCl (8.0), and CaCl2 (1.3). The serosal Krebs buffer contained 10 mmol/liter glucose, and the mucosal Krebs buffer contained 10 mmol/liter mannitol. Buffers were aerated and mixed by using a gas lift system (5% CO2 and 95% O2). Tissue responses were measured by clamping the potential difference (PD) to 0 mV by applying an Isc with a voltage-clamp apparatus (EVC-4000, World Precision Instruments, Sarasota, FL). Isc was monitored throughout the experiment as the indicator of net active electrolyte transport across the tissue (15). After a 20-min equilibration period, the viability of the tissues was assessed by delivering an electrical field stimulation (100 V, pulse duration 500 μs, 25 Hz, 3 s) with a dual-impedance stimulator (Harvard Apparatus). Drugs were then added to the serosal side of the tissues, and the changes in Isc were determined. At the end of all experiments, carbachol (1 μM) was applied to the serosal side of the tissues. Changes in Isc induced by the drugs were expressed as a percentage of the carbachol response. Concentration-effect curves [n = 6 for each point ± SEM. (bars)] were established for SL-NH2, tc-NH2, Cit-NH2, and trypsin. The effects of the addition of LSIRGL (LS)-NH2: an inactive form of the SL-NH2 peptide), PGE2 (1 μM), and thrombin (5 units/ml; 50 nM) to the serosal side of the tissues also were observed.
The effect of a previous addition of a proteinase-inhibitor mixture for mammalian cells (Sigma P 8340, containing 4–2-aminoethyl benzenesulfonyl fluoride/pepstatin A/trans-epoxysuccinyl-l-leucylamino-4-guanidino butane/bestatin/leupeptin/aprotinin) on PAR-AP-induced Isc response was observed. Paired tissues from the same animal were matched on the basis of basal conductance. The serosal side of one member of each pair was exposed to the proteinase inhibitor mixture (P 8340, 1 μl/20 mg of tissue) and the other member of the pair was exposed to vehicle. Ten minutes later, SL-NH2 (15 and 30 μM), tc-NH2 (40 and 60 μM), or Cit-NH2 (80 and 100 μM) were added to the serosal side of both tissues (n = 6 for each concentration used) and the Isc response to the peptides were compared, in the presence or in the absence of proteinase inhibitors.
To determine whether the SL-NH2 responses were desensitized by trypsin (and vice versa), the intestinal tissue was first exposed to either trypsin (100 units/ml; 200 nM) or SL-NH2 (80 μM). The serosal side of the chamber was then washed twice and either SL-NH2 (80 μM) or trypsin (100 units/ml; 200 nM), respectively, was then added to the serosal side of the tissues.
The effects of various inhibitors on the Isc response to SL-NH2 were studied: indomethacin (10 μM), a nonselective cycloxygenase (COX) inhibitor, SC-58125 (3 μM), a COX-2 inhibitor, and the neural blocker tetrodotoxin (TTX; 1 μM). These inhibitors were added to the serosal side of jejunal tissue 20 min before the addition of SL-NH2 (80 and 40 μM).
To confirm the chloride dependency of the Isc response to SL-NH2, experiments were conducted with chloride-free Krebs buffer, as described (16). In this experiment, after a 20-min equilibration period, SL-NH2 was added to the serosal side of the chamber and the Isc response was recorded.
HPLC Analysis of PAR-APs Recovered from the Bioassay Systems.
Previous work had established that peptide hydrolysis does not occur during the course of vascular assays of PAR-APs (18, 26). We used a comparable HPLC analysis to assess possible peptide hydrolysis in the Ussing chamber assay. Immediately after observing a jejunal response, the peptide-containing medium was withdrawn from the tissue and quick frozen for subsequent HPLC analysis. Tissue-exposed solutions were analyzed by HPLC by using a microbondpak C-18 analytical column (Waters, Mississauga, ON, Canada) with a 0–50% gradient of acetonitrile in 0.1% vol/vol aqueous trifluoroacetic acid (TFA), begun 5 min after the sample application to the column (flow rate of 1 ml/min of the 0.1% TFA eluant). The linear acetonitrile gradient, run over the course of 60 min, resulted in the elution of standard peptide samples at reproducible times for each peptide, ranging from 20 to 50 min. Peptide elution was monitored by measuring absorption at 215 nm.
Aorta Relaxation Assay.
Immediately after killing, animals were anticoagulated by the injection of heparin (1,000 units in 2 ml of isotonic saline) into the left ventricular circulation. Clot-free samples derived from the aorta were dissected free from adhering tissue and ring preparations (≈2 mm × 2 mm) were cut for use in the bioassay. Aorta ring tissue was equilibrated for 1 h at 37°C in a gassed (5% CO2, 95% O2) Krebs–Henseleit buffer, pH 7.4, of the following composition (mM): NaCl (118), KCl (4.7), CaCl2 (2.5), MgCl2 (1.2), NaHCO3 (25), KH2PO4 (1.2), and glucose (10). As described (10), the relaxant actions of the PAR2APs and trypsin were measured in endothelium-intact rings that were precontracted with 1 μM phenylephrine. For the construction of concentration–relaxation curves, the relaxant responses to increasing concentrations of PAR2APs and trypsin were expressed as a percentage of the relaxation caused by 1 μM acetylcholine (% Ach) and monitored both before and after the exposure of the tissue to all test concentrations of the peptides. Agonists were added directly to the organ bath (4 ml) and ring tension was monitored by using either Grass- or Statham force-displacement transducers.
Calcium-Signaling Assay by Using PAR2-Transfected KNRK Cells.
The rat PAR2 receptor (10) was subcloned into pcDNA3 (Invitrogen) and transfected into Kirsten Sarcoma virus-transformed rat kidney epithelial cells (KNRK; American Tissue Type Culture Collection) by using the Lipofectamine method, according to the manufacturer’s instructions (GIBCO/BRL). Transfected cells were subcloned in geneticin-containing medium (0.6 mg/ml) to yield a permanent cell line (PAR2-KNRKs) expressing ≈75,000 receptors/cell (B.A.-A., S. Mokashi, and M.D.H., unpublished data). A comparable cell line (PAR2-KNRKb) also was obtained by using the viral LNCX vector (17). Routinely, PAR2-KNRK cells were grown in a geneticin-containing (0.6 mg/ml) DMEM supplemented with 5% (vol/vol) fetal calf serum by using 80-cm2 plastic T-flasks; cells were propagated without the use of trypsin. Background KNRK cells were similarly grown in the absence of cytocidal antibiotic. For the calcium-signaling assay, cells grown just to the point of confluence were harvested by suspension in EDTA-containing calcium-free isotonic PBS. Disaggregated cells were pelleted by centrifugation and were resuspended in 1 ml of DMEM/10% fetal calf serum for loading with the calcium indicator (Molecular Probes) at a final concentration of 22 μM (25 μg/ml) fluo-3AM ester (Molecular Probes). Indicator uptake was achieved over a 20- to 25-min incubation period at room temperature in the presence of 0.25 mM sulfinpyrazone, at which time cells were washed twice by centrifugation and resuspension (106 cells/ml stock suspension) in assay buffer, pH 7.4, of the following composition (mM): NaCl (150), KCl (3), CaCl2 (1.5), Hepes (20), Glucose (10), and sulfinpyrazone (0.25). Fluorescence measurements, reflecting elevations of intracellular calcium were done at 24°C, using a Perkin–Elmer fluorescence spectrometer with an excitation wavelength of 480 nm and emission recorded at 530 nm. Cells (2 ml of ≈3 × 105 cells/ml) were stirred in a thermostated plastic cuvette to which agonist stock solutions were added directly for monitoring trypsin- or peptide-induced changes in fluorescence. To construct fluorescence-yield concentration-effect curves, the signals elicited by agonists in replicate cell suspensions, were expressed as percentage of the fluorescence peak height caused by 2 μM ionophore A23187 (Sigma). This concentration of ionophore was at the plateau of its concentration-effect curve for a fluorescence response. Measurements of PAR-APs-stimulated calcium signals were done both in the absence and presence of either amastatin (10 μM) or the same mixture of proteinase inhibitors (P8340) used in the Ussing chamber assay (above).
Peptides and Other Reagents.
All peptides, prepared by solid phase synthesis, were obtained either from the peptide synthesis facility of the University of Calgary Faculty of Medicine (D. McMaster) or through the courtesy of L. Leblond via the peptide synthesis facility at BioChem Therapeutic (Laval, Quebec, Canada). The composition and purity of all peptides were confirmed by HPLC analysis, mass spectral analysis, and amino acid analysis. Stock solutions, prepared in 25 mM Hepes buffer, pH 7.4, were analyzed by quantitative amino acid analysis to verify peptide concentration and purity. Porcine trypsin (14,900 units/mg, cat no. T7418), TTX, carbachol, indomethacin, and amastatin were from Sigma. A maximum specific activity of 20,000 units/mg was used to calculate the approximate molar concentrations of trypsin in the bioassay systems (upper axes in Fig. 3).
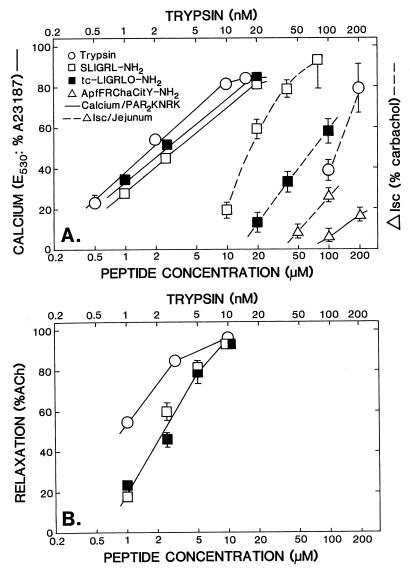
Figure 3.
Concentration-effect curves for PAR-APs and trypsin. (A). The PAR2-KNRK calcium assay and the Isc assay and (B) the AR assay. The three bioassay responses: (i) Δ Isc, (A) (right-hand ordinate, Δ Isc in percentage of carbachol response) broken lines; (ii) PAR2-KNRK cell calcium signaling, (A) (left-hand ordinate, E530 in percentage of A23187 response) solid lines; and (iii) AR, (B) (left-hand ordinate, in percentage of acetylcholine response; %ACh) were measured for increasing concentrations of PAR-APs (□, SL-NH2, ▪, tc-NH2, ▵, Cit-NH2; and ○, trypsin) as outlined in Methods. The concentrations of trypsin (nM) are shown on the upper abcissa. Each data point represents the average response (± SEM bars) observed at each concentration for three to six independently conducted experiments with tissues or cells derived from two to six different animals or cell culture preparations. Error bars smaller than the symbols are not shown.
RESULTS
Isc Response of Jejunal Serosa to PAR-APs, Trypsin, and Thrombin.
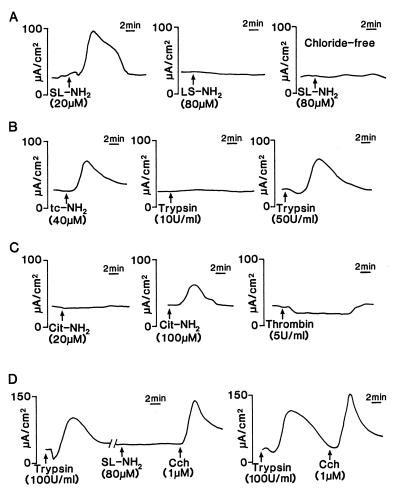
The serosal application of SL-NH2, the PAR2AP peptide based on the proteolytically revealed rat PAR2 receptor sequence, evoked an increase in Isc in all preparations (n = 40). This response began within 3 min of the addition of SL-NH2, quickly reached a peak, and fell to baseline during the subsequent 10 min (Fig. 1A). LS-NH2, the inactive form of the SL-NH2 peptide, had no effect on Isc (Fig. 1A). The Isc response to 80 μM of SL-NH2 was abolished when chloride-free buffer was used (Fig. 1A). Another PAR2AP, tc-NH2, induced a similar Isc response, but much higher concentrations of this peptide were required (Fig. 1B). Low concentrations of trypsin (0.5, 1, and 10 units/ml; 1 to 20 nM) failed to reproduce the Isc response obtained with PAR2AP (only 10 units/ml trypsin is shown in Fig. 1B). However, much higher concentrations (50 and 100 units/ml; 100 and 200 nM) of trypsin induced an Isc response (see Fig. 1B for 50 units/ml).
Figure 1.
Representative tracings of the Isc response of rat jejunum to the serosal addition of (A) SL-NH2 (20 μM) and LS-NH2 (80 μM), when tissues were bathed in normal Krebs buffer, or SL-NH2 (80 μM) in tissues bathed with chloride- (Cl−) free Krebs buffer. (B) tc-NH2 (40 μM) and trypsin (10 and 50 units/ml; 20 and 100 nM) in normal Krebs buffer. (C) Cit-NH2 (20 and 100 μM) and thrombin (5 units/ml; 50 nM) in normal Krebs buffer. (D) SL-NH2 (80 μM) after trypsin (100 units/ml; 200 nM), and trypsin alone (100 units/ml; 200 nM), followed by carbachol (Cch, 1 μM) in normal Krebs buffer. The scale for time and Δ Isc (in μA/cm2) is shown respectively to the right and to the left of each tracing. All panels show a tracing for an individual tissue preparation that is representative of six independently conducted experiments.
In contrast, the selective PAR1AP Cit-NH2 at concentrations more than sufficient to activate PAR1 (20 μM; M.D.H., A. Kawabata, M.S., and L. Leblond, unpublished data) caused no effect on Isc (Fig. 1C). Nonetheless, an Isc increase was observed by using very high concentrations of Cit-NH2 (50 and 100 μM) (shown in Fig. 1C for 100 μM). The high concentrations were, nonetheless, lower than those required to activate the cloned rat PAR2 expressed in KNRK cells (see Fig. 3A). The addition of thrombin to the serosal side of the chambers did not provoke any increase in Isc, but a small decrease could be detected (Δ Isc = 11.37 ± 3.45 μA/cm2, n = 6) (Fig. 1C).
Crossdesensitization of SL-NH2-Induced Jejunal Isc Response by Trypsin.
The repetitive and cumulative exposure of the tissues at short time intervals (<10 min) to high concentrations of either SL-NH2 peptide (80 and 100 μM) or trypsin (100 units/ml; 200 nM) resulted in a desensitization of the Isc response (data not shown). To determine whether the SL-NH2–Isc response could be desensitized by trypsin (and vice versa), a response of jejunal tissues was first obtained by the addition of 100 units/ml trypsin (200 nM). Then, after a return of Isc to the baseline, the tissues were washed and exposed to SL-NH2 (80 μM). The tissues desensitized to trypsin were not able to respond further to SL-NH2, even at high concentrations (80 μM) of the peptide (Fig. 1D). Pretreating the tissues with SL-NH2 (80 μM) partially desensitized the subsequent increase in Isc in response to 100 units/ml trypsin (not shown). After desensitization of the tissues to either trypsin or SL-NH2, the Isc response to carbachol was, nonetheless, intact (Fig. 1D and data not shown).
Effects of Inhibitors on the Isc Response Induced by SL-NH2.
To determine whether the SL-NH2-induced Isc response was mediated by the neuronal release of stored agonists, we preincubated the jejunal tissues with TTX, a sodium channel blocker that inhibits axonal conduction and action potentials. TTX (1 μM) completely inhibited the Isc response to electrical field stimulation but did not affect the SL-NH2-induced Isc response (81.79 ± 12.9% of the carbachol response for an Isc response induced by 40 μM of SL-NH2 in the presence of TTX vs. 78.57 ± 8.5% for the same Isc response in the absence of TTX) (mean ± SD for six independent experiments).
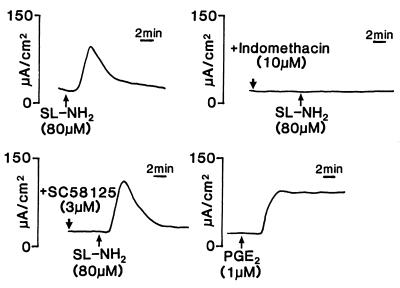
To determine whether the Isc response of the intestinal tissues to SL-NH2 was mediated by the activation of cycloxygenase and the release of prostaglandins, we pretreated the jejunal tissues either with indomethacin (10 μM), a nonselective COX inhibitor, or with SC-58125 (3 μM), a specific COX-2 inhibitor. The Isc response to 80 μM of SL-NH2 was completely abolished in the presence of 10 μM indomethacin, but the SL-NH2-induced response was unchanged in the presence of SC-58125 (85.15 ± 11.3% of the carbachol response with SC-58125 present vs. 92.67 ± 26.9% of the carbachol response in the absence of SC-58125) (Fig. 2 in which the tracings are representative of six independent experiments). The addition of PGE2 (1 μM) to the serosal side of the Ussing chamber caused an increase in Isc, as did SL-NH2. However, the Isc response to PGE2 was sustained as opposed to the transient effect of SL-NH2 (Fig. 2).
Figure 2.
Representative tracings of the Isc response of rat jejunum, to the serosal addition of SL-NH2 (80 μM) in the presence or in the absence of indomethacin (10 μM), SC-58125 (3 μM) and to the serosal addition of PGE2 (1 μM). The scale for time and Δ Isc (in μA/cm2) is shown respectively to the right and to the left of each tracing. All panels show a tracing for an individual tissue preparation that is representative of six independently conducted experiments.
Concentration-Effect Curves for PAR-APs and Trypsin in the Three Bioassays.
The concentration-effect curves for the changes in Isc induced by SL-NH2, tc-NH2, Cit-NH2, and trypsin are shown in Fig. 3A. An increased Isc response was induced by SL-NH2 at concentrations from 10 to 80 μM; tc-NH2 was less potent than SL-NH2 to induce the same response. Isc responses from 40 to 80% of the carbachol response were induced by 100–200 nM (50–100 units/ml) trypsin (the same magnitude of response caused by trypsin was obtained with 20–40 μM SL-NH2; Fig. 3A). Concentrations of 50–100 μM of Cit-NH2 were necessary to induce an Isc response in this bioassay. On a molar basis, the order of potencies of the agonists in the Ussing chamber Isc assay was: trypsin≫SL-NH2>tc-NH2>Cit-NH2 (as summarized in Table 1). These relative potencies were in stark contrast to the relative potencies of the same agonists in the PAR2-KNRK cell calcium-signaling assay and in the AR assay (see below; Fig. 3 and Table 1).
Table 1.
Activity ratios (REC) for PAR-APs and trypsin in the ISC, PAR2-KNRK, and aorta relaxation assays
| Relative activity ratio (REC)* | |||
|---|---|---|---|
| assay | |||
| Agonist | ΔISC | PAR2KNRK | Aorta relaxation |
| SL-NH2 | 1 | 1 | 1 |
| tc-NH2 | 3.4 ± 0.4 | 0.8 ± 0.01 | 1.0 |
| Cit-NH2 | 8.5 ± 0.4 | 460 ± 8 | ND |
| Trypsin, ×103 | 7.4 ± 0.1 | 0.5 ± 0.02 | 0.5 ± 0.02 |
Activity ratios (REC) for each agonist, relative to the concentration of SL-NH2 (REC 1) causing an equivalent biological response, were calculated as described (18) from the concentration-effect curves shown in Fig. 3, according to the formula: REC = (concentration of agonist for a given response in the linear portion of its concentration-effect curve)/(concentration of SL-NH2 required to give the equivalent biological response). A value >1.0 designates an agonist with a potency lower than that of SL-NH2. Values for trypsin were multiplied by 103. Each value represents an average (± SEM) obtained from measurements done at three to seven levels of response along the concentration-effect curves for each agonist shown in Fig. 3. ND, not determined.
The potency of tc-NH2 to activate a calcium signal in rat KNRK cells expressing rat PAR2 was equivalent to that of SL-NH2 (Fig. 3A), and the inactive form of the SL-NH2 peptide (LS-NH2 peptide) had no effect (not shown). In the PAR2-KNRK cell assay, 5–10 nM (2.5–5 units/ml) trypsin caused an activation of PAR2 that was equivalent to the maximal activation caused by PAR2APs. At comparatively high concentrations of Cit-NH2 (100–200 μM), which were more than two orders of magnitude higher than those required for maximal activation of PAR1 (not shown), the selective PAR1AP Cit-NH2 caused a low activation of the PAR2-mediated calcium signal (≈20% of the maximal response caused by SL-NH2; Fig. 3A, curve on far-right). In the nontransfected KNRK cells, none of the agonists at the concentrations shown in Fig. 3, caused a calcium signal (data not shown). Thus, in the PAR2-KNRK cell calcium-signaling assay, the potency order of the agonists was: trypsin≫>tc-NH2 ≅ SL-NH2≫>Cit-NH2 (as summarized in Table 1). Comparable results were obtained with a PAR2-KNRKb cell line, obtained by using the viral LNCX vector, instead of the pcDNA3 expression vector (not shown).
With tissues derived from the same strain of rat (Hooded Lister) used for the Ussing chamber experiments, the AR assay showed that the potency of SL-NH2 was the same as that for tc-NH2 (Fig. 3B), and the LS-NH2 peptide was inactive (not shown). Results comparable with those for the PAR2-KNRK cell assay were obtained for the relative potency of the two PAR2APs (SL-NH2 ≅ tc-NH2) in the AR assay, with tissues derived not only from the Hooded Lister strain of rats (Fig. 3B), but also from male Sprague–Dawley rats (not shown). Trypsin at concentrations of 5–10 nM (2.5–5 units/ml) caused a near-maximal relaxation of the tissue (Fig. 3B). Thus, in the relaxation assay, the relative potencies of the agonists were: trypsin≫>SL-NH2 ≅ tc-NH2 (summarized in Table 1). Because of the complex action of Cit-NH2, the selective PAR1AP, in the aorta tissue that contains both PAR1 and PAR2 (PAR1 activation causes both contraction and relaxation, ref. 19), this peptide was not evaluated in the Hooded Lister AR assay.
The relative potencies of trypsin, SL-NH2, and tc-NH2 in the AR assay were, therefore, in good accord with the potency order in the PAR2-KNRK cell calcium-signaling assay but were entirely out of keeping with the potency order observed in the intestinal Isc assay (Table 1). The concentration range for the activity of Cit-NH2, relative to the concentration range for SL-NH2 activity, also was very different in the PAR2-KNRK cell assay (≈460:1, Table 1) compared with the activity relative to SL-NH2 in the jejunal Isc assay (≈8.5:1, Table 1). Moreover, for all agonists, the concentration range of activity in the AR and the PAR2-KNRK cell assays was approximately an order of magnitude lower than the concentration range necessary for the same agonists in the jejunal Isc assay.
Susceptibility to Degradation of PAR-APs.
To determine that the different Isc responses of intestinal tissues to SL-NH2, tc-NH2, and Cit-NH2 were not caused by differences in susceptibility to local degradation by cell surface proteinases, peptide degradation in Ussing chambers was assessed by HPLC analysis. In addition, the PAR-AP-induced Isc responses were observed in the presence or in the absence of a mammalian proteinase inhibitor cocktail. Even in the absence of proteinase inhibitors, the peptides (SL-NH2, tc-NH2, and Cit-NH2) were recovered intact from the serosal buffer of Ussing chambers at the end of an experiment, and no other degradation product was detected by HPLC analysis. No difference was observed for SL-NH2-, tc-NH2-, or Cit-NH2-induced Isc response in the presence or in the absence of proteinase inhibitors for two concentrations of the tested peptides. The same mixture of proteinase inhibitors (2.5 μl of P 8340/2 ml of cell suspension) as well as amastatin (10 μM) were added to the PAR2-KNRK cells and the PAR2-mediated calcium signal induced by SL-NH2, tc-NH2, or Cit-NH2 measured. The fluorescence yield was not affected by the presence or the absence of these proteinase inhibitors in the assay cuvette.
DISCUSSION
One of the main findings of our study was that the administration of PAR-APs and trypsin to the serosal side of the rat jejunal preparation in Ussing chambers caused a prompt change in active ion transport, as reflected by the increase in Isc. The SL-NH2-induced Isc effect was caused by active chloride transport because the response was abolished in chloride-free buffer. COX-1-derived prostaglandins appeared to act as mediators of this effect because indomethacin, but not a selective COX-2 inhibitor (SC58125), completely inhibited the SL-NH2-induced response. PGE2 caused an activation of transport that was sustained, as compared with the transient increase caused by SL-NH2. The difference in the responses to PGE2 and SL-NH2 may suggest the production of distinct prostanoids in response to SL-NH2. Alternatively, it could be that the high concentration (1 μM) or overall amount of PGE2 in the bath results in sustained receptor activation and/or saturation of the mechanisms responsible for PGE2 metabolism, events which are unlikely to occur after endogenous release in uninflamed tissue. That TTX did not block the Isc response to SL-NH2, but did block the response caused by electrical field stimulation, indicated that SL-NH2 was acting directly on the serosal side of the tissue and not via the neural release of stored neurotransmitters such as acetylcholine.
The peptide structure–activity data strongly suggest that the action of the PAR-APs to regulate transport was mediated via a receptor distinct from PAR2. Clearly, neither of the two thrombin receptors (PAR1 and PAR3; refs. 1, 2, 20) were involved in the increase in Isc that we monitored because thrombin had an effect opposite to that obtained with PAR2APs and because the PAR1-selective agonist Cit-NH2 failed to activate ion transport at concentrations well above those that are required to activate PAR1. The low activity of Cit-NH2 in stimulating changes in Isc at relatively high concentrations (50–100 μM) could possibly be ascribed to the low activity of this analog at the PAR2 receptor, but its different potency relative to SL-NH2 in the jejunal Isc assay, compared with that in the PAR2-KNRK cell assay (Table 1), points to the activation of a receptor other than PAR2.
The lack of activity of the reverse peptide LS-NH2 in the Isc assay and the ability of trypsin to stimulate intestinal transport might suggest that PAR2 itself was involved in this tissue response. However, the relative potencies for trypsin and SL-NH2 in the Isc assay, compared with the other two assays, pointed strongly to the activation of a receptor different from PAR2. The clearest difference between PAR2 and the receptor responsible for regulating jejunal ion transport can be seen in comparing the structure–activity profile for the PAR-APs and trypsin in the Isc assay, with the profiles observed in the PAR2-KNRK cell assay and in the AR assay (Fig. 3 and Table 1). In the two quite distinct PAR2 assays (PAR2-KNRK cell and AR), one involving the cloned rat PAR2 receptor expressed in a cultured cell system and the other using an intact tissue, tc-NH2 was approximately equipotent (AR assay) or even slightly higher in potency (PAR2-KNRK assay) than the parent PAR2AP SL-NH2. Yet, in the jejunal Isc assay, tc-NH2 was only approximately one-third as potent as SL-NH2 (Fig. 3A and Table 1). Moreover, as noted above, Cit-NH2 also showed a distinct potency relative to SL-NH2 in the Isc assay, compared with the PAR2-KNRK cell assay. Furthermore, the concentration range over which the PAR2APs and trypsin activated PAR2 in the AR assay and the PAR2-KNRK calcium-signaling assay was approximately one (PAR2APs) or two (trypsin) orders of magnitude lower than the concentration required to activate the jejunal Isc response (Fig. 3). Classically, such differences in agonist potency profiles have been used to define receptor subfamilies, such as those for adrenoreceptor agonists (21) or histamine (22). Although the tc-NH2 peptide was synthesized in the hope of generating a PAR2 antagonist (comparable derivatives are antagonists of PAR1; ref. 23), this analog proved to be a PAR2 agonist. Thus, it has not yet been possible to obtain selective PAR2 antagonists to complement the structure–activity data we have obtained with the PAR-AP agonists. Nonetheless, the low relative potency of tc-NH2 compared with SL-NH2 and the distinct relative potencies of trypsin and Cit-NH2 in the Isc assay (Fig. 3 and Table 1), point clearly to a receptor different from PAR2.
Although the requirement for high concentrations of trypsin in the Isc assay might possibly be accounted for by the presence of high levels of serpin-like trypsin inhibitors in the tissue, this possibility would not be in accord with our previous assays with intact intestinal preparations (9, 10, 24) or vascular preparations (9, 10), in which trypsin was able to activate PAR2 fully at concentrations in the same range as those shown in Fig. 3 (0.5–10 units/ml; 1–20 nM, for the PAR2-KNRK cell and AR assays). Furthermore, our previous experience with tissue bioassays has shown that peptide degradation (e.g., by aminopeptidase action 25), as assessed by HPLC analysis, does not appear to be a factor in assigning relative potencies to PAR-APs (18, 26). In the Ussing chamber assay, no degradation of the peptides was detected by HPLC analysis. Moreover, both in the Ussing chamber assay and in the PAR2-KNRK cell assay, the proteinase inhibitors did not affect the responses to different concentrations of the PAR-APs. Thus, the differences in the peptide potencies observed in these bioassays were unlikely to be caused by a local peptide degradation by cell surface proteinases. We therefore believe it to be unlikely that either elevated tissue serpins or tissue proteolysis of the PAR-APs could account for the distinct concentration range of activity for trypsin and the PAR-APs and for the clearly distinct relative potencies of SL-NH2, tc-NH2, and Cit-NH2 in the jejunal Isc assay, compared with the PAR2-KNRK cell calcium-signaling and AR assays.
We believe it is of significance: (i) that trypsin added at quite high concentrations (50–100 units/ml or 100–200 nM) did increase Isc upon serosal application and (ii) that this action of trypsin desensitized the tissue to SL-NH2, but not to a second agonist (carbachol), that also acts via a G protein-coupled receptor (Fig. 1D). These data indicate that in the jejunal Isc assay, SL-NH2 was working via a receptor that, like PAR2 and other PARs, can be activated/desensitized by a serine proteinase. Possibly, mast cell tryptase may activate this receptor in the setting of intestinal inflammation. It is also significant that PAR2AP-activated intestinal transport was blocked by indomethacin, in keeping with the ability of trypsin (10 μM; 5,000 units/ml) and SL-NH2 (100 μM) to cause PGE2 secretion when applied to the luminal side of everted sacs of rat jejunum (13). The very high concentrations of trypsin and SL-NH2 that were required to stimulate luminal prostaglandin secretion would be more than sufficient to activate the “serosa-side” receptor described here, that is responsible for modulating intestinal transport. We believe our data demonstrate, by a pharmacological approach, the presence in rat jejunal tissues of a receptor for PAR-APs, distinct from PARs 1, 2, and 3, that can be activated by a serine proteinase. Our findings add one more response, the regulation of intestinal transport, to the repertoire of physiological roles that PARs may play in vivo.
Acknowledgments
We are grateful to Dr. Dennis McMaster at the University of Calgary Peptide Synthesis Facility and to Dr. Lorraine Leblond at BioChem Therapeutic, Laval, Quebec, Canada, for assistance with provision of synthetic peptides for our work. We also are indebted to Dr. S. Mokashi for help with the isolation of one of the PAR2-expressing KNRK cell lines used for this study, to Dr. A. Kawabata for help with the calcium-signaling assays, to Dr. S. Robbins for the provision of the viral LNCX expression vector, and to Bernard Renaux for performing the HPLC analysis. These studies were supported in large part by operating grants from the Medical Research Council of Canada (to M.D.H. and J.L.W.), the Crohn’s and Colitis Foundation of Canada (to W.K.M.), and by ancillary funds for peptide synthesis provided by an Natural Sciences and Engineering Research Council (Canada)/National Research Council of Canada partnership grant, in conjunction with BioChem Therapeutic. N.V. was supported by a postdoctoral fellowship from the Canadian Association of Gastroenterology/Abbott Laboratories. Dr. J. Wallace is a Medical Research Council Senior Scientist and an Alberta Heritage Foundation for Medical Research Scientist.
ABBREVIATIONS
- AR
aorta ring relaxation
- Cit
Ala-parafluoroPhe-Arg-cyclohexylAla-Citrulline-Tyr
- COX
cycloxygenase
- Isc
short circuit current
- LS
LSIGRL
- PAR
proteinase-activated receptor
- PAR-APs
PAR-activating peptides
- PAR2-KNRK cells
rat KNRK cells expressing cloned rat PAR2
- PGE2
prostaglandin E2
- SL
SLIGRL
- tc-
trans-cinnamoyl-LIGRLO
- TFA
trifluoroacetic acid
- TTX
tetrodotoxin
Footnotes
To whom reprint requests should be addressed. e-mail: mhollenb@acs.ucalgary.ca.
References
- 1.Rasmussen U G, Vouret-Craviari V, Jallat S, Schlesinger Y, Pages G, Pavirani A, Lecocq J P, Pouyssegur J, Obberghen-Schilling E. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- 2.Vu T K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 3.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nystedt S, Larsson A-K, Aberg H, Sundelin J. J Biol Chem. 1995;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin S R, Vu T K, Hung D T, Wheaton V I. J Clin Ivest. 1992;89:351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackhart B D, Emilsson K, Nguyen D, Teng W, Martelli A J, Nystedt S, Sundelin J, Scarborough R M. J Biol Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- 7.Hollenberg M D, Saifeddine M, Al-Ani B, Kawabata A. Can J Physiol Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- 8.Böhm S K, Kong W, Brömme D, Smeekens S P, Anderson D C, Connolly A, Kahn M, Nelken N A, Coughlin S R, Payan D G, et al. Biochem J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ani B, Saifeddine M, Hollenberg M D. Can J Physiol Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- 10.Saifeddine M, Al-Ani B, Cheng C-H, Wang L, Hollenberg M D. Br J Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwa J J, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Circ Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- 12.Emilsson K, Wahlestedt C, Sun M-K, Nystedt S, Owman C, Sundelin J. J Vasc Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- 13.Kong W, McConalogue K, Khitin L M, Hollenberg M D, Payan D G, Bohm S K, Bunnett N W. Proc Natl Acad Sci USA. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConalogue K, Kong W, Böhm S K, Gamp P D, Dery O, Bunnett N W. Gastroenterology. 1997;112:1171. (abstr.). [Google Scholar]
- 15.Cooke H J. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. New York: Raven; 1989. pp. 1307–1350. [Google Scholar]
- 16.MacNaughton W K, Gall D G. Eur J Biochem. 1991;200:17–23. doi: 10.1016/0014-2999(91)90660-i. [DOI] [PubMed] [Google Scholar]
- 17.Miller A D, Rosman G J. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 18.Tay-Uyboco J, Poon M C, Ahmad S, Hollenberg M D. Br J Pharmacol. 1995;115:569–578. doi: 10.1111/j.1476-5381.1995.tb14970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laniyonu A A, Hollenberg M D. Br J Pharmacol. 1995;114:1680–1686. doi: 10.1111/j.1476-5381.1995.tb14957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara H, Connoly A, Zeng D, Kahn M, Zheng Y, Timmons C, Tram T, Coughlin S. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 21.Ahlquist R P. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 22.Black J W, Duncan W A M, Durant C J, Ganellin C R, Parsons E M. Nature (London) 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 23.Bernatowicz, M. S., Klimas, C. E., Hartl, K. S., Peluso, M., Allegretto, N. J. & Seiler, S. M. J. Med. Chem. 39, 4879–4887. [DOI] [PubMed]
- 24.Yang S G, Laniyonu A A, Saifeddine M, Moore G J, Hollenberg M D. Life Sci. 1992;51:1325–1332. doi: 10.1016/0024-3205(92)90631-x. [DOI] [PubMed] [Google Scholar]
- 25.Coller, B. S., Ward, P., Ceruso, M., Scudder, L. E., Kutok, J. & Prestwich, G. D. (19972) Biochemistry31, 11713–11720. [DOI] [PubMed]
- 26.Hollenberg M D, Laniyonu A A, Saifeddine M, Moore G J. Mol Pharmacol. 1993;43:921–930. [PubMed] [Google Scholar]