Abstract
Objective:
The auditory Event-Related Potentials (ERP) component P50 to sound onset and offset have been reported to be similar, but their magnetic homologue has been reported absent to sound offset. We compared the spatio-temporal distribution of cortical activity during P50 to sound onset and offset, without confounds of spectral change.
Methods:
ERPs were recorded in response to onsets and offsets of silent intervals of 0.5 s (gaps) appearing randomly in otherwise continuous white noise and compared to ERPs to randomly distributed click pairs with half second separation presented in silence. Subjects were awake and distracted from the stimuli by reading a complicated text. Measures of P50 included peak latency and amplitude, as well as source current density estimates to the clicks and sound onsets and offsets.
Results
P50 occurred in response to noise onsets and to clicks, while to noise offset it was absent. Latency of P50 was similar to noise onset (56 msec) and to clicks (53 msec). Sources of P50 to noise onsets and clicks included bilateral superior parietal areas. In contrast, noise offsets activated left inferior temporal and occipital areas at the time of P50. Source current density was significantly higher to noise onset than offset in the vicinity of the temporo-parietal junction.
Conclusions:
P50 to sound offset is absent compared to the distinct P50 to sound onset and to clicks, at different intracranial sources. P50 to stimulus onset and to clicks appears to reflect preattentive arousal by a new sound in the scene. Sound offset does not involve a new sound and hence the absent P50.
Significance:
Stimulus onset activates distinct early cortical processes that are absent to offset.
Keywords: Event-Related Potentials, Gaps in noise, Change detection, Low-resolution electromagnetic tomography, Functional imaging
1. Introduction
The auditory P50 component is the earliest (around 50 msec), the smallest in amplitude, the most variable and consequently the least studied of the auditory Event Related Potentials (ERP). Early reports on long-latency evoked potentials typically reported its presence, as part of the obligatory exogenous “vertex potential” (e.g., Davis and Zerlin 1966) or “P1-N1-P2 complex” (e.g., Knight et al. 1980; Naatanen and Picton 1987), but parametric effects were rarely elaborated.
The earliest report on human auditory evoked potentials (Davis 1939) reported a response to onset as well as offset of a tone. A later study had better control of the acoustic properties of the onset and offset of the tone (Davis and Zerlin 1966) and reported the on-response and the off-response to be “very similar”, consisting of a P1-N1-P2 vertex potential. In contrast, the auditory neuromagnetic P50 field has been reported to be absent from offset responses but present and indistinguishable in its sources from N100 in response to stimulus onset (Hari et al., 1987; Pantev et al., 1996). This neuromagnatic response, P40m, peaking about 40 ms after stimulus onset, preceded a prominent field in the opposite direction at about 100 ms (N100m). Both deflections could be explained by cortical activity within the Sylvian fissure (Hari et al., 1987). Striking similarities were found between the N100m of the on- and off-responses in their latency, estimated sources in the supratemporal plane and in their amplitude dependence on stimulus rate (Hari et al., 1987). However, only the on-response was preceded by P40m (Hari et al., 1987; Pantev et al., 1996), suggesting that N100 is not dependent on a preceding P50 (Hari et al., 1987). Moreover, while N100 seems to reflect cortical activity related to any abrupt change in the auditory environment, e.g., sound onset as well as offset (Hari et al., 1987), P50 was suggested to reflect a distinct process evoked only by stimulus onset. This contradiction between the early reports on the electric P50 and the recent reports on the magnetic P40m have not been resolved.
A possible confound of studies on brain responses to sound onsets is the spectral change accompanying onset – from zero energy in silence to the spectral energy contained in the stimulus, resulting in spectral splatter introduced by sound envelope. Similarly, offsets of sounds are accompanied by spectral changes associated with the spectral splatter of the diminishing stimulus envelope. Brain responses to such spectral change could affect and override brain responses that are specific to onset and offset of sound, obscuring their differences. One way to overcome this limitation is to use interruptions in white noise: the wide spectral content of white noise remains flat even when abrupt gaps are introduced because both the abrupt envelope of gaps and white noise have the same spectrum. Comparisons of brain potentials to gap onset and offset, therefore, reveal the differences between the responses to offset and onset of sound without confounds of an associated spectral change.
The N100 component to sound onset (gap offset) is single-peaked whereas to sound offsets (gap onsets) it is a double-peaked N-Complex. The first constituent of the N-Complex (N1a) begins its downward slope at ∼50 msec and peaks at ∼100 msec, is frontal in distribution and similar to N100 of clicks. The following peak (N1b) occurs at ∼150 msec with a central/temporal scalp distribution, with distinct sources and time courses of their activity (Michalewski et al., 2005; Pratt et al., 2005). Whereas P50 to sound offset may be absent, the subsequent negativity (N100) has a more complex configuration (consisting of N1a and N1b) to sound offset compared to sound onset (consisting of a single-peaked N100). An absence of P50 to sound offset may thus be a result of the opposite polarity N-complex to sound offset summating with P50 to obscure it. This suggestion could be verified by comparing the sources of brain activity at the time of P50 and N100 to sound onset and offset.
The purpose of this study was to compare the auditory P50 and its intracranial sources in response to stimulus offset and onset and to compare them to the P50 in response to two clicks with similar time separations as the onset and offset of sound.
2. Methods
The detailed description of procedures to study potentials to onsets and offsets of noise (offsets and onsets of gaps) and to compare them with their counterparts in response to brief transient sounds (clicks) are provided in an earlier report (Pratt et al., 2005).
2.1. Subjects
Thirteen, right handed, normal hearing subjects, 18 to 25 years old participated in the study. Subjects were paid for their participation and all procedures were approved by the institutional review board for experiments involving human subjects (Helsinki Committee).
2.2. Stimuli
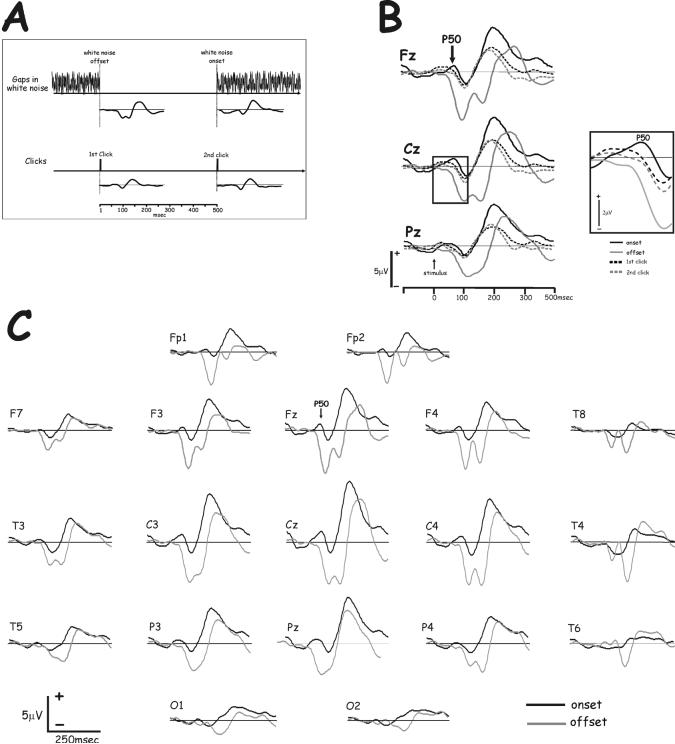
Two types of binaural stimuli were presented separately through earphones (Sony MDR-CD770): (1) Binaural continuous white noise with randomly distributed gaps of different durations; and (2) Binaural click pairs presented in silence such that the first and second click of each pair corresponded in timing to gap onsets and offsets, respectively, in the noise condition (Fig. 1A). Thus, each offset and onset in the noise had a correspondingly timed click.
Fig. 1.
A. Binaural stimuli used in this study. Top: Binaural continuous white noise with randomly distributed gaps; Bottom: Click pairs presented with inter stimulus intervals identical to the gap durations and inter-gap intervals. Potentials associated with noise offset, noise onset, first and second clicks in the pair were recorded.
B. Grand averaged waveforms (13 subjects) of potentials to noise offsets and noise onsets. In addition to the marked differences in N100, note the clearly defined P50 to noise onset, particularly at midline central and frontal electrodes, and its diminution to an earlier inflection from baseline in response to noise offset.
C. Potentials in response to noise onset, noise offset, first and second clicks of click pairs with the same 500 msec interclick intervals. Note the similar latencies and waveforms of potentials to noise onsets and to the first and second clicks in the pairs, comprising a P50 followed by an N100-P160 sequence. In contrast, the potentials to noise offset, in addition to the bifid N-Complex, had no P50 peak. The inset shows enlarged P50 waveforms to the different stimuli. Note that waveforms evoked by clicks are intermediate between the waveforms to noise onset and offset. The definition of P50 peak to clicks in this grand average is inferior to that of individual subjects, due to intersubject latency jitter.
2.2.1. Noise onsets and offsets
White noise was presented continuously throughout the noise condition, with randomly distributed short gaps of up to 20 msec durations (“short gaps”) and longer gaps with a variable average duration of 500 msec (“long gaps”). Noise durations between gaps were 1500 msec. This report relates to the long gaps only. The variable duration of the long gaps provided sufficient temporal separation between the potentials evoked by gap onset (noise offset) and offset, as well as temporal jitter of gap offset (noise onset), precluding interference of noise offset and onset responses in the averaged waveform. One hundred random repetitions of the long gaps were presented. The spectral content of the noise was flat within 10 dB across the frequency range 100-10,000 Hz, and the gaps had abrupt (square) onsets and offsets resulting in a similarly flat spectrum. Noise intensity was 65 dBnHL. In this report the potentials to gap onset will be referred to as the noise offset potentials and those to gap offset as the noise onset evoked potentials (Fig. 1A).
2.2.2. Click pairs
Clicks were generated by transducing 100 μsec square electric pulses in the earphones. Spectral content of the clicks and white noise was the same: flat with a 10 dB increase in energy between 2 and 4 kHz. Click intensity was 65 dBnHL. Click pairs with interclick intervals of up to 20 msec (short intervals), and 500 ms were presented with interpair intervals of 1500 msec. Click timing was adjusted such that first and second click positions along the train of clicks corresponded to offsets and onsets of noise, respectively, in the noise condition (Fig. 1A). One hundred repetitions of the pairs with 500 msec intervals were randomly repeated. Only potentials evoked by onsets and offsets of half second gaps and click intervals of 500 msec were analyzed in this study.
2.3. Procedure
Twenty-two 9 mm silver disc electrodes were placed at: FP1, F7, F3, Fz, F4, F8, FP2, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2, according to the 10-20 system, 1 cm above the left and right mastoids (A1 and A2), as well as the middle of the chin, which served as reference, and below the left eye, which was referenced to Fz, to control for eye movements (EOG). In total, EEG was recorded from 21 electrodes referenced to the center of the chin and EOG was recorded from one diagonal differential recording below the left eye referenced to Fz. An electrode on the left forearm served as ground. Impedance at each electrode was maintained below 5 kOhms.
Subjects were then seated in a comfortable reclining armchair in a sound proof chamber and listened to the two types of stimuli in turn: gaps in noise and click pairs in silence. Subjects were instructed to read a complicated text on which they were to be examined, while stimuli were presented (not attending to sounds).
2.4. Data acquisition
Potentials from the EEG (X100,000) and EOG (X20,000) channels were amplified, digitized with a 12-bit A/D converter at a rate of 256 samples/sec, filtered (0.1 to 100Hz, 6 dB/octave slopes) and stored for off-line analysis. EEG processing began with segmentation of the continuous EEG to epochs beginning 100 ms before until 1000 ms after noise offset, noise onset or click onsets. Eye movement correction (Attias et al., 1993) and artifact rejection (±150 μV) followed segmentation. Average waveforms were then computed relative to noise offset, relative to noise onset, as well as relative to the first click in the pair and relative to the second click in the pair. These averages were computed for each subject, as well as across subjects to obtain grand mean waveforms. After averaging, the data were low-pass filtered (FIR rectangular filter with a low-pass cutoff at 24 Hz) and baseline (average amplitude across the 100 ms before stimulus onset) corrected.
2.5. ERP data analysis
ERP analysis included P50 peak latency and amplitude comparisons among noise onsets, offsets and clicks, as well as comparisons of the respective source current densities and their distributions. Except for small amplitude differences, most probably due to marginal refractoriness from the 500 msec interclick interval, potentials to the first and second clicks in pairs were almost identical. Therefore, P50 to noise onset and offset were statistically compared only to their counterpart in response to the first click of the click pairs.
2.5.1. Peak analysis
The amplitude and latency of P50 to noise onset, first and second click and noise offset were measured for each subject in each channel. Because P50 varied in its definition among the stimulus conditions, the following guidelines were used to define it: (1) when a peak was observed in the latency interval of 35-90 msec this peak was defined as P50 (such a peak was typically defined in response to clicks and to noise onset), and (2) when a peak could not be identified in this latency range (typically in response to noise offset), P50 was defined at the point the waveform departed from baseline before N100. Group grand averaged waveforms for each stimulus condition determined this latency range for peak identification.
ERP peak amplitudes and latencies were subjected to a repeated measures analysis of variance (ANOVA) with Geisser-Greenhouse correction for violation of sphericity and Bonferroni corrections for multiple comparisons. Factors were: Stimulus type with 3 levels (noise offset, noise onset and first click in pair) and electrode group with 3 levels (Frontal – FP1, FP2, Fz; Central – C3, Cz, C4; Temporo-parietal - T3, T4, Pz). To determine the significance of P50 amplitudes relative to baseline, amplitude was assessed across all electrodes for four stimulus conditions: P50 to noise onset, P50 to noise offset, baseline preceding noise onset and baseline preceding noise onset. Probabilities below 0.05, after Geisser-Greenhouse corrections, were considered significant.
2.5.2. Functional imaging
Standardized Low Resolution Electromagnetic Tomographic Analysis (sLORETA, Pascual-Marqui, 1994; Pascual-Marqui, 2002) was applied on the 21-channel ERP records to image the estimated source current density throughout the duration of P50 in response to noise onsets, offsets and clicks and to compare the current density distributions among stimuli.
sLORETA is a functional brain imaging method that estimates the distribution of current density in the brain given by the minimum norm solution. Localization inference is based on standardized values of the current density estimates. The solution space is restricted to cortical gray matter and hippocampus. A total of 6430 voxels at 5mm spatial resolution are registered to the Stereotaxic Atlas of the Human Brain (Talairach and Tournoux, 1988). In this study, Statistical non-Parametric Mapping (SnPM) was used to assess differences in current density distributions to onset and offset of noise during P50. The SnPM method estimates the probability distribution by using a randomization procedure, corrects for multiple comparisons and has the highest possible statistical power (Nichols and Holmes, 2002). SnPM, in the context of electrophysiological functional imaging, was validated in previous studies by comparing its results with more conventional ANOVA results (Laufer and Pratt, 2003; Sinai and Pratt, 2003).
Specifically, in our study we used the pseudo-t statistic which reduced noise in the data by averaging over adjacent voxels (Nichols and Holmes, 2002). In order to trace time segments of significant differences between responses we compared them on a time-frame by time-frame, voxel-by-voxel basis for the duration of P50. A time-segment was designated significant only if it contained at least 5 contiguous significant (p<0.01) time frames. We employed this procedure to reduce the probability that time frames assigned with significance by chance alone due to alpha inflation would be included in the analysis. Average current density values were then obtained across the contiguous significant time-frames to obtain a single sLORETA solution consisting of 6430 voxels representing the entire time segment that was found significant. The procedure outlined above was employed in order to trace significant time segments, utilizing the high temporal resolution of sLORETA, while extending (to the time domain) the method originally used by Nichols and Holmes (2002), of averaging significant t-values over space only.
3. Results
3.1. Waveforms to noise onset and offset
Clearly different evoked potentials were obtained in response to noise onsets and offsets, with the most obvious difference - a bifid N-Complex (N1a and N1b) to noise offsets and a single peaked N100 to noise onsets (Fig. 1B). These differences have been detailed in previous reports (Michalewski et al., 2005; Pratt et al., 2005, 2007). In addition, P50 was also clearly different to noise onset and offset: a clear positive component (P50) was observed approximately 50 msec after noise onset (and clicks) with the largest amplitudes at the midline frontal and central electrodes. In contrast, P50 was absent in response to noise offset and, at times, marked by a negative inflection from baseline (Fig. 1C).
3.2. Component comparisons across stimulus conditions
Potentials evoked by noise onset and noise offset were compared to each other as well as to potentials to the correspondingly timed first and second clicks of the click pairs. In general, the latencies of potentials to noise onsets and to the first and second clicks in the pairs were not significanly different, each comprising of a P50, N100-P160 sequence. In contrast, the potentials to noise offset had no P50 (Fig. 1C). Currrent density distributions associated with P50 were significantly different among stimulus conditions (Fig. 2) and different than those of N100 and the N-Complex.
Fig. 2.

Average source current density distributions in the time period of P50 to noise onset, offset and to clicks. In reponse to noise onset (top) activity involved bilateral superior central regions (Brodmann areas 18, 19, 20, 23, 38, 7 and 31) while to noise offset (middle) left inferior temporal and occipital regions (Brodmann areas 18, 20, 28, 6 and 13) were activated. The P50 to clicks (bottom) involved all the above areas, but the most active areas (Brodman areas 47, 31 and 20) was in the left inferior frontal and temporal cortices. Note the different calibrations of the current density color bars, underscoring the significantly lower activity to noise offset.
3.2.1. Waveform comparisons
The latency of P50 was not significantly affected by the stimulus type (noise onset, noise offset or clicks) that evoked it. When pairs of stimulus conditions were compared, P50 latency to noise onset (56 msec) was significantly longer [F(1,12)= 4.81, p<0.05] than to noise offset (47 msec), but not significantly different than to clicks [53 msec; p>0.05]. P50 amplitude was significantly affected by stimulus type [F(2,22)=9.01, p<0.002] with post-hoc analysis indicating that in response to noise onset it was larger (0.63μV) than the inflection to noise offset (−0.25μV), but not significantly different than to clicks (0.49μV). Amplitude to noise offset was thus significantly different than to noise onset or to clicks.
To determine whether the amplitude to noise offset reflected a diminished peak (that was significantly larger than its baseline) or an absent peak (which was not significantly different than baseline), amplitudes during 4 types of events: P50 to noise onset, the corresponding period to noise offset and their respective baselines were assessed. Analysis of variance procedures revealed a significant effect of event type on amplitude ([F(3,33)=7.38, p<0.001]. Post-hoc procedures indicated that only P50 to noise onset was significantly larger than the baseline preceding it, larger than baseline preceding noise offset and larger than P50 to noise offset. In contrast, the corresponding amplitude following noise offset was not significantly different than its baseline (p>0.05).
3.2.2. Current density comparisons
Source current density estimates for the period of occurrence of the P50 component to noise onset and offset revealed differences in current density distributions (Figs. 2 and 3A) and their time courses (Fig. 3B). Source current density distributions involved bilateral superior parietal and central regions (Brodmann areas 18, 19, 20, 23, 38, 7 and 31) in reponse to noise onset (Fig. 2 top) and left inferior temporal and occipital regions (Brodmann areas 18, 20, 28, 6 and 13) to noise offset (Fig. 2 middle). P50 to clicks involved all these areas, but the most active areas (Brodmann areas 47, 31 and 20) were in left inferior frontal and temporal cortices (Fig. 2 bottom).
Fig. 3.

A. Statistical non-parametric t-value mapping of current density differences during P50 between noise onset and offset. At the peak of P50 current density was significantly higher in response to noise onset than to offset in the vicinity of Brodmann area 40 as well as the general location of Brodmann areas 39, 31 and 13. The t-value for statistical significance is indicated on the color bar.
B. The time courses of activity in the areas most differentially activated between noise onset and offset. In response to noise onset, activity in BA 39 and 40 peaked slightly before the scalp recorded peak of P50, in BA 31 - slightly after the peak, while in BA13 it peaked slightly before and slightly after P50. In the plots to noise offset, ‘peak’ denotes the point of inflection from baseline. Note that in response to offset, activity in these areas was unchanged and low throughout the duration of P50, increasing only toward the very end of this period.
Statistical non-parametric t-value mapping of significant current density differences between P50 to noise onset and offset confirmed differences in the distribution of brain activity between these conditions. During the peak of P50, current density was significantly higher in response to noise onset than to offset in the vicinity of Brodmann area 40 (Fig. 3A) as well as the general location of Brodmann areas 39, 31 and 13. This significant difference extended over 5 time frames, beginning 12 msec before the peak of P50 until 4 msec after it. Towards the very end of this period, activity in these areas was higher to noise offset than to onset, most probably because of activity associated with N1a of the subsequent N-Complex.
Following the time course of activity in the differentially activated areas (Fig. 3B) revealed activity in BA 39 and 40 that peaked 16 msec before the peak of the surface-recorded P50 in response to noise onset. Activity in BA 31 peaked 4 msec after P50 peak, while in BA13 activity peaked 16 msec before and 12 msec after the peak, with lower activity during the peak of P50. In contrast, in response to noise offset, activity in these areas was unchanged and low throughout the duration, and only began increasing toward the end of this period, surpassing activity to sound onset at the very end of P50, most probably in association with the onset of N1a.
3.3. Summary
Activity during P50 to noise offset was no different than baseline before the stimulus. The P50 component to noise onset was associated with distinct spatio-temporal patterns of activity. The response to the transient clicks combined features of both noise onset and offset but was more similar to noise onset, as indicated by their similar waveforms and scalp distributions.
4. Discussion
In this study the P50 potentials to onset and offset of noise were compared to each other, as well as to the better studied potentials to short transient stimuli (clicks), while subjects were not attending to the stimuli. A clear P50 was recorded in response to noise onsets and to clicks, but it was absent to noise offset. P50 latencies and amplitudes to onset were not different than to clicks but latencies were longer and amplitudes larger than to noise offset.
Refractoriness is highly unlikely to account for the amplitude differences between P50 to noise onset and offset: the practically identical P50 amplitudes to the first and second clicks of the pairs, which corresponded in timing to noise offset and onset, indicate that there were no refractory effects on the amplitude or latency of P50 in the intervals used in this study. Moreover, because noise offset (at the beginning of gaps) always preceded noise onset (at the end of the gap), if there were refractoriness effects, they would have reduced P50 to noise onset and not to offset.
The apparent discrepancy between the results of this study, showing very little adaptation effects on P50, and earlier studies showing P50 adaptation in pairs of clicks (e.g., Schall et al., 1997; Skinner et al., 1999; Rasco et al., 2000; Uc et al. 2003; Kisley et al., 2003a) may result from different degrees of adaptation at different stimulus intervals. Earlier studies on P50 adaptation in pairs of stimuli used intervals that extended to shorter intervals than the 500 msec used in this study. One of them reported that in healthy subjects an interval of 100 ms, but not 500 ms, reduced P50 amplitude to the second stimulus in the pair (Schall et al., 1997). Another study reported an age-related effect at the 250 msec interval, but not at the 500 msec interval (Rasco et al., 2000). Thus, our results of only a marginal effect of adaptation with a 500 msec interclick interval are actually compatible with earlier reports.
Sources of the scalp activity during P50 to noise onset and during the corresponding time in response to noise offset were significantly different: noise offsets were associated with weak left inferior temporal and occipital activation whereas noise onsets and clicks, although different from each other, both activated mostly bilateral superior parietal areas. Source current density was significantly higher to noise onset in the vicinity of the temporo-parietal junction. These findings suggest that early brain responses to onset and to offset of sound are distinct.
4.1. Earlier studies on P50
Although P50 is the least studied of the auditory ERPs, some early reports detailed P50 latency and amplitude values stating that they were not affected by factors such as age (Barnet et al., 1975) or attention (Picton and Hillyard, 1974). More recently, reports described extensive changes in P50 with maturation, beginning with its domination of the P50-N100-P160 complex in young children (Sharma et al., 1997; Ceponiene et al., 2002) to its small amplitude in adults. The normal maturation of P50 (Sharma et al., 1997) has been used to determine a period of about 3.5 years during which the human central auditory system remains maximally plastic and therefore optimal for cochlear implantation (Sharma et al., 2002). The latency of P50 was used as the indicator of auditory system maturation and the effects of deprivation due to deafness on auditory function (Eggermont et al., 1997). P50 was found to increase in amplitude in normal aging and increase more with cognitive decline (Golob et al., 2007). Maturation of P50 evoked by pairs of clicks has also been studied to define sensory adaptation or gating in schizophrenia (Erwin et al., 1994; Schall et al., 1997; Kisley et al., 2003a), autism (Buchwald et al., 1992), Parkinson's disease (Teo et al., 1998) and major depression (Franks et al., 1983). Adults with sensory hypersensitivity without additional health or mental problems have been reported to have less robust P50 suppression (Kisley et al., 2004) alongside “over-inclusion” of irrelevant sounds into their focus of attention.
The generators of P50 have been attributed to the primary auditory cortex at Heschl's gyrus (Wood and Woolpaw, 1982; Reite et al., 1988; Pool et al., 1989; Liegeois-Chauvel et al., 1994; Huotilainen et al, 1998; Ponton et al., 2002), with the earlier work describing P50 as part of the middle-latency potentials Pb. However, more recent work suggests that these are distinct components, with P50 involving generators that also include the hippocampus, planum temporale and the lateral temporal cortex (Howard et al., 2000; Liegeois-Chauvel et al., 1999) and neocortical areas (Grunwald et al., 2003; Kisley et al., 2003b).
The variety of conditions affecting P50 could be indicative of the brain processes reflected by this component. A number of studies reported P50 sensitivity to reticular formation non-specific cholinergic activation (Buchwald et al., 1991) and consequently to levels of arousal (Erwin and Buchwald, 1986; de Lugt et al., 1996), sensory activation and a variety of disorders. Sensory gating (Skinner et al., 1999) and habituation (Gillette et al., 1997; Pitman et al., 1999) of P50 was found impaired in subjects with Post-Traumatic Stress Disorder (PTSD) compared to controls, indicating dysregulation of sensory processing in PTSD. Such decreased gating was also observed in normal adolescents compared to normal older subjects (Rasco et al., 2000). The amplitude of P50 was found attenuated in autism (Buchwald et al., 1988; 1992), Alzheimer's disease (Buchwald et al., 1989; Green et al., 1992; Fein et al., 1994; O'Mahony et al., 1994), Huntington's disease (Uc et al., 2003), Attention Deficit Hyperactivity Disorder (ADHD) (Kemner et al., 1996) and narcolepsy (Boop et al., 1994), suggesting decreased reticular arousal by sound. P50 was reported to be diminished and prolonged or absent in Parkinson's disease, improving following posterior ansa-pallidotomy, except in one patient who showed mild worsening attributed to post-operative sleepiness (Mohamed et al., 1996). Increased P50 amplitudes in mild cognitive impairment identified individuals who will subsequently convert to dementia (Irimajiri et al., 2005; Golob et al., 2007). Similar relationships have been identified in HIV-1 infection, correlating with indices of disease progression (Schroeder et al., 1994). The amplitude of P50 in an auditory task was reported to be significantly increased in Irritable Bowel Syndrome (IBS) patients compared to controls, compatible with a generalized preattentive increase in central nervous system reactivity in this disorder (Berman et al., 2002).
A common denominator of all these earlier reports is cortical arousal which is: (1) sensitive to sleep; (2) involves ascending activation by reticular formation; and (3) can be predictive of subsequent processing of sound, independent of attention. These findings therefore suggest P50 to be associated with multiple generators involved in preattentive arousal by sound and gating its subsequent processing.
4.2. Comparison of early brain reponses to noise offset, onset and to clicks
The ERPs to sound onset and offset, particularly in the context of gaps in noise, are different. The potentials to noise onset (gap offset) are similar to the potentials to transients, consisting of a clear P50-N100-P160 sequence with a single-peaked N100 (Michalewski et al, 2005; Pratt et al., 2005). In contrast, the potentials to noise offset (gap onset) include a double-peaked N-Complex (N1a and N1b) followed by P160 (Michalewski et al, 2005; Pratt et al., 2005), which, as shown in this study, are not preceded by a P50.
Neuromagnetic studies have shown a P50 field to stimulus onset which was indistinguishable in its sources from N100, whereas to stimulus offset P50 was absent (Hari et al., 1987; Pantev et al., 1996). These findings were interpreted to suggest that while N100 seems to reflect cortical activity related to any abrupt change in the auditory environment (Hari et al., 1987), P50 reflects a distinct process which is unique to stimulus onset. This would imply a unique source activity during stimulus onset P50 that is absent in response to stimulus offset and is distinct from that of N100.
The absent P50 in response to sound offset compared to its presence to sound onset may have an alternative explanation. In contrast to P50's absence in response to sound offset, the subsequent negativity (N100) is more complex and double-peaked to sound offset than to onset. The absent P50 may therefore be explained by the N100 activity to sound offset that extends earlier than to sound onset. This explanation would imply that P50 to onset and offsets share the same generators, but the offset response is overwhelmed by temporally overlapping activity from N-Complex generators. These alternative explanations can be validated by comparing the sources of P50 to noise onset and offset to each other and to the sources of N100.
The results of this study showed distinct sources for P50 to noise onset and offset, which were different than those of N100 and the N-Complex (Pratt et al., 2005). In response to noise onset, the time course of activity in BA 39 and 40 peaked slightly before P50, activity peaked slightly after - in BA 31 while in BA 13 it peaked slightly before and slightly after P50 and decreased during the peak of P50. In contrast, in response to noise offset, activity in these areas was unchanged and low throughout this time. Thus, the distinct sources and time courses of activity suggest that P50 reflects brain processes that are present to noise onset and absent to offset and are also distinct from those underlying N100.
The sources of P50 to clicks, although more similar to those of noise onset, were a composite involving the generators activated by both onset and offset. This is congruent with the onset and offset of sound associated with short transient clicks. Thus, P50 to clicks is a composite of onset and offset responses, with overlapping activity evoked by both. This is reflected in the waveforms of P50 to clicks, which are intermediate between the waveforms to stimulus onset and offset (Fig. 1C, inset). Accordingly, the latency of P50 to clicks was slightly shorter than to sound onset, being biased by the shorter latency of the offset-evoked contributions.
4.3. Processes associated with P50 to noise onset and offset
The differences in morphology and sources of P50 to noise onset and offset appear to reflect distinct brain processes to onset and offset that are different from those associated with the N-Complex and N100. The auditory P50 has been most often studied in response to transient stimuli such as tone pips or clicks. It has been typically associated with auditory cortex activation (Wood and Woolpaw, 1982; Huotilainen et al, 1988; Reite et al., 1988; Pool et al., 1989; Liegeois-Chauvel et al., 1994; Ponton et al., 2002), but in addition more complex generators have been indicated, including the hippocampus, planum temporale and the lateral temporal cortex (Liegeois-Chauvel et al., 1994; Howard et al., 2000; Liegeois-Chauvel et al., 1999) and neocortical areas (Grunwald et al., 2003; Kisley et al., 2003b). Moreover, P50 habituates at intervals as long as 500 msec, in contrast to the Auditory Middle Latency potentials that are optimally recorded with much shorter intervals of 100 msec. This difference in habituation suggests that P50 is not part of primary auditory cortical processing, and its sources would therefore not be expected to be confined to the temporal lobe.
P50 was found sensitive to reticular formation non-specific cholinergic activation (Buchwald et al., 1991) and hence to levels of arousal (Erwin and Buchwald, 1986; de Lugt et al., 1996) and sensory activation (Kisley et al., 2004). More specifically, P50 was reported present during waking and REM sleep but not slow wave sleep (Erwin and Buchwald, 1986), i.e., present during states driven by ascending reticular projections with no attentional involvement. The blocking of P50 by a muscarinic cholinergic antagonist (Buchwald et al, 1991), suggests it is generated by ascending reticular cholinergic projections.
P50 was attenuated in conditions involving decreased arousal by – and processing of sound, such as autism (Buchwald et al., 1988; 1992), Alzheimer's disease (Buchwald et al., 1989; Green et al., 1992; Fein et al., 1994; O'Mahony et al., 1994), Huntington's disease (Uc et al., 2003), ADHD (Kemner et al., 1996) and narcolepsy (Boop et al., 1994). Increased P50 amplitudes have been reported in mild cognitive impairment involving memory and language functions (Irimajiri et al., 2005; Golob et al., 2007), in HIV-1 infection (Schroeder et al., 1994), but also in normal elderly subjects (Smith et al., 1980). The increased amplitude of P50 in mild cognitive impairment (MCI) patients with memory and language difficulties (Golob et al., 2007) is of particular interest. P50 has been reported to be sensitive to reticular formation non-specific cholinergic activation (Buchwald et al., 1991). Dementia is known to involve decreased cholinergic function, yet the MCI patients paradoxically presented with enhanced, rather than diminshed P50 amplitudes as would be expected with impaired cholinergic activation. Notably, this increased amplitude was only observed in a subset of these patients with language difficulties. Thus, the alterations in P50 amplitude appear to be related to aspects of auditory processing in addition to the non specific ascending activation. Earlier reports on factors affecting P50 suggested that the differences between P50 to stimulus onset and offset may be related to specific aspects of auditory processing. This specificity is supported by reports of diminished P50 habituation in disorders that involve auditory hallucinations such as schizophrenia (Erwin et al., 1994; Schall et al., 1997; Kisley et al., 2003a) or altered sensory perception such as autism (Buchwald et al., 1992) and less robust suppression of P50 in sensory hypersensitivity (Kisley et al., 2004). The common aspect of the abnormalities that affect P50, in addition to general arousal, is altered control of brain activation by auditory stimuli.
The brain areas that were differentially activated by noise onset and offset during P50 include mostly the vicinity of the supramarginal and angular gyri (BA 39 and 40), at the temporo-parietal junction, as well as the general location of the dorsal posterior cingulate (BA 31). These areas have been associated with aspects of spatial orienting, including motion sensitivity (Luks and Simpson, 2004), action planning (Ruby et al., 2002) and multisensory integration (Matsuhashi et al., 2004; Lenggenhager et al., 2006). These areas have also been implicated in stimulus-driven reorienting of attention in processing of competing stimuli (Corbetta et al., 2002; Thiel et al., 2004; Meister et al., 2006), temporal and spatial orienting and exploration (Coull et al., 2001; Himmelbach et al., 2006), directing attention to salient events (Marois et al., 2000; Astafiev et al., 2006, Gomot et al., 2006) across all modalities, even when they are behaviorally neutral (Downar et al., 2002). Our findings show these areas to be active in response to noise onset but not to noise offset, even when subjects were not attending to the sounds. All this suggests that P50 to noise onset reflects preattentive arousal by - and integration of the new sound into the multisensory scene in which the subject is immersed. Noise offset does not induce these processes and hence the absence of P50. The brain response to termination of an ongoing stimulus occurs about 100 msec later and manifests in the N-Complex as N1b (Pratt et al., 2005). In response to clicks these processes begin but are abruptly terminated because of the short duration of this transient stimulus resulting in a clear P50 and a single-peaked N100.
4.4. Summary
The results of this study show that P50 to stimulus onset involves cortical processes with spatio-temporal distributions that are absent to sound offset. The aspects of auditory processing associated with P50 that are absent to noise offset appear to be preattentive arousal by a new sound in the scene.
Acknowledgements
We are grateful to an anonymous reviewer of an earlier publication (on the N-Complex to gaps in noise) who drew our attention to the differences in P50 to sound offset and onset. This study was partially supported by the U.S.-Israel Binational Science Foundation, by grant DC 02618 from the National Institutes of Health and by the Rappaport Family Institute for Research in the Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporoparietal junction are independent of response selection. Eur J Neurosci. 2006;23:591–596. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Attias J, Urbach D, Gold S, Shemesh Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear Res. 1993;71:106–113. doi: 10.1016/0378-5955(93)90026-w. [DOI] [PubMed] [Google Scholar]
- Barnet AB, Ohlrich ES, Weiss IP, Shanks B. Auditory evoked potentials during sleep in normal children from ten days to three years of age. Electroenceph clin Neurophysiol. 1975;39:29–41. doi: 10.1016/0013-4694(75)90124-8. [DOI] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Chang L, Fitzgerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97:2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- Boop FA, Garcia-Rill E, Dykman R, Skinner RD. The P1: insights into attention and arousal. Pediat Neurosurg. 1994;20:57–62. doi: 10.1159/000120765. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin RJ, Schwfel J, Tanguay P. Abnormal P1 potentials in autistic subjects. Neurosci Abst. 1988;14:771. [Google Scholar]
- Buchwald JS, Erwin RJ, Van Lancker D, Cummings JL. Midlatency auditory evoked responses: differential abnormalities of P1 in Alzheimer's disease. Electroenceph clin Neurophysiol. 1989;74:378–384. doi: 10.1016/0168-5597(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Rubinstein EH, Schwafel J, Stranburg RJ. Midlatency auditory evoked responses: differential effects of a cholinergic agonist and antagonist. Electroenceph clin Neurophysiol. 1991;80:303–309. doi: 10.1016/0168-5597(91)90114-d. [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P. Midlatency auditory evoked responses: P1 abnormalities in adult autistic subjects. Electroenceph clin Neurophysiol. 1992;84:164–171. doi: 10.1016/0168-5597(92)90021-3. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, Frith CD. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex. 2001;11:73–84. doi: 10.1093/cercor/11.1.73. [DOI] [PubMed] [Google Scholar]
- Davis H, Zerlin S. Acoustic relations of the human vertex potential. Journ Acoust Soc Am. 1966;39:109–116. doi: 10.1121/1.1909858. [DOI] [PubMed] [Google Scholar]
- Davis PA. Effects of acoustic stimuli on the waking human brain. J Neurophysiol. 1939;2:494–499. [Google Scholar]
- de Lugt DR, Loewy DH, Campbell KB. The effect of sleep onset on event related potentials with rapid rates of stimulus presentation. Electroenceph clin Neurophysiol. 1996;98:484–492. doi: 10.1016/0013-4694(96)94726-4. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW, Don M, Waring MD, Kwong B. Maturational delays in cortical evoked potentials in cochlear implant users. Acta Otolaryngol. 1997;117:161–163. doi: 10.3109/00016489709117760. [DOI] [PubMed] [Google Scholar]
- Erwin R, Buchwald JS. Midlatency auditory evoked responses: differential effects of sleep in the human. Electroenceph Clin Neurophysiol. 1986;65:383–392. doi: 10.1016/0168-5597(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Shtasel D, Gur RE. Effects of medication history on midlatency auditory evoked responses in schizophrenia. Schizophr Res. 1994;11:251–258. doi: 10.1016/0920-9964(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, van Dyke C. The auditory P50 response is normal in Alzheimer's disease when measured via a paired click paradigm. Electroenceph Clin Neurophysiol. 1994;92:536–45. doi: 10.1016/0168-5597(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R. Neurophysiological studies of sensory gating in mania: comparison with schizophrenia. Biol Psychiat. 1983;18:989–1005. [PubMed] [Google Scholar]
- Gillette GM, Skinner RD, Rasco LM, Fielstein EM, Davis DH, Pawelak JE, Freeman TW, Karson CN, Boop FA, Garcia-Rill E. Combat veterans with posttraumatic stress disorder exhibit decreased habituation of the P1 midlatency auditory evoked potential. Life Sci. 1997;61:1421–1434. doi: 10.1016/s0024-3205(97)00688-7. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, Baron-Cohen S. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Green JB, Flagg L, Freed DM, Schwankhaus JD. The middle latency auditory evoked potential may be abnormal in dementia. Neurology. 1992;42:1034–1036. doi: 10.1212/wnl.42.5.1034. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von-Oertzen J, Fernandez G, Schaller C, Elger CE. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Hari R, Pelizzone M, Makela JP, Hallstrom J, Leinonen L, Lounasmaa OV. Neuromagnetic responses of the human auditory cortex to on- and offsets of noise bursts. Audiology. 1987;26:31–43. doi: 10.3109/00206098709078405. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Erb M, Karnath HO. Exploring the visual world: the neural substrate of spatial orienting. Neuroimage. 2006;32:1747–1759. doi: 10.1016/j.neuroimage.2006.04.221. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, Jaaskelainen IP, Pekkonen E, Naatanen R. Combined mapping of human auditory EEG and MEG responses. Electroenceph clin Neurophysiol. 1998;108:370–379. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Irimajiri R, Golob EJ, Starr A. Auditory brain-stem, middle- and long-latency evoked potentials in mild cognitive impairment. Clin Neurophysiol. 2005;116:1918–1929. doi: 10.1016/j.clinph.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag RJ, Camfferman G, van Engeland H. Event-related brain potentials in children with attention-deficit and hyperactivity disorder: effects of stimulus deviancy and task relevance in the visual and auditory modality. Biol Psychiatry. 1996;40:522–534. doi: 10.1016/0006-3223(95)00429-7. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Olincy A, Robbins E, Polk SD, Adler LE, Waldo MC, Freedman R. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiol. 2003a;40:29–38. doi: 10.1111/1469-8986.00004. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003b;14:693–697. doi: 10.1097/00001756-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41:604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal and temporal-parietal lesions on the auditory evoked potential in man. Electroenceph clin Neurophysiol. 1980;50:112–124. doi: 10.1016/0013-4694(80)90328-4. [DOI] [PubMed] [Google Scholar]
- Laufer I, Pratt H. Evoked potentials to auditory movement sensation in duplex perception. Clin Neurophysiol. 2003;114:1316–1331. doi: 10.1016/s1388-2457(03)00083-x. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Smith ST, Blanke O. Functional and neural mechanisms of embodiment: importance of the vestibular system and the temporal parietal junction. Rev Neurosci. 2006;17:643–657. doi: 10.1515/revneuro.2006.17.6.643. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroenceph clin Neurophysiol. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, deGraaf JB, Laguitton V, Chauvel P. Specialization of left auditory cortex for speech perception in man depends on temporal coding. Cereb Cortex. 1999;9:484–496. doi: 10.1093/cercor/9.5.484. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV. Preparatory deployment of attention to motion activates higher-order motion-processing brain regions. Neuroimage. 2004;22:1515–1522. doi: 10.1016/j.neuroimage.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M, Ikeda A, Ohara S, Matsumoto R, Yamamoto J, Takayama M, Satow T, Begum T, Usui K, Nagamine T, Mikuni N, Takahashi J, Miyamoto S, Fukuyama H, Shibasaki H. Multisensory convergence at human temporo-parietal junction - epicortical recording of evoked responses. Clin Neurophysiol. 2004;115:1145–1160. doi: 10.1016/j.clinph.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Meister IG, Wienemann M, Buelte D, Grunewald C, Sparing R, Dambeck N, Boroojerdi B. Hemiextinction induced by transcranial magnetic stimulation over the right temporoparietal junction. Neuroscience. 2006;142:119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Nguyen TT, Kong Y-Y, Zeng F-G. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116:669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Mohamed AS, Iacono RP, Yamada S. Normalization of middle latency auditory P1 potential following posterior ansa-pallidotomy in idiopathic Parkinson's disease. Neurol Res. 1996;18:516–520. doi: 10.1080/01616412.1996.11740464. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D, Rowan M, Feely J, Walsh JB, Coakley D. Primary auditory pathway and reticular activating system dysfunction in Alzheimer's disease. Neurology. 1994;44:2089–2094. doi: 10.1212/wnl.44.11.2089. [DOI] [PubMed] [Google Scholar]
- Pantev C, Eulitz C, Hampson S, Ross B, Roberts LE. The auditory evoked “off” response: sources and comparison with the “on” and the “sustained” responses. Ear Hear. 1996;17:255–265. doi: 10.1097/00003446-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low resolution brain electromagnetic tomography (sLORETA): technical details. Methods & Findings Exp & Clin Pharmacol. 2002;24D:5–12. [PubMed] [Google Scholar]
- Picton TY, Hillyard SA. Human auditory evoked potentials: II. Effects of attention. Electroenceph clin Neurophysiol. 1974;36:191–199. doi: 10.1016/0013-4694(74)90156-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4:234–241. doi: 10.153/SCNP00400234. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol. 2002;113:407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Pool KD, Finitzo T, Hong CT, Rogers J, Pickett RB. Infarction of the superior temporal gyrus: a description of auditory evoked potential latency and amplitude topology. Ear Hear. 1989;10:144–152. [PubMed] [Google Scholar]
- Pratt H, Bleich N, Mittelman N. The composite N1 component to gaps in noise. Clin Neurophysiol. 2005;116:2648–2663. doi: 10.1016/j.clinph.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michalewski HJ, Bleich N, Mittelman N. The N1 complex to gaps in noise: Effects of preceding noise duration and intensity. Clin Neurophysiol. 2007;118:1078–1087. doi: 10.1016/j.clinph.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rasco L, Skinner RD, Garcia-Rill E. Effect of age on sensory gating of the sleep state-dependent P1/P50 midlatency auditory evoked potential. Sleep Res Online. 2000;3:97–105. [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50-msec-latency auditory evoked field component. Electroenceph clin Neurophysiol. 1988;70:490–498. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Ruby P, Sirigu A, Decety J. Distinct areas in parietal cortex involved in long-term and short-term action planning: a PET investigation. Cortex. 2002;38:321–339. doi: 10.1016/s0010-9452(08)70663-4. [DOI] [PubMed] [Google Scholar]
- Schall U, Schon A, Zerbin D, Bender S, Eggers C, Oades RD. A left temporal lobe impairment of auditory information processing in schizophrenia: an event-related potential study. Neurosci Lett. 1997;229:25–28. doi: 10.1016/s0304-3940(97)00403-5. [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Handelsman L, Torres L, Dorfman D, Rinaldi P, Jacobson J, Wiener J, Ritter W. Early and late cognitive event-related potentials mark stages of HIV-1 infection in the drug-user risk group. Biol Psychiatry. 1994;35:54–69. doi: 10.1016/0006-3223(94)91168-1. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel-consonant syllables. Electroenceph clin Neurophysiol. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sinai A, Pratt H. High-resolution time course of hemispheric dominance revealed by low-resolution electromagnetic tomography. Clin Neurophysiol. 2003;114:1181–1188. doi: 10.1016/s1388-2457(03)00087-7. [DOI] [PubMed] [Google Scholar]
- Skinner RDM, Rasco BA, Fitzgerald J, Karson CN, Matthew M, Williams DK, Garcia-Rill E. Reduced sensory gating of the P1 potential in rape victims and combat veterans with posttraumatic stress disorder. Depression and Anxiety. 1999;9:122–130. doi: 10.1002/(sici)1520-6394(1999)9:3<122::aid-da4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Smith DB, Michalewski HJ, Brent GA, Thompson LW. Auditory averaged evoked potentials and aging: factors of stimulus, task and topography. Biol Psychol. 1980;11:135–51. doi: 10.1016/0301-0511(80)90048-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Teo C, Rasco L, Skinner RD, Garcia-Rill E. Disinhibition of the sleep state-dependent p1 potential in Parkinson's disease-improvement after pallidotomy. Sleep Res Online. 1998;1:62–70. [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21:318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Uc EY, Skinner RD, Rodnitzky RL, Garcia-Rill E. The midlatency auditory evoked potential P50 is abnormal in Huntington's disease. J Neurol Sci. 2003;212:1–5. doi: 10.1016/s0022-510x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Wood CC, Wolpaw JR. Scalp distribution of human auditory evoked potentials: II Evidence for multiple sources and involvement of auditory cortex. Electroenceph clin Neurophysiol. 1982;54:25–38. doi: 10.1016/0013-4694(82)90228-0. [DOI] [PubMed] [Google Scholar]