Abstract
Chromatin immunoprecipitation (ChIP) is a widely used technique for quantifying protein–DNA interactions in living cells. This method commonly uses fixed (crosslinked) chromatin that is fragmented by sonication (X-ChIP). We developed a simple new ChIP procedure for the immunoprecipitation of sonicated chromatin isolated from osteoblasts in the absence of crosslinking (N-ChIP). The use of noncrosslinked chromatin allowed development of a new modification of the ChIP assay: the combination of N-ChIP and competition with double-stranded oligonucleotides containing specific binding sites for individual transcription factors (Competitive N-ChIP). Using this approach, we were able to discriminate between individual binding sites for the Runx2 transcription factor in the osteocalcin and bone sialoprotein genes that cannot be resolved by traditional X-ChIP. N-ChIP assays were also able to detect several other types of chromatin interactions including those with Dlx homeodomain factors and nuclear proteins such as Sin3a that lack an intrinsic DNA-binding motif and, therefore, bind to chromatin via interactions with other proteins.
INTRODUCTION
Transcriptional control of gene expression is a major mechanism for regulating cellular activity and differentiation. Dysregulation of certain transcription factors plays a significant role in the etiology of many diseases including disorders of skeletal development and cancer. In bone, mutations in the Runx2 transcription factor cause cleidocranial dysplasia, a human disorder characterized by hypoplastic clavicles, patent fontanelles, supernumerary teeth and short stature (1) while translocations of Runx3 (AML-1) are associated with acute myelogenous leukemias (2). Therefore, an understanding of transcription factors and their targets is of central interest to bone biology and medicine.
A considerable effort has been devoted toward the development of methods for identifying in vivo chromatin-binding sites for transcription factors. Using a bioinformatics approach, sequence-based methods for defining putative transcription factor-binding sites have been developed and hundreds of consensus binding sequences defined (3). However, the predictive ability of this approach is limited by a number of factors including DNA methylation, nucleosomal positioning and adjacent DNA sequences that affect binding specificity (4,5). For example, based on sequence information, we identified a putative Runx2 binding site in the bone sialoprotein promoter that contains a perfect consensus Runx2-binding region. Furthermore, this site binds Runx2 with high affinity and specificity in vitro. However, it is not occupied by Runx2 in vivo as measured by chromatin immunoprecipitation (ChIP) assays and is devoid of enhancer activity (6). A second major concern with computer-based approaches is that many true positives may be overlooked if the transcription factor is recruited to the DNA via a site that does not exactly match a consensus site or by protein–protein interactions (7). Thus, the lack of a strict consensus can make it difficult to identify true target promoters using only sequence analysis software.
ChIP is a powerful and increasingly used technique for determining the in vivo binding sites of transcription factors as well as identifying epigenetic changes in chromatin. There are two main types of ChIP assays that differ primarily in how the input chromatin is prepared: X-ChIP and N-ChIP. The X-ChIP method, by far the most commonly used approach, utilizes formaldehyde-fixed chromatin that is fragmented by sonication or enzymatically (8–11), while N-ChIP uses native, unfixed chromatin solubilized by micrococcal nuclease digestion (12,13). Both methods have advantages and disadvantages (12). X-ChIP reduces the possibility of protein dissociation from chromatin, although excess crosslinking prevents chromatin from being fragmented to the desired size and can disrupt epitopes necessary for antibody recognition (10). On the other hand, the N-ChIP/nuclease digestion approach provides a gentler means of shearing chromatin, but is not generally useful for detecting nonhistone proteins associated with internucleosomal regions of DNA that are preferentially sensitive to nuclease treatment.
Here we report a simple and efficient ChIP procedure that utilizes unfixed, native chromatin fragmented by sonication and demonstrate that this method can be used to detect chromatin binding by the bone-related Runx2 transcription factor and other osteoblast-associated nuclear factors including those that do not bind directly to DNA. In addition, we developed a modification of the N-ChIP approach involving oligonucleotide competition. This approach allows discrimination between chromatin sites that cannot be directly resolved by classical ChIP assays. Using this approach, we were able to resolve adjacent Runx2-binding sites in two osteoblast-related genes encoding osteocalcin (Ocn) and bone sialoprotein (Bsp).
MATERIALS AND METHODS
Cell culture
Subclone 4 MC3T3-E1 (MC-4) cells were maintained in ascorbic acid-free alpha minimal essential medium (α-MEM; Life Technologies, Inc., Grand Island, NY, U.S.A.), 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT, U.S.A.) and 1% penicillin/streptomycin as previously described (14,15). Osteoblast differentiation was induced by the addition of 50 µg/ml ascorbic acid (AA) to the medium for 5–6 days (16).
Antibodies
The anti-Runx2 (M-70; sc-10758), anti-Dlx6 (G20; sc-18154) and the control preimmune goat and rabbit IgGs were purchased from Santa Cruz Biotechnology. The anti-Sin3A (cat# 06-913) and anti-acetyl Histone 3 (cat# 06-599) were obtained from Millipore Upstate (Lake Placid, NY).
Native ChIP assays
N-ChIP assays were performed using differentiated MC-4 cells. Cells were washed twice with cold PBS and harvested by gentle scraping in PBS containing protease inhibitors [1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% v/v Protease Inhibitor Cocktail (PIC) (Sigma)]. Cell pellets were resuspended in Lysis Buffer [0.25% Triton X-100, 10 mM Tris pH 8, 10 mM EDTA (pH 8), 0.5 mM EGTA (pH8), 200 mM NaCl and protease inhibitors (PMSF and PIC)] and homogenized with three to five strokes of a Teflon pestle homogenizer. After centrifugation at 200 g for 5 min, crude nuclei were resuspended in sonication buffer [10 mM Tris–HCl (pH 8), 1 mM EDTA, 0.1% SDS and PIC (Sigma)] and sonicated for 10 s. 5–10 times at 4°C to reduce the average DNA length to 0.4–0.5 kb. Cellular debris were removed by high-speed centrifugation (>13 000 r.p.m. in a microcentrifuge) for 30 min at 4°C. The amount of soluble chromatin was quantified using the PicoGreen ds DNA Quantitation Assay and a phage lambda DNA standard (Molecular Probes, Invitrogen) according to manufacture's instructions. Chromatin containing 10 µg DNA (input) was used as starting material in each ChIP reaction. Samples were diluted to 1ml with IP buffer (10 mM Tris–HCl, pH 8, 150 mM NaCl, 1% Triton X-100, 0.1% w/v sodium deoxycolate, 100 µg/ml BSA, 10 µg/ml yeast tRNA, and protease inhibitors, PMSF and PIC). Prior to immunoprecipitation, input chromatin was pre-cleared by adding 50 µl of protein A/G-agarose (Santa Cruz Biotechnology) and rotating the tubes for 1 h at 4°C. The supernatant was recovered by 1 min centrifugation at 5000 r.p.m. in a microcentrifuge. ChIP reactions were initiated by the addition of 2 µg of specific antibody (Runx2, Dlx6, Sin3A or Ac-H3) or control antibody (rabbit or goat IgG) and samples were incubated overnight at 4°C. Immune complexes were isolated by adding 50 µl of protein A/G-agarose, rotating for 1 h at 4°C and collecting beads by centrifugation for 1 min at 5000 r.p.m. Beads were washed three times with 1 ml of IP buffer, twice with TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8 and PMSF) and the immunocomplexes were eluted by resuspending the beads in 150 µl of elution buffer [10 mM Tris–HCl, 10 mM EDTA (pH 8), 1% SDS] and heating at 65°C for 15 min The supernatant was recovered by centrifugation at 5000 r.p.m. for 1 min and the samples were digested with 0.2 mg/ml Proteinase K for 2 h at 45°C. ChIP-DNA was purified using the QIAquick Nucleotide Removal Kit (Qiagen) according to manufacture's instructions. Fractions of the purified ChIP DNA (5%) or Inputs (0.02–0.05%) were used for PCR analysis. The PCR reaction was performed with AmpliTaq Gold DNA Polymerase (Applied Biosystems) for 30 cycles of 30 s at 95°C, 30 s at 60°C and 15 s at 72°C in a thermocycler (PTC 200, MJ Research, U.S.A). Primers used (P1-8) are listed in Table 1. PCR products were separated on 3% agarose gels containing 0.5 µg/ml of ethidium bromide and the DNA bands visualized with ultraviolet light. For the N-ChIP competition assays, the ds-oligonucleotides shown in Table 1 were added to the immunoprecipitation reactions just before incubation with the antibody. All ChIP assays were repeated at least three times.
Table 1.
Oligonucleotides used in this study
| Oligo name | Sequence |
|---|---|
| C-OSE2 | CAGCTGCAATCACCAACCACAGCATC |
| M-OSE2 | CAGCTGCAATCACCAAgaACAGCATC |
| C-R1 | GATTCTCTGGTGAGAACCCACAGCCTG |
| M-R1a | GATTCTCTGGTGAGAACgaACAGCCTG |
| M-R1b | GATTCTCTtcTGAGAACCCACAGCCTG |
| C-R2 | CACCCTTCAATTAAATCCCACAATGCA |
| M-R2 | CACCCTTCAATTAAATCgaACAATGCA |
| C-R3 | TCTTTTGTGGTTCTCTATTTTATTTTT |
| M-R3 | TCTTTTGTtcTTCTCTATTTTATTTTT |
| P1 | CTCAGTGGGTCAAACCCAAAG |
| P2 | CGTCCACTCCCAGAGCCTT |
| P3 | TGCCTCCATAAGATCCGGTT |
| P4 | CCCACAATGGGCTAGGCTC |
| P5 | CTGCCAGGCTTCCTGCTAGT |
| P6 | TACAGATGCCAAGCCCAGC |
| P7 | GCCTCAGTTGAATAAACATGAAA |
| P8 | TCCTCACCCTTCAATTAAATCCCACAA |
| OP1 | AGTGATGTGTCATGAGGTTTTTGC |
| OP2 | TAACCACAAAACCAGAGGAGGAA |
Note that for double stranded (ds) oligos used in N-ChIP competition assays, only one strand is shown. Mutated bases in the oligos are designated using bold lowercase letters.
X-ChIP assays used the same protocol described above except that before scraping in PBS, cells were first cross-linked with 1.1% formaldehyde for 10 min followed by neutralization with 2.5 M glycine (5% v/v). Electrophoresis mobility shift assays (EMSA) were carried out as previously described(6).
Real-time PCR
The quantification of ChIP-DNA by Real-time PCR was performed using the TaqMan Universal PCR master mix (Applied Biosystems, Foster City, CA) as well as the combination of primers P1-P2 or P3-P4 described in Table 1 and one of the following TaqMan probes that detect the OSE2a (P1-P2) and OSE2b (P3-P4) respectively:
(i) OSE2a-(FAM/TAMRA probe)- CAGCTGCAATCACCAACCACAGCA and (ii) OSE2b-(FAM/TAMRA probe)- TCCCCACCAACCACAAGAAATGCC.
RESULTS
Development of an N-ChiP method using mechanically sheared chromatin
The idea of conducting ChIP assays with noncrosslinked chromatin originated in the course of studies to analyze interactions between the Runx2 transcription factor and its cognate sequences in the promoters of genes expressed during osteoblast differentiation. Our initial hypothesis was that, if interactions of Runx2 with chromatin were strong enough, they should survive the sonication procedure used to shear chromatin and be detectable by ChIP in the absence of crosslinking. Under these conditions, chromatin proteins would remain in the native state, which would facilitate subsequent analysis or purification. In addition, noncrosslinked chromatin could be used to study protein–DNA interactions in native chromatin using oligonucleotide competition.
We first tested this hypothesis using the osteocalcin (Ocn) promoter. Runx2 is a key regulator of osteoblast-specific gene expression via its binding to consensus Runt domain protein-binding elements present in Ocn and other osteoblast-associated genes (17,18). Mouse Ocn contains two such sites designated OSE2a and OSE2b located at −130 and −605 bp, respectively, relative to the transcription start site (Figure 1A). Previous work showed differences in the in vitro affinity of these sites for Runx2 and in their relative ability to contribute to Ocn promoter activity (19). Therefore, Ocn is a good model system to test the specificity of the N-ChIP approach as well as to compare interactions between a transcription factor and two very similar, but functionally distinct regulatory elements.
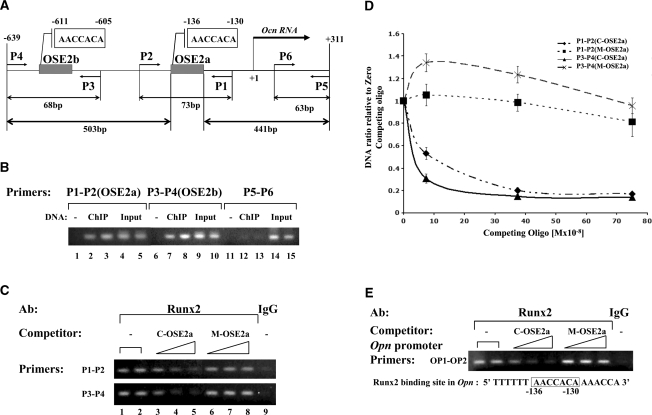
Figure 1.
Analysis of RUNX2 binding to the Ocn and Opn promoters in differentiated MC-4 cells using competitive N-ChIP. (A) Schematic representation of Runx2-binding sites in the proximal murine Ocn promoter. The positions of the RUNX2-binding sites, OSE2a and OSE2b, relative to the transcription start site are shown. P1, P2, P3, P4, P5 and P6 represent the different PCR primers used for the analysis of ChIP DNA (see Table 1). P1-P2 and P3-P4 were used to analyze the Runx2 binding to OSE2a and OSE2b, respectively. The sizes of the fragments they amplify are indicated at the bottom of the figure. (B) Primers pairs (P1, P2) and (P3, P4) specifically detect Runx2 interactions with OSE2a and OSE2b, respectively. Shown is the analysis of PCR products obtained with two independent X-ChIP-DNA samples obtained using Runx2 antibody and different primer pairs: P1-P2, P3-P4 and P5-P6 (see panel A). Control PCRs were done with duplicate samples of Input-DNAs and without DNA for each primer combination. Note that the P5-P6 primer pair amplifies a DNA fragment 441-bp away from OSE2a and that these primers do not produce a PCR product with ChIP-DNA (lanes 12 and 13). In contrast, the fragment amplified by the P3-P4 primer pair is a similar distance (503 bp) from OSE2a and, in this case the PCR is clearly positive (lanes 7 and 8). (C) N-ChIP competition assay for the Ocn promoter. The C-OSE2a double-stranded oligonucleotide or the mutant M-OSE2a was added at increasing concentrations (75, 375 and 750 nM) to immunoprecipitation reactions. A control reaction was also performed with normal rabbit IgG. The gels are ethidium bromide-stained agarose gels of the PCR products obtained with ChIP DNAs using the Ocn promoter-primers P1-P2 (top) and P3-P4 (bottom). (D) Real-time PCR analysis of the N-ChIP-competition assay. ChIP-DNAs were quantified by real-time PCR using two primer-pairs, P1-P2 and P3-P4, and their respective TaqMan probes. Results are expressed as ChIP-DNA ratio relative to zero competing-oligo from two independent experiments. The legend indicates the primer pair used in the PCR and, in parenthesis, the competing-oligo used for each ChIP-DNA set of samples. (E) N-ChIP competition assay for the Opn promoter. ChIP-DNAs were analyzed by PCR using the Opn promoter primers OP1 and OP2. These primers correspond to the region near the Runx2-binding site in the Opn promoter (−130 to −136) having the same sequence as the OSE2 site (AACCACA, lower panel).
Before commencing N-ChIP analysis of Ocn, we first conducted a control study to evaluate whether ChIP assays are capable of resolving binding of Runx2 to OSE2a versus OSE2b sites. Because the average size of chromatin used in ChIP is 400–500 bp and OSE2a and OSE2b are only 500 bp apart, it is possible that both sites might be precipitated on the same chromatin fragment, thereby precluding their separate analysis. To address this issue, sheared, formaldehyde cross-linked chromatin was prepared and X-ChIP assays were carried out using a specific anti-Runx2 antibody and the primer pairs shown in Figure 1A and Table 1. Primer pairs were selected to amplify OSE2a (P1, P2), an OSE2b-containing fragment 503-bp upstream from OSE2a (P3, P4) as well as a third region located a similar distance (441 bp) downstream from OSE2a in the Ocn transcribed region that does not contain any Runx2-binding sites (P5, P6). We reasoned that if chromatin fragments large enough to contain both OSE2a and OSE2b were present in ChIP reactions, similarly sized fragments containing OSE2a and the region amplified by P5/P6 should also be present. If so, all three primer pairs would give a positive PCR signal after ChIP with the Runx2 antibody. As shown in Figure 1B, this was not seen. Instead, positive ChIP signals were obtained for P1/P2 and P3/P4 primer pairs, but not for P5/P6. Based on this result, we conclude that Runx2 is separately bound to both OSE2a and OSE2b and that occupancy of these sites can be individually analyzed on ChIP using P1/P2 and P3/P4 primers.
To conduct N-ChIP assays, chromatin isolation and shearing was conducted as for X-ChIP except that the formaldehyde cross-linking step was omitted (see Materials and Methods section). Results of a representative N-ChIP assay for the Ocn promoter using P1, P2 and P3, P4 PCR primers is shown in Figure 1C. Interactions of Runx2 with both OSE2 sites are easily detected (lanes 1, 2 and 9). PCR band intensities were comparable to those obtained with X-ChIP (Figure 1B), indicating that Runx2 forms a stable complex with chromatin that is able to survive isolation and shearing procedures.
Competitive ChIP assay of Runx2 binding to chromatin using OSE2-containing oligonucleotides
Because Runx2 is not crosslinked to chromatin in N-ChIP, it exists in an equilibrium between chromatin bound and free states. Therefore, it should be possible to preferentially dissociate Runx2 from chromatin with an excess of specific competitor double-stranded oligonucleotide containing an intact OSE2a site. Competitor oligo (C-OSE2a) was added to immunoprecipitation reactions just before incubation with antibodies. As shown in Figure 1C lanes 3–5, increasing concentrations of C-OSE2a we were able to gradually dissociate Runx2 from both OSE2a and OSE2b chromatin sites. In contrast, the same concentrations of mutant oligo, M-OSE2a [contains a 2-bp mutation in the OSE2 site that abolishes Runx2 binding in vitro (15)], was not able to displace Runx2 (lanes 6, 7 and 8). To quantitatively evaluate competition experiments, we analyzed ChIP results using Real-time PCR. Figure1D summarizes these results expressed as ChIP DNA ratio relative to zero competing oligo from triplicate independent experiments, using both primer pairs (P1, P2) or (P3, P4). The real-time results confirmed that only the specific oligo (C-OSEa) was able to compete Runx2 away from OSE2a and OSE2b chromatin sites. Of further interest, differences were also noted when oligo competition was compared between OSE2a and b sites. Specifically, a 75 nM concentration of C-OSE2a more effectively competed Runx2 from the OSE2b chromatin site (amplified by P3, P4 primers) than from the OSE2a site (primers P1, P2). This is consistent with previously reported EMSA results showing that even though the core runt-binding site sequences of OSE2a and b are identical, Runx2 binds to OSE2b with lower affinity than OSE2a (19).
To further validate the N-ChIP competition assay, we examined a second osteoblast-related gene, osteopontin (Opn). Like Ocn, Opn is transcriptionally regulated by Runx2 via binding to a site located in the proximal promoter at −136 bp (20). This site has the same core sequence (AACCACA) as OSE2a and b (see Figure 1E, lower panel). PCR primers (OP1, OP2) were designed to specifically amplify DNA from this promoter region. As shown in Figure 1E (upper panel), N-ChIP was able to detect in vivo association of Runx2 with this site and, furthermore, binding was specifically competed with the C-OSE2a (but not M-OSE2a) oligo.
Detection of other chromatin complexes with N-ChIP
To assess the degree to which N-ChIP can be used to detect the association of other transcriptional regulators with osteoblast chromatin, we carried out the experiment depicted in Figure 2. Native sheared chromatin was immunoprecipitated using antibodies specific to Runx2, Dlx6, mSin3A or acetylated-histone 3 and proximal Ocn promoter sequences were amplified using P1 and P2 primers (see Figure 1A). As negative controls, we used preimmune IgG (from rabbit and goat). Specific association with a chromatin fragment in the Ocn promoter was revealed not only for Runx2 and the acetylated histone H3, but also for two other transcriptional regulators, Dlx6 and Sin3A (Figure 2, lanes 2 and 3). The specific interactions of Dlx6 and Sin3A with the Ocn promoter were also detected by X-ChIP (data not shown). Dlx6 and the related Dlx5 and Dlx3 belong to a subfamily of homeodomain-containing transcription factors that play important roles in osteoblast-specific gene expression and skeletal development (21,22). In fact, specific siRNA for Dlx6 that substantially reduced its mRNA levels, also dramatically inhibited the expression of the Ocn gene (Roca and Franceschi, unpublished data). Dlx proteins are known to specifically bind a homeodomain protein-binding element in the proximal Ocn promoter(23,24). Of particular interest, Sin3A was also found to associate with chromatin using the N-ChIP method. Sin3A is a global transcriptional regulator that has no intrinsic DNA-binding activity (25), thus its interaction with the chromatin occurs via protein–protein interactions that are preserved in the absence of crosslinking. By flexibly interacting with different kinds of DNA-binding proteins, many complexes can be assembled around a Sin3A core, and by adding new components, the specific activities of other factors may be drastically modified, providing a combinatorial tool for both positive and negative transcriptional regulation (25).
Figure 2.
N-ChIP successfully detects interactions of multiple nuclear factors with osteoblast chromatin. Chromatin fragments were immunoprecipitated using antibodies raised to the following proteins; Runx2, Dlx6, mSin3A and acetylated-histone3. A negative controls using nonspecific normal rabbit or goat IgGs were also included. The PCR analysis was performed using the primer pair P1-P2 in the downstream region of the Ocn promoter were the OSE2a site is located.
Application of N-ChIP for the analysis of Runx2-binding sites in the murine bone sialoprotein (Bsp) promoter
In a previous study, we analyzed Runx2 binding elements in the 2.5-kb promoter region of the Bsp gene (6). In that paper, we used X-ChIP to examine the in vivo association of Runx2 with proximal (−209 bp) and distal (−1335 bp) promoter regions (Figure 3A) in osteoblast-like MC-4 cells. First, we discovered that a putative binding site (R3 in Figure 3A) that perfectly matches the OSE2 Runx2-enhancer in the Ocn promoter is not associated with Runx2 in vivo, although it is able to specifically bind Runx2 in vitro (26). In contrast, using ChIP assays we observed a direct binding of Runx2 to the proximal promoter region delineated by P7 and P8 primers (Figure 3A). Two adjacent Runx2-binding sequences (R1 and R2, Figure 3A) were identified in this region. Mutation of both R1 and R2 sites was required to abolish Runx2 association with this region of chromatin as measured by X-ChIP. Thus, Runx2 is bound to both R1 and R2 sites in vivo (6).
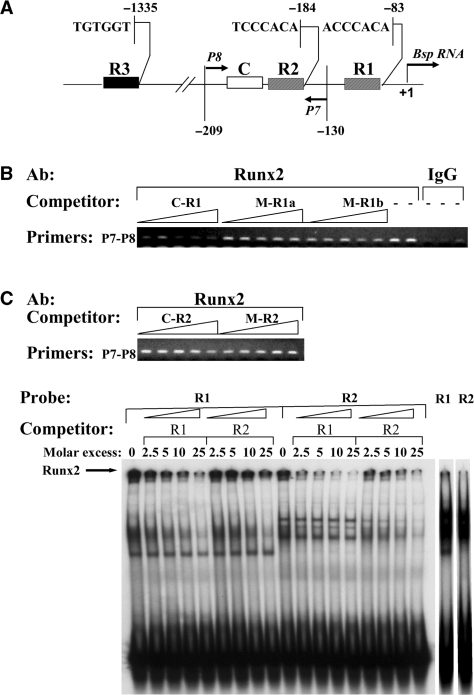
Figure 3.
N-ChIP analysis of Runx2-binding sites in the Bsp promoter. (A) Schematic representation of the Runx2-binding sites in the proximal murine Bsp promoter. The putative Runx2-binding sites R1, R2 and the previously described R3 site (−1335) are indicated. C denotes a homeodomain-protein-binding site that interacts with the Runx2 bound to the R2 site. Primers P7-P8 amplify the downstream region of the Bsp promoter that includes sites R1 and R2. (B) N-ChIP competition assay for the Bsp promoter. The N-ChIP competition assays were performed using ds-oligos containing the intact sites R1 and R2 (oligos: C-R1, C-R2) or their respective mutant oligos (M-R1a, M-R1b, M-R2) as described in the text. Competing ds-oligos were used at different concentrations: 75, 225, 375, 525 and 750 nM. Note that two mutant oligos were made to the R1 site (a and b). The M-R1a oligo contains a mutation in the Runx2 consensus while M-R1b has a mutation in the adjacent 5′ region (see Table 1). Control reactions were included with no-competing oligo and using nonspecific normal rabbit IgG. The gels are ethidium bromide-stained agarose gels of the PCR products obtained with ChIP-DNAs using the primers P7-P8. The experiment was repeated two times with similar results. (C) Electrophoresis mobility shift assay showing that Runx2 binds to the R2 site from the Bsp promoter with lower affinity than to R1. Runx2 binding to R1 and R2 containing ds oligonucleotides was analyzed by EMSA combined with supershift using Runx2 antibody. Labeled (probe) R1 or R2 was incubated with MC4 nuclear extracts and the competition experiment was performed in each case with unlabeled (cold) wild-type oligos (R1 and R2). No competition was seen with oligos containing mutations in the Runx2-binding sites (data not shown). In the assay, MC-4 nuclear extracts were pre-incubated with Runx2 antibody prior to their incubation with the mixture of probe and the cold-competing oligos. The experiment was performed with the indicated molar excess of cold-competing oligos. The sequences of oligos R1, R2, mR1 and mR2 as well as the details of the EMSA appear described in our previous paper (6). The black arrow indicates the supershifted Runx2-bound complexes. Also shown is the gel shift pattern for R1 and R2 probes in the absence of anti-Runx2 antibody and competing oligonucleotides (right 2 lanes).
Having studied this promoter in detail, we wanted to know what new information could obtained using the competitive N-ChIP method. First, we confirmed our previous X-ChIP result (6) by showing that Runx2 does not interact with the R3 site after N-ChIP (data not shown). Thus, even in the absence of crosslinking, Runx2 remains associated with its in vivo sites in the Bsp promoter and does not alter its distribution to become associated with a cryptic nonphysiological site. The competitive N-ChIP method was then used to analyze Runx2 binding to the proximal promoter region (in or adjacent to the fragment amplified by primers P7-P8). The ds-oligos used for competitive ChIP assays contained the intact sites R1 and R2 (C-R1, C-R2) and the corresponding mutants (M-R1a, M-R2) each containing a 2-bp mutation in the Runx2 consensus sequence that abolished Runx2 binding in vitro (6,26). Furthermore, in the case of R1, we also included an oligo (designated M-R1b) containing a 2-bp mutation in the adjacent region 5′ to the R1 site (see Table 1). The results of a representative ChIP-competition experiment using increasing concentrations of oligonucleotides (75, 225, 375, 525 and 750 nM) are shown in Figure 3B. The wild type R1 oligo (C-R1) was able to displace Runx2 from chromatin; however neither the R2 oligo (C-R2) nor any of the mutant oligos including the R1b oligo had any detectable competition activity. We interpret these results as follows: Because R1 and R2 are only separated by 54 bp, the P7-P8 primer pair amplifies DNA fragments containing both sites. Therefore, the fact that C-R1 oligo is able to inhibit the ChIP signal indicates that this oligo is displacing Runx2 from both R1 and R2 sites. In contrast, C-R2 was unable to disrupt Runx2 binding. Therefore, the affinity of Runx2 for the R2 site must be weaker than for R1. This interpretation is supported by results from EMSA analysis using ds oligos containing each site (Figure 3C). As shown, C-R1 was able to efficiently compete binding of Runx2 to labeled oligos containing either R1 or R2 sites (shown with supershift assays in the presence of anti-Runx2 antibody). In contrast, C-R2 could only compete Runx2 binding to labeled R2 oligo; it was a much weaker competitor of binding to R1. Densitometric analysis of the supershifted species showed that a 10-fold molar excess of C-R1 reduced binding to labeled R1 and R2 oligos by 58 and 89%, respectively, while the same molar excess of C-R2 reduced binding to R1 oligo by only 32% and to R2 oligo by 65%. Consistent with these results, our previous functional analysis of R1 and R2 sites indicated that R2 is active only in cooperation with an adjacent homeodomain-protein-binding site known to bind the Dlx5 transcription factor (C-site in Figure 3A), which may stabilize the Runx2 bound to R2 (6).
DISCUSSION
Here we describe a new method for the immunoprecipitation of chromatin isolated from cells in the absence of crosslinking (N-ChIP) as well as the use of oligonucleotide competition as a means of assessing the specificity and relative affinity of transcription factors for specific enhancer sites in target genes. We specifically used this assay to evaluate interactions between Runx2 and its cognate binding sites in regulatory regions of Ocn and Bsp, two genes that are tightly regulated by Runx2 in osteoblasts. Quantification of results for Ocn using real-time PCR allowed us to compare differences in Runx2-binding affinity between OSE2a and OSE2b enhancers in chromatin. For Bsp, we observed even more striking differences in relative affinity of Runx2 for R1 and R2 sites (Figure 3B). Results obtained with competitive ChIP assays are consistent with our previous functional studies as well as EMSA competition experiments. Taken together, our results indicate that competitive N-ChIP can be used to detect protein–nucleic acid and protein–protein interactions on native chromatin, a finding that may have many applications in the study of gene regulation.
In the absence of crosslinking, the interaction of a nuclear factor with chromatin, like all noncovalent interactions, is defined by an equilibrium between bound and free factor. This lack of covalent attachment introduces the possibility that nuclear factors might dissociate from chromatin or become associated with nonphysiological sites during chromatin isolation and shearing. However, in many cases, chromatin complexes can be sufficient stable to be detected without crosslinking. The best example of this is the nucleosome, which is held together by high-affinity histone–DNA and histone–histone interactions (27). As shown in the present work, certain bone-related transcription factors (Runx2, Dlx6 and mSin3a) also form stable complexes with their cognate DNA-binding sites in chromatin. For example, minimal dissociation of Runx2 from chromatin was seen in N-ChIP samples even after overnight incubation at 4°C in the absence of competing oligonucleotides (Figure 1B, lanes 1 and 2). In fact, comparable PCR signals were obtained when X-ChIP and N-ChIP samples were compared (Figure 1A versus 1B). Only when OSE2-containing oligos were added did we see dissociation of Runx2 from chromatin. In this case, oligos were added in vast (∼50 000-fold) excess relative to the number of Runx2-binding sites present in chromatin. Under these conditions, the added oligo can trap Runx2 as it dissociates from chromatin. In contrast, when no oligo or oligo with a mutated OSE2 site was added, no Runx2 trapping occurred and the Runx2-chromatin complex was preserved (Figures 1B and 2). We also think it unlikely that Runx2 can dissociate from chromatin and nonspecifically re-associate with nonphysiological sites during N-ChIP. The Bsp promoter gave us a good opportunity to address this issue. This promoter contains three sites, designated R1, R2 and R3 (at −83, −184 and −1335 bp, respectively) that all bind Runx2 when examined by EMSA in vitro. However, as was previously shown by X-ChIP, only R1 and R2 bind Runx2 in vivo. Similarly, occupancy of R1 and R2 was clearly detected by N-ChIP while no Runx2 binding was detected in the −1335-bp region (Figure 3B), arguing against any Runx2 redistribution taking place even in the absence of crosslinking.
Although we have not applied N-ChIP analysis to nonbone derived cells, it is likely that this approach will be widely applicable to the study of protein–chromatin interactions in many other cell types. Because affinities for chromatin are likely to be highly variable, results of N-ChIP must obviously be confirmed with X-ChIP whenever a new factor is being examined.
The competitive N-ChIP approach we describe may have a number of applications related to the study of transcriptional regulation. These include the following:
(i) Measuring the affinity of nuclear factors for chromatin. As shown in the present work, competitive ChIP can be used to compare the relative affinity of transcription factors for previously known enhancers in the context of native chromatin. This may be particularly informative in cases where a given nuclear factor interacts at multiple sites within the same gene that may be selectively active as levels of factor fluctuate. We show examples of this in both Ocn and Bsp promoters (Figures 1 and 3). In both cases, differences in Runx2 association with two distinct enhancers could be related to differences in transcriptional activity. Thus, in Ocn we detected a relatively higher affinity interaction between Runx2 and OSE2a when compared with OSE2b. Since OSE2a and OSE2b are separated by ∼500 bp, their individual interactions with Runx2 could be clearly resolved by ChIP using separate PCR primers. Previous studies also showed preferential binding of Runx2 to OSE2a versus OSE2b using EMSA analysis and provided evidence for preferential regulation of Ocn expression via OSE2a in cell culture and in vivo (19). A similar situation was found for the Bsp promoter. In this case, however, the two Runx2 sites are separated by only 54 bp, making it impossible to directly resolve their separate interactions by ChIP/PCR. However, differences in binding affinity could be inferred from the observation that only R1 oligo was able to inhibit the ChIP signal. Since this could only happen if Runx2 binding was competed at both sites, R1 must be able to block both interactions. In contrast, R2 was unable to disrupt Runx2 binding. As was the case for Ocn, these observations are consistent with EMSA analysis and previous functional studies (6). This type of analysis may prove quite useful for resolving protein–chromatin interactions on other genes as well.
(ii) Identification of new binding sites/enhancers. Competitive N-ChIP will be particularly helpful in the analysis transcription factors for which the DNA-binding motif is currently unknown or has not been previously characterized. In this case, the gene under investigation or the entire genome could first be screened to find transcription factor binding regions that could then be further resolved using competitive N-ChIP assays with oligonucleotides corresponding to consensus binding sites within specific regions of interest. Finally, mutational analysis could be used to functionally characterize these sites. This approach could be used with traditional ChIP/PCR analysis as well as ChIP on Chip or high-throughput sequencing strategies (27). Although the size of oligos used in the present study was ∼30 bp, this size could be increased or decreased to simplify the screening procedure.
(iii) Identification of binding sites for nuclear proteins that do not directly interact with DNA. We demonstrated that N-ChIP can detect binding of Sin3A to chromatin (Figure 2). Because this protein lacks a DNA-binding motif, it can only interact with chromatin by first binding to nuclear factors that have inherent DNA-binding activity. The ability to detect this type of protein–protein interaction using N-ChIP obviously depends on the strength of the interaction being studied with only high-affinity interactions being able to survive chromatin isolation and shearing procedures. For those interactions that do survive, competitive N-ChIP could be used to identify DNA sequences required for association of the factor of interest with chromatin, and this information could then be used to infer which DNA-binding factors account for the observed chromatin association.
(iv) Isolation of transcription factor complexes. Our observation that Runx2 can be displaced from chromatin by double-stranded oligonucleotides containing a Runx2-binding site suggests a possible strategy for purification of transcription factor complexes. This approach would involve initial isolation of chromatin fragments containing a particular transcription factor using either antibodies or expression of an affinity-tagged factor followed by displacement of the transcription factor and associated proteins using a specific oligonucleotide.
In summary, we developed a simple and efficient N-ChIP procedure that, when combined with oligonucleotide competition, will be an important tool for studying chromatin structure and function.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
This study was funded by National Institutes of Health (DE11723 to R.T.F.). Funding to pay the Open Access publication charges for this article was provided by NIH DE11723.
Conflict of interest statement. None declared.
REFERENCES
- 1.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 2.Meyers S, Downing JR, Hiebert SW. Identification of AML-1 and the (8;21) translocation protein (AML- 1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol. Cell. Biol. 1993;13:6336–6345. doi: 10.1128/mcb.13.10.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert T, Wells J, Funk JO, Pullner A, Raschke EE, Stelzer G, Meisterernst M, Farnham PJ, Eick D. The chromatin structure of the dual c-myc promoter P1/P2 is regulated by separate elements. J. Biol. Chem. 2001;276:20482–20490. doi: 10.1074/jbc.M100265200. [DOI] [PubMed] [Google Scholar]
- 5.Burnett E, Christensen J, Tattersall P. A consensus DNA recognition motif for two KDWK transcription factors identifies flexible-length, CpG-methylation sensitive cognate binding sites in the majority of human promoters. J. Mol. Biol. 2001;314:1029–1039. doi: 10.1006/jmbi.2000.5198. [DOI] [PubMed] [Google Scholar]
- 6.Roca H, Phimphilai M, Gopalakrishnan R, Xiao G, Franceschi RT. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J. Biol. Chem. 2005;280:30845–30855. doi: 10.1074/jbc.M503942200. [DOI] [PubMed] [Google Scholar]
- 7.Schlisio S, Halperin T, Vidal M, Nevins JR. Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. EMBO J. 2002;21:5775–5786. doi: 10.1093/emboj/cdf577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon MJ, Varshavsky A. Formaldehyde-mediated DNA-protein crosslinking: a probe for in vivo chromatin structures. Proc. Natl Acad. Sci. USA. 1985;82:6470–6474. doi: 10.1073/pnas.82.19.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon MJ, Larsen PL, Varshavsky A. Mapping protein-DNA interactions in vivo with formaldehyde: evidence that histone H4 is retained on a highly transcribed gene. Cell. 1988;53:937–947. doi: 10.1016/s0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 10.Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- 11.Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem. Sci. 2000;25:99–104. doi: 10.1016/s0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill LP, Turner BM. Immunoprecipitation of native chromatin: NChIP. Methods. 2003;31:76–82. doi: 10.1016/s1046-2023(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 13.Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J. Neurochem. 2005;94:324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 15.Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol. Endocrinol. 1997;11:1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 17.Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geoffroy V, Ducy P, Karsenty G. A PEBP2 alpha/AML-1-related factor increases osteocalcin promoter activity through its binding to an osteoblast-specific cis-acting element. J. Biol. Chem. 1995;270:30973–30979. doi: 10.1074/jbc.270.52.30973. [DOI] [PubMed] [Google Scholar]
- 19.Frendo JL, Xiao G, Fuchs S, Franceschi RT, Karsenty G, Ducy P. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J. Biol. Chem. 1998;273:30509–30516. doi: 10.1074/jbc.273.46.30509. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Morii E, Komori T, Kawahata H, Sugimoto M, Terai K, Shimizu H, Yasui T, Ogihara H, Yasui N, et al. Transcriptional regulation of osteopontin gene in vivo by PEBP2alphaA/CBFA1 and ETS1 in the skeletal tissues. Oncogene. 1998;17:1517–1525. doi: 10.1038/sj.onc.1202064. [DOI] [PubMed] [Google Scholar]
- 21.Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan MQ, Tare RS, Lee SH, Mandeville M, Morasso MI, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by Dlx3 and a homeodomain transcriptional network. J. Biol. Chem. 2006;281:40515–40526. doi: 10.1074/jbc.M604508200. [DOI] [PubMed] [Google Scholar]
- 23.Newberry EP, Latifi T, Towler DA. Reciprocal regulation of osteocalcin transcription by the homeodomain proteins Msx2 and Dlx5. Biochemistry. 1998;37:16360–16368. doi: 10.1021/bi981878u. [DOI] [PubMed] [Google Scholar]
- 24.Hassan MQ, Javed A, Morasso MI, Karlin J, Montecino M, van Wijnen AJ, Stein GS, Stein JL, Lian JB. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol. Cell. Biol. 2004;24:9248–9261. doi: 10.1128/MCB.24.20.9248-9261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstein RA, Ekwall K. Sin3: a flexible regulator of global gene expression and genome stability. Curr. Genet. 2005;47:1–17. doi: 10.1007/s00294-004-0541-5. [DOI] [PubMed] [Google Scholar]
- 26.Benson MD, Aubin JE, Xiao G, Thomas PE, Franceschi RT. Cloning of a 2.5 kb murine bone sialoprotein promoter fragment and functional analysis of putative Osf2 binding sites. J. Bone Miner. Res. 1999;14:396–405. doi: 10.1359/jbmr.1999.14.3.396. [DOI] [PubMed] [Google Scholar]
- 27.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]