Abstract
Complex birdsong is a classic example of a sexually selected ornamental trait. In many species, females prefer males with large song repertoires, possibly because repertoire size is limited by the size of song control nuclei which reflect developmental success. We investigated whether song repertoire size was indicative of brain area and male quality in song sparrows (Melospiza melodia) by determining if repertoire size was related to the volume of song control nucleus HVC, as well as several morphological, immunological and genetic indices of quality. We found that males with large repertoires had larger HVCs and were in better body condition. They also had lower heterophil to lymphocyte ratios, indicating less physiological stress and a robust immune system as measured by the number of lymphocytes per red blood cell. Song repertoire size also tended to increase with neutral-locus genetic diversity, as assessed by mean d2, but was not related to internal relatedness. Our results suggest several mechanisms that might explain the finding of a recent study that song sparrows with large song repertoires have higher lifetime fitness.
Keywords: song repertoire size, HVC, body condition, immunity, genetic diversity, song sparrow
1. Introduction
Song repertoires are a classic example of an ornamental trait produced by sexual selection (Searcy 1992; Andersson 1994). Numerous laboratory (Searcy 1984; Baker et al. 1986) and field studies (Hasselquist et al. 1996; Reid et al. 2004) have demonstrated a female preference for large song repertoires. An adaptive explanation for this female preference is that repertoire size reliably signals male quality whereby males with large repertoires may provide direct benefits to females, such as superior parental care or territorial defences and/or indirect benefits in the form of good genes for their offspring (Searcy 1992). Indeed, song repertoire size has been correlated positively with several phenotypic traits important for survival and reproduction such as body size and mass (Lampe & Espmark 1994; Doutrelant et al. 2000). Furthermore, sedge warbler (Acrocephalus schoenobaenus) males with small song repertoires were more likely to be parasitized by blood protozoans (Buchanan et al. 1999).
Nowicki et al. (1998) suggested that song repertoire complexity may reliably indicate male quality if males that experience more developmental stress or that are less resilient to this stress produce poor phenotypes overall coupled with specific deficiencies in the volume of song control nuclei that are required to learn and produce complex song repertoires. Recent experimental evidence shows that birds under less developmental stress grow larger song nuclei, particularly HVC (Nowicki et al. 2002; Buchanan et al. 2004), the volume of which is associated with song repertoire size both between songbird species (DeVoogd et al. 1993; Szekely et al. 1996) and between males within species (Garamszegi & Eens 2004). Moreover, nutritionally stressed European starling (Sturnus vulgaris) fledglings had a suppressed humoral immune response and developed smaller song repertoires as adults (Buchanan et al. 2003).
Genetic factors such as inbreeding and heterosis can have strong effects on developmental stability whereby genetically diverse individuals often undergo more stable and successful phenotypic development (reviewed in Møller & Swaddle (1997)). Therefore, if repertoire size is indicative of developmental stability, males with large repertoires should be more genetically diverse. Recent studies have supported this hypothesis. In an island population of song sparrows, inbred males had the smallest song repertoires (Reid et al. 2005a), and song repertoire size was positively correlated with genetic diversity in sedge warblers (Marshall et al. 2003).
Song sparrow females prefer males with large repertoires (Searcy 1984; Reid et al. 2004) and in this species song repertoire size predicts male lifetime reproductive success (Reid et al. 2005b). If song complexity is indicative of developmental success then males with large repertoires should have large song nuclei (Nowicki et al. 1998). In song sparrows, a recent study found that HVC volume was selectively reduced in juveniles given a food-restricted diet during their early development (MacDonald et al. 2006). The researchers, however, had no data to determine if HVC volume was associated with song repertoire size in adults. In this paper, we examine song repertoire size and HVC volume in adult male song sparrows. Next, we examine whether song repertoire size was related to several morphological and physiological indices of male quality including body size, body condition, and immunological and haematological profiles. Finally, we examine if several indices of individual genetic diversity are related to song repertoire size, which is expected if genetic diversity promotes developmental homeostasis and song complexity is a reliable signal of developmental quality.
2. Material and methods
(a) Study area and species
We studied a breeding population of song sparrows at the Queen's University Biological Station in eastern Ontario. Data were collected from late April to mid-August in 2002–2004. All territorial males and most females were captured in potter traps or mist nets and given unique colour-band combinations for individual recognition.
(b) Song repertoires
Song sparrow song repertoires comprise several distinct and highly stereotyped song types. Territorial males typically repeat each song type numerous times before switching to a bout of a different song type. Singing males were recorded using a Marantz PMD222 or a Sony TCM 5000 EV portable cassette recorder and a Sennheiser ME66 microphone. We considered a repertoire recorded in full when either 300 or more continuous songs or more than 18 song bouts were sampled (n=75), or when we compiled 425 or more songs from non-continuous recording sessions (n=5; following Cassidy 1993). All recorded songs were digitized and classified into song types by visual inspection of their spectrograms using Syrinx (John Burt, www.syrinxpc.com).
(c) HVC volume
Brains were collected in July 2003 from 20 adult males. Each male was captured immediately after his repertoire was recorded, anesthetized with isoflurane vapours and killed by rapid decapitation. Whenever possible, unpaired males were chosen as subjects to minimize effects on dependant offspring. We immediately removed the brain, froze it in powdered dry ice and then stored it at −80°C upon return to the laboratory. Frozen brains were cut in 40 μm coronal sections and every second section that contained the nucleus HVC was thaw-mounted onto a microscope slide. Slides were fixed by methanol immersion for 10 min, Nissl stained with thionin, dehydrated in a graded series of ethanol and then protected with cover slips affixed with Permount (Fisher Scientific). For each slide, we took an image of the HVC in both brain hemispheres using a bright field microscope equipped with a digital camera. The boundary of the HVC was traced in each image and its area determined using Spot Insight imaging software. The HVC volume was estimated by combining the area measurements using the formula for a truncated cone. We were unable to measure HVC volume for two males due to improper alignment of the brain during sectioning, and song repertoire size could not be determined for two additional males due to malfunction in the recording apparatus during sampling.
(d) Morphology
Morphological measurements were collected from 39 males in 2002, 67 males in 2003 and 33 males in 2004. We measured tarsus length and wing chord to the nearest 0.1 mm using dial callipers, and body mass to the nearest 0.2 g using a spring scale. Body fat in the furcular depression was scored using a five-point scale (following Helms & Drury 1960).
(e) Immunological profile
We obtained haematological and immunological profiles from 22 adult male birds in 2002. Blood smears were prepared in the field using a small blood sample (approx. 20 μl) taken from each male's brachial vein. The smears were fixed in 100% methanol for 1 min then stored at room temperature for several months before applying Wright–Giemsa stain (Leukostat; Fisher Scientific). Slides were examined with a light microscope using a 100×oil immersion objective lens. For each slide, blood cell counts were taken from 20 digital images of adjacent rectangular fields of view (150×112 μm) in the centre of the microscope's field of view. The total numbers of normal red blood cells (RBCs, mean±s.e.=3293.86±75.41), polychromatic (immature) RBCs (83.18±10.07), lymphocytes (=6.86±0.86) and heterophils (=3.68±0.71) in the 20 images were counted. Less prevalent white blood cells such as eosinophils and monocytes were not included in analyses. White blood cell counts were measured as a ratio to RBCs to control for volume of blood used in each smear. The number of polychromatic relative to normal RBCs (polychromasia) was measured as an index of RBC regeneration (Campbell 1995). We also calculated the heterophil to lymphocyte (H/L) ratio for each slide. The H/L ratio is a widely used indicator of physiological stress in birds (Maxwell 1993), which increases with heat shock proteins (Moreno et al. 2002) and with exposure to a variety of stressors in many different avian species (Ilmonen et al. 2003; Souorsa et al. 2004).
(f) Genetic diversity
We collected small blood samples (less than 50 μl) from 70 adult male sparrows and blotted each onto a square of high wet-strength filter paper. A drop of 0.5 M EDTA was added immediately after collection as a preservative. The blots were dried and stored desiccated in airtight containers for several months. Genomic DNA was extracted using an ammonium acetate-based protocol to salt out proteins (Laitinen et al. 1994). Microsatellite alleles were amplified by polymerase chain reaction (PCR) at the loci Mme 2, Mme 7 (Jeffery et al. 2001) and Escμ 1 (Hanotte et al. 1994); one primer at each locus was dye labelled, and PCR products were analysed on a Beckman Coulter CEQ 8000 according to the manufacturer's protocol.
Genetic diversity was measured using two complementary indices. Internal relatedness (IR; Amos et al. 2001) estimates the degree of recent inbreeding in an individual's pedigree. IR assesses parental similarity based on each individual's degree of allele sharing (homozygosity), taking into account the allele frequencies in the population (Amos et al. 2001). A second metric of genetic diversity, mean d2, attempts to measure diversity resulting from longer-term processes due to past population history (Coulson et al. 1998; but see Tsitrone et al. 2001). Mean d2 was calculated as the squared difference in repeat units of the two alleles at each locus, averaged across all loci.
(g) Statistical analyses
We used a Pearson correlation to test whether song repertoire size increased with HVC volume and a multiple linear regression to determine whether morphological size or body condition was related to song repertoire size. Male morphology differed significantly between years after controlling for Julian date (MANCOVA, Wilks' λ8,250=0.85, p=0.01) and subsequent post hoc tests (Tukey's HSD) showed that males had significantly more body fat in 2002 than in 2003 (p=0.012). To control for these year effects, we regressed mass then fat scores on Julian date for each year separately, using linear or quadratic regressions (following Sokal & Rohlf 2001). A separate principal components analysis (PCA) for each year was conducted using standardized z-scores of the four morphological variables. Each PCA extracted two components accounting for 37.1 and 25.7% of the morphological variability (62.8% cumulative) among individuals in 2002, 41.7 and 22.6% (64.3% cumulative) in 2003 and 43.2 and 31.6% (74.8% cumulative) in 2004. In each year, mass and tarsus length had very high loadings on PC1 (mass: all loadings greater than 0.73; tarsus length: all loadings greater than 0.74) and fat had very high loadings on PC2 (all loadings greater than 0.88). PC1 and PC2 were thus interpreted as representative of body size and body condition in each year, respectively. PC1 and PC2 scores from each year were pooled. If an individual male was in the dataset for more than 1 year, morphological data were randomly selected for one year to avoid pseudoreplication. A multiple linear regression was used to determine whether song repertoire size was related to relative lymphocyte or heterophil counts, and Spearman rank order correlations were used to determine if repertoire size was related to polychromasia or the H/L ratio. We used Pearson correlation to test whether song repertoire size changed with mean d2 and IR.
Non-parametric tests were used only when residuals were not normally distributed. All multiple regressions used sequential backward elimination of predictors with p>0.1. Statistics concerning eliminated variables are based on their reintroduction to the final models. Morphological and genetic analyses were conducted on data collected from all males used in this study. Blood cell counts and HVC volumes were measured on a subset of these males in 2002 and 2003, respectively. Analyses were conducted using SPSS v. 13.0 and Statistica v. 5.5 and all tests were two-tailed.
3. Results
Song repertoire sizes ranged from 5 to 13 song types and averaged 8.5±0.20 s.e. song types for our 74 recorded males and did not differ across years (one-way ANOVA, F2,71=1.6, p=0.21). Our sampling effort captured the complete song repertoires of males since sampling more than 300 consecutive songs (we sampled up to 528 songs) or more than 18 song bouts (up to 56 bouts) did not lead to an increase in estimated repertoire size (Spearman rank order correlations, rs=0.03, n=35, p=0.85 and rs=0.13, n=41, p=0.41, respectively).
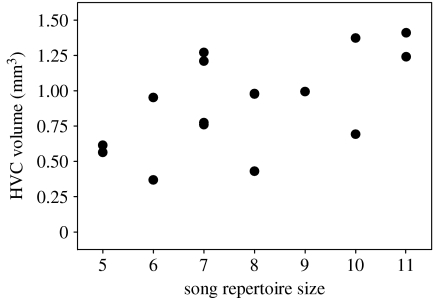
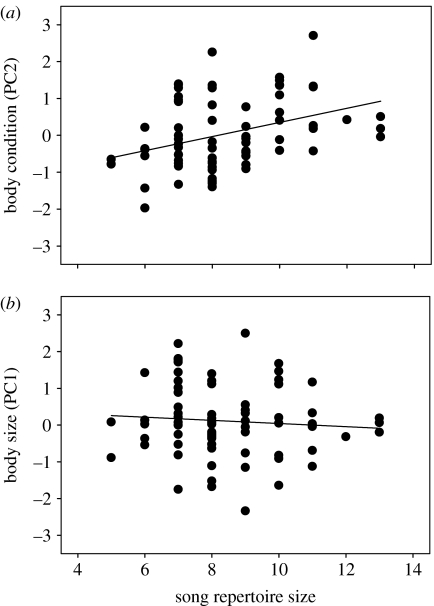
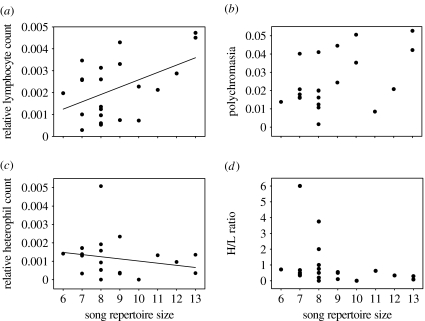
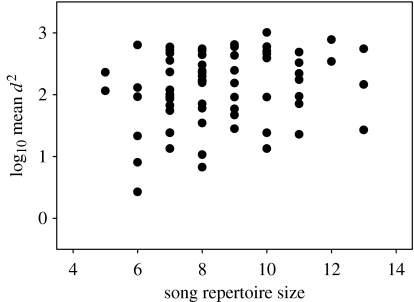
The volume of the song control nucleus HVC (mean=0.91±0.83 mm3) was positively correlated with song repertoire size (r=0.59, n=16, p=0.017; figure 1). Song repertoire size also was significantly related to male condition. Males with larger song repertoires were in better condition (multiple regression, R2=0.14, F1,71=11.1, p=0.001; β=0.7, t=3.3, n=73, p=0.001; figure 2a) but of similar size (β=−0.1, t=−0.9, n=73, p=0.35; figure 2b) than those with small repertoires. In addition, males with large repertoire sizes had immunological profiles consistent with them possessing robust immune systems. Specifically, males with larger song repertoires had more lymphocytes (multiple regression, R2=0.24, F1,20=6.26, n=22, p=0.02; figure 3a) and polychromatic RBCs (rs=0.47, n=22, p=0.03; figure 3b), but had a similar number of heterophils (β=−0.24, t=−1.26, n=22, p=0.22; figure 3c). Males with larger song repertoires had lower H/L ratios, indicating that they suffered less from physiological stress (rs=−0.48, n=22, p=0.03; figure 3d). More genetically diverse males as measured by mean d2 tended to have larger song repertoires (r=0.22, n=70, p=0.066; figure 4), but IR did not vary with song repertoire size (rs=0.053, n=70, p=0.66).
Figure 1.
Relationship between song repertoire size and HVC volume for 16 adult males (r=0.59, p=0.017).
Figure 2.
Relationship between song repertoire and morphology from 73 adult male birds. Song repertoire size was (a) significantly related to body condition but (b) not body size.
Figure 3.
Relationship between song repertoire size and white blood cell counts per normal RBC from blood smears collected from 22 adult male sparrows in 2002. (a) relationship between relative lymphocyte count and song repertoire size (β=0.49, t=2.5, p=0.02), (b) relationship between relative polychromasia and song repertoire size (rs=0.47, p=0.03), (c) relationship between relative heterophil count and song repertoire size (β=−0.24, t=−1.26, p=0.22) and (d) relationship between H/L ratio and song repertoire size (rs=−0.48, p=0.03).
Figure 4.
Relationship between song repertoire size and log10 transformed mean d2 for 70 adult male birds (r=0.22, p=0.066).
4. Discussion
We demonstrate that song repertoire complexity is related to the size of song control nuclei and the overall physiological condition of adult male song sparrows. MacDonald et al. (2006) showed previously that food restriction of developing song sparrows had a negative effect on HVC volume in this species. Our findings in conjunction with MacDonald et al. (2006) support the developmental stress hypothesis, wherein the development of a critical song control nucleus can be impaired by stressors experienced early in life thereby curtailing the degree of song complexity a male can produce as an adult.
Female song sparrows prefer males with large song repertoires in laboratory (Searcy 1984) and field studies (Reid et al. 2004). Reid et al. (2005b) have recently supported an adaptive explanation for this preference by showing that males with larger repertoires successfully fledge a higher proportion of young over their lifetime. Our results mechanistically link song repertoire size with the fitness benefits discovered by Reid et al. (2005b) by demonstrating that song repertoire size is indicative of HVC volume and general phenotypic quality in male song sparrows as predicted by the developmental stress hypothesis.
Nestling and fledgling birds undergo development of much of their general phenotype, including morphology and many physiological systems, concurrently with song system development (Nowicki et al. 1998). Therefore, song complexity should correlate with male phenotypic quality in general because stressors experienced early in life should affect the entire organism. In song sparrows, developmental stress in the form of food restriction affects many morphological and physiological traits, producing structurally smaller fledglings with reduced mass and fat deposits, as well as increased levels of plasma corticosterone and blood glucose than ad libitum fed birds (Kempster et al. 2007). Theoretically, resources should first be allocated to traits critical for survival before making a significant investment in an ornamental trait (Andersson 1986). It follows then that only individuals who have developed a strong overall phenotype should have robust song nuclei, which enable them to learn a large number of song types. Our results concur with this idea. We found that males with large song repertoires had larger HVCs, were in better body condition and experienced fewer symptoms of physiological stress, which was evident in their lower H/L ratios, higher lymphocyte numbers and higher rate of RBC regeneration.
To our knowledge, a relationship between song repertoire size and adult male body condition has not previously been investigated, though males with large repertoires were heavier in pied flycatchers (Ficedula hypoleuca, Lampe & Espmark 1994). Several mechanisms could explain this relationship. One possibility is that song repertoire size may signal the successful development of neural systems in general, song control nuclei comprising one such system. Neural systems responsible for foraging and prey detection or learning to forage efficiently also may be superior in males with large song repertoires. Nutritional stress during development impairs associative learning for food rewards in red-legged kittiwakes (Rissa brevirostris, Kitaysky et al. 2005) and spatial learning of food cache sites in western scrub-jays (Aphelocoma californica, Pravosudov et al. 2005). Although developmental stress can have a pronounced negative effect specifically on HVC development (Buchanan et al. 2004), it also reduces the volume of the entire telencephalon (Nowicki et al. 2002). In western scrub-jays, developmental stress reduced the volume and number of neurons in the hippocampus, a brain region important for spatial memory (Pravosudov et al. 2005). Therefore, males that experience a great deal of developmental stress or that are particularly susceptible to it probably will have smaller brains overall in addition to a diminished song system incapable of supporting a large song repertoire.
Males with large song repertoires had more lymphocytes per RBC, which may indicate one of two things. First, the elevated lymphocyte levels in males with large repertoires may be indicative of parasitic infection in these males as lymphocytosis is often the most significant and immediate haematological effect of endoparasitic infection (Garvin et al. 2003). Second, males with large repertoires may have a more robust immune response. Several lines of evidence support the latter interpretation. First, song repertoire size was greatest among males in the best body condition, which would not be expected if these males were more heavily infected with parasites. Second, males with large repertoires had lower heterophil to lymphocyte (H/L) ratios, suggesting that they suffered less physiological stress. The positive correlation between polychromasia and song repertoire size indicates that males with large song repertoires were able to replace RBCs lost to infection and natural cell death at a higher rate (Campbell 1995). Reid et al. (2005a) previously found that smaller song repertoires were associated with an impaired cell-mediated immune response in an isolated island song sparrow population, presumably as a consequence of inbreeding depression. Our results from a migratory and outbred population support their finding of a relationship between song repertoire size and immune function and suggest that this relationship is not confined to inbred insular populations.
Inferences about the strength of heterozygosity/fitness correlations based on a small number of loci are at best problematic (Tsitrone et al. 2001; Slate et al. 2004). Nonetheless, the positive trend between song repertoire size and mean d2 described here corroborates several recent studies demonstrating a relationship between genetic diversity and the expression of sexually selected traits (e.g. Foerster et al. 2003; Marshall et al. 2003). Such findings support recent models of sexual selection stressing the importance of non-additive genetic variance (Neff & Pitcher 2005). Moreover, it is interesting that repertoire size in this population tended to increase with mean d2 but not IR. These measures of genetic diversity are thought to reflect processes operating on different temporal scales (Coulson et al. 1998; Tsitrone et al. 2001). In this large, migratory and non-isolated population of song sparrows, the observed relationship between genetic diversity (mean d2) and repertoire size may thus stem from relatively ancient events rather than from current levels of inbreeding.
Although the precise developmental mechanism underlying the relationship between genetic diversity and repertoire size is beyond the scope of this study, developmental stability may be implicated in linking genetic diversity to song repertoire size (Reid et al. 2005b). In many species, heterozygosity has been linked to developmental stability (as inferred by bilateral symmetry, reviewed in Brown (1997)). In songbirds then, genetic diversity may improve developmental homeostasis in the face of nutritional or other stressors, permitting development of larger brain nuclei and more complex song as well as many other fitness-critical traits.
Acknowledgments
All procedures were conducted in accordance with Canadian Council on Animal Care guidelines and were approved by the University of Western Ontario Council on Animal Care.
We would like to thank the Queen's University Biological Station for providing us access to an excellent study area. Invaluable fieldworkers were Julia Panczuk, Mariechen Riefesel, Alexandra Hernandez and Tyler Stevens, and Brendan Chapman helped section brains and measure HVC volumes. We are grateful to Michael Clinchy for his advice and Bryan Neff, Chris Guglielmo, David Sherry and four anonymous reviewers for their comments on a previous version of the manuscript. Thanks to Kathy Wynne-Edwards. Funding for this research was provided by the National Science and Engineering Research Council of Canada and Premier's Research Excellence Awards.
References
- Amos W, Worthington Wilmer J, Fullard K, Burg T.M, Croxall J.P, Bloch D, Coulson T. The influence of parental relatedness on reproductive success. Proc. R. Soc. B. 2001;268:2021–2027. doi: 10.1098/rspb.2001.1751. doi:10.1098/rspb.2001.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Evolution of condition dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution. 1986;40:804–816. doi: 10.1111/j.1558-5646.1986.tb00540.x. doi:10.2307/2408465 [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Baker M.C, Bjerke T.K, Lampe H, Yngve E. Sexual response of female great tits to variation in size of males' song repertoires. Am. Nat. 1986;128:491–498. doi:10.1086/284582 [Google Scholar]
- Brown J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997;8:60–65. doi:10.1093/beheco/8.1.60 [Google Scholar]
- Buchanan K.L, Catchpole C.K, Lewis J.W, Lodge A. Song as an indicator of parasitism in the sedge warbler. Anim. Behav. 1999;57:307–314. doi: 10.1006/anbe.1998.0969. doi:10.1006/anbe.1998.0969 [DOI] [PubMed] [Google Scholar]
- Buchanan K.L, Spencer K.A, Goldsmith A.R, Catchpole C.K. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proc. R. Soc. B. 2003;270:1149–1156. doi: 10.1098/rspb.2003.2330. doi:10.1098/rspb.2003.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K.L, Leitner S, Spencer K.A, Goldsmith A.R, Catchpole C.K. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc. R. Soc. B. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. doi:10.1098/rspb.2004.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T.W. 2nd edn. Iowa State University Press; Ames, IO: 1995. Avian hematology and cytology. [Google Scholar]
- Cassidy, A. L. E. V. 1993 Song variation and learning in island populations of song sparrows. PhD thesis, University of British Columbia.
- Coulson T.N, Pemberton J.M, Albon S.D, Beaumont M, Marshall T.C, Slate J, Guinness F.E, Clutton-Brock T.H. Microsatellites reveal heterosis in red deer. Proc. R. Soc. B. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. doi:10.1098/rspb.1998.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoogd T.J, Krebs J.R, Healy S.D, Purvis A. Relations between song repertoire size and the volume of brain nuclei related to song—comparative and evolutionary analyses amongst oscine birds. Proc. R. Soc. B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. doi:10.1098/rspb.1993.0129 [DOI] [PubMed] [Google Scholar]
- Doutrelant C, Blondel J, Perret P, Lambrechts M.M. Blue tit song repertoire size, male quality and interspecific competition. J. Avian Biol. 2000;31:360–366. doi:10.1034/j.1600-048X.2000.310312.x [Google Scholar]
- Foerster K, Delhey K, Johnson A, Lifjeld J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z, Eens M. Brain space for a learned task: strong intraspecific evidence for neural correlates of singing behaviour in songbirds. Brain Res. Rev. 2004;44:187–193. doi: 10.1016/j.brainresrev.2003.12.001. doi:10.1016/j.brainresrev.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Garvin M.C, Homer B.L, Greiner E.C. Epizootiology of Haemoproteus danilewskyi (Haemosporina: Haemoproteidae) in blue jays (Cyanocitta cristata) in southcentral Florida. J. Wildl. Dis. 2003;39:161–169. doi: 10.7589/0090-3558-39.1.1. [DOI] [PubMed] [Google Scholar]
- Hanotte O, Zanon C, Pugh A, Greig C, Dixon A, Burke T. Isolation and charaterization of microsatellite loci in a passerine bird: the reed bunting Emberiza schoeniclus. Mol. Ecol. 1994;3:529–530. doi: 10.1111/j.1365-294x.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Bensch S, von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. doi:10.1038/381229a0 [Google Scholar]
- Helms C.W, Drury W.H. Winter and migratory fat field studies on some North American buntings. Bird Banding. 1960;31:1–40. [Google Scholar]
- Ilmonen P, Hasselquist D, Langefors A, Wiehn J. Stress, immunocompetence and leukocyte profiles of pied flycatchers in relation to brood size manipulation. Oecologia. 2003;136:148–154. doi: 10.1007/s00442-003-1243-2. doi:10.1007/s00442-003-1243-2 [DOI] [PubMed] [Google Scholar]
- Jeffery K.J, Keller L.F, Arcese P, Bruford M.W. The development of microsatellite loci in song sparrows, Melospiza melodia (Aves) and genotyping errors associated with good quality DNA. Mol. Ecol. Notes. 2001;1:11–13. doi:10.1046/j.1471-8278.2000.00005.x [Google Scholar]
- Kempster B, Zanette L, Longstaffe F.J, MacDougall-Shackleton S.A, Wingfield J.C, Clinchy M. Do stable isotopes reflect nutritional stress? Results from a laboratory experiment on song sparrows. Oecologia. 2007;151:365–371. doi: 10.1007/s00442-006-0597-7. doi:10.1007/s.0042-006.0597.7 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Kitaiskaia E.V, Piatt J.F, Wingfield J.C. A mechanistic link between chick diet and decline in seabirds? Proc. R. Soc. B. 2005;273:445–450. doi: 10.1098/rspb.2005.3351. doi:10.1098/rspb.2005.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen J, Samarut J, Hölttä E. A nontoxic and versatile protein salting-out method for isolation of DNA. Biotechniques. 1994;17:316–322. [PubMed] [Google Scholar]
- Lampe H.M, Espmark Y.O. Song structure reflects male quality in pied flycatchers, Ficedula hypoleuca. Anim. Behav. 1994;47:869–876. doi:10.1006/anbe.1994.1118 [Google Scholar]
- MacDonald I.F, Kempster B, Zanette L, MacDougall-Shackleton S.A. Nutritional stress impairs development of song-control brain regions in juvenile male and female song sparrows (Melospiza melodia) Proc. R. Soc. B. 2006;273:2559–2564. doi: 10.1098/rspb.2006.3547. doi:10.1098/rspb.2006.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.C, Buchanan K.L, Catchpole C.K. Sexual selection and individual genetic diversity in a songbird. Proc. R. Soc. B. 2003;270:S248–S250. doi: 10.1098/rsbl.2003.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M. Avian blood leukocyte responses to stress. Worlds Poult. Sci. J. 1993;49:34–42. doi:10.1079/WPS19930004 [Google Scholar]
- Møller A.P, Swaddle J.P. Oxford University Press; Oxford, UK: 1997. Developmental stability and evolution. [Google Scholar]
- Moreno J, Merino S, Martinez J, Sanz J.J, Arriero E. Heterophil/lymphocyte ratios and heat-shock protein levels are related to growth in nestling birds. Ecoscience. 2002;9:434–439. [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Nowicki S, Peters S, Podods J. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 1998;38:179–190. [Google Scholar]
- Nowicki S, Searcy W.A, Peters S. Brain development, song learning and mate choice in birds: a review and experimental test of the ‘nutritional stress hypothesis’. J. Comp. Physiol. A. 2002;188:1003–1014. doi: 10.1007/s00359-002-0361-3. doi:10.1007/s00359-002-0361-3 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V, Lavenex P, Omanska A. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behav. Neurosci. 2005;119:1368–1374. doi: 10.1037/0735-7044.119.5.1368. doi:10.1037/0735-7044.119.5.1368 [DOI] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Cassidy A.L.E.V, Hiebert S.M, Smith J.N.M, Stoddard P.K, Marr A.B, Keller L.F. Song repertoire size predicts initial mating success in male song sparrows, Melospiza melodia. Anim. Behav. 2004;68:1055–1063. doi:10.1016/j.anbehav.2004.07.003 [Google Scholar]
- Reid J.M, Arcese P, Cassidy A.L.E.V, Marr A.B, Smith J.N.M, Keller L.K. Hamilton–Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia) Proc. R. Soc. B. 2005a;272:481–487. doi: 10.1098/rspb.2004.2983. doi:10.1098/rspb.2004.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Cassidy A.L.E.V, Heibert S.M, Smith J.N.M, Stoddard P.K, Marr A.B, Keller L.F. Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia) Am. Nat. 2005b;165:299–310. doi: 10.1086/428299. doi:10.1086/428299 [DOI] [PubMed] [Google Scholar]
- Searcy W.A. Song repertoire size and female preferences in song sparrows. Behav. Ecol. Sociobiol. 1984;14:281–286. doi:10.1007/BF00299499 [Google Scholar]
- Searcy W.A. Song repertoire and mate choice in birds. Am. Zool. 1992;32:71–80. [Google Scholar]
- Slate J, David P, Dodds K.G, Veenvliet B.A, Glass B.C, Broad T.E, McEwan J.C. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity. 2004;93:255–265. doi: 10.1038/sj.hdy.6800485. doi:10.1038/sj.hdy.6800485 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J.Biometry3rd edn2001W. H. Freeman; New York, NY [Google Scholar]
- Souorsa P, Helle H, Koivounen V, Huhta E, Nikula A, Hakkarainen H. Effects of forest fragment size on physiological stress and immunocompetence in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris): an experiment. Proc. R. Soc. B. 2004;271:435–440. doi: 10.1098/rspb.2003.2620. doi:10.1098/rspb.2003.2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely T, Catchpole C.K, DeVoogd A, Marchl Z, DeVoogd T.J. Evolutionary changes in a song control area of the brain (HVC) are associated with evolutionary changes in song repertoire among European warblers (Sylvidae) Proc. R. Soc. B. 1996;263:607–610. doi:10.1098/rspb.1996.0091 [Google Scholar]
- Tsitrone A, Rousset F, David P. Heterosis, marker mutational processes and population inbreeding history. Genetics. 2001;159:1845–1859. doi: 10.1093/genetics/159.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]