Abstract
Social life is generally associated with an increased exposure to pathogens and parasites, due to factors such as high population density, frequent physical contact and the use of perennial nest sites. However, sociality also permits the evolution of new collective behavioural defences. Wood ants, Formica paralugubris, commonly bring back pieces of solidified coniferous resin to their nest. Many birds and a few mammals also incorporate green plant material into their nests. Collecting plant material rich in volatile compounds might be an efficient way to fight bacteria and fungi. However, no study has demonstrated that this behaviour has a positive effect on survival. Here, we provide the first experimental evidence that animals using plant compounds with antibacterial and antifungal properties survive better when exposed to detrimental micro-organisms. The presence of resin strongly improves the survival of F. paralugubris adults and larvae exposed to the bacteria Pseudomonas fluorescens, and the survival of larvae exposed to the entomopathogenic fungus Metarhizium anisopliae. These results show that wood ants capitalize on the chemical defences which have evolved in plants to collectively protect themselves against pathogens.
Keywords: host defence, self-medication, parasite resistance, social insects
1. Introduction
The detrimental impact of parasites and pathogens can be particularly high in social animals for several reasons. First, high host density and frequent social contacts increase the probability of parasite transmission (Anderson & May 1979; Côté & Poulin 1995; Nunn et al. 2000). For instance, the prevalence of ectoparasites increases with colony size in birds (Brown & Brown 1996). Second, most social animals breed in the same nests during successive years, which favours parasite survival and specialization (Møller & Erritzøe 1996; McCoy et al. 2003). Colonial birds and mammals have a high prevalence of parasites (Brown & Brown 1996; Rozsa et al. 1996; Christe et al. 2003a) and coloniality promotes parasite specialization and speciation in birds (Tella 2002; Tripet et al. 2002). Social insects also live in fixed nests, often for many generations (Crozier & Pamilo 1996). Third, most social animals live in family groups. As a result, the genetic diversity in the social groups is generally low, which may facilitate the spread and adaptation of parasites within groups (Hamilton 1987; Sherman et al. 1988).
Social animals have evolved sophisticated behavioural defences to counteract the detrimental effect of parasites and pathogens, including parasite avoidance, grooming and waste management (Giorgi et al. 2001; Boomsma et al. 2005). Another way to keep parasites at bay might be to incorporate fresh plant material rich in secondary volatile compounds into the nest material (Hart 2005). Wood ants Formica paralugubris collect pieces of solidified resin from coniferous trees (mostly spruce, Picea abies) that they mix with their nest material. Resin is commonly found on the nest surface and within the mound where the brood is raised, and large mounds can contain up to 20 kg of resin (Christe et al. 2003b). Coniferous resin contains a complex mixture of terpenes that protects wounded trees against bacterial and fungal infections (Cowan 1999; Phillips & Croteau 1999; Martin et al. 2002), and the presence of resin inhibits the growth of bacteria and fungi in ant nest material (Christe et al. 2003b). However, the impact of resin on ant survival has not been investigated so far.
Many cavity nesting birds and a few mammals also add fresh plant material to their nests (Clark & Mason 1985; Hemmes et al. 2002; Petit et al. 2002; Hart 2005; Gwinner & Berger 2006; Rajasekar et al. 2006). A possible explanation is that plant collection is a sexually selected trait, and in a few bird species, the plants indeed increase the attractiveness of males (Brouwer & Komdeur 2004; Gwinner & Berger 2006; Veiga et al. 2006). Interestingly, this hypothesis does not apply to the social insects in which males do not construct the nest and usually mate away from it. An alternative hypothesis is that plant compounds have an anti-parasite effect. In birds, some studies have found that green plant material has a negative impact on mites, mosquitoes or fleas (Lafuma et al. 2001; Hart 2005; Shutler & Campbell 2007), but others failed to detect this effect (Gwinner et al. 2000; Brouwer & Komdeur 2004). So far, no study has demonstrated that the use of fresh plant material increases survival.
Here, we test whether the presence of resin improves the survival of wood ants challenged with two micro-organisms that are likely to be detrimental to the ants, the Gram-negative bacteria Pseudomonas fluorescens and the generalist entomopathogenic fungus, Metarhizium anisopliae. Pseudomonas fluorescens are common bacteria colonizing soil, water and plant surfaces. Although P. fluorescens are generally considered benign or weakly pathogenic, some strains have a strong detrimental effect on insects. For example, a strain produces toxins that are lethal to larvae and pupae of mosquitoes (Prabakaran et al. 2003), another strain produces extracellular chitinases and kills ladybird beetle (Epilachna vigintioctpunctata) larvae when ingested (Otsu et al. 2004), and P. fluorescens is often associated with septicaemia in honeybees (Apis mellifera Schmid-Hempel 1998). Metarhizium anisopliae is a virulent fungus that infects many insect species (Hajek & Leger 1994) and is very common in Switzerland (Keller et al. 2003). Metarhizium anisopliae is frequently used as an experimental pathogen of ants and termites (Traniello et al. 2002; Hughes & Boomsma 2004), and it has been found to naturally infect ants, although its prevalence in the field is not well known and might be low (Schmid-Hempel 1998; Hughes et al. 2004). In full-factorial design experiments, we investigated whether the presence of resin increased the survival of adult and larval ants exposed to either P. fluorescens or M. anisopliae.
2. Material and methods
(a) Micro-organisms inhibited by resin
We first tested the effect of resin against a strain of P. fluorescens that we isolated from nest material sampled in our study population (see below), and against a strain of M. anisopliae var. anisopliae that was isolated from codling moth larvae in Austria (ATCC number 90448). The resin inhibited P. fluorescens in vitro. We suspended three bacterial colonies in 5 ml of 0.9% NaCl, and spread 0.5 ml of this solution on Müller–Hinton agar plates. After 30 min, we placed four pieces of resin (diameter 6.4±1.0 mm, mean±s.e.) on the plates. After incubating the plates at 30°C for 24 h, the inhibition halos around the pieces of resin were 2.36±0.58 mm wide (mean±s.e.). The resin also inhibited the vegetative growth and sporulation of M. anisopliae in vitro. We spread 100 μl of a 0.1% Tween 80 solution containing M. anisopliae spores at a concentration of 18×106 spores ml−1 on a selective medium containing dodine, cycloheximide and chloramphenicol (Lacey 1997). We placed four pieces of resin (diameter 6.0±0.7 mm, mean±s.e.) on the plates and incubated them at 25°C. After 7 days, the pieces of resin were surrounded by 3.6±0.4 mm (mean±s.e.) wide areas clear of hyphae and in which all spores had failed to germinate. After 17 days, the plates were covered with a layer of green spores, except for large areas (9.0±1.0 mm wide, mean±s.e.) around the pieces resin that were clear of spores.
(b) Sampling of ants
Wood ants, F. paralugubris, were sampled from nests located near the Chalet à Roch in the Swiss Jura Mountains (Chapuisat et al. 1997). In this population, there are many queens per nest and the workers are not aggressive towards each other (Chapuisat et al. 2005; Holzer et al. 2006). At least 70 queens, thousands of workers and nest material were collected within eight nests (other than the one from which the Pseudomonas strain was isolated). These samples from the eight nests were thoroughly mixed together, resulting in one large homogenous mix of workers, queens and nest material that was placed in one large stock colony. Adult workers and larvae born in the stock colony were randomly allocated to treatment groups. The pieces of resin used in the experiments also came from this stock.
(c) Ant survival
We performed full-factorial design experiments to measure the impact of resin and potential pathogens on ant survival. We recorded the survival of adult workers or larvae subjected to the following four conditions: first, a control with no resin and no microbial challenge; second, a treatment with resin but no microbial challenge, to examine whether the resin by itself is toxic or beneficial; third, a treatment with a microbial challenge in the absence of resin, to reveal the pathogenicity of the bacteria or fungus; and fourth, a treatment with the same microbial challenge in the presence of resin, to examine whether the resin improves the survival of ants under the threat of infection.
In the experiments with adult workers, single workers were kept in 5.5 cm diameter Petri dishes with a filter paper at the bottom. The ants were provided with ad libitum 8% sugar water. Each control and treatment group had 20 replicates. In the treatments with resin, one piece (0.06 g) of resin was placed on the filter paper in the Petri dish. We tested two separate microbial challenges in two independent, full-factorial experiments, so the total number of replicates (single adult workers) was 160. In the Pseudomonas challenge, 0.5 ml of peptone solution containing P. fluorescens at a concentration of 4×109 CFU ml−1 was deposited in the centre of the filter paper, close to but not on the resin or ant. In the Metarhizium challenge, 0.5 ml of peptone solution containing spores of M. anisopliae var. anisopliae at a concentration of 8.3×106 spores ml−1 was used in the same way. The challenge-free controls received 0.5 ml of peptone solution. The entire filter paper became damp, so that the individuals probably came into direct contact with the micro-organisms. The infection by Pseudomonas may occur through oral or cuticular contact, whereas spores (conidia) of Metarhizium adhere to the surface of the insect, germinate and produce hyphae that penetrate the cuticle (Hajek & Leger 1994). To confirm that the infection with Metarhizium was effective, a sample of corpses was surface-sterilized and placed on a malt extract agar at 25°C. Out of 20, 12 (60%) corpses from the infected treatment produced green conidia, whereas none of the 20 corpses from the control group did.
The impact of resin and potential pathogens on the survival of larvae was tested by placing three larvae in small artificial nests made of 9 cm circular plastic boxes with a filter paper covering the bottom of the nest and a central layer of plaster connected to a small underneath water reservoir (Passera et al. 1988). The nest was covered with a dark plastic flowerpot and placed in a 19×14 cm foraging arena lined with fluon to prevent escape. We provided sugar water and a protein-rich jelly made of honey, eggs and agar. Fifteen workers fed and tended the larvae. We performed two separate experiments, one with the Pseudomonas challenge and another with the Metarhizium challenge, but used the same challenge-free replicates (20 with and 20 without resin) for both experiments, resulting in a total number of replicates (experimental nests with three larvae) of 120. The design of these experiments was similar to the one with single adult workers, except for the amount of resin placed in the nests (0.2 g) and the concentrations of micro-organisms (1011 CFU ml−1 and 8.3×107 spores ml−1 in the Pseudomonas and Metarhizium challenges, respectively), which were adapted to the larger box size and higher number of individuals.
All experiments took place at 25°C. We recorded the survival of single adult workers and larvae on a daily basis, over 20 and 11 days, respectively. We removed corpses to prevent contamination. Differences in the survival of single adult ants were tested using Kaplan–Meier analyses with Mantel–Cox log-rank tests, as implemented in the computer program Statview v. 4.51 (Abacus Concepts, Berkeley, CA). Differences in the survival of larvae in groups were tested with repeated measures ANOVA, using the R software (v. 1.5.1, http://www.r-project.org/). The repeated measure was the daily number of live larvae in each box, over 10 days.
3. Results
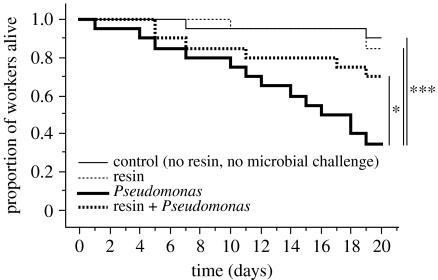
The presence of resin improved the survival of single adult workers exposed to the bacteria P. fluorescens (figure 1), but not to the fungus M. anisopliae (figure 2). The experimental challenge with P. fluorescens strongly reduced the survival of adult workers in the absence of resin (figure 1, Pseudomonas versus control, Kaplan–Meier analysis: Χ12=13.4, p<0.001). The presence of resin significantly increased the survival of adults exposed to P. fluorescens (figure 1, resin+Pseudomonas versus Pseudomonas, Χ2=4.6, p=0.03).
Figure 1.
Effect of resin on the survival of adult wood ants challenged with the bacteria P. fluorescens. Asterisks indicate significant differences in survival between the controls with no resin and no bacterial challenge (thin continuous line), treatment group with resin only (thin dashed line), treatment group with the bacterial challenge only (bold continuous line) and treatment group with the bacterial challenge in presence of resin (bold dashed line; Kaplan–Meier analyses with Mantel–Cox log-rank tests, *p<0.05, ***p<0.001).
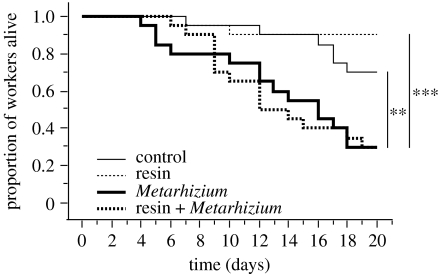
Figure 2.
Effect of resin on the survival of adult wood ants challenged with the fungus M. anisopliae. Asterisks indicate significant differences in survival between the controls with no resin and no fungal challenge (thin continuous line), treatment group with resin only (thin dashed line), treatment group with the fungal challenge only (bold continuous line) and treatment group with the fungal challenge in presence of resin (bold dashed line; Kaplan–Meier analyses with Mantel–Cox log-rank tests, **p<0.01, ***p<0.001).
The challenge with M. anisopliae also induced a significant mortality of adult workers in the absence of resin (figure 2, Metarhizium versus control, Χ2=7.4, p=0.007). However, the presence of resin did not affect the survival of adults exposed to M. anisopliae (figure 2, resin+Metarhizium versus Metarhizium, Χ2=0.02, p=0.89). In the absence of a microbial challenge, the resin had no significant effect on the survival of adult workers (figures 1 and 2, resin versus control, Χ2=0.2, p=0.66 and Χ2=2.0, p=0.16, respectively).
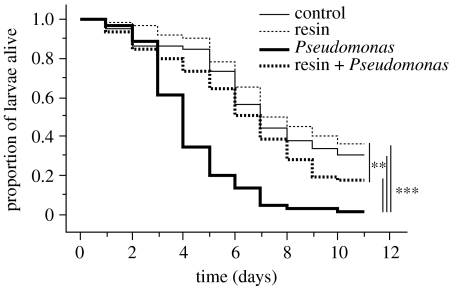
The presence of resin strongly improved the survival of larvae challenged with either P. fluorescens or M. anisopliae (figures 3 and 4). The exposure to P. fluorescens had a strong negative effect on the survival of larvae in the absence of resin (figure 3, Pseudomonas versus control, repeated measures ANOVA: F1,38=29.3, p<0.001), but this effect was significantly reduced in the presence of resin (figure 3, resin+Pseudomonas versus Pseudomonas, F1,38=20.7, p<0.001).
Figure 3.
Effect of resin on the survival of larval wood ants challenged with P. fluorescens. Asterisks indicate significant differences in survival between the controls with no resin and no bacterial challenge (thin continuous line), treatment group with resin only (thin dashed line), treatment group with the bacterial challenge only (bold continuous line) and treatment group with the bacterial challenge in presence of resin (bold dashed line; repeated measures ANOVA, **p<0.01, ***p<0.001).
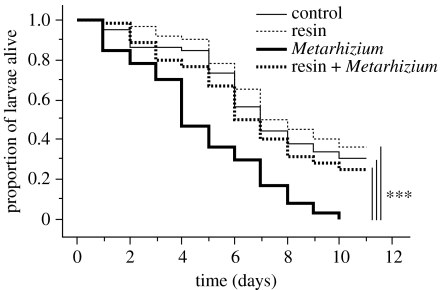
Figure 4.
Effect of resin on the survival of larval wood ants challenged with M. anisopliae. Asterisks indicate significant differences in survival between the controls with no resin and no fungal challenge (thin continuous line), treatment group with resin only (thin dashed line), treatment group with the fungal challenge only (bold continuous line) and treatment group with the fungal challenge in presence of resin (bold dashed line; repeated measures ANOVA, ***p<0.001).
The challenge with M. anisopliae also induced a significant mortality of larvae in the absence of resin (figure 4, Metarhizium versus control, F1,38=17.9, p<0.001). In contrast to adults, the presence of resin significantly improved the survival of larvae exposed to M. anisopliae (figure 4, resin+Metarhizium versus Metarhizium, F1,38=13.6, p<0.001). Finally, the resin had no significant effect on the survival of larvae in the absence of a microbial challenge (figures 3 and 4, resin versus control, F1,38=0.75, p=0.39).
4. Discussion
These experiments demonstrate that the pieces of solidified coniferous resin that wood ants commonly bring to their nests protect their larvae against the detrimental impact of two common micro-organisms. Single adult workers also better survived a bacterial challenge in the presence of resin. This is the first evidence that the collection of plant compounds with antibiotic and antifungal properties by wild animals increases their survival when they are exposed to harmful micro-organisms under laboratory conditions.
The bacteria P. fluorescens and fungi M. anisopliae were pathogenic to both adult and larval ants in the experimental conditions that we tested. The doses of micro-organisms were high, but the exposition by contacting a damp filter paper is probably benign in comparison with direct topical application or oral ingestion. The conditions were likely to be stressful, both for isolated adult workers and for larvae in small experimental colonies. Indeed, the survival of larvae was low, even in absence of microbial challenge.
The important point of this experiment is that the presence of resin greatly enhanced the survival of adult and larval ants challenged with P. fluorescens, as well as the survival of larval ants challenged with M. anisopliae. In contrast, the presence of resin had no beneficial or toxic effect on adult or larval ants in the absence of a microbial challenge. Moreover, the resin inhibited both micro-organisms in vitro (§2). Together, these results strongly suggest that the presence of resin protects the ants against detrimental micro-organisms.
Under field conditions, the resin is likely to be efficient against multiple other pathogens of ants. This is because the resin is rich in terpenes and other oleic compounds that have a broad spectrum of antibacterial and antifungal properties (Cowan 1999; Phillips & Croteau 1999). Overall, the protection that resin confers against the detrimental effect of bacterial and fungal pathogens, particularly during the sensitive brood stage, is likely to be important for the productivity and persistence of wood ant colonies.
The precise mode of action of resin in ant colonies remains to be studied. The presence of resin limits the growth of bacteria and fungi in nest material, which might be mediated by fumigation through volatile compounds or direct contact (Christe et al. 2003b). In addition, the workers might also contact the resin, either directly or indirectly through nestmates or volatile compounds, which might result in topical application or ingestion of active substances, or even in a modulation of immune defences (Gwinner et al. 2000). The effect of resin may also depend on the dose and mode of infection of each pathogen, and there is no reason to expect that the resin will protect the ants against all micro-organisms in all conditions. Indeed, the presence of resin did not significantly increase the survival of single adult workers exposed to spores of M. anisopliae. The resin might still have a positive effect on adults in less stringent conditions, for example, under lower doses or when multiple workers groom each other to remove spores (Hughes et al. 2002; Yanagawa & Shimizu 2007).
Our study shows that wood ants use plant chemical defences to collectively protect themselves against detrimental micro-organisms and improve the public health in their societies. These results illustrate that if social life increases the exposure to parasites, it also offers novel and efficient means of defence (Boomsma et al. 2005). Wood ants form very large social groups with high rates of contacts. Moreover, they feed on animal prey and live in nests that are perennial, warm and humid, which may further facilitate the spread of epidemics (Christe et al. 2003b). However, cooperation in large groups also permits efficient exploitation of natural resources and control of the physical environment of the nest to limit the impact of parasites and pathogens.
Acknowledgments
This research complies with the ethical guidelines for animal research in Switzerland. We thank Centre de conservation de la faune et de la nature of the canton de Vand for permission to collect ants.
We thank Simone Degiacomi, Lorenzo De Stefani and Ester Pellegrini for their important contribution to this study during their undergraduate research training, and Grégoire Castella, Laurent Keller, Claus Wedekind, Jonathan Yearsley and two anonymous reviewers for their comments on the manuscript. This research was supported by grants 3100A0-104118 and 3100A0-108263 from the Swiss National Science Foundation.
References
- Anderson R.M, May R.M. Population biology of infectious diseases: part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. doi:10.1038/280361a0 [DOI] [PubMed] [Google Scholar]
- Boomsma J.J, Schmid-Hempel P, Hughes W.O.H. Life histories and parasite pressure across the major groups of social insects. In: Fellowes M.D.E, Holloway G.J, Rolff J, editors. Insect evolutionary ecology. CABI Publishing; Wallingford, UK: 2005. pp. 139–175. [Google Scholar]
- Brouwer L, Komdeur J. Green nesting material has a function in mate attraction in the European starling. Anim. Behav. 2004;67:539–548. doi:10.1016/j.anbehav.2003.07.005 [Google Scholar]
- Brown C.R, Brown B.M. The University of Chicago Press; Chicago, IL; London, UK: 1996. Coloniality in the cliff swallow: the effect of group size on social behavior. [Google Scholar]
- Chapuisat M, Goudet J, Keller L. Microsatellites reveal high population viscosity and limited dispersal in the ant Formica paralugubris. Evolution. 1997;51:475–482. doi: 10.1111/j.1558-5646.1997.tb02435.x. doi:10.2307/2411120 [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Bernasconi C, Hoehn S, Reuter M. Nestmate recognition in the unicolonial ant Formica paralugubris. Behav. Ecol. 2005;16:15–19. doi:10.1093/beheco/arh128 [Google Scholar]
- Christe P, Giorgi M.S, Vogel P, Arlettaz R. Differential species-specific ectoparasitic mite intensities in two intimately coexisting sibling bat species: resource-mediated host attractiveness or parasite specialization? J. Anim. Ecol. 2003a;72:866–872. doi:10.1046/j.1365-2656.2003.00759.x [Google Scholar]
- Christe P, Oppliger A, Bancalà F, Castella G, Chapuisat M. Evidence for collective medication in ants. Ecol. Lett. 2003b;6:19–22. doi:10.1046/j.1461-0248.2003.00395.x [Google Scholar]
- Clark L, Mason J.R. Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia. 1985;67:169–176. doi: 10.1007/BF00384280. doi:10.1007/BF00384280 [DOI] [PubMed] [Google Scholar]
- Côté I.M, Poulin R. Parasitism and group size in social animals: a meta analysis. Behav. Ecol. 1995;6:159–165. [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier, R. H. & Pamilo, P. 1996 Evolution of social insect colonies: sex allocation and kin selection Oxford series in ecology and evolution. Oxford, UK: Oxford University Press.
- Giorgi M.S, Arlettaz R, Christe P, Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proc. R. Soc. B. 2001;268:2071–2075. doi: 10.1098/rspb.2001.1686. doi:10.1098/rspb.2001.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner H, Berger S. Parasite defence in birds: the role of volatiles. Acta Zool. Sinica. 2006;52:280–283. [Google Scholar]
- Gwinner H, Oltrogge M, Trost L, Nienaber U. Green plants in starling nests: effects on nestlings. Anim. Behav. 2000;59:301–309. doi: 10.1006/anbe.1999.1306. doi:10.1006/anbe.1999.1306 [DOI] [PubMed] [Google Scholar]
- Hajek A.E, Leger R.J., St Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994;39:293–322. doi:10.1146/annurev.en.39.010194.001453 [Google Scholar]
- Hamilton W.D. Kinship, recognition, disease, and intelligence: constraints of social evolution. In: Ito Y, Brown J.L, Kikkawa J, editors. Animal societies: theories and facts. Japan Science Society Press; Tokyo, Japan: 1987. pp. 81–102. [Google Scholar]
- Hart B.L. The evolution of herbal medicine: behavioural perspectives. Anim. Behav. 2005;70:975–989. doi:10.1016/j.anbehav.2005.03.005 [Google Scholar]
- Hemmes R.B, Alvarado A, Hart B.L. Use of California bay foliage by wood rats for possible fumigation of nest-borne ectoparasites. Behav. Ecol. 2002;13:381–385. doi:10.1093/beheco/13.3.381 [Google Scholar]
- Holzer B, Chapuisat M, Kremer N, Finet C, Keller L. Unicoloniality, recognition and genetic differentiation in a native Formica ant. J. Evol. Biol. 2006;19:2031–2039. doi: 10.1111/j.1420-9101.2006.01133.x. doi:10.1111/j.1420-9101.2006.01133.x [DOI] [PubMed] [Google Scholar]
- Hughes W.O.H, Boomsma J.J. Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution. 2004;58:1251–1260. doi: 10.1554/03-546. [DOI] [PubMed] [Google Scholar]
- Hughes W.O.H, Eilenberg J, Boomsma J.J. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. B. 2002;269:1811–1819. doi: 10.1098/rspb.2002.2113. doi:10.1098/rspb.2002.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W.O.H, Thomsen L, Eilenberg J, Boomsma J.J. Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Invertebr. Pathol. 2004;85:46–53. doi: 10.1016/j.jip.2003.12.005. doi:10.1016/j.jip.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Keller S, Kessler P, Schweizer C. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metarhizium anisopliae. Biocontrol. 2003;48:307–319. doi:10.1023/A:1023646207455 [Google Scholar]
- Lacey L.A. Academic Press; San Diego, CA: 1997. Manual of techniques in insect pathology. [Google Scholar]
- Lafuma L, Lambrechts M.M, Raymond M. Aromatic plants in bird nests as a protection against blood-sucking flying insects? Behav. Processes. 2001;56:113–120. doi: 10.1016/s0376-6357(01)00191-7. doi:10.1016/S0376-6357(01)00191-7 [DOI] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J. Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol. 2002;129:1003–1018. doi: 10.1104/pp.011001. doi:10.1104/pp.011001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K.D, Boulinier T, Tirard C, Michalakis Y. Host-dependent genetic structure of parasite populations: differential dispersal of seabird tick host races. Evolution. 2003;57:288–296. doi: 10.1111/j.0014-3820.2003.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Møller A.P, Erritzøe J. Parasite virulence and host immune defense: host immune response is related to nest reuse in birds. Evolution. 1996;50:2066–2072. doi: 10.1111/j.1558-5646.1996.tb03592.x. doi:10.2307/2410763 [DOI] [PubMed] [Google Scholar]
- Nunn C.L, Gittleman J.L, Antonovics J. Promiscuity and the primate immune system. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. doi:10.1126/science.290.5494.1168 [DOI] [PubMed] [Google Scholar]
- Otsu Y, et al. Stable phylloplane colonization by entomopathogenic bacterium Pseudomonas fluorescens KPM-018P and biological control of phytophagous ladybird beetles Epilachna vigintioctopunctata (Coleoptera: Coccinellidae) Biocontrol Sci. Techn. 2004;14:427–439. doi:10.1080/09583150410001683538 [Google Scholar]
- Passera L, Keller L, Suzzoni J.-P. Control of brood male production in the Argentine ant Iridomyrmex humilis (Mayr) Insectes Soc. 1988;35:19–33. doi:10.1007/BF02224135 [Google Scholar]
- Petit C, Hossaert-McKey M, Perret P, Blondel J, Lambrechts M.M. Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecol. Lett. 2002;5:585–589. doi:10.1046/j.1461-0248.2002.00361.x [Google Scholar]
- Phillips M.A, Croteau R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999;4:184–190. doi: 10.1016/s1360-1385(99)01401-6. doi:10.1016/S1360-1385(99)01401-6 [DOI] [PubMed] [Google Scholar]
- Prabakaran G, Paily K.P, Padmanabhan V, Hoti S.L, Balaraman K. Isolation of a Pseudomonas fluorescens metabolite/exotoxin active against both larvae and pupae of vector mosquitoes. Pest Manag. Sci. 2003;59:21–24. doi: 10.1002/ps.610. doi:10.1002/ps.610 [DOI] [PubMed] [Google Scholar]
- Rajasekar R, Chattopadhyay B, Sripathi K. Depositing masticated plant materials inside tent roosts in Cynopterus sphinx (Chiroptera: Pteropodidae) in southern India. Acta Chiropt. 2006;8:269–274. doi:10.3161/1733-5329(2006)8[269:DMPMIT]2.0.CO;2 [Google Scholar]
- Rozsa L, Rekasi J, Reiczigel J. Relationship of host coloniality to the population ecology of avian lice (Insecta: Phthiraptera) J. Anim. Ecol. 1996;65:242–248. doi:10.2307/5727 [Google Scholar]
- Schmid-Hempel P. Princeton University Press; Princeton, NJ: 1998. Parasites in social insects. [Google Scholar]
- Sherman P.W, Seeley T.D, Reeve H.K. Parasites, pathogens, and polyandry in social Hymenoptera. Am. Nat. 1988;131:602–610. doi: 10.1086/286127. doi:10.1086/284809 [DOI] [PubMed] [Google Scholar]
- Shutler D, Campbell A.A. Experimental addition of greenery reduces flea loads in nests of a non-greenery using species, the tree swallow Tachycineta bicolor. J. Avian Biol. 2007;38:7–12. [Google Scholar]
- Tella J.L. The evolutionary transition to coloniality promotes higher blood parasitism in birds. J. Evol. Biol. 2002;15:32–41. doi:10.1046/j.1420-9101.2002.00375.x [Google Scholar]
- Traniello J.F.A, Rosengaus R.B, Savoie K. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl Acad. Sci. USA. 2002;99:6838–6842. doi: 10.1073/pnas.102176599. doi:10.1073/pnas.102176599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F, Christe P, Møller A.P. The importance of host spatial distribution for parasite specialization and speciation: a comparative study of bird fleas (Siphonaptera: Ceratophyllidae) J. Anim. Ecol. 2002;71:735–748. doi:10.1046/j.1365-2656.2002.00639.x [Google Scholar]
- Veiga J.P, Polo V, Vinuela J. Nest green plants as a male status signal and courtship display in the spotless starling. Ethology. 2006;112:196–204. doi:10.1111/j.1439-0310.2006.01148.x [Google Scholar]
- Yanagawa A, Shimizu S. Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. Biocontrol. 2007;52:75–85. doi:10.1007/s10526-006-9020-x [Google Scholar]