Abstract
The dramatic colours of biological communication signals raise questions about how animals perceive suprathreshold colour differences, and there are long-standing questions about colour preferences and colour categorization by non-human species. This study investigates preferences of foraging poultry chicks (Gallus gallus) as they peck at coloured objects. Work on colour recognition often deals with responses to monochromatic lights and how animals divide the spectrum. We used complementary colours, where the intermediate is grey, and related the chicks' choices to three models of the factors that may affect the attractiveness. Two models assume that attractiveness is determined by a metric based on the colour discrimination threshold either (i) by chromatic contrast against the background or (ii) relative to an internal standard. An alternative third model is that categorization is important. We tested newly hatched and 9-day-old chicks with four pairs of (avian) complementary colours, which were orange, blue, red and green for humans. Chromatic contrast was more relevant to newly hatched chicks than to 9-day-old birds, but in neither case could contrast alone account for preferences; especially for orange over blue. For older chicks, there is evidence for categorization of complementary colours, with a boundary at grey.
Keywords: colour, vision, behaviour, Gallus gallus, chicks
1. Introduction
In colour vision, we have a relatively good understanding of when two spectra can be discriminated and how thresholds differ according to viewing conditions and between species. Discrimination thresholds are straightforward to measure experimentally and can be related to low-level visual mechanisms, such as photoreceptors and chromatic opponent neurons (Goldsmith & Butler 2003, 2005; Kelber et al. 2003). Where colour stimuli exceed the discrimination threshold, it is more difficult to account for their appearance to non-human species. Higher-level mechanisms may be involved and experiments are harder to interpret. These concerns are relevant to interpretation of the dramatic colours and patterns of visual displays used by animals and plants (Bennett et al. 1994). For example, there are questions about why certain colours are more prevalent than others in aposematic signals, or among fruit and flowers (Grant 1966; Willson & Whelan 1990; Giurfa et al. 1995; Rowe & Guilford 1996), and what makes a signal attractive or memorable (Roper 1990; Rowe & Guilford 1996; Lindstrom et al. 2001).
A familiar problem raised in comparing humans with other species is that of categorical perception. When humans identify red with ‘danger’ or green with ‘go’, we refer to a colour category. Colour names are commonly used in the literature on biological communication and visual behaviour, which can carry the implication that animals recognize similar categories. Categorization is fundamental to the way humans classify colour and other perceptual continuums (Harnad 1987; Boynton & Olsen 1990). It is unclear whether animals do the same (Harnad 1987; Nelson & Marler 1989).
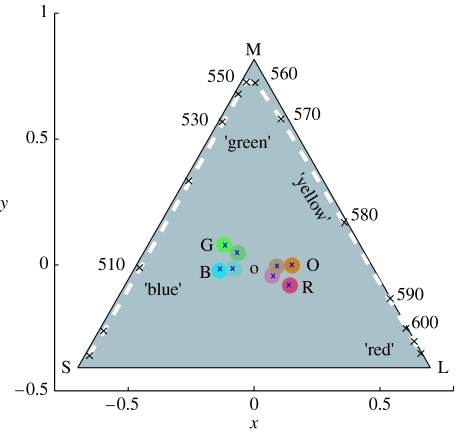
Wright & Cumming (1971) made an interesting study of colour naming (i.e. categorization) of monochromatic lights by pigeons (Columba livia). In a match-to-sample procedure, the birds initially learnt to respond to a light that matched an example wavelength (512, 572 or 655 nm). In the tests, the sample wavelength was novel and birds had to choose between the two nearest training lights. This procedure identified two wavelengths 540 and 600 nm that were equally likely to be matched to 512/572 or 572/655 nm standards. To move from a measure of similarity to evidence of categorization, the standard lights were blue-shifted by approximately 20 nm (to 473, 555 and 633 nm). Crucially, there was no corresponding shift in the ‘ambiguous’ wavelengths suggesting that they lie at categorical boundaries. Figure 2 indicates the locations of Wright and Cumming's lights in a chicken cone space (similar in pigeon).
Figure 2.
Chromaticity coordinates of the colours used in these experiments (table 2 and §2). Saturation is defined as being proportional to the distance from the origin, and hue to the angle relative to the x-axis (table 2). Locations of the eight colours used are plotted (crosses) within circles that approximate to the actual colour used. These colours are saturated and unsaturated versions of orange (O), blue (B), red (R) and green (G). Orange/blue and red/green are complementary pairs of colours. The dashed line plots the monochromatic locus in this receptor space.
Notwithstanding evidence such as that from pigeon (Wright & Cumming 1971) several authors have argued that colour categorization requires language (Davidoff 2001), or that cognitive capacities associated with the neocortex, mean that there are likely to be fundamental differences in colour perception between ourselves and groups such as bird and insects (Yoshioka et al. 1996; Stoerig 1998; Freedman et al. 2001). Consistent with this view, many studies suggest that for groups such as bees and birds the efficacy of colour signals correlates with the magnitude measured in terms of contrast or saturation (Andersson 1994; Lunau et al. 1996; Gamberale-Stille 2001; Lindstro¨m et al. 2001). By comparison, it may be that certain colours are preferred because they are perceptually ‘pure’ (or ideals of their categorical class), as Poralla & Neumeyer (2006) suggest for goldfish (Carassius auratus).
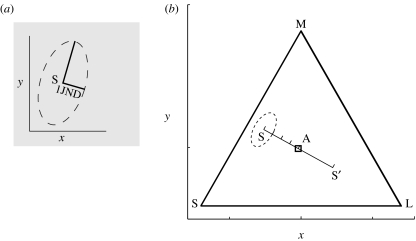
How then should one describe suprathreshold stimuli in a way that is relevant to animal colour vision (Bennett et al. 1994; Endler & Mielke 2005)? A straightforward measure of stimulus strength is by a metric based on the discrimination threshold (figure 1; Kelber et al. 2003; Dyer & Chittka 2004). This measure is reflected in Fechner's law, which asserts that the strength of a sensation, S, caused by a stimulus of intensity, I, against a background intensity, I0, is given by S=k log(I/I0), where k is a constant, that is dependent upon the discrimination thresholds or just noticeable difference (JND). For colour vision, the definition of I is not obvious. This is because chromaticity (i.e. hue and saturation) and achromatic brightness make separate contributions to the discrimination thresholds that depend upon the size of the stimulus and the light level (Wyszecki & Stiles 1982; Kelber et al. 2003). Schrödinger (1920) applied a version of Fechner's law to a model of colour space (see also Wyszecki & Stiles 1982) to predict large differences in chromaticity from the JND (figure 1). We refer to this measure as discriminability. The key implication of Schrödinger's models and its successors (Wyszecki & Stiles 1982) is that two signals with equal magnitudes in terms of JNDs will have equal strength. The model applies quite well to human judgements of colour difference (Wyszecki & Stiles 1982, ch. 7), but self-evidently cannot account for categorization (Boynton & Olson 1990).
Figure 1.
A model of suprathreshold colour differences based on receptor responses, see also Wyszecki & Stiles (1982). (a) For stimulus S located at x, y in a Cartesian perceptual space (e.g. a chromaticity diagram), we can define a region within which other stimuli are not discriminable; the distance from x, y to the edge of this ellipse is 1JND. Schrödinger (1920) proposed that the perceived magnitude of suprathreshold difference is proportional to the (minimum) number of JNDs separating two colours. (b) In general, discrimination thresholds at each location in a perceptual space need to be determined empirically (Wyszecki &Stiles 1982). However, if colour thresholds are set by noise in photoreceptors, then four loci plotted in a space, whose axes are determined by receptor excitations, produce threshold ellipses (a) that are approximately uniform across the space (Vorobyev & Osorio 1998; Kelber et al. 2003). It follows that a given vector corresponds to a fixed number of JNDs, so we can predict that complementary colours S and S′ have equal colour differences from the achromatic point A. The present study shows that this prediction does not hold for chicks, and hence the response strength to a given colour is affected by some factor other than discriminability.
While Wright & Cumming (1971) studied pigeon categorization of monochromatic lights, this study tests poultry chicks' perception of large colour differences by comparing their spontaneous responses to pairs of complementary colours (figure 2; Hess & Gogel 1954; Osorio et al. 1999b). When complementary colours are viewed against an achromatic (i.e. grey) background (experiments 1, 2 and 4), they present equal and opposite signals, defined in terms of receptor responses (figures 1 and 2; table 2). If the effects of achromatic brightness are controlled (table 2 and figure 2; Osorio et al. 1999c), it is possible to separate the effects of chromatic contrast from those of other aspects of colour. Behavioural preferences can then be related to three models of the processes that may underlie perception of large colour signals. Two of these models assume that signal strength is determined by a metric based on the JND (figure 1). The relevant measure may either be the chromatic contrast against the background or the difference from an (innate or learnt) internal standard. An alternative possibility is that a JND-based metric does not hold; for example, if there were relatively sharp distinctions between ranges of colour that share approximately equivalent value, as would be expected for colour categorization.
Table 2.
Estimated receptor responses to the eight stimulus colours used for chicken short (S), medium (M) and long (L) wavelength-sensitive single cones and (D) double cones, relative to a barium sulphate reflectance standard under the same illumination (see also Osorio et al. 1999c). The response to the achromatic background was close to 0.3 for the four cone types. Values given are for the highest intensity colour, but intensities were varied at random over a uniform distribution with a contrast range of 0.3 (Osorio et al. 1999c). x and y chromaticity coordinates were calculated according to the formula given in the legend to figure 2. Aspects of colour that correspond to human ‘hue’ and ‘saturation’ are defined by polar coordinate frame centred on the origin. Saturation corresponds to the distance of the colour from the origin, and hue to the angle relative to a line running in the negative direction from the origin and parallel to the x-axis. The UV sensitive receptor was inactive because a coloured filter (Schott G475) blocked short-wavelength illumination (Osorio et al. 1999c). Examples of reflectance spectra that are close to the orange and blue used here and were produced by the similar Epson printer inks are illustrated by Miklósi et al. (2002; figure 1a,b).
| S | M | L | D | hue (°) | sat. | x | y | |
|---|---|---|---|---|---|---|---|---|
| saturated colours | ||||||||
| orange | 0.19 | 0.28 | 0.37 | 0.31 | 180 | 0.15 | 0.1515 | 0.0000 |
| blue | 0.45 | 0.33 | 0.25 | 0.30 | 353 | 0.14 | −0.1373 | −0.0159 |
| green | 0.28 | 0.29 | 0.16 | 0.23 | 34 | 0.14 | −0.1162 | 0.0783 |
| red | 0.165 | 0.17 | 0.285 | 0.22 | 204 | 0.16 | 0.1507 | −0.0669 |
| unsaturated colours | ||||||||
| orange | 0.27 | 0.33 | 0.40 | 0.36 | 183 | 0.092 | 0.092 | −0.004 |
| blue | 0.46 | 0.37 | 0.32 | 0.35 | 351 | 0.087 | −0.086 | −0.014 |
| green | 0.30 | 0.31 | 0.22 | 0.27 | 36 | 0.084 | −0.068 | 0.049 |
| red | 0.22 | 0.22 | 0.30 | 0.25 | 210 | 0.088 | 0.076 | −0.044 |
2. Material and methods
(a) Subjects
We used male poultry chicks (Gallus gallus; ISA Brown) from a commercial hatchery. Chicks were housed in pairs in 240×270 mm cages. The room was held at 21°C with a 12 : 12 L : D cycle for the main room illumination. Bedding was changed daily and all equipment thoroughly washed at 60°C between batches of chicks. Experiments were conducted and chicks maintained as specified by UK Animal Welfare Legislation and Guidelines.
For experiment 1, chicks were kept in the dark for up to 40 h after hatching (chicks survive on yolk for 2 days). These birds were tested directly on emergence into the light and hence were visually naive. Keeping chicks in this way is normal in commercial husbandry; there is no evidence that they are impaired either on emergence or subsequently.
In experiments 2–4, chicks were 9 days old. The birds were maintained in standard conditions where water and chick starter crumbs were continuously available. There was however 2 h of food deprivation before an experiment started. Chicks were housed, trained and tested in pairs as they become distressed when isolated. We did not separate the responses of the chicks in a pair.
(b) Stimuli
Colours were defined by estimated excitations of chicken short (S, blue), medium (M, green) and long (L, red) wavelength-sensitive single cone photoreceptors (table 2; Osorio et al. 1999c), normalized to a 100% reflective surface. Excitations of S, M and L cones give three parameters qs, qm and ql. Chromaticity is represented by the projection of a colour locus in a three-dimensional receptor space onto a plane given by (fig. 2a; Osorio et al. 1999c)
UV cones were excluded by filtering the experimental illumination with a Schott BG475 filter (Osorio et al. 1999c). Exclusion of UV has no effect on our conclusions, but its presence could affect the relative attractiveness to birds of colours that are alike to the human eye.
If chromaticities are specified by polar coordinates in the x, y space, the angle about the achromatic point corresponds approximately to hue, and the radius to saturation. We use the terms in a geometrical sense, without implying that chicks recognize hue and saturation qualities of colour. A pair of colours with x, y loci equal and opposite distances from the achromatic point are complementary; that is, an additive 50 : 50 mixture is achromatic. According to the model of colour differences outlined in figure 1, complementary colours are equally discriminable from the grey.
Each experiment used two sets of hue, which are named orange/blue and red/green, according to their appearance to humans (figure 2 and table 2). For each hue, there were two levels of saturation, designated ‘unsaturated’ and ‘saturated’. Saturated and unsaturated colours formed complementary pairs. The saturated colours were approximately double the unsaturated distance from the achromatic point. This allowed us to distinguish the effect of saturation (difference from grey) from that of hue. Comparisons were made separately for the orange/blue and red/green hue pairs, so that any one test included four colours, plus the achromatic colour. Miklósi et al. (2002, figure 1) illustrate reflectance spectra of colours that were near to the saturated orange and blue used here.
To minimize achromatic artefacts, we start from the assumption that double cones give an avian luminance signal (Livingstone & Hubel 1988; Osorio et al. 1999b; Jones & Osorio 2004; Goldsmith & Butler 2005). The mean excitation of the chicken double cones by complementary pairs of experimental colours was approximately equal (table 2). Remaining achromatic effects were masked by achromatic noise of uniform intensity distribution and contrast range of 0.3 (Osorio et al. 1999c).
(c) Experiment 1
Pairs of day-old chicks were taken from dark holding boxes and placed on a floor of laminated paper that was printed with randomly placed 10 mm diameter coloured circles over a grey background (for a description and illustration of the background see Osorio et al. (1999a)). Circles of 10 mm were used because there is evidence that the responses of chicks to targets greater than 5 mm are based on chromaticity, while responses to targets less than 2 mm in diameter are based on luminance (Osorio et al. 1999b). We tested responses to orange/blue (n=33 pairs) or red/green (n=33 pairs). Each treatment included each of two hues at two levels of saturation. The first colour selected (i.e. pecked) by one of the two chicks was recorded and the test was then terminated.
(d) Experiments 2–4
Here, the stimuli were coloured paper cones (25 mm length and 7.5 mm diameter). The printed (Epson StylusPro) patterns consisted of 2 mm×6 mm rectangular tiles (Osorio et al. 1999c). Stimuli were either all grey (i.e. achromatic) or had 30% coloured tiles, which were placed at random locations on a grey background. Both grey and coloured tiles had fixed chromaticities (figure 2), but to minimize effects of achromatic contrast their intensities varied with a contrast range of 0.3 (see above).
The experiment was run on day 9 post-hatching. Training and testing took place in a 0.4 m×0.3 m arena, which was illuminated with a filtered (Schott BG475) 250 W quartz–halogen lamp. Initially, chicks were acclimatized to the stimuli by training with six grey stimuli that contained chick starter crumbs.There were two 6-min training sessions, separated by 30 min.
An unrewarded test took place 1 h after the second training session. During this interval, the chicks had water but not food. In experiments 2 and 4, the background tiles were achromatic. In experiment 3, the background was unsaturated orange for the orange/blue tests and unsaturated red for the red/green tests, so that blue and green stimuli contrasted more with the background than orange and red. Experiment 2 used 10 pairs of chicks (n=10) for each treatment, while for experiments 3 and 4, there were six pairs (n=6).
In all tests, pairs of chicks were placed in the training arena with 10 empty stimuli. These consisted of two each of the following: (i) all-grey containers and (ii) both saturated (S) and unsaturated (U) forms of two colours, either orange and blue or red and green. The first 10 selections (i.e. pecks) of each pair of chicks were used to score colour preferences. Repeat pecking at a stimulus without an intervening search, or the obvious copying of the choice of a partner was not counted (Osorio et al. 1999c). All analyses combined the responses of the two chicks.
In experiment 1, differences between preferences for colours were analysed using Wilcoxon signed-rank tests. In experiments 2–4, sign tests were used to analyse the number of selections for each colour. A Mann–Whitney U-test was used to compare effects of treatment (i.e. test colours) on responses to achromatic stimuli. All tests were two-tailed.
3. Results
The eight experimental colours (figure 2 and table 2) were defined by their locations in polar coordinates in the x, y space, with the origin at the grey background tiles (and hence achromatic by definition). The four hue angles are identified as orange, blue, red and green according to their appearance to the human eye. Each of these four hues was presented in saturated (S) or unsaturated (U) forms as defined by their (relative) distance from the origin. Saturated colours were approximately twice as far as unsaturated colours from the origin. Blues and oranges, and reds and greens, respectively, lay close to lines through the origin, so that saturated orange and saturated blue were approximately complementary, as were saturated red and green. The same complementary relationships applied to the unsaturated colours. An experimental test included four colours, namely US and S versions either of orange and blue or of green and red. Table 3 summarizes experimental data.
Table 3.
Summary of the data from chick colour preference experiments. For experiment 1, one selection was made by each pair of chicks, and we give the total number of selections for each colour. For experiments 2–4, the mean number of selections for each colour from the first 10 pecks is given. n is the number of pairs of chicks in each experiment. Colours are specified in table 2 and figure 2. sat., saturated; unsat., unsaturated.
| experiment | n | orange | blue | grey | red | green | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sat. | unsat. | sat. | unsat. | sat. | unsat. | sat. | unsat. | sat. | sat. | ||
| 1 | 33 | 23 | 3 | 6 | 1 | n.a. | n.a. | 13 | 1 | 17 | 2 |
| 2 | 10 | 5 | 2.8 | 1 | 1 | 0.2 | 1 | 4.5 | 2 | 1.6 | 0.9 |
| 3 | 6 | 4.67 | 3.33 | 0.33 | 0.83 | 0.83 | 0.33 | 7.83 | 0.5 | 0.83 | 0.5 |
| 4 | 6 | 5 | 4 | 0.67 | 0.17 | 0.17 | 0.33 | 5.33 | 2 | 1.67 | 1.67 |
(a) Experiment 1
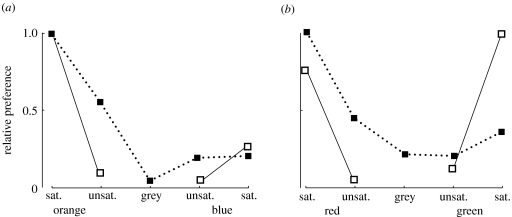
Day-old chicks, which had been kept in the dark prior to the experiment, were often hesitant to peck. However, those that did peck at the dots printed on the floor showed significant preferences. We recorded only the first response for each bird, so that records are fully independent. For the orange/blue treatment, 23 selections (70%) were for saturated orange (table 3 and figure 3). Unsurprisingly then orange was preferred to blue and saturated colours preferred to unsaturated (Wilcoxon signed-rank tests; saturated orange versus unsaturated orange: Z=−3.922, p<0.001; saturated orange versus saturated blue: Z=−3.157, p=0.002).
Figure 3.
Relative preferences of chicks to colours on the (a) orange to blue and (b) red to green axes for newly hatched (solid line) and 9-day-old (dotted line) birds. Newly hatched chicks (experiment 1) are substantially more sensitive to chromatic contrast (or saturation) than the older subjects (experiment 2). Data plotted are for the total number of pecks in experiment 1 and the mean number of pecks per pair of chicks for experiment 2. The responses to grey were not measurable in experiment 1. Further details and statistics are given in the text.
For the red/green group, there was no effect of hue but a significant effect of saturation. Out of 33 selections, 13 were for saturated red and 17 for saturated green. Saturated red was preferred to unsaturated red (Wilcoxon signed-rank test; Z=−3.207, p=0.001) and saturated green was preferred to unsaturated green (Wilcoxon signed-rank test; Z=−3.441, p=0.001).
(b) Experiment 2
This resembled experiment 1, but tested 9-day-old chicks with coloured paper containers. Stimuli printed on the containers were random tilings of (on average) 70% grey and 30% coloured tiles (Osorio et al. 1999c). The colours were the same as in experiment 1. Now orange was selected 3.9 times more frequently than blue, and grey was still less attractive (table 3 and figure 3; sign tests; saturated orange/saturated blue: n=9, k=1, p=0.004; unsaturated orange/unsaturated blue: n=9, k=1, p=0.021; saturated orange/grey: n=10, k=0, p=0.002; saturated orange/unsaturated blue: n=10, k=0, p=0.002; unsaturated orange/grey: n=9, k=1, p=0.004). In contrast to the younger birds, there was no difference between the two types of orange (sign test; saturated orange/unsaturated orange: n=6, k=2, p>0.2, n.s.) and no effect of saturation.
For red and green, there was a significant effect of hue, but was weaker than for the orange/blue treatment, and there was no effect of saturation. Reds were selected 2.6 times more frequently than greens (sign tests; saturated red/saturated green: n=8, k=1, p=0.039; saturated red/unsaturated green: n=9, k=1, p=0.021; saturated red/grey: n=9, k=1, p=0.004). Unsaturated red stimuli were not significantly preferred to any of the other colours.
(c) Experiment 3
This resembled experiment 2, except that the background colour of the food containers and the arena floor was not achromatic, but the unsaturated version of the preferred hue; that is, either unsaturated orange or unsaturated red. Here again there was no effect of saturation (or chromatic contrast; table 3). In the orange/blue treatment, saturated orange was strongly preferred to other hues (sign tests; saturated orange/saturated blue, saturated orange/unsaturated blue and saturated orange/grey: n=6, k=0, p=0.031), although there was no difference between the two types of orange (sign test; saturated orange/unsaturated orange: n=3, k=2, p>0.5, n.s.).
In the red/green treatment, saturated red was strongly preferred to the other colours (sign tests; saturated red/unsaturated red, saturated red/saturated green, saturated red/unsaturated green and saturated red/grey: n=6, k=0, p=0.031). This finding reinforces the point that chromatic contrast does not determine the attractiveness of a colour.
(d) Experiment 4
This experiment investigated the effect of elevating background contrast. Luminance contrast is a common feature of aposematic patterns and other visual displays. In experiments similar to those reported here, luminance contrast enhances the attractiveness of novel stimuli for chicks (Osorio et al. 1999a). In experiment 4, rather than having grey tiles with intensity ranging randomly over a contrast range of 0.3, the achromatic background tiles were of two intensities with reflectances 0.2 and 0.7 (i.e. mean contrast 0.56). The test colours remained the same as in other tests (figure 2).
As with experiment 2, orange was again preferred to blue (table 3; sign tests; saturated orange/saturated blue, saturated orange/unsaturated blue and saturated orange/grey: n=6, k=0, p=0.031), but there was not a significant difference between the two types of orange (sign test; saturated orange/unsaturated orange: n=3, k=2, p>0.5, n.s.). For red/green, saturated red was preferred to unsaturated green and grey (sign tests; saturated red/unsaturated green and saturated red/grey: n=6, k=0, p=0.031), but there were no significant differences between any other pairs of colours.
In experiments 2–4, the overall mean number of selections of grey was not significantly affected by experimental treatment (Mann–Whitney U-test; U=193.00, n1=n2=22, p>0.1, n.s.).
4. Discussion
This study uses a simple behavioural test and stimuli that are well defined in terms of avian receptor signals to investigate bird's responses to colour. Stimuli were selected to minimize the effects of achromatic contrast. A simple model (figure 1) predicts that signal strength should be based on discriminability of the colour from its background. This requires no specific knowledge of colour. It is perhaps not surprising that discriminability should be more relevant to newly hatched than to older chicks (figure 3). Nonetheless, this model cannot account for the newly hatched chicks' preference for orange over blue (table 2 and figure 3). The preference for orange persists in 9-day-old chicks, which also prefer red to green. The preference for orange is robust to the effects of changing the background (experiment 3) and the luminance contrast within the pattern (experiment 4). Preferences for orange and red are perhaps expected from other work on birds (table 1), but they are not a major concern here. Preferences vary genetically (Kovach & Wilson 1988), and for older birds they are influenced by general experience (Miklósi et al. 2002).
Table 1.
Results of previous avian colour preference experiments.
| species | age (day(s)) | experiment | colour biases observed | reference |
|---|---|---|---|---|
| white rock chicks | 1 | innate pecking preferences | bimodal preference for orange and blue | Hess (1956) |
| Pekin ducklings | 1 | preference for green and yellowish-green | ||
| bobwhite quail chicks | 1 | pecking at coloured dots | preference for green and blue-green; aversion to red and orange | Kear (1964) |
| domestic chicks | 1 | approach behaviour | preference for red or yellow over blue | Taylor et al. (1969) |
| Rhode Island Red chicks | 3 | approach behaviour | aversion to green; white, blue, yellow and red equally preferred | Kovach (1970) |
| white leghorn chicks | 1 | approach behaviour | preference for red over green or yellow | Salzen et al. (1971) |
| white leghorn chicks | 1 | pecking preferences | bimodal preference for orange-red and blue-violet | Fischer et al. (1975) |
| male zebra finches | n.a. | predation on two morphs of a bug | aversion to red; grey morph preferred | Sillén-Tullberg (1985) |
| domestic chicks | 1–2 | predation on painted mealworms | aversion to black-and-yellow stripes, black and red; relative preference for other stripes and olive green prey | Roper & Cook (1989) |
| domestic chicks | 4–5 | predation on painted mealworms | aversion to red relative to brown | Roper (1990) |
| bobwhite quail chicks | 1 | innate preferences for food | descending preferences: blue>green>yellow>red | Mastrota & Mench (1995) |
A possible reason why discriminability from the background does not entirely account for the chicks' colour preferences is that they have an innate (or learnt) standard that biases their decisions. This seems likely and could be influenced by the colour of their food or the environment (Miklósi et al. 2002). What is less clear is whether the preference is based on a simple JND-based metric of the difference between the standard and the observed colour, or if colour categorization is also involved. At least the differences between blue and orange (figures 2 and 3; table 2) in this study give some evidence for categorization.
Categorization implies that a set of discriminable stimuli have a common value (or meaning) that differs from the value of another set of stimuli. The demarcation between categories on the perceptual continuum (e.g. in colour space; figures 1 and 2) can be sharp (Harnad 1987; Boynton & Olsen 1990; Davidoff 2001). Our data are not conclusive, but there is evidence that older chicks are less sensitive to chromatic contrast than the day-old birds, and the effect of saturation was not significant in this study (experiment 2; table 2 and figure 3a). Experiment 3, where backgrounds were either orange or red, strongly indicates that chromatic contrast is not the main factor determining stimulus strength. Likewise, experiment 4, which used high-contrast backgrounds but otherwise resembled experiment 2, shows a strong colour preference but little effect of saturation (figure 3 and table 3). Overall, there is a clear suggestion that the achromatic point (grey) is a categorical boundary on the line in colour space (figure 2) between the complementary pairs of test colours.
Evidence for chicks treating the achromatic point as a categorical boundary was also found in an earlier work on colour generalization (Jones et al. 2001). For this latter study, the chicks learnt to associate food with two different colours and were then tested on their preferences for the intermediate colour (defined in the receptor space; figure 2). If this test colour was chromatic (orange, turquoise or purple), then the chicks responded to it strongly. There was no such preference for grey intermediate lying between complementary yellow and blue. It was argued (Jones et al. 2001) that upon training chicks learn to group (or categorize) pairs of colours such as red and yellow or blue and green, as belonging to a common kind, in which case the intermediate is placed in the same category. That they will not group the complementary pair in this way implied a perceptual constraint on forming a link across the achromatic point.
Overall, the evidence from this study, from our earlier work on colour learning (Jones et al. 2001) and from pigeons (Wright & Cumming 1971) makes good case that chicks can impose sharp (categorical) boundaries on the colour continuum, but the evidence is not compelling. However, problems in establishing evidence for colour categorization in non-human species such as bees, fishes and monkeys (Giurfa et al. 1995; Fagot et al. 2006; Poralla & Neumeyer 2006) are perhaps not surprising, given the difficulties in understanding the mechanistic basis for and ontogeny of human unique hues and colour names (Boynton & Olsen 1990; Davidoff 2001; Wuerger et al. 2005; Philipona & Regna 2006).
5. Conclusion
Colour is highly salient to humans, and essential to both how we recognize and evaluate objects. Colour signals probably have a similar value to other species, which has led to wide recognition of their significance in biological communication (Bennett et al. 1994; Endler & Mielke 2005). In view of our relatively good knowledge of receptor mechanisms and how they set discrimination thresholds in non-human species (Vorobyev & Osorio 1998; Goldsmith & Butler 2003, 2005), there are good arguments for basing measures of colour signals on low-level metrics related to the discrimination threshold (Endler & Mielke 2005). However, it has long been known that the relationship between threshold and the perceived magnitude of evaluation of suprathreshold signals is complicated (Stevens 1957), and such measures obviously fail for categorical perception (Harnad 1987). In our previous works (Vorobyev & Osorio 1998; Osorio et al. 1999a,b,c; Jones & Osorio 2004), we have drawn attention to the different behavioural uses of chromatic and achromatic visual signals in bird vision. Here, we have shown how using well-defined chromatic stimuli we can begin to investigate what aspects of colour stimulus controls the simplest of visual tasks, namely spontaneous pecking at possible food items.
Acknowledgments
We thank R. J. Andrew and T. J. Roper for their help and advice. The work was funded by the BBSRC.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Norris K.N. Sexual selection and the mismeasure of color. Am. Nat. 1994;144:848–860. doi:10.1086/285711 [Google Scholar]
- Boynton R.M, Olson C.X. Salience of chromatic basic color terms confirmed by three measures. Vision Res. 1990;30:1311–1317. doi: 10.1016/0042-6989(90)90005-6. doi:10.1016/0042-6989(90)90005-6 [DOI] [PubMed] [Google Scholar]
- Davidoff J. Language and perceptual categorisation. Trends Cogn. Sci. 2001;5:382–387. doi: 10.1016/s1364-6613(00)01726-5. doi:10.1016/S1364-6613(00)01726-5 [DOI] [PubMed] [Google Scholar]
- Dyer A.G, Chittka L. Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. J. Comp. Physiol. A. 2004;190:759–763. doi: 10.1007/s00359-004-0547-y. [DOI] [PubMed] [Google Scholar]
- Endler J.A, Mielke P.W. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 2005;86:405–431. doi:10.1111/j.1095-8312.2005.00540.x [Google Scholar]
- Fagot J, Goldstein J, Davidoff J, Pickering A. Cross-species differences in color categorization. Psychon. Bull. 2006;13:275–280. doi: 10.3758/bf03193843. [DOI] [PubMed] [Google Scholar]
- Fischer G.J, Morris G.L, Ruhsam J.P. Color pecking preferences in white leghorn chicks. J. Comp. Physiol. Psychol. 1975;88:402–406. doi: 10.1037/h0076227. doi:10.1037/h0076227 [DOI] [PubMed] [Google Scholar]
- Freedman D.J, Riesenhuber M, Poggio T, Miller E.K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–315. doi: 10.1126/science.291.5502.312. doi:10.1126/science.291.5502.312 [DOI] [PubMed] [Google Scholar]
- Gamberale-Stille G. Benefit by contrast: an experiment with live aposematic prey. Behav. Ecol. 2001;12:768–772. doi:10.1093/beheco/12.6.768 [Google Scholar]
- Giurfa M, Núñez J, Chittka L, Menzel R. Colour preferences of flower-naive honeybees. J. Comp. Physiol. A. 1995;177:247–259. doi:10.1007/BF00192415 [Google Scholar]
- Grant K.A. A hypothesis concerning the prevalence of red coloration in California hummingbird flowers. Am. Nat. 1966;100:85–97. doi:10.1086/282403 [Google Scholar]
- Goldsmith T.H, Butler B.K. The roles of receptor noise and cone oil droplets in the photopic spectral sensitivity of the budgerigar Melopsittacus undulatus. J. Comp. Physiol. A. 2003;189:135–142. doi: 10.1007/s00359-002-0385-8. [DOI] [PubMed] [Google Scholar]
- Goldsmith T.H, Butler B.K. Color vision of the budgerigar Melopsittacus undulatus: hue matches, tetrachromacy, and intensity discrimination. J. Comp. Physiol. A. 2005;19:933–951. doi: 10.1007/s00359-005-0024-2. doi:10.1007/s00359-005-0024-2 [DOI] [PubMed] [Google Scholar]
- Harnad S. Cambridge University Press; New York, NY: 1987. Categorical perception: the groundwork of cognition. [Google Scholar]
- Hess E.H. Natural preferences of chicks and ducklings for objects of different colors. Psychol. Rep. 1956;2:477–483. [Google Scholar]
- Hess E.H, Gogel W.C. Natural preferences of the chick for objects of different colors. J. Psychol. 1954;38:483–493. [Google Scholar]
- Jones C.D, Osorio D. Discrimination of oriented visual textures by poultry chicks. Vision Res. 2004;44:83–89. doi: 10.1016/j.visres.2003.08.014. doi:10.1016/j.visres.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Jones C.D, Osorio D, Baddeley R.J. Colour categorization by domestic chicks. Proc. R. Soc. B. 2001;268:2077–2084. doi: 10.1098/rspb.2001.1734. doi:10.1098/rspb.2001.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kear J. Colour preference in young Anatidae. Ibis. 1964;106:361–369. [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. doi:10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Kovach J.K. Effectiveness of different colors in the elicitation and development of approach behaviour in chicks. Behaviour. 1970;38:154–168. [Google Scholar]
- Kovach J.K, Wilson G. Genetics of color preferences in quail chicks: major genes and variable buffering by background genotype. Behav. Genet. 1988;18:645–661. doi: 10.1007/BF01082315. doi:10.1007/BF01082315 [DOI] [PubMed] [Google Scholar]
- Lindström L, Rowe C, Guilford T. Pyrazine odour biases food selection in avian predators against conspicuously coloured predators. Proc. R. Soc. B. 2001;268:1–4. doi:10.1098/rspb.2000.1322 [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, colour, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. doi:10.1126/science.3283936 [DOI] [PubMed] [Google Scholar]
- Lunau K, Wacht S, Chittka L. Colour choices of naive bumble bees and their implications for colour perception. J. Comp. Physiol. A. 1996;178:477–489. doi:10.1007/BF00190178 [Google Scholar]
- Mastrota F.N, Mench J.A. Colour avoidance in northern bobwhites, effects of age, sex and previous experience. Anim. Behav. 1995;50:519–526. doi:10.1006/anbe.1995.0266 [Google Scholar]
- Miklósi A, Gonda Z.S, Osorio D, Farzin A. The effects of the visual environment on responses to colour by domestic chicks. J. Comp. Physiol. A. 2002;188:135–140. doi: 10.1007/s00359-002-0284-z. doi:10.1007/s00359-002-0284-z [DOI] [PubMed] [Google Scholar]
- Nelson D.A, Marler P. Categorical perception of a natural stimulus continuum: birdsong. Science. 1989;244:976–978. doi: 10.1126/science.2727689. doi:10.1126/science.2727689 [DOI] [PubMed] [Google Scholar]
- Osorio D, Jones C.D, Vorobyev M. Accurate memory for colour but not pattern contrast in chicks. Curr. Biol. 1999a;9:199–202. doi: 10.1016/s0960-9822(99)80089-x. doi:10.1016/S0960-9822(99)80089-X [DOI] [PubMed] [Google Scholar]
- Osorio D, Miklósi A, Gonda Z.S. Visual ecology and perception of coloration patterns by domestic chicks. Evol. Ecol. 1999b;13:673–689. doi:10.1023/A:1011059715610 [Google Scholar]
- Osorio D, Vorobyev M, Jones C.D. Colour vision of domestic chicks. J. Exp. Biol. 1999c;202:2951–2959. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
- Philipona D.L, O'Regan K. Color naming, unique hues, and hue cancellation predicted from singularities in reflection properties. Visual Neurosci. 2006;23:331–339. doi: 10.1017/S0952523806233182. doi:10.1017/S0952523806233182 [DOI] [PubMed] [Google Scholar]
- Poralla J, Neumeyer C. Generalization and categorization of spectral colors in goldfish II. Experiments with two and six wavelengths. J. Comp. Physiol. A. 2006;192:469–479. doi: 10.1007/s00359-005-0082-5. doi:10.1007/s00359-005-0082-5 [DOI] [PubMed] [Google Scholar]
- Roper T.J. Responses of domestic chicks to artificially coloured insect prey: effects of previous experience and background colour. Anim. Behav. 1990;39:466–473. doi:10.1016/S0003-3472(05)80410-5 [Google Scholar]
- Roper T.J, Cook S.E. Responses of chicks to brightly coloured insect prey. Behaviour. 1989;110:276–293. [Google Scholar]
- Rowe C, Guilford T. Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature. 1996;383:520–522. doi:10.1038/383520a0 [Google Scholar]
- Salzen E.A, Lily R.E, McKeown J.R. Colour preference and imprinting in domestic chicks. Anim. Behav. 1971;19:542–547. doi:10.1016/S0003-3472(71)80109-4 [Google Scholar]
- Schrödinger E. Grundlinien einer theorie der Farbenmetric im Tagessehen. Annalen der Physik. 1920;63:81–520. [Google Scholar]
- Sillén-Tullberg B. The significance of coloration per se, independent of background, for predator avoidance of aposematic prey. Anim. Behav. 1985;33:1382–1384. doi:10.1016/S0003-3472(85)80211-6 [Google Scholar]
- Stevens S.S. On the psychophysical law. Psychol. Rev. 1957;64:153–181. doi: 10.1037/h0046162. doi:10.1037/h0046162 [DOI] [PubMed] [Google Scholar]
- Stoerig P. Wavelength information processing versus color perception: evidence from blindsight and color-blind sight. In: Backhaus W.G.K, Kliegl R, Werner J.S, editors. Color vision: perspectives from different disciplines. Walter de Gruyter; Berlin, Germany: 1998. pp. 131–147. [Google Scholar]
- Taylor A, Sluckin W, Hewitt R. Changing colour preferences of chicks. Anim. Behav. 1969;17:3–8. doi: 10.1016/0003-3472(69)90003-7. doi:10.1016/0003-3472(69)90105-5 [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson M.F, Whelan C.J. The evolution of fruit color in fleshy-fruited plants. Am. Nat. 1990;136:790–809. doi:10.1086/285132 [Google Scholar]
- Wright A.A, Cumming W.W. Color-naming functions for the pigeon. J. Exp. Anal. Behav. 1971;15:7–17. doi: 10.1901/jeab.1971.15-7. doi:10.1901/jeab.1971.15-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerger S.M, Atkinson P, Cropper S. The cone inputs to the unique-hue mechanisms. Vision Res. 2005;45:3210–3223. doi: 10.1016/j.visres.2005.06.016. doi:10.1016/j.visres.2005.06.016 [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles W.S. 2nd edn. Wiley; New York, NY: 1982. Color science. [Google Scholar]
- Yoshioka T, Dow B.M, Vautin R.G. Neuronal mechanisms of color categorization in areas V1, V2 and V4 of macaque monkey visual cortex. Behav. Brain Res. 1996;76:51–70. doi: 10.1016/0166-4328(95)00183-2. doi:10.1016/0166-4328(95)00183-2 [DOI] [PubMed] [Google Scholar]