Abstract
Biased operational sex ratios (OSRs) can drive sexual selection on members of the over-represented sex via competition for mates, causing higher variance and skew in reproductive success (RS) among them if an individual's quality is a persistent characteristic. Alternatively, costs of reproduction may degrade breeding performance, creating the opportunity for members of the limiting sex to switch mates adaptively, effectively homogenizing variance and skew in RS among the sex in excess. We tested these two contrasting models in a male-biased population of the Nazca booby (Sula granti) with demonstrated costs of reproduction with data on total RS over a 14-year period. Variances and skews in RS were similar, and males changed from breeder to non-breeder more frequently than females. Under the persistent individual quality model, females should mate only with high quality males, and non-breeding males should seldom enter the breeding pool, yet 45% of non-breeding males (re)entered the breeding pool each year on average. Many Nazca booby females apparently exchange a depleted male for a new mate from the pool of current non-breeder males. Our evidence linking serial monogamy to costs of reproduction is novel and suggests selection on female mating preferences based on an interaction between at least two life-history components (OSR and reproductive effort).
Keywords: mate choice, operational sex ratio, cost of reproduction, serial monogamy, divorce, multi-state mark-recapture models
1. Introduction
A biased operational sex ratio (OSR; relative ratio of individuals ready to mate: males/(males+females); Enders 1993; Kvarnemo & Ahnesjö 1996, 2002) should drive sexual selection on members of the over-represented sex via competition for mates, and can logically be expected to cause higher variance and skew in reproductive success (RS) than in the limiting sex (Emlen & Oring 1977; Andersson 1994; Shuster & Wade 2003). For example, as the limiting sex in a male-biased OSR, females should have more opportunity than males do to choose among competing potential mates, and when females consistently recognize and mate with high quality males, and reject low quality males, they induce a higher variance and skew in mean mating success of males. Members of the limiting sex presumably have easy access to potential mates, so even the least competitive members may have mating successes similar to those of the most competitive members (Kvarnemo & Ahnesjö 2002; Shuster & Wade 2003). Numerous theoretical and empirical studies (Andersson 1994) as well as recent experimental manipulation of the sex ratio during a single breeding season (Jones et al. 2004, 2005; Mills et al. 2007) have supported this idea.
If the pattern of RS seen in a single season is to persist over the lifetimes of iteroparous organisms, then the mating pool must consistently exclude the same individuals of the sex in excess. This assumes that quality is a persistent characteristic of individuals as proposed in optimal divorce/re-mating strategy models (McNamara & Forslund 1996; Dubois et al. 2004). Under a persistent individual quality model, high quality members of the sex in excess tend to remain ‘in the game’, and low quality members tend to remain excluded. This pattern is expected especially in long-lived species where reproductive output may improve with age and/or experience (see Clutton-Brock (1988) for a review; Wooller et al. 1992; Forslund & Pärt 1995; Anderson & Apanius 2003; van de Pol et al. 2006) and with the length of the pair bond (Black et al. 1996; Pyle et al. 2001; van de Pol et al. 2006).
Alternatively, breeding quality may not be a persistent trait of individuals, but may change throughout the lifespan, declining in old age (see Clutton-Brock (1988) for a review; Anderson & Apanius 2003; van de Pol et al. 2006), with temporary infection (although susceptibility to infection has been proposed as a fixed trait; Hamilton & Zuk 1982) or with reproductive effort (Reid 1987; Sæther et al. 1993; Jacobsen et al. 1995; Weimerskirch et al. 1995; Pyle et al. 1997; Erickstad et al. 1998; Golet et al. 1998, 2004; Kalmbach et al. 2004; Townsend & Anderson in press). In this situation, an individual could switch mates adaptively if its current mate's quality dropped below the value of those in the unmated pool (Ens et al. 1996; Dubois et al. 2004). Divorce is also expected to increase in frequency with the increasing availability of unpaired individuals (see Choudhury (1995) for a review). With a biased OSR and temporally variable breeding quality, members of the over-represented sex might rotate in and out of the breeding pool, via the effect of choice exerted by the other sex.
In this paper, we consider the interaction of the sex ratio and costs of reproduction in the mating system of Nazca boobies (Sula granti). Our study population at Punta Cevallos, Isla Española, Galápagos Islands has shown a consistent male bias (0.589, 95% CI 0.589–0.589 (a highly precise point estimate); Townsend & Anderson in press), arising during the post-fledging subadult period (Maness et al. 2007), over the course of a 21-year ongoing study. Sex-specific costs of reproduction may override the effect of OSR on mating system through increased mortality risk or increased need to recover from breeding (‘time out’; Clutton-Brock & Parker 1992) in the sex providing more parental care (Kokko & Monoghan 2001). Although costs of reproduction exist in Nazca boobies, they do not appear to be sex specific. Mothers and fathers incur similar survival costs of reproduction (Townsend & Anderson in press); during breeding, the sexes lose similar amounts of mass and neither sex exhibits decreased immune-mediated self-maintenance (Apanius et al. in press). Behavioural evidence indicates that all adults present in the breeding colony attempt to enter the breeding pool (T. J. Maness 2007, unpublished data). Therefore, the adult sex ratio of our study population apparently reflects the OSR, both are male-biased, and confounding effects of differential costs of reproduction (i.e. Kokko & Monoghan 2001) on evolution of the mating system apparently do not exist.
Even in monogamous species like the Nazca booby, a long-lived pelagic seabird with obligate biparental care and no extra-pair fertilization (Anderson & Boag 2006), we expect an unbalanced OSR to induce a greater variance and skew in RS in the over-represented sex, if the same competitive individuals of that sex consistently acquire mates of the limiting sex. However, male quality might not be persistent, given the known costs of reproduction borne by breeders (Townsend & Anderson in press), so we also considered an alternative model of mate choice, serial monogamy (sensu Baeyens 1981), in which females change mates adaptively between consecutive breeding efforts. In this model, based on transient individual quality rooted in costs of reproduction, females exchange a depleted male for one that has not recently bred. Females using this strategy exploit their status as the limiting sex, and as the larger sex (females are 16% heavier; Anderson 1993) they can control social interactions, to maximize the parental contribution of their mate. Ideally, a female should switch mates before her mate's performance begins to decline in order to maximize her reproductive output. Under this model, male variance in RS might not exceed that of females, despite the sex ratio bias, because the rotation in mating imposed on males by females would tend to homogenize RS among males. In this study, we tested both this ‘rotation’ model and the ‘persistent individual quality’ model.
Given the observed male-biased OSR, the model of persistent individual quality makes several predictions: (i) males should have higher variance and (ii) skew in RS than females and (iii) breeding state transition probabilities (breeder to non-breeder) should be similar between the sexes because ‘winner’ males and females should remain in the breeding category while ‘loser’ males should remain non-breeders. A female may switch mates between consecutive breeding attempts, but her new mate should come from the current pool of high quality breeders. Following the same logic, (iv) the number of years the individuals breed consecutively (breeding bout length) should be similar among breeding males and females. The rotation model predicts the opposite on all four points, with males having a similar or lower variance and skew in RS, higher breeding state transition probabilities and shorter breeding bout lengths. Additionally, the rotation model predicts that the RS of divorced males (an inverse proxy for condition) in the year prior to divorce should be higher than that of retained males if a successful breeding attempt induces higher costs of reproduction than does a failed attempt.
2. Material and methods
(a) Variance and skew in RS
To test predictions about variance and skew in RS, we calculated the mean, variance and skew in the total number of fledglings produced by each known-age adult from each of eight hatch-year cohorts breeding in the 14 breeding seasons from 1992 to 1993 through 2005 to 2006 (see Huyvaert & Anderson (2004) and Townsend & Anderson (in press) for details of fieldwork and study site). Adults in these cohorts fledged during the breeding seasons beginning in 1984–1987 and 1992–1995. The oldest birds were 21 years old at the end of the study, while the youngest were 10 years old. Successful reproduction is apparently rare after approximately 20 years of age (Anderson & Apanius 2003), so these estimates do not reflect lifetime RS for longer lived individuals in the younger cohorts. In addition, we did not begin collecting comprehensive RS data until the 1992–1993 breeding season, so we do not have the early breeding history of the oldest cohorts. Since cohorts differed in number of years available for reproduction and the mean and variance of RS were positively correlated (figure 1 in the electronic supplementary material), a Wilcoxon matched pairs test was used for within-cohort comparisons of standardized variances (variance divided by the squared mean; also known as the opportunity for selection (I); Crow 1958; Wade 1979; Wade & Arnold 1980; Shuster & Wade 2003) of males and females. Recent studies (Jones et al. 2005; Mills et al. 2007) have supported the use of the opportunity for selection based on Bateman's principle (Bateman 1948) as an index of mating competition.
Reproductive skew was compared using the B index (Nonacs 2000). The B index calculates the observed variance and then subtracts the expected variance if every group member has an equal probability of gaining a resource (e.g. mate). B values of zero indicate randomly distributed resources, while significantly positive or negative values indicate more skewed or more equally shared resources, respectively. We used the Skew Calculator 2003 (Nonacs 2003a) to determine B index values, their 95% CI and level of statistical significance.
Much debate exists in the sexual selection literature over the best measure of inequality (Kokko et al. 1999; Nonacs 2000, 2003b; Fairbairn & Wilby 2001; Jones et al. 2005; Mills et al. 2007), leading Kokko et al. (1999) to advocate the use of multiple measures. Most measures correlate with each other (Nonacs 2003b; Mills et al. 2007), but the B index was reliable under a wide range of assumptions and allows the comparison of groups of different sizes and productivities (Nonacs 2003b). We calculated 12 additional inequality measures (detailed in table 1 in the electronic supplementary material) and the Spearman rank correlations between all 13 inequality indices (table 2 in the electronic supplementary material). Frequency distributions of RS of males and females within cohorts are presented in figure 2 in the electronic supplementary material.
(b) Breeding state transition probabilities
We examined sex-specific variability in breeding state (non-breeder or breeder) transitions using multi-state mark-recapture model selection implemented in Program Mark (Cooch & White 2005). Individual encounter histories were established for the four oldest cohorts for a 13-year period (1992–2004). In these encounter histories, individuals could occupy one of three categories in a particular year: not seen, non-breeder or breeder. The 2005–2006 breeding season was not included in this analysis because we monitored only successful breeding events in this year. Candidate models included survival (s) probability, resight (p) probability and breeding state transition (ψ) probability parameters; each could remain constant (.) or vary by group (g), sex (r) or year (t). We used Akaike's Information Criterion (AIC) for model selection and ranking (Burnham & Anderson 2002). In practice, we used QAICC, a version of AIC incorporating adjustment of the variance inflation factor, based on an estimate of median c-hat (; Cooch & White 2005). Models with the lowest QAICC values were assumed to better explain variation in the data.
(c) Breeding bout lengths
Breeding bout lengths were determined for the same individuals over the same 13-year period used to test breeding state transition probabilities; individuals which never bred were excluded. A bout length was calculated as the number of consecutive years an individual bred (incubated one or more eggs for any length of time). If an individual had two or more breeding bouts during the 13-year period, then the mean bout length was used for that individual. The total number of breeding bouts was also determined. Overall male and female mean bout lengths and total number of breeding bouts were compared with separate t-tests.
(d) RS of divorced and retained males
RS within year (X) was determined for subsequently divorced or retained males (in year X+1) in the four oldest cohorts over a 9-year period (1992–2001). Males were classified as retained (those that bred with the same female in years X and X+1) or divorced (bred with different females in years X and X+1 or males that rotated out of the breeding pool for 1 or more years and then returned to the breeding pool). Individuals which never bred were excluded. To allow divorced males the opportunity to resume breeding again, only males (retained or divorced) that survived till year X+4 were included in the analysis; hence, retentions and divorces from the last four breeding seasons were excluded. Many males could not be categorized as retained or divorced because they bred with unbanded females, so mean RS for all uncategorized males was determined as well. Mean RS (fledglings per male per year; a mean proportion) of retained, divorced, and all other males was compared with an ANOVA because assumptions of normality and homogeneity of variance were met. Additionally, divorced males were further subdivided into six categories by non-breeding gap length (0, 1, 2, 3, 4 or more years, still out) to determine the proportion of males falling into each category.
(e) Statistical analyses
Most statistical tests were performed using Statistica (v. 6.1 Statsoft, Inc., Tulsa, OK, USA), except Mark analyses were performed using Program Mark (White & Burnham 1999; Cooch & White 2005).
3. Results
(a) Variance and skew in RS
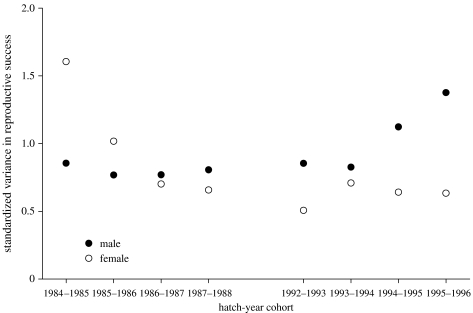
Standardized variance (I) in RS of males and females did not differ (Z=0.980, p=0.327), although the data suggested an age trend; males had higher values at younger ages, while females had higher values at older ages (figure 1).
Figure 1.
Standardized variances in RS of males and females within a hatching cohort. The trend (n.s.) towards higher values for young males and older females may be explained by younger males having fewer breeding attempts than females (T. J. Maness 2007, unpublished data), amplifying the contrast in RS between successful and unsuccessful males, and by the rotation of mating partners by females homogenizing variance in RS among older males.
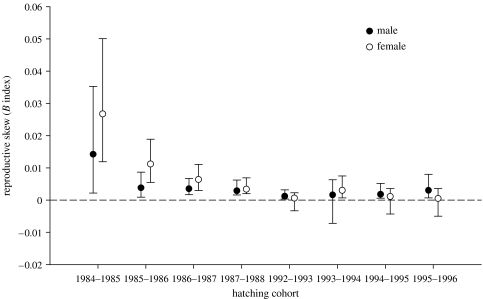
Following correction with the false discovery method (Benjamini & Hochberg 1995; Curran-Everett 2000) for multiple comparisons, the B index values indicated that male RS was more skewed than would be expected from a random process (values significantly greater than zero) in seven of eight cohorts, while female RS was more skewed in five of the eight cohorts (figure 2). Female RS tended to be less skewed in younger cohorts. No significant difference in reproductive skew between males and females was found in any cohort (figure 2). Therefore, one sex did not exhibit more reproductive skew than the other, although inspection of the distributions for males and females suggests a tendency for higher skew in females, not males (figure 2 in the electronic supplementary material).
Figure 2.
Relationship between male and female reproductive skew (B index, Nonacs 2000) within a hatching cohort. Despite a male sex ratio bias, no difference in reproductive skew was found between the sexes. Error bars represent 95% CI. The dashed line indicates the B value for randomly distributed reproduction.
(b) Breeding state transition probabilities
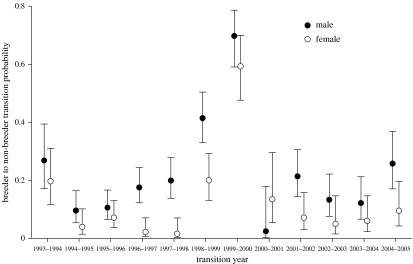
Model ranking using QAICC gave support for an influence of sex on breeding state transition probability (table 1). The likelihood of the best model that did not include a sex effect on breeding state transition probability (ψ(g*t)) indicated that it received essentially no support relative to the best model overall, which included a sex effect ((ψ(r*g*t)); table 1). The difference between the two best models (ΔQAICC) was less than 2, so these two were considered to have similar explanatory power (Burnham & Anderson 2002). Both top models included a sex effect as well as group (g) and year (t) effects on breeding state transition probability. Real parameter estimates derived from weighted averaging of these two models showed that males had higher breeder to non-breeder transition probabilities than females did in 11 out of 12 years; 95% CIs indicated significant differences in 8 of the 12 years (figure 3). Females had higher non-breeder to breeder transition probabilities than males did in 10 out of 12 years; 95% CIs indicated a significant difference in 5 of the 12 years (figure 3 in the electronic supplementary material). Transition probabilities were year dependent, but the mean non-breeder to breeder transition probability for males was 0.452 (±0.161 MSE); thus, few males should be excluded from breeding at some point during their lifetimes. The sex ratio bias of our population suggests that approximately 33% of males should be non-breeders under the persistent individual quality model, but only 8.2% (95% CI=5.6–11.8%) of males never bred based on estimates from our mark-recapture models.
Table 1.
QAICC rankings of multi-state models developed in Program Mark. (Survival probabilities (s), recapture probabilities (p) and breeding state transition probabilities (ψ) were modelled as constant (.) or were allowed to vary as a function of sex (r), group (g; breeder/non-breeder), time (t; year), or by incorporating interactions (*) of these factors.)
| model | QAICC | ΔQAICC | QAICC weight | model likelihood | parameters | Qdev |
|---|---|---|---|---|---|---|
| s(g) p(g) ψ(r*g*t) | 5277.19 | 0.00 | 0.57 | 1.00 | 52 | 2087.35 |
| s(g) p(r*g) ψ(r*g*t) | 5277.71 | 0.53 | 0.43 | 0.77 | 54 | 2083.77 |
| s(g) p(g*t) ψ(r*g*t) | 5296.56 | 19.38 | 0.00 | 0.00 | 74 | 2061.30 |
| s(r*g*t) p(r*g*t) ψ(r*g*t) | 5314.67 | 37.48 | 0.00 | 0.00 | 140 | 1940.06 |
| s(g) p(r*g*t) ψ(r*g*t) | 5327.55 | 50.37 | 0.00 | 0.00 | 98 | 2042.16 |
| s(g) p(g*t) ψ(g*t) | 5375.90 | 98.71 | 0.00 | 0.00 | 50 | 2190.16 |
| s(g) p(g) ψ(g*t) | 5835.04 | 557.85 | 0.00 | 0.00 | 28 | 2694.18 |
| s(g) p(r*g*t) ψ(r*t) | 5861.85 | 584.66 | 0.00 | 0.00 | 74 | 2626.59 |
| s(.) p(.) ψ(.) | 6238.87 | 961.68 | 0.00 | 0.00 | 3 | 3148.41 |
Figure 3.
Real parameter estimates of breeder to non-breeder transition probabilities of males and females modelled over a 13-year period. Males had significantly higher transition probabilities in most years. Probabilities increased dramatically for both sexes after the breeding failures during the strong El Niño-Southern Oscillation event of 1997–1998. Most birds did not breed during 1999–2000, but females that did breed in that season were more likely than males were to become non-breeders in the following year. Error bars denote 95% CI.
(c) Breeding bout lengths
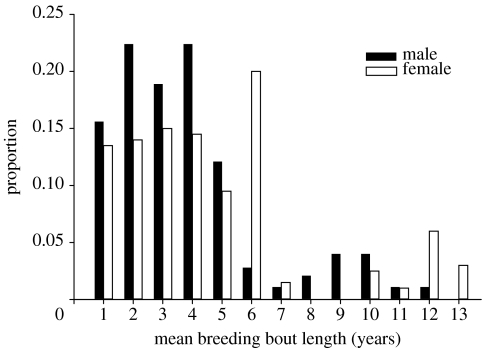
Breeding bout length was significantly shorter in males (males=3.44±0.12 years, n=282, females=4.70±0.22 years, n=201; t=−5.35, p<0.0001; figure 4). Males also had significantly more breeding bouts than females (males=1.85±0.05, females=1.65±0.05; t=2.59, p=0.01). Breeding bouts were ended for most birds by poor environmental conditions in 1999–2000 (see high breeder to non-breeder transition probabilities in figure 3), truncating the bouts of many females, but not males, at 6 years (figure 4). These females had bred continuously since the beginning of the study. As a result, our estimates of bout length and number probably understate the continuity of breeding of females more than males.
Figure 4.
The mean number of years of male and female Nazca boobies bred consecutively (bout length) shown as a proportion. Females had significantly longer breeding bout lengths than males (t=−5.35, p<0.0001).
(d) RS of divorced and retained males
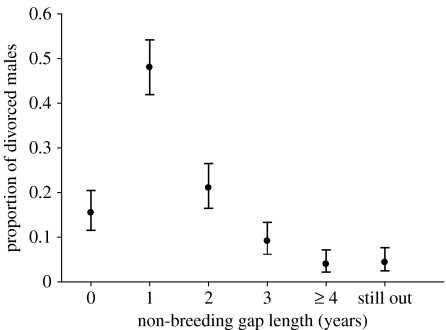
The mean RS of retained (0.416±0.097), divorced (0.412±0.060), and all uncategorized (0.403±0.092) males in the year prior to divorce/retention did not differ (F2,24=0.006, p=0.994). Most divorced males resumed breeding after 1 year of non-breeding and few males obtained a new mate in the year of divorce (figure 5).
Figure 5.
The number of divorced males, expressed as a proportion, categorized by non-breeding gap length determined from cohorts that fledged from the 1984–1985 to the 1987–1988 cohorts over a 9-year period (1992–2001). ‘0’ indicates that divorced males bred with a new female with no interruption in breeding status. ‘Still out’ indicates that the divorced male was still alive (in 2005–2006), but had yet to resume breeding at the time of this study. Most divorced males resumed breeding after a gap of 1 year. Error bars indicate 95% CI.
4. Discussion
Males did not exhibit the higher variance and skew in RS predicted by the persistent individual quality model of mate selection in our male-biased study population. Instead, these values were homogenized in males because males rotated in and out of the breeding pool, as indicated by their higher breeder to non-breeder transition probabilities (figure 3), greater than zero non-breeder to breeder transition probabilities (figure 3 in the electronic supplementary material), shorter and more numerous breeding bouts (figure 4), and short periods outside the breeding pool (figure 5). Non-breeding males had nearly a 50% chance to (re)enter the breeding pool per year, and few males were excluded from breeding completely. Since females bred more regularly than males (figures 3 and 4), some females must switch mates between successive breeding attempts to continue their longer breeding bouts, and we found that approximately 38% of Nazca booby pairs divorce each year (T. J. Maness 2007, unpublished data).
Nazca booby males could voluntarily skip breeding attempts as some procellariiform seabirds do (Bradley et al. 2000; Dobson & Jouventin 2007); however, evidence obtained from a behavioural study conducted in the 2003–2004 breeding season on this Nazca booby population (T. J. Maness 2007, unpublished data) clearly refutes this idea. Every Nazca booby male present in a subsection of the ‘Study Area’ (detailed in Townsend & Anderson (in press)) in the 2003–2004 breeding season performed mate attraction behaviours (described in Nelson (1978)). Of 111 males that bred in the same area in the previous season (2002–2003), 22 became non-breeders in 2003–2004; 13 of these non-breeders were seen displaying in 2003–2004, while the remaining nine individuals have not been seen in the four yearly censuses (described in Huyvaert & Anderson (2004)) conducted since 2002–2003. Therefore, 100% of males breeding in 2002–2003 and present in 2003–2004 attempted to obtain a mate by performing mate attraction behaviours, while 100% of 2002–2003 breeders not seen in 2003–2004 are most probably deceased.
Alternatively, costs of reproduction provide a basis for serial monogamy, imposed by females. Assuming that breeding induces ‘fecundity’ costs of reproduction (as well as the demonstrated survival costs; Townsend & Anderson in press) in male Nazca boobies, females could mate-switch adaptively, replacing a temporarily degraded male for a current non-breeder in better condition. Females, but not males, would have this option in a male-biased population. Typically, female Nazca boobies in our study population simply leave the nest site held by their current mate and join another elsewhere (T. J. Maness 2007, unpublished data). In such a mating system, few males would mate consistently, and few males would be excluded from the mating pool, even under a male-biased OSR. Our data support this interpretation. Since females breed more often than males, one might expect that they would suffer higher mortality rates, but Townsend & Anderson (in press) found similar mortality rates between the sexes. This apparent contradiction may be explained by sex differences during the non-breeding season, when females vacate the breeding colony (presumably the site of negative density-dependent effects). In contrast, most males remain, defending nest sites that they will use in future breeding seasons, so males may have less opportunity to recover condition between breeding seasons (D. J. Anderson 2007, unpublished data).
This evidence linking serial monogamy to sex ratio bias is novel and suggests selection on female mating preferences based on an interaction between at least two life-history components: OSR and reproductive effort. A within-sex, variance-based measure of sexual selection, like the opportunity for selection (Crow 1958; Wade 1979; Wade & Arnold 1980; Shuster & Wade 2003) indicates that Nazca booby males do not appear to be under stronger sexual selection than females are, despite the biased OSR. Yet, males had more trouble securing a mate each breeding season as indicated by behavioural observations as well as breeder to non-breeder transition probabilities (figure 3), and females have good reason to remain choosy. Our novel findings suggest that the use of variance-based measures of intensity of selection may only be correct when restricted to a single breeding season.
Mate rotation influenced by OSR may explain divorce in successful pairs, an area seldom addressed in divorce theory. Most birds (90% of species; Lack 1968) are socially monogamous, but far fewer species form persistent pair bonds (Black 1996). Selective factors favouring mate fidelity include obligate biparental care (Williams 1966; Lack 1968; Emlen & Oring 1977) and the ‘mate familiarity effect’ (improved joint reproductive performance from experience with each other; Black 1996). Long lifespan is also positively associated with high mate fidelity (Ens et al. 1996). From these considerations, long-lived seabirds are expected to form persistent pair bonds, and most do (Ens et al. 1996): albatrosses form some of the longest pair-bonds known in any animal taxon (Jouventin et al. 1999; Tickell 2000). Still, variation exists in divorce rate, even between different populations of the same long-lived species (Ens et al. 1996).
Many hypotheses regarding divorce fall under the ‘better option’ model, whereby individuals may leave a partnership to obtain a higher quality mate, and thus to improve their RS (reviewed in Choudhury (1995) and Ens et al. (1996)). The rotation model suggests that divorced males should be more successful than retained males because higher costs of reproduction may be incurred by successful breeders. Most hypotheses regarding divorce predict worse performance in divorced males (Choudhury 1995; Ens et al. 1996): in Nazca boobies, previous performances of divorced and retained males were similar. The finding that RS was not higher in divorced males, as expected under the rotation model, may be explained by several possibilities which are not mutually exclusive: females might follow a mixture of divorce strategies; more than 1 year of successful breeding may be needed for some males to become depleted; and successful breeding may not induce higher costs than attempted breeding, especially if the nest failed late in the breeding period (Townsend & Anderson (in press) detected this effect for survival costs of reproduction). It should be noted that most hypotheses regarding divorce do not predict equal performance between divorced and retained mates either.
If costs of reproduction are ubiquitous in iteroparous organisms, as suggested by life-history theory (Stearns 1992), then any bias in the OSR should allow the members of the limiting sex the opportunity to improve their reproductive output through mate rotation. This implies that offspring-provisioning ability, rather than persistent genetic quality (or experience), may be more important to potential mates in some instances, particularly in altricial species with prolonged parental care. If the genetic quality of potential mates varies little among individuals, then the degrading effects of reproductive effort may make switching mates more beneficial than staying with an experienced mate. van de Pol et al. (2006) found that pairs of oystercatchers (Haematopus ostralegus) that had been together for many years (more than 10 years) had lower RS than did newly formed pairs. Individuals of both sexes whose mates were experimentally removed, even ones with pair-bonds in excess of 10 years, improved their reproductive output after pairing with a new mate. The authors concluded that divorce would seem to be advantageous for either member of very old pairs (van de Pol et al. 2006), yet few pairs actually separate (Ens et al. 1993; Heg et al. 1993, 2003; van de Pol et al. 2006). We suggest that the territorial requirements of this population (Ens et al. 1992, 1995; Heg et al. 1993) create an effectively even OSR, regardless of any bias in the adult sex ratio, because few openings in the breeding pool exist. Leaving a mate will probably lead to non-breeding status (van de Pol et al. 2006). Mate changing in oystercatchers may, thus, be constrained by their even OSR, such that they cannot take advantage of re-pairing with a new or refreshed mate whose condition has not declined with recent reproductive effort. Comparisons of standardized variances in RS between the sexes and divorce rates in other monogamous species with biased sex ratios are needed to assess the generality of the novel rotation re-mating strategy found in Nazca boobies.
Acknowledgments
We thank the Galápagos National Park Service for permission to work in the Park; the Charles Darwin Research Station and TAME Airline for logistical support; the National Geographic Society, the Oak Foundation, the Mead Foundation, and Wake Forest University for research funding; our many competent assistants and colleagues for their work in producing our long-term databases; J. Awkerman and V. Apanius for their statistical guidance; and the members of the Anderson laboratory group, K. Huyvaert, and an anonymous reviewer and Peter Nonacs for their comments on an earlier draft. This material is based upon work supported by the National Science Foundation under grants no. DEB 93045679, DEB 9629539, DEB 98-06606 and DEB 0235818 to D.J.A.
Supplementary Material
The relationship between mean reproductive success and variance in reproductive success
Frequency distribution of total reproductive success for male and female Nazca boobies
Real parameter estimates of non-breeder to breeder transition probabilities of males and females modelled over a 13-year period
Values for 13 inequality measures for male and female Nazca boobies from eight different hatching cohorts
Spearman rank order correlations between the 13 indices of inequality described in supplementary table 1
References
- Anderson D.J. Masked booby (Sula dactylatra) In: Poole A, Gill F, editors. Birds of North America no. 73. The American Ornithologists' Union; Washington, DC: 1993. pp. 1–16. [Google Scholar]
- Anderson D.J, Apanius V. Actuarial and reproductive senescence in a long-lived seabird: preliminary evidence. Exp. Gerontol. 2003;38:757–760. doi: 10.1016/s0531-5565(03)00104-9. doi:10.1016/S0531-5565(03)00104-9 [DOI] [PubMed] [Google Scholar]
- Anderson D.J, Boag P.T. No extra-pair fertilization observed in Nazca Booby (Sula granti) broods. Wilson J. Ornithol. 2006;118:244–247. doi:10.1676/05-106.1 [Google Scholar]
- Andersson M.B. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Apanius, V. A., Westbrock, M. W. & Anderson, D. J. In press. Parental effort, plastic offspring growth and immunoglobulin G homeostasis in a long-lived seabird, the Nazca Booby Sula granti Ornithol. Monogr.
- Baeyens G. Functional aspects of serial monogamy—the magpie pair-bond in relation to its territorial system. Ardea. 1981;69:145–166. [Google Scholar]
- Bateman A.J. Intrasexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the flase discovery rate—a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- Black J.M. Introduction: pair bonds and partnerships. In: Black J.M, editor. The study of monogamy. The study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 3–20. [Google Scholar]
- Black J.M, Choudhury S, Owen M. Do barnacle geese benefit from lifelong monogamy? In: Black J.M, editor. Partnerships in birds. The study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 91–117. [Google Scholar]
- Bradley J.S, Wooller R.D, Skira I.J. Intermittent breeding in the short-tailed shearwater Puffinus tenuirostris. J. Anim. Ecol. 2000;69:639–650. doi:10.1046/j.1365-2656.2000.00422.x [Google Scholar]
- Burnham K.P, Anderson D.R. 2nd edn. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information-theoretical approach. [Google Scholar]
- Choudhury S. Divorce in birds: a review of the hypotheses. Anim. Behav. 1995;50:413–429. doi:10.1006/anbe.1995.0256 [Google Scholar]
- Clutton-Brock T.H. The University of Chicago Press; Chicago, IL: 1988. Reproductive success. [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 1992;67:437–456. doi:10.1086/417793 [Google Scholar]
- Cooch, E. & White, G. 2005 ProgramMark: a gentle introduction 5th edn. See http://www.phidot.org/software/mark/docs/book/
- Crow J.F. Some possibilities for measuring selection intensities in man. Hum. Biol. 1958;30:1–13. [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Dobson F.S, Jouventin P. How slow breeding can be selected in seabirds: testing Lack's hypothesis. Proc. R. Soc. B. 2007;274:275–279. doi: 10.1098/rspb.2006.3724. doi:10.1098/rspb.2006.3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois F, Wajnberg É, Cézilly F. Optimal divorce and re-mating strategies for monogamous female birds: a simulation model. Behav. Ecol. Sociobiol. 2004;56:228–236. doi:10.1007/s00265-004-0780-y [Google Scholar]
- Emlen S.T, Oring L.W. Ecology, sexual selection, and the evolution of mating. systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. doi:10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Enders M.M. The effect of male size and operational sex ratio on male mating success in the common spider mite, Tetranychus urticae Kock (Acari: Tetranychidae) Anim. Behav. 1993;46:835–846. doi:10.1006/anbe.1993.1269 [Google Scholar]
- Ens B.J, Kersten M, Brenninkmeijer A, Hulscher J.B. Territory quality, parental effort and reproductive success of oystercatchers (Haematopus ostralegus) J. Anim. Ecol. 1992;61:703–715. doi:10.2307/5625 [Google Scholar]
- Ens B.J, Safriel U.N, Harris M.P. Divorce in the long-lived and monogamous oystercatcher, Haematopus ostralegus: incompatibility or choosing the better option? Anim. Behav. 1993;45:1199–1217. doi:10.1006/anbe.1993.1142 [Google Scholar]
- Ens B.J, Weissing F.J, Drent R.H. The despotic distribution and deferred maturity—2 sides of the same coin. Am. Nat. 1995;146:625–650. doi:10.1086/285818 [Google Scholar]
- Ens B.J, Choudhury S, Black J.M. Mate fidelity and divorce in monogamous birds. In: Black J.M, editor. Partnerships in birds: the study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 344–401. [Google Scholar]
- Erickstad K.E, Fauchald P, Tveraa T, Steen H. On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology. 1998;79:1781–1788. doi:10.2307/176796 [Google Scholar]
- Fairbairn D.J, Wilby A.E. Inequality of opportunity: measuring the potential for sexual selection. Evol. Ecol. Res. 2001;3:667–686. [Google Scholar]
- Forslund P, Pärt T. Age and reproduction in birds—hypotheses and tests. Trends Ecol. Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. doi:10.1016/S0169-5347(00)89141-7 [DOI] [PubMed] [Google Scholar]
- Golet G.H, Irons D.B, Estes J.A. Survival costs of chick rearing in Black-legged Kittiwakes. J. Anim. Ecol. 1998;67:827–841. doi:10.1046/j.1365-2656.1998.00233.x [Google Scholar]
- Golet G.H, Schmutz J.K, Irons D.B, Estes J.A. Determinants of reproductive costs in the long-lived Black-legged Kittiwake: a multiyear experiment. Ecol. Monogr. 2004;74:353–372. [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds—a role for parasites. Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Heg D, Ens B.J, Burke T, Jenkins L, Kruijt J.P. Why does the typically monogamous oystercatcher (Haematopus ostralegus) engage in extra-pair copulations? Behaviour. 1993;126:247–289. [Google Scholar]
- Heg D, Bruinzeel L.W, Ens B.J. Fitness consequences of divorce in the oystercatcher, Haematopus ostralegus. Anim. Behav. 2003;66:175–184. doi:10.1006/anbe.2003.2188 [Google Scholar]
- Huyvaert K.P, Anderson D.J. Limited dispersal by Nazca boobies (Sula granti) J. Avian Biol. 2004;35:46–53. doi:10.1111/j.0908-8857.2004.03131.x [Google Scholar]
- Jacobsen K.O, Erikstad K.E, Sæther B.E. An experimental-study of the costs of reproduction in the Kittiwake Rissa tridactyla. Ecology. 1995;76:1636–1642. doi:10.2307/1938164 [Google Scholar]
- Jones A.G, Arguello J.R, S J. Molecular parentage analysis in experimental newt populations: the response of mating system measures to variation in the operational sex ratio. Am. Nat. 2004;164:444–456. doi: 10.1086/423826. doi:10.1086/423826 [DOI] [PubMed] [Google Scholar]
- Jones A.G, Rosenqvist G, Berglund A, Avise J.C. The measurement of sexual selection using Bateman's principles: an experimental test in the sex-role-reversed pipefish Syngnathus typhle. Integr. Comp. Biol. 2005;45:874–884. doi: 10.1093/icb/45.5.874. doi:10.1093/icb/45.5.874 [DOI] [PubMed] [Google Scholar]
- Jouventin P, Lequette B, Dobson F.S. Age-related mate choice in the wandering albatross. Anim. Behav. 1999;57:1099–1106. doi: 10.1006/anbe.1999.1083. doi:10.1006/anbe.1999.1083 [DOI] [PubMed] [Google Scholar]
- Kalmbach E, Nager R, Griffiths R, Furness R. Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. Proc. R. Soc. B. 2004;268:2175–2179. doi: 10.1098/rspb.2001.1793. doi:10.1098/rspb.2001.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Monoghan P. Predicting the direction of sexual selection. Ecol. Lett. 2001;4:159–165. doi10.1046/j.1461-0248.2001.00212.x [Google Scholar]
- Kokko H, Mackenzie A, Reynolds J.D, Lindström J, Sutherland W.J. Measures of inequality are not equal. Am. Nat. 1999;72:358–382. doi: 10.1086/303235. doi:10.1086/303235 [DOI] [PubMed] [Google Scholar]
- Kvarnemo C, Ahnesjö I. The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol. 1996;11:404–408. doi: 10.1016/0169-5347(96)10056-2. doi:10.1016/0169-5347(96)10056-2 [DOI] [PubMed] [Google Scholar]
- Kvarnemo C, Ahnesjö I. Operational sex ratios and mating competition. In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 366–382. [Google Scholar]
- Lack D. Chapman and Hall; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Maness T.J, Westbrock M.A, Anderson D.J. Ontogenic sex ratio variation in Nazca Boobies ends in male-biased adult sex ratio. Waterbirds. 2007;30:10–16. doi:10.1675/1524-4695(2007)030[0010:OSRVIN]2.0.CO;2 [Google Scholar]
- McNamara J.M, Forslund P. Divorce rates in birds: predictions from an optimization model. Am. Nat. 1996;147:609–640. doi:10.1086/285869 [Google Scholar]
- Mills S.C, Grapputo A, Koskela E, Mappes T. Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc. R. Soc. B. 2007;274:143–150. doi: 10.1098/rspb.2006.3639. doi:10.1098/rspb.2006.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.B. Oxford University Press; Oxford, UK: 1978. The Sulidae: gannets and boobies. [Google Scholar]
- Nonacs P. Measuring and using skew in the study of social behavior and evolution. Am. Nat. 2000;156:577–589. doi: 10.1086/316995. doi:10.1086/316995 [DOI] [PubMed] [Google Scholar]
- Nonacs, P. 2003aSkew Calculator 2003, v1.2. See http://www.eeb.ucla.edu/Faculty/Nonacs/SKEW%20CALCULATOR%202003.htm
- Nonacs P. Measuring the reliability of skew indices: is there one best index? Anim. Behav. 2003b;65:615–627. doi:10.1006/anbe.2003.2096 [Google Scholar]
- Pyle P, Nur N, Sydeman W.J, Emslie S.D. Cost of reproduction and the evolution of deferred breeding in the western gull. Behav. Ecol. 1997;8:140–147. doi:10.1093/beheco/8.2.140 [Google Scholar]
- Pyle P, Sydeman W.J, Hester M. Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin's auklets. J. Anim. Ecol. 2001;70:1088–1097. doi:10.1046/j.0021-8790.2001.00567.x [Google Scholar]
- Reid W.V. The cost of reproduction in the glaucous-winged gull. Oecologia. 1987;74:458–467. doi: 10.1007/BF00378945. doi:10.1007/BF00378945 [DOI] [PubMed] [Google Scholar]
- Sæther B.E, Andersen R, Pedersen H.C. Regulation of parental effort in a long-lived seabird—an experimental manipulation of the cost of reproduction in the Antarctic Petrel, Thalassoica Antarctica. Behav. Ecol. Sociobiol. 1993;33:147–150. doi:10.1007/BF00216594 [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Stearns S.C. Oxford University Press; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Tickell W.L.N. Yale University Press; New Haven, CT: 2000. Albatrosses. [Google Scholar]
- Townsend, H. M. & Anderson, D. J. In press. Long-term assessment of costs of reproduction in Nazca boobies (Sula granti) using multi-state mark-recapture models. Evolution
- van de Pol M, Heg D, Bruinzeel L.W, Kuijper B, Verhulst S. Experimental evidence for a causal effect of pair-bond duration on reproductive performance in oystercatchers (Haematopus ostralegus) Behav. Ecol. 2006;17:982–991. doi:10.1093/beheco/arl036 [Google Scholar]
- Wade M.J. Sexual selection and variance in reproductive success. Am. Nat. 1979;114:742–747. doi: 10.1086/424531. doi:10.1086/283520 [DOI] [PubMed] [Google Scholar]
- Wade M.J, Arnold S.J. The intensity of sexual selection in relation to male sexual behavior, female choice, and sperm precedence. Anim. Behav. 1980;28:446–461. doi:10.1016/S0003-3472(80)80052-2 [Google Scholar]
- Weimerskirch H, Chastel O, Ackermann L. Adjustment of parental effort to manipulated foraging ability in a pelagic seabird, the thin-billed Prion Pachyptila belcheri. Behav. Ecol. Sociobiol. 1995;36:11–16. doi:10.1007/s002650050119 [Google Scholar]
- White G.C, Burnham K.P. Program Mark: survival estimation from populations of marked animals. Bird Study. 1999;46(Suppl.):120–139. [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection. [Google Scholar]
- Wooller R.D, Bradley J.S, Croxall J.P. Long-term population studies of seabirds. Trends Ecol. Evol. 1992;7:111–114. doi: 10.1016/0169-5347(92)90143-Y. doi:10.1016/0169-5347(92)90143-Y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The relationship between mean reproductive success and variance in reproductive success
Frequency distribution of total reproductive success for male and female Nazca boobies
Real parameter estimates of non-breeder to breeder transition probabilities of males and females modelled over a 13-year period
Values for 13 inequality measures for male and female Nazca boobies from eight different hatching cohorts
Spearman rank order correlations between the 13 indices of inequality described in supplementary table 1