Abstract
The origin of specific insect genotypes that enable efficient use of agricultural plants is an important subject not only in applied fields like pest control and management but also in basic disciplines like evolutionary biology. Conventionally, it has been presupposed that such pest-related ecological traits are attributed to genes encoded in the insect genomes. Here, however, we report that pest status of an insect is principally determined by symbiont genotype rather than by insect genotype. A pest stinkbug species, Megacopta punctatissima, performed well on crop legumes, while a closely related non-pest species, Megacopta cribraria, suffered low egg hatch rate on the plants. When their obligate gut symbiotic bacteria were experimentally exchanged between the species, their performance on the crop legumes was, strikingly, completely reversed: the pest species suffered low egg hatch rate, whereas the non-pest species restored normal egg hatch rate and showed good performance. The low egg hatch rates were attributed to nymphal mortality before or upon hatching, which were associated with the symbiont from the non-pest stinkbug irrespective of the host insect species. Our finding sheds new light on the evolutionary origin of insect pests, potentially leading to novel approaches to pest control and management.
Keywords: Megacopta punctatissima, Megacopta cribraria, Candidatus Ishikawaella capsulata, symbiont capsule, plant adaptation, pest evolution
1. Introduction
In many herbivorous insects, different populations of the same species often use different food plants, which are referred to as host races, biotypes or ecotypes. Formation of such intraspecific plant specialization must have evolved through acquisition of a new food plant by a local population of the insect. In the case that the new food plant is an agricultural plant, the insect population will be recognized as an emergent pest. Hence, the origin of specific insect genotypes that enable efficient use of agricultural plants is an important subject not only in applied fields like pest control and management but also in basic disciplines like evolutionary biology (Via 1990; Berlocher & Feder 2002; Karban & Agrawal 2002; Coyne & Orr 2004; Shoonhoven et al. 2005). Conventionally, it has been presupposed that such ecological traits are attributed to genes encoded in the insect genomes (Feder et al. 1988; Hawthorne & Via 2001).
However, recent studies have revealed that facultative bacterial symbionts may substantially affect various ecological traits of herbivorous insects. For example, several species of facultative symbionts play important biological roles for the pea aphid Acyrthosiphon pisum in specific ecological contexts, including tolerance to high temperature (Montllor et al.2002; Russell & Moran 2006), resistance to parasitoid wasps (Oliver et al. 2003, 2005), resistance to pathogenic fungi (Scarborough et al. 2005), broadening of food plant range (Tsuchida et al. 2004), and others. Here, an idea arises that the symbiont could be involved in emergence of insect pest. However, although many pest insects of agricultural, economical and medical importance are known to be associated with bacterial symbionts (Bourtzis & Miller 2003, 2006), there have been no studies demonstrating that the symbiont is responsible for pest status of its host insect.
Many stinkbugs (Insecta: Heteroptera) are, by sucking plant sap and tissues, known as notorious agricultural pests (Schaefer & Panizzi 2000). These plant-feeding stinkbugs are generally in close association with gut symbiotic bacteria: they have a specialized midgut section bearing a number of caecal evaginations, in which a copious amount of symbiont is harboured (Buchner 1965). When experimentally deprived of the symbiont, host stinkbugs suffer retarded growth and high mortality (Buchner 1965; Abe et al. 1995; Fukatsu & Hosokawa 2002; Hosokawa et al. 2006). Probably the host stinkbugs are provided by the symbionts with essential nutrients that are lacking in their diet, as aphids are nutritionally dependent on the endocellular symbiont Buchnera aphidicola (Douglas 1989; Baumann et al. 2000).
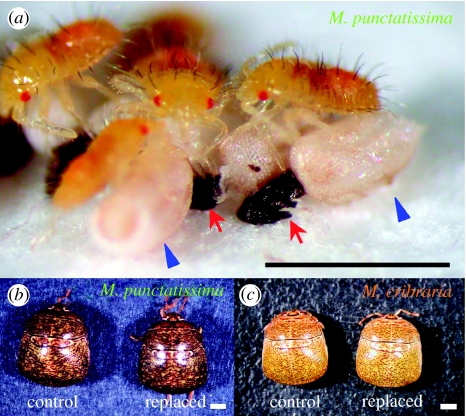
Stinkbugs of the family Plataspidae harbour an obligate γ-proteobacterial symbiont ‘Candidatus Ishikawaella capsulata’ in the cavity of crypt-bearing posterior midgut (Hosokawa et al. 2006). The plataspid stinkbugs have been known for their unique mechanism for vertical transmission called ‘symbiont capsule’. When adult females lay eggs on their host plant, small brownish particles are always deposited under the egg mass. The particles encase a copious amount of the symbiont inside, and hatchlings from the eggs orally acquire the symbiont from the capsule (Schneider 1940; Müller 1956; Fukatsu & Hosokawa 2002; Hosokawa et al. 2005, 2006; figure 1a; electronic supplementary material, movie 1). When deprived of the symbiont capsule, the symbiont-free insects suffer abnormal coloration, retarded growth, smaller body size, higher mortality and sterility (Müller 1956; Fukatsu & Hosokawa 2002; Hosokawa et al. 2006). Hence, despite the extracellular location in the gut cavity, the symbionts are regarded as obligate mutualistic associates for the host stinkbugs. The symbiont phylogeny shows a perfect agreement with the host phylogeny, indicating strict host–symbiont cospeciation over evolutionary time (Hosokawa et al. 2006). A number of plataspid species have been reported as pests of various legumes and other agricultural plants (Tomokuni 1993; Schaefer & Panizzi 2000).
Figure 1.
Pest and non-pest plataspid stinkbugs. (a) Newborn nymphs of Megacopta punctatissima probing capsules for symbiont acquisition. Arrows and arrowheads indicate symbiont capsules and eggshells, respectively. (b) Pest species M. punctatissima. (c) Non-pest species Megacopta cribraria. Normal adult females with their original symbiont (control) and manipulated adult females whose symbiont was experimentally replaced by the heterospecific one (replaced) are shown. Scale bars, 1 mm.
In this study, by making use of the unique transmission system mediated by symbiont capsules, we experimentally demonstrated that pest status of a plataspid stinkbug is determined by the symbiont genotype rather than by the insect genotype.
2. Material and methods
(a) Insect sampling and rearing
Freshly laid egg masses of Megacopta punctatissima were collected from Pueraria lobata at Tsukuba, Ibaraki, Japan. Egg masses of Megacopta cribraria were obtained from sexually mature females that had been collected from Pueraria montana at Naha, Okinawa, Japan. Each of the field-collected females of M. cribraria was allowed to oviposit in a Petri dish containing several pea pods (Pisum sativum). To avoid pseudoreplication, all of the egg masses of M. cribraria used for experiments were derived from different females. These insects were maintained in climatic chambers at 25°C in a long-day regimen (16 h light–8 h dark).
(b) Experimental manipulation
Only newly laid egg masses containing 24 or more eggs and 7 or more capsules were used for experiments. All of these egg masses exhibited normal egg hatch rates ranging from 80 to 100%. To minimize confounding effects of the insect genetic backgrounds, two experimental groups were produced from each of the egg masses for control treatment and symbiont-replacing treatment, respectively. Each of the egg masses was separated into individual eggs and capsules by using fine forceps under a binocular microscope. To sterilize remnant symbiont cells possibly adhering to the egg surface, the eggs were treated with 70% ethanol for 5 min, 4% formalin for 30 min and 70% ethanol for 10 s, and were dried in air. For control treatment, randomly chosen 12 eggs and 7 capsules from an egg mass were realigned like a natural egg mass and were glued on a piece of filter paper. For symbiont-replacing treatment, the other 12 eggs from the egg mass were realigned with 7 heterospecific capsules in the same way. These experimental egg masses were individually kept in a Petri dish with a wet cotton ball.
(c) Fitness measurement
For each of the stinkbug species, nymphs from 18 sets (families) of a pair of experimental egg masses were subjected to fitness measurements. After a day of egg hatch, 10 nymphs were randomly chosen from each of the egg masses and reared on a potted soya bean plant (Glycine max). These insects were examined for growth rate (nymphal period), adult emergence rate (%) and adult body size (thorax width).
Adult insects that emerged from the 17 out of 18 sets for M. punctatissima and those from the 16 out of 18 sets for M. cribraria were examined for their reproductive performance. Each of several (1–5) pairs of a male and a female from the same egg masses was separately kept in a Petri dish and provided with pea pods. The dishes were inspected everyday and the pea pods were periodically replaced with fresh ones. All egg masses laid by each of the females were collected during 30 days after the first oviposition. Each of the egg masses was kept in a Petri dish and inspected, and the eggs were categorized into hatched eggs, fertilized unhatched eggs and unfertilized eggs. Nymphal body segments and/or red eyespots were seen in fertilized eggs but not in unfertilized eggs. Nymphs that died during hatching were counted as fertilized unhatched eggs. For each of the females, total number of eggs, egg fertilization rate (%) and egg hatch rate (%) were calculated. Females that died without producing eggs were excluded from the analyses. Proportions of such females were statistically not different between the control and symbiont-replaced treatments (5 out of 62 control females versus 5 out of 58 symbiont-replaced females in M. punctatissima, Fisher's exact probability test, p=1; 4 out of 45 control females versus 6 out of 43 symbiont-replaced females in M. cribraria, Fisher's exact probability test, p=0.517).
(d) Cloning and sequencing
DNA was extracted from isolated symbiont capsules by using a QIAamp tissue mini kit (QIAGEN), from which a 1.7 kbp segment of eubacterial groE gene was amplified by polymerase chain reaction (PCR) with primers Gro-F1 (5′-ATG GCA GCW AAA GAC GTA AAT TYG G-3′) and Gro-R1 (5′-TTA CAT CAT KCC GCC CAT GC-3′). The PCR product was cloned and sequenced as described previously (Kikuchi & Fukatsu 2003). The groE sequences of the symbiont from M. punctatissima and M. cribraria were deposited in the DNA Data Bank of Japan database with accession numbers AB231904 and AB264337, respectively.
(e) Symbiont quantification
We constructed seven and six sets of control and symbiont-replaced egg masses for M. punctatissima and M. cribraria, respectively. After a day of egg hatch, 10 nymphs from each of the experimental egg masses were individually subjected to DNA extraction and symbiont quantification. The symbiont titres were measured in terms of bacterial groE gene copies by using a quantitative PCR technique as described previously (Koga et al. 2003). Since the symbiont groE gene sequences were completely identical between M. punctatissima and M. cribraria (accession nos. AB231904 and AB264337, respectively), the same primers MEGAgro-F1 (5′-GGT GCT GCC ACT GAA GTT GA-3′) and MEGAgro-R1 (5′-CCG CTA CAC GCA CTA ACG C-3′) and the same probe MEGAgro-P1 (5′-TGA AGA AGG TGT CGT TCC CGG AGG-3′) were used.
(f) Statistics
To statistically evaluate the differences between the control and the symbiont-replacing treatment, the Wilcoxon signed-rank test was adopted for adult emergence rate, and a generalized linear model (GLM) framework was applied to the other fitness parameters (McCullagh & Nelder 1989). In the GLM analyses, for egg fertilization rates and egg hatch rates, we used binominal error or, if overdispersion was detected, quasi-binominal error (Crawley 1993, 2005), whereas for the other data, an appropriate error distribution was selected from normal, Poisson, gamma, inverse normal and negative binomial errors according to the Akaike information criterion. Two terms, namely symbiont types (control and replaced) and family, were included in the models, and the effects of the terms were evaluated by an analysis of deviance (Crawley 1993). All the statistical analyses were conducted by using a software R v. 2.3.1 (R Development Core Team 2006).
3. Results and discussion
(a) Closely related pest and non-pest plataspid stinkbugs
The plataspid stinkbug M. punctatissima (figure 1b) is commonly found in the mainland Japan, while a closely related stinkbug M. cribraria (figure 1c) is distributed across the southwestern islands of Japan. They are classified into different species morphologically: e.g. M. cribraria is smaller in size and paler in colour than M. punctatissima (figure 1b,c). However, they are no doubt close genetically and biologically: their mitochondrial 16S rRNA genes showed 99.3% (1565/1576 nucleotide sites) sequence identity (Hosokawa et al. 2006); reciprocal crosses between the species resulted in F1 offspring with intermediate morphological traits; and crosses between the F1 insects could produce F2 offspring (T. Hosokawa 2005, unpublished data). The main host plants of M. punctatissima and M. cribraria are wild leguminous vines P. lobata and P. montana, respectively, while these insects also use other leguminous plants (Tomokuni 1993). In particular, M. punctatissima has been known as pest of soya bean, pea and other legumes. The insects often gregariously infest the plants and damage the crops, and without spraying, lay eggs and proliferate in the legume fields (Kono 1990; Tomokuni 1993; Endo et al. 2002). On the other hand, M. cribraria scarcely causes such serious problems in Japan, although infestation on soya bean and other legumes has been occasionally reported (Tomokuni 1993). What is the basis of the difference between the pest and non-pest insects?
(b) Fitness parameters of pest and non-pest stinkbugs on crop legumes
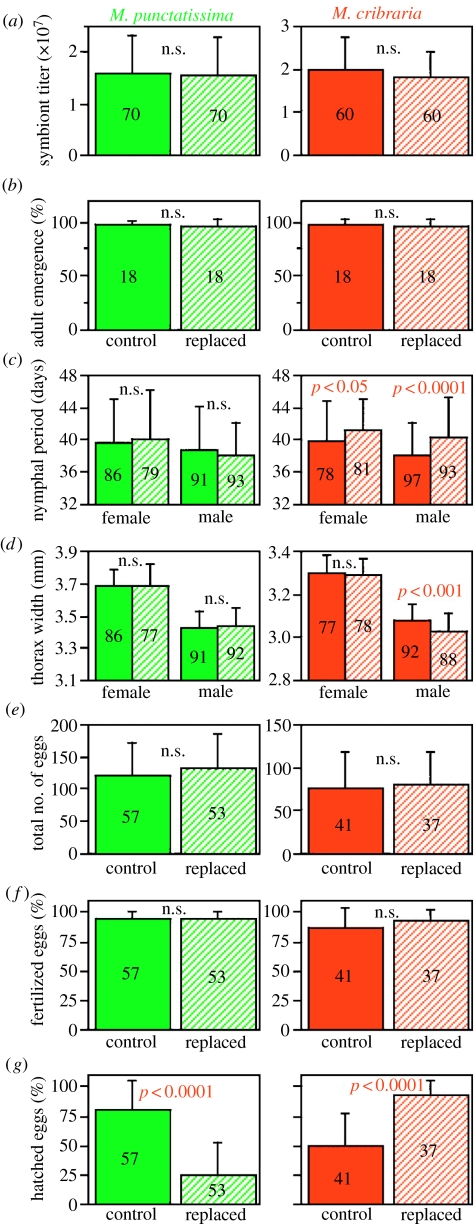
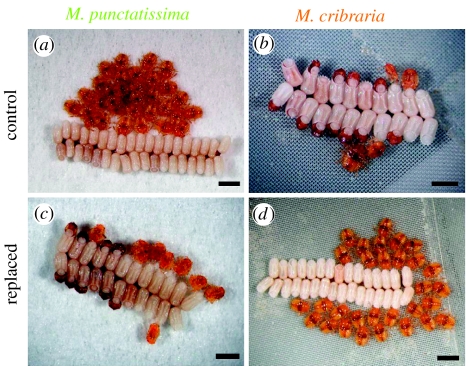
In order to address the question, we evaluated the general performance of the pest species M. punctatissima and the non-pest species M. cribraria on potted soya bean plants and pea pods. Both species normally grew to adult and laid eggs (figure 2b–e). The eggs were certainly fertilized (figure 2f). However, egg hatch rates were strikingly different between the species; around 80% in M. punctatissima in contrast to only 50% in M. cribraria (figure 2g). The difference was statistically significant (median test, p<0.0001). A characteristic mortality symptom was observed in the egg masses of M. cribraria, wherein many nymphs failed to escape from the eggshell and died (figure 3b). These dead nymphs were generally frail in size and morphology, probably due to developmental abnormality (data not shown).
Figure 2.
Fitness measurements of normal and symbiont-replaced plataspid stinkbugs. (a) Symbiont titre acquired by newborn nymphs, in terms of symbiont groE gene copies per insect. (b) Adult emergence rate (%). (c) Growth rate, in terms of nymphal period (days). (d) Adult body size, in terms of thorax width (mm). (e) Total number of eggs produced by an adult female. (f) Fertilization rate of eggs (%). (g) Hatch rate of eggs (%). Means and standard deviations are shown. Sample sizes are indicated on columns. Statistically significant differences between control treatment and symbiont-replacing treatment are shown in red.
Figure 3.
Mortality symptom observed with hatchlings of plataspid stinkbugs reared on the crop legumes. (a,c) Megacopta punctatissima, (b,d) M. cribraria, (a,b) egg masses laid by normal females and (c,d) egg masses laid by symbiont-replaced females. Scale bars, 1 mm.
(c) Low egg hatch rate of non-pest stinkbug on crop legumes
These results indicated that the non-pest species M. cribraria suffers low egg hatch rate on the crop legumes while the pest species M. punctatissima does not, which is probably relevant to their different pest status. The crop legumes, although used under the laboratory condition, are unsuitable host plants for M. cribraria.
(d) Experimental symbiont exchange between pest and non-pest stinkbugs
In most of the obligate endosymbiotic systems in insects, such as those in aphids and tsetse flies, the host and the symbiont are structurally, functionally and developmentally integrated into an almost inseparable biological entity (Buchner 1965; Douglas 1989; Baumann et al. 2000; Braendle et al. 2003). Thus, it has been practically impossible to manipulate these obligate host–symbiont associations experimentally. However, the unique capsule-mediated transmission system in the plataspid stinkbugs enabled us to attempt such experiments despite the obligate nature of the symbiosis. The host eggs and the symbiont capsules were separated by using forceps under a binocular microscope, and the eggs of M. punctatissima were combined with the capsules of M. cribraria, and vice versa. The hatchlings readily accepted the heterospecific capsules and ingested the content. Quantitative PCR assays confirmed that the nymphs certainly acquired the heterospecific symbiont cells, and the acquired amount was equivalent to that of the conspecific symbiont cells (figure 2a).
(e) Fitness parameters of pest and non-pest stinkbugs on crop legumes after symbiont exchange
The symbiont-replaced insects normally grew to adult and laid eggs (figure 2b–e). Although symbiont-eliminated adults of these stinkbugs were reported to severely suffer abnormal coloration and reduced body size (Fukatsu & Hosokawa 2002; Hosokawa et al. 2006), the symbiont-replaced adults were almost indistinguishable from the control adults (figure 1b,c), except that growth rate and body size of M. cribraria were slightly but significantly reduced in association with the symbiont replacement (figure 2c,d). In both the species, the eggs laid by the symbiont-replaced adults were certainly fertilized (figure 2f), but egg hatch rates were strikingly different between the species; around 90% in M. cribraria in contrast to only 25% in M. punctatissima (figure 2g). The difference was statistically significant (median test, p<0.0001). In the egg masses deposited by the symbiont-replaced females of M. punctatissima, many nymphs failed to escape from the eggshell and died (figure 3c), which was reminiscent of the symptom observed with the egg masses deposited by the normal females of M. cribraria (figure 3b).
(f) Pest status of Megacopta punctatissima determined by symbiont genotype
In summary, the fitness measurements on the crop legumes in combination with the symbiont-replacing experiments demonstrated that (i) M. punctatissima that originally exhibited a normal egg hatch rate suffered a low egg hatch rate when infected with the symbiont from M. cribraria, (ii) M. cribraria that originally exhibited a low egg hatch rate restored a normal egg hatch rate when infected with the symbiont from M. punctatissima, (iii) the mortality symptom in hatchlings was similar irrespective of the stinkbug species and was associated with the symbiont from M. cribraria, and (iv) hence, the normal egg hatch rate and the good performance of the stinkbugs on the crop legumes were attributed to the symbiont from M. punctatissima. These results strongly suggest that the pest status of M. punctatissima is principally determined by the symbiont genotype rather than by the insect genotype.
(g) Evolutionary origin of pest-related symbiont genotype?
The mechanism whereby the symbiont from M. punctatissima can support the normal development of the host insects on the crop legumes is unknown. In plataspid stinkbugs, the host insect phylogeny perfectly agreed with the symbiont phylogeny, indicating stable host–symbiont association over evolutionary time (Hosokawa et al. 2006). Among the plataspid stinkbugs phylogenetically analysed, M. punctatissima and M. cribraria are the closest genetically (Hosokawa et al. 2006). The symbionts from M. punctatissima and M. cribraria are also very close genetically as their host insects are: their 16S rRNA genes showed 99.9% (1308/1309 nucleotide sites) sequence identity, and their genome size was estimated to be 0.82 Mbp in common (Hosokawa et al. 2006). It appears probable, although speculative, that mutations occurring in the symbiont genomes after the host speciation have modulated their capability of using different host plants, which predisposed the host insects to potentially become pests or not.
(h) What underlies symbiont-mediated fitness effect on host insect: plant-specific difference or general difference in vigour?
For herbivorous insects, being pests basically requires the ability to use an alternative suboptimal host plant (Karban & Agrawal 2002; Shoonhoven et al. 2005). For M. punctatissima and M. cribraria, wild leguminous vines Pueraria spp. are the main optimal host plants. Both species perform well on their original host plant, attaining over 90% egg hatch rates under natural conditions (T. Hosokawa 2005, unpublished data). Meanwhile, soya bean and other crop legumes are regarded as auxiliary suboptimal host plants for these insects. We found that normal M. cribraria and symbiont-replaced M. punctatissima suffer low egg hatch rates on the crop legumes (figures 2g and 3), indicating that the symbiont from M. cribraria is responsible for the poor performance of the insects on suboptimal host plants. In other words, the symbiont from M. punctatissima was superior to the symbiont from M. cribraria in supporting normal development of the host insects on the suboptimal host plants. There are two mechanisms as to how these symbionts differ in their effects on the host fitness. One mechanism is a plant-specific difference, wherein the symbiont from M. punctatissima physiologically performs better for the host insects than the symbiont from M. cribraria specifically on the suboptimal host plants. The other mechanism is a general difference in vigour, wherein the symbiont from M. punctatissima generally shows better performance for the host insects than the symbiont from M. cribraria, and the difference becomes obvious on the suboptimal host plants but not on the optimal host plants. If the former scenario is true, we should consider the possibility that the biological trait of the symbiont from M. punctatissima might have been selected for in the mainland Japan where the crop legumes are widely cultivated. On the other hand, if the latter scenario is true, the possibility becomes less likely, and the difference might be attributed to more general aspects of the symbiont genomes. To verify which of these mechanisms better accounts for the phenomena, further experimental studies, in particular fitness measurements of the symbiont-manipulated insects on the optimal host plants, are needed.
(i) Plant specialization of herbivorous insect mediated by obligate symbiont
Recent studies have revealed that some facultative microbial symbionts substantially affect various ecological traits of herbivorous insects including plant specialization (Montllor et al. 2002; Oliver et al. 2003, 2005; Tsuchida et al. 2004; Scarborough et al. 2005; Leonardo & Mondor 2006; Russell & Moran 2006). Our discovery indicates that even obligate symbionts that play essential biological roles for their host may also affect plant specialization, and suggests that such symbionts could potentially be causal agents of emergent insect pests. It is currently unknown how prevalent similar cases of symbiont-mediated plant specialization are in natural and agricultural ecosystems. In this context, it is of both evolutionary and practical importance to survey the correlation between symbiont genotypes and host races/biotypes/ecotypes in various insect–microbe symbiotic systems.
(j) Perspective for pest control and management
A number of agriculturally, economically and medically notorious insect pests harbour symbiotic micro-organisms (Bourtzis & Miller 2003, 2006). In some of these cases, the symbionts have been suggested as possible agents for controlling the pests by using paratransgenic approach (Durvasula et al. 1997; Ben Beard et al. 2002), symbiont-driven population replacement (Dobson 2003; Sinkins & Gould 2006) and incompatible insect technique (Zabalou et al. 2004). Strikingly, it was reported that the most widely applied biological insecticide, Bacillus thuringiensis, is effective to lepidopteran larvae only when the insects harbour a gut microbial community (Broderick et al. 2006), illuminating profound relevance of insect gut bacteria to pest control. The gut symbiotic bacteria of the plataspid stinkbugs provide a model system for understanding the mechanisms underlying the symbiont-mediated pest evolution, which would potentially lead to novel means of pest control and management. Functional and genomic analyses of the stinkbug symbiont would lead to further insights into how the symbionts affect such ecological traits of the host insects.
Acknowledgments
We thank S. Ohno and D. Haraguchi for collecting samples, and E. Kasuya for statistics. This study was financially supported by the National Institute of Advanced Industrial Science and Technology (AIST) and the 21st COE Program ‘Research Centre for Integrated Science’ (E-5) at the University of Tokyo. T.H. and Y.K. were supported by the Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists.
Supplementary Material
Movie 1. Two newborn nymphs of Megacopta punctatissima are sucking symbiotic bacteria from brownish symbiont capsules. A newly-emerged nymph is coming up from the behind, pushing one of the nymphs to make its own space, probing the capsule with its proboscis, and starting ingestion of the symbiont
References
- Abe Y, Mishiro K, Takanashi M. Symbiont of brown-winged green bug, Plautia stali Scott. Jpn J. Appl. Entomol. Zool. 1995;39:109–115. [Google Scholar]
- Baumann P, Moran N.A, Baumann L. Bacteriocyte-associated endosymbionts of insects. In: Dworkin M, editor. The prokaryotes. Springer; New York, NY: 2000. pp. 1–55. [Google Scholar]
- Ben Beard C, Cordon-Rosales C, Durvasula R.V. Bacterial symbionts of the triatominae and their potential use in control of chagas disease transmission. Annu. Rev. Entomol. 2002;47:123–141. doi: 10.1146/annurev.ento.47.091201.145144. doi:10.1146/annurev.ento.47.091201.145144 [DOI] [PubMed] [Google Scholar]
- Berlocher S.H, Feder J.L. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu. Rev. Entomol. 2002;47:773–815. doi: 10.1146/annurev.ento.47.091201.145312. doi:10.1146/annurev.ento.47.091201.145312 [DOI] [PubMed] [Google Scholar]
- Bourtzis K, Miller T.A. CRC Press; Boca Raton, FL: 2003. Insect symbiosis. [Google Scholar]
- Bourtzis K, Miller T.A. CRC Press; Boca Raton, FL: 2006. Insect symbiosis II. [Google Scholar]
- Braendle C, Miura T, Bickel R, Shingleton A.W, Kambhampati S, Stern D.L. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 2003;1:e21. doi: 10.1371/journal.pbio.0000021. doi:10.1371/journal.pbio.0000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick N.A, Raffa K.F, Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl Acad. Sci. USA. 2006;103:15 196–15 199. doi: 10.1073/pnas.0604865103. doi:10.1073/pnas.0604865103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P. Interscience; New York, NY: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer Associates; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Crawley M.J. Blackwell Scientific; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2005. Statistics. An introduction using R. [Google Scholar]
- Dobson S.L. Reversing Wolbachia-based population replacement. Trends Parasitol. 2003;19:128–133. doi: 10.1016/s1471-4922(03)00002-3. doi:10.1016/S1471-4922(03)00002-3 [DOI] [PubMed] [Google Scholar]
- Douglas A.E. Mycetocyte symbiosis in insects. Biol. Rev. 1989;64:409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Durvasula R.V, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield R.B, Richards F.F, Beard C.B. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc. Natl Acad. Sci. USA. 1997;94:3274–3278. doi: 10.1073/pnas.94.7.3274. doi:10.1073/pnas.94.7.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo N, Wada T, Mizutani N, Takahashi M. Possible resistance and tolerance of a soybean breeding line, Kyukei 279 to the common cut worm, Spodoptera litura and soybean stink bugs. Kyushu Plant Prot. Res. 2002;48:68–71. [Google Scholar]
- Feder J.L, Chilcote C.A, Bush G.L. Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature. 1988;336:61–64. doi:10.1038/336061a0 [Google Scholar]
- Fukatsu T, Hosokawa T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug Megacopta punctatissima. Appl. Environ. Microbiol. 2002;68:389–396. doi: 10.1128/AEM.68.1.389-396.2002. doi:10.1128/AEM.68.1.389-396.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D.J, Via S. Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature. 2001;412:904–907. doi: 10.1038/35091062. doi:10.1038/35091062 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Meng X.Y, Fukatsu T. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol. Ecol. 2005;54:471–477. doi: 10.1016/j.femsec.2005.06.002. doi:10.1016/j.femsec.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. Strict host–symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:e377. doi: 10.1371/journal.pbio.0040337. doi:10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Agrawal A.A. Herbivore offences. Annu. Rev. Ecol. Syst. 2002;33:641–664. doi:10.1146/annurev.ecolsys.33.010802.150443 [Google Scholar]
- Kikuchi Y, Fukatsu T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl. Environ. Microbiol. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. doi:10.1128/AEM.69.10.6082-6090.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. B. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. doi:10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono S. Spatial distribution of three species of stink bugs attacking soybean seeds. Jpn J. Appl. Entomol. Zool. 1990;34:89–96. [Google Scholar]
- Leonardo T.E, Mondor E.B. Symbiont modifies host life-history traits that affect gene flow. Proc. R. Soc. B. 2006;273:1079–1084. doi: 10.1098/rspb.2005.3408. doi:10.1098/rspb.2005.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder J.A. 2nd edn. Chapman & Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- Montllor C.B, Maxmen A, Purcell A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 2002;27:189–195. doi:10.1046/j.1365-2311.2002.00393.x [Google Scholar]
- Müller H.J. Experimentelle studien an der symbiose von Coptosoma scutellatum Geoffr. (Hem. Heteropt.) Z. Morphol. Ökol. Tiere. 1956;44:459–482. [Google Scholar]
- Oliver K.M, Russell J.A, Moran N.A, Hunter M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. doi:10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K.M, Moran N.A, Hunter M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl Acad. Sci. USA. 2005;102:12 795–12 800. doi: 10.1073/pnas.0506131102. doi:10.1073/pnas.0506131102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for statistical computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. See http://www.R-project.org. [Google Scholar]
- Russell J.A, Moran N.A. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. B. 2006;273:603–610. doi: 10.1098/rspb.2005.3348. doi:10.1098/rspb.2005.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough C.L, Ferrari J, Godfray H.C. Aphid protected from pathogen by endosymbiont. Science. 2005;310:1781. doi: 10.1126/science.1120180. doi:10.1126/science.1120180 [DOI] [PubMed] [Google Scholar]
- Schaefer C.W, Panizzi A.R. CRC Press; Boca Raton, FL: 2000. Heteroptera of economic importance. [Google Scholar]
- Schneider G. Beiträge zur Kenntnis der symbiontischen Einrichtungen der Heteropteren. Z. Morphol. Ökol. Tiere. 1940;36:565–644. doi:10.1007/BF01261001 [Google Scholar]
- Shoonhoven L.M, van Loon J.J.A, Dicke M. Oxford University Press; Oxford, UK: 2005. Insect–plant biology. [Google Scholar]
- Sinkins S.P, Gould F. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 2006;7:427–435. doi: 10.1038/nrg1870. doi:10.1038/nrg1870 [DOI] [PubMed] [Google Scholar]
- Tomokuni M. Zenkoku Noson Kyoiku Kyokai; Tokyo, Japan: 1993. A field guide to Japanese bugs. [Google Scholar]
- Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science. 2004;303:1989. doi: 10.1126/science.1094611. doi:10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- Via S. Ecological genetics and host adaptation in herbivorous insects: the experimental study of evolution in natural and agricultural systems. Annu. Rev. Entomol. 1990;35:421–446. doi: 10.1146/annurev.en.35.010190.002225. doi:10.1146/annurev.en.35.010190.002225 [DOI] [PubMed] [Google Scholar]
- Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl Acad. Sci. USA. 2004;101:15 042–15 045. doi: 10.1073/pnas.0403853101. doi:10.1073/pnas.0403853101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Two newborn nymphs of Megacopta punctatissima are sucking symbiotic bacteria from brownish symbiont capsules. A newly-emerged nymph is coming up from the behind, pushing one of the nymphs to make its own space, probing the capsule with its proboscis, and starting ingestion of the symbiont