Abstract
Aspergillus ustus is a very common species in foods, soil and indoor environments. Based on chemical, molecular and morphological data, A. insuetus is separated from A. ustus and revived. A. insuetus differs from A. ustus in producing drimans and ophiobolin G and H and not producing ustic acid and austocystins. The molecular, physiological and morphological data also indicated that another species, A. keveii sp. nov. is closely related but distinct from A. insuetus. Aspergillus section Usti sensu stricto includes 8 species: A. ustus, A. puniceus, A. granulosus, A. pseudodeflectus, A. calidoustus, A. insuetus and A. keveii together with Emericella heterothallica.
Keywords: actin, Aspergillus, β-tubulin, calmodulin, extrolite profiles, ITS, phylogenetics, polyphasic taxonomy.
INTRODUCTION
Aspergillus ustus is a very common filamentous fungus found in foods, soil and indoor air environments (Samson et al. 2002). This species is considered as a rare human pathogen that can cause invasive infection in immunocompromised hosts. However, A. ustus has been noted increasingly as causes of invasive aspergillosis in tertiary care centres in the US (Malani & Kaufman 2007). Up to date, 22 invasive aspergillosis cases have been reported to be caused by A. ustus (Verweij et al. 1999; Pavie et al. 2005; Panackal et al. 2006; Yildiran et al. 2006). Several studies indicate that A. ustus isolates are resistant to amphotericin B, echinocandins and azole derivatives (Verweij et al. 1999; Pavie et al. 2005; Gene et al. 2001; Garcia-Martos et al. 2005). Other species related to A. ustus can also cause human or animal infections. Aspergillus granulosus was found to cause disseminated infection in a cardiac transplant patient (Fakih et al. 1995), while A. deflectus has been reported to cause disseminated mycosis in dogs (Robinson et al. 2000; Kahler et al. 1990; Jang et al. 1986).
A. ustus is a variable species. Raper & Fennell (1965) stated that “not a single strain can be cited as wholly representative of the species as described”. Indeed, A. ustus isolates may vary in their colony colour from mud brown to slate grey, with colony reverse colours from uncoloured through yellow to dark brown (Raper & Fennell 1965; Kozakiewicz 1989). Molecular data also indicate that this species is highly variable; RAPD analysis carried out in various laboratories could be used to detect clustering of the isolates (Rath et al. 2002; Panackal et al. 2006), and sequence analysis of parts of the ribosomal RNA gene cluster also detected variability within this species (Henry et al. 2000; Peterson 2000; Hinrikson et al. 2005).
We examined a large set of A. ustus isolates and related species originating from environmental and clinical sources to clarify the taxonomic status of the species, and to clarify the taxonomy of Aspergillus section Usti. The methods used include sequence analysis of the ITS region (intergenic spacer region and the 5.8 S rRNA gene of the rRNA gene cluster), and parts of the β-tubulin, calmodulin and actin genes, analysis of extrolite profiles, and macro- and micromorphological analysis of the isolates.
MATERIALS AND METHODS
Morphological examination. The strains examined are listed in Table 1. Both clinical and environmental strains were grown as 3-point inoculations on Czapek yeast agar (CYA), malt extract agar (MEA), creatine agar (CREA) and yeast extract sucrose agar (YES) at 25 °C, and on CYA at 37 °C for 7 d (medium compositions according to Samson et al. 2004). For micro morphological examination light microscopy (Olympus BH2 and Zeiss Axioskop 2 Plus) was employed.
Table 1.
Isolates in Aspergillus section Usti and related species examined in this study.
| Species | Strain No. | Source |
|---|---|---|
| A. calidoustus | CBS 112452 | Indoor air, Germany |
| A. calidoustus | CBS 113228 | ATCC 38849; IBT 13091 |
| A. calidoustus | CBS 114380 | Wooden construction material, Finland |
| A. calidoustus | CBS 121601T | Bronchoalveolar lavage fluid, proven invasive aspergillosis, Nijmegen, The Netherlands† |
| A. calidoustus | CBS 121602 | Bronchial secretion, proven invasive aspergillosis, Nijmegen, The Netherlands† |
| A. calidoustus | CBS 121589 | Autopsy lung tissue sample, proven invasive aspergillosis, Nijmegen, The Netherlands† |
| A. calidoustus | CBS 121603 | Elevator shaft in hospital, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121604 | Patient room, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121605 | Laboratory, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121606 | Sputum, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121607 | Feces, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121608 | Bronchoalveolar lavage, Nijmegen, The Netherlands |
| A. calidoustus | 7843 | Pasteur Institute, Paris, France |
| A. calidoustus | 8623 | Oslo, Norway |
| A. calidoustus | 9331 | Mouth wash, Nijmegen, The Netherlands |
| A. calidoustus | 9371 | Mouth wash, Nijmegen, The Netherlands |
| A. calidoustus | 9420 | Bronchial secretion, Nijmegen, The Netherlands |
| A. calidoustus | 9692 | Hospital ward, Nijmegen, The Netherlands |
| A. calidoustus | V02-46 | Tongue swab, Nijmegen, The Netherlands |
| A. calidoustus | V07-21 | Bronchial secretion, Nijmegen, The Netherlands |
| A. calidoustus | V17-43 | Bronchial secretion, Nijmegen, The Netherlands |
| A. calidoustus | V22-60 | Skin biopsy, Nijmegen, The Netherlands |
| A. calidoustus | CBS 121609 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | 907 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | 908 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | 64 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | 67 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | CBS 121610 | Post-cataract surgery endophthalmitis, Turkey |
| A. calidoustus | 351 | Osteorickets |
| A. calidoustus | 482 | Post-cataract surgery endophthalmitis |
| A. calidoustus | CBS 121611 | Patient 4, Washington, U.S.A. |
| A. calidoustus | CBS 121616 | Environmental, Washington, U.S.A. |
| A. calidoustus | FH 165 | Patient 5b, Washington, U.S.A. |
| A. calidoustus | CBS 121614 | Patient 5a, Washington, U.S.A. |
| A. calidoustus | CBS 121615 | Patient 6, Washington, U.S.A. |
| A. calidoustus | CBS 121613 | Patient 2, Washington, U.S.A. |
| A. calidoustus | CBS 121612 | Patient 1, Washington, U.S.A. |
| A. calidoustus | FH 91 | Patient 1a, Washington, U.S.A. |
| A. calidoustus | NRRL 26162 | Culture contaminant, Peoria, U.S.A. |
| A. calidoustus | NRRL 281 | Thom 5634 |
| A. calidoustus | NRRL 277 | Thom 5698.754, Green rubber |
| A. granulosus | CBS 588.65T | Soil, Fayetteville, Arkansas, U.S.A. |
| A. granulosus | CBS 119.58 | Soil, Texas, U.S.A. |
| A. granulosus | IBT 23478 = WB 1932 = IMI 017278iii = CBS 588.65 | Soil, Fayetteville, Arkansas, U.S.A. |
| A. insuetus | CBS 107.25T | South Africa |
| A. insuetus | CBS 119.27 | Unknown |
| A. insuetus | CBS 102278 | Subcutaneous infection left forearm and hand of 77-year-old woman |
| A. keveii | CBS 209.92 | Soil, La Palma, Spain |
| A. keveii | CBS 561.65 | Soil, Panama |
| A. keveii | IBT 10524 = CBS 113227 = NRRL 1254 | Soil, Panama |
| A. keveii | IBT 16751 = DMG 153 | Galápagos Islands, Ecuador, D.P. Mahoney |
| A. pseudodeflectus | CBS 596.65 | Sugar, U.S.A., Louisiana |
| A. pseudodeflectus | CBS 756.74T | Desert soil, Egypt, Western Desert |
| A. puniceus | CBS 122.33 | Unknown |
| A. puniceus | 9377 | Mouth wash, Nijmegen, Netherlands |
| A. puniceus | V41-02 | Faeces, Nijmegen, Netherlands |
| A. puniceus | NRRL 29173 | Indoor air, Saskatoon, Canada |
| A. puniceus | CBS 495.65T | Soil, Zarcero Costa Rica |
| A. puniceus | CBS 128.62 | Soil, Louisiana, U.S.A. |
| A. ustus | CBS 116057 | Antique tapestries, Krakow, Poland |
| A. ustus | CBS 114901 | Carpet, The Netherlands |
| A. ustus | CBS 261.67T | Culture contaminant, U.S.A. |
| A. ustus | CBS 133.55 | Textile buried in soil, Netherlands |
| A. ustus | CBS 239.90 | Man, biopsy of brain tumor, Netherlands |
| A. ustus | CBS 113233 | IBT 14495 |
| A. ustus | CBS 113232 | IBT 14932 |
| A. ustus | NRRL 285 | Soil, Iowa, U.S.A. |
| A. ustus | NRRL 280 | Bat dung, Cuba |
| A. ustus | NRRL 1609 | Bat dung, Cuba |
| A. ustus | NRRL 29172 | Indoor air, Edmonton, Canada |
| E. heterothallica | CBS 489.65T | soil, Costa Rica |
| E. heterothallica | CBS 488.65 | soil, Costa Rica |
These samples were taken from the same patient (Verweij et al. 1999)
Extrolite analysis. Extrolites were analysed by HPLC using alkylphenone retention indices and diode array UV-VIS detection as described by Frisvad & Thrane (1987), with minor modifications as described by Smedsgaard (1997). Standards of ochratoxin A and B, aflavinine, asperazine, austamide, austdiol, kotanin and other extrolites from the collection at Biocentrum-DTU were used to compare with the extrolites from the species under study.
Isolation and analysis of nucleic acids. The cultures used for the molecular studies were grown on malt peptone (MP) broth using 10 % (v/v) of malt extract (Brix 10) and 0.1 % (w/v) bacto peptone (Difco), 2 mL of medium in 15 mL tubes. The cultures were incubated at 25 °C for 7 d. DNA was extracted from the cells using the Masterpure™ yeast DNA purification kit (Epicentre Biotechnol.) according to the instructions of the manufacturer. Fragments containing the ITS region were amplified using primers ITS1 and ITS4 as described previously (White et al. 1990). Amplification of part of the β-tubulin gene was performed using the primers Bt2a and Bt2b (Glass 1995). Amplifications of the partial calmodulin and actin genes were set up as described previously (Hong et al. 2005). Sequence analysis was performed with the Big Dye Terminator Cycle Sequencing Ready Reaction Kit for both strands, and the sequences were aligned with the MT Navigator software (Applied Biosystems). All the sequencing reactions were purified by gel filtration through Sephadex G-50 (Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated in double-distilled water and analyzed on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Data analysis. The sequence data was optimised using the software package Seqman from DNAStar Inc. Sequence alignments were performed by using CLUSTAL-X (Thompson et al. 1997) and improved manually. The neighbour-joining (NJ) method was used for the phylogenetic analysis. For NJ analysis, the data were first analysed using the Tamura-Nei parameter distance calculation model with gamma-distributed substitution rates (Tamura & Nei 1993), which were then used to construct the NJ tree with MEGA v. 3.1 (Kumar et al. 2004). To determine the support for each clade, a bootstrap analysis was performed with 1000 replications.
For parsimony analysis, the PAUP v. 4.0 software was used (Swofford 2000). Alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed for all data sets using the heuristic search option with 100 random taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1000 bootstrap replications (Hillis & Bull 1993). An Aspergillus versicolor isolate was used as outgroup in these experiments. Unique sequences of the ITS, actin, calmodulin and β-tubulin gene sequences have been deposited in the GenBank under accession numbers EU076344-EU76377.
RESULTS
Phylogenetic analyses
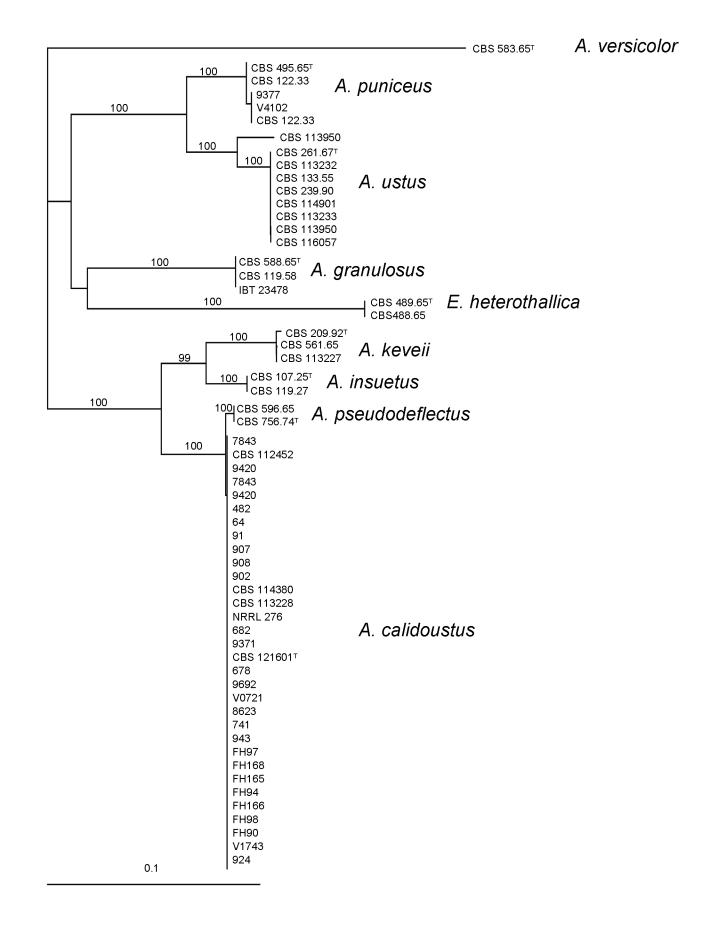
For the molecular analysis, four genomic regions, the ITS region, and parts of the actin, calmodulin and β-tubulin genes were amplified and sequenced. Phylogenetic analysis of the data was carried out using the neighbour-joining technique and parsimony analysis. The trees obtained by the different approaches were identical, neighbour-joining trees based on the different data sets are shown in Figs 1, 2, 3 and 4. During analysis of part of the β-tubulin gene, 487 characters were analyzed, 111 of which were found to be parsimony informative. The topology of the tree is the same as that of one of the more than 104 maximum parsimony trees constructed by the PAUP program (length: 216 steps, consistency index: 0.8148, retention index: 0.9679). The calmodulin data set included 474 characters, with 172 parsimony informative characters (1 MP tree, tree length: 360, consistency index: 0.8083, retention index: 0.9550). The actin data set included 406 characters, with 161 parsimony informative characters (3 MP trees, tree length: 292, consistency index: 0.8870, retention index: 0.9633). The ITS data set included 482 characters, 26 of which were parsimony informative (>104 MP trees, tree length: 71, consistency index: 0.9155, retention index: 0.9781).
Fig. 1.
Neighbour-joining tree based on β-tubulin sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 2.
Neighbour-joining tree based on calmodulin sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 3.
Neighbour-joining tree based on actin sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Fig. 4.
Neighbour-joining tree based on ITS sequence data of Aspergillus section Usti. Numbers above branches are bootstrap values. Only values above 70 % are indicated.
Molecular data revealed that Aspergillus section Usti consists of eight species: A. ustus, A. puniceus, A. granulosus, A. pseudodeflectus, A. calidoustus, A. insuetus and a new species including CBS 209.92 and some other isolates. We propose the name A. keveii sp. nov. for this set of isolates. The trees based on ITS, calmodulin and β-tubulin sequence data indicated that also E. heterothallica belongs to this section, although actin sequence data did not support this finding.
Morphological and physiological studies
Phenotypic comparison of the different members of the section Usti showed that eight taxa could be distinguished. Various characters showed to be valuable for differentiation (see also Table 2). One of the main criteria is the growth rate on CYA at 37 °C. A. calidoustus, A. pseudodeflectus and A. granulosus had high growth rates at this temperature, while E. heterothallica only grew restrictedly. The other members of this section were unable to grow at 37 °C, which reduces the potential of these species to become opportunistic human pathogens. The growth rate and the mycelium colour on creatin agar (CREA) also proved to be a good tool to differentiate between the species examined. Some species, like A. ustus, A. puniceus, A. insuetus and A. keveii have a good growth on this medium. Since sporulation on this medium is often inhibited, this medium was also useful to determine the colour of the mycelium. The colours varied from bright yellow by A. puniceus and E. heterothallica to faint yellow in A. ustus to colourless in the other species. Another useful character was the use of the Ehrlich test to detect the presence of indol metabolites. This feature gave, with the exception of A. keveii, very clear-cut results. Besides these features, the colony diam on YES was also suitable to differentiate between A. insuetus and the other species.
Table 2.
Overview of morphological criteria to differentiate between the members of Aspergillus section Usti.
| Species | CYA37 (mm) | YES (mm) | Ehrlich reaction | Reaction on CREA | Conidial colour on MEA** |
|---|---|---|---|---|---|
| A. ustus | No growth | 43-49 | None | Good growth, faint yellow mycelium | Hair brown |
| A. puniceus | No growth | 48-53 | None | Moderate to good growth, yellow mycelium | Olive brown |
| A. calidoustus | 20-35 | 36-41 | Violet | Weak to moderate growth, hyaline mycelium | Brownish grey |
| A. insuetus | No growth | 23-30 | Violet | Good growth, hyaline mycelium | (Brownish) grey to light grey |
| A. keveii | No growth | 40-46 | Violet* | Good growth, hyaline mycelium | Brownish grey / pinkish brown |
| A. pseudodeflectus | 15-20 | 20-30 | None | Weak to moderate growth, hyaline mycelium | No sporulation |
| A. granulosus | 30-35 | 35-40 | Violet | Weak growth, hyaline mycelium | Buff to greyish brown |
| E. heterothallica | 5-10 | 38-42 | None | Weak growth, bright yellow mycelium | No sporulation |
All have violet reaction, except CBS 113227
Colour according Methuen handbook of colours
Extrolite profiles
Aspergillus ustus has been claimed to produce a range of extrolites including austdiol (Vleggaar et al. 1974), Austin (Chexal et al. 1976), austocystins (Steyn & Vleggaar 1974; Kfir et al. 1986), brevianamide A (Steyn 1973), sterigmatocystin (Rabie et al. 1977), austalides (de Jesus et al. 1987), austamide (Steyn 1971), dehydroaustin (Scott et al. 1986), pergillin (Cutler et al. 1980), dehydropergillin (Cutler et al. 1981), phenylahistin (Kanoh et al. 1997), ophiobolins G & H (Cutler et al. 1984), drimans (Hayes et al. 1996), diacetoxyscirpenol (Tuomi et al. 2000) and ustic acid (Raistrick & Strickings 1951).
The mycotoxins and other extrolites found to be produced by the examined species in this study are listed in Table 3. Species assigned to section Usti could clearly be divided in three chemical groups based on the extrolites produced by them. A. ustus, A. granulosus and A. puniceus produced ustic acids in common. A. ustus and A. puniceus also produced austocystins and versicolorins. In the second chemical group, A. pseudodeflectus produced drimans (Hayes et al. 1996) in common with the other species in this group, and also several unique unknown compounds. A. calidoustus isolates produced drimans and ophiobolins in common with A. insuetus and A. keveii, but also produced austins not identified in other species of section Usti. A. insuetus isolates also produced pergillin, while A. keveii together with some other isolates produced nidulol. In the third chemical group, E. heterothallica has been reported to produce emethallicins A-F (Kawahara et al. 1989, 1990a, 1990b), 5”-hydroxyaveranthin (Yabe et al. 1991), emeheterone (Kawahara et al. 1988), emesterones A & B (Hosoe et al. 1998), 5”-hydroxyaveranthin (Yabe et al. 1991), Mer-NF8054X (Mizuno et al. 1995). This latter compound is an 18,22-cyclosterol derivative, and was also identified in an A. ustus isolate (Mizuno et al. 1995). Apart from this chemical similarity Emericella heterothallica appear to be quite different from the anamorphic species in section Usti, in agreement with actin sequencing data. Austamide, deoxybrevianamide E and austdiol could not be detected in any of the strains examined here and the strain producing these mycotoxins should be reexamined.
Table 3.
Extrolites produced by species assigned to Aspergillus section Usti.
| Species | Extrolites produced |
|---|---|
| Chemical group I | |
| A. ustus | Ustic acids, austocystins (and versicolorins), austalides, a compound related to sterigmatocystin, nidulol |
| A. granulosus | Ustic acids, a compound resembling sterigmatocystin, nidulol, drimans |
| A. puniceus | Ustic acids, austocystins (and versicolorins), phenylahistin, a compound related to sterigmatocystin, nidulol |
| Chemical group II | |
| A. pseudodeflectus | Drimans, unknown compounds |
| A. calidoustus | Drimans, ophiobolins G and H, austins |
| A. insuetus | Drimans, ophiobolins G and H, pergillin-like |
| A. keveii | Drimans, ophiobolins G and H, nidulol |
| Chemical group III | |
| E. heterothallica | Emethallicins A, B, C, D, E & F, emeheterone, emesterones A & B, 5″-hydroxyaveranthin, Mer-NF8054X, sterigmatocystin, versicolorins |
Comparing the extrolites profiles of section Usti with other sections within subgenus Nidulantes, nidulol and versicolorins are also produced by members of sections Versicolores and Nidulantes (Cole & Schweikert 2003). Interestingly, versicolorins and 5”-hydroxyaveranthin are intermediates of the aflatoxin biosynthetic pathway and also produced by species assigned to Aspergillus section Flavi and Ochraceorosei (Yabe et al. 1991; Frisvad et al. 2005). However, while the versicolorins are precursors of sterigmatocystin in section Ochraceorosei, Versicolores and Nidulantes, they are precursors of austocystins in section Usti.
Section Usti contains the only Aspergillus species known to produce pergillins, ophiobolins, austins, austocystins, ustic acids, drimans, Mer-NF8054X, austalides, deoxybrevianamides and austamide and thus this section is chemically unique. We have not examined the species for production of emethallicins, emesterones and emeheterones, as standards of these compounds were not available.
DISCUSSION
Raper and Fennell (1965) classified A. ustus in the Aspergillus ustus group together with four other species: A. panamensis, A. puniceus, A. conjunctus and A. deflectus. Later, Kozakiewicz (1989) revised the taxonomy of the group, and included A. ustus, A. pseudodeflectus, A. conjunctus, A. puniceus, A. panamensis and A. granulosus into the A. ustus species group, and established the A. deflectus group including A. deflectus, A pulvinus and A. silvaticus based on morphological studies. Klich (1993) treated A. granulosus as member of section Versicolores, and found that A. pseudodeflectus is only weakly related to this section based on morphological treatment of section Versicolores. Peterson (2000) transferred most species of section Usti to section Nidulantes based on sequence analysis of part of the 28 S rRNA gene. On his cladogram, A. ustus, A. pseudodeflectus, A. granulosus and A. puniceus form a well-supported branch closely related to A. versicolor and its allies, while A. deflectus is on another branch related to A. elongatus and A. lucknowensis. Peterson (2000) transferred A. conjunctus, A. funiculosus, A. silvaticus, A. panamensis and A. anthodesmis to section Sparsi. Recently Varga et al. (submitted) studied large numbers of isolates from clinical and other sources using molecular, morphological and physiological approaches. Phylogenetic analysis of partial β-tubulin, calmodulin, actin and ITS sequences indicated that none of the clinical isolates recognised previously as A. ustus belong to the A. ustus species. All but two of these isolates formed a well-defined clade related to A. pseudodeflectus based on sequence analysis of protein coding regions. Morphological and physiological examination of the isolates indicated that they are able to grow above 37 °C, in contrast with A. ustus isolates, and give a positive Ehrlich reaction, in contrast with related species including A. granulosus, A. ustus, and A. pseudodeflectus. These isolates were described as A. calidoustus.
Aspergillus ustus (Bainier) Thom & Church was redescribed by Thom & Church (1926) based on Sterigmatocystis usta Bainier. In this manual, A. insuetus (Bainier) Thom & Church was also accepted based on S. insueta Bainier (Thom & Chuch, 1926), but later A. insuetus was abandoned (Thom and Raper, 1945) and included in the broad description of A. ustus in Raper and Fennell (1965). Our studies clarified that A. insuetus is a valid species which can be distinguished from A. ustus and other species assigned to Aspergillus section Usti. A. insuetus could be separated from the other members of the section Usti by various phenotypic characters. The most important one is the slower growth rate on YES agar and clear differences in extrolite profiles (Table 2). This finding was supported by all the different data sets used to characterise section Usti. The molecular data showed that this species is more related to A. calidoustus and A. pseudodeflectus than A. ustus. Also different extrolite patterns were observed. There were many differences between A. ustus and A. insuetus, and, like the molecular data, this species was mostly related to A. calidoustus and A. pseudodeflectus. The main difference between the latter species was the production of a pergillin-like compound by A. insuetus (Table 3).
Our polyphasic taxonomic approach revealed that Aspergillus section Usti includes eight species: A. ustus, A. puniceus, A. granulosus, A. pseudodeflectus, A. calidoustus, A. insuetus and A. keveii sp. nov. The phylogenetic trees based on ITS, calmodulin and β-tubulin sequence data indicated that E. heterothallica also belongs to this section. This species has similar morphology of the conidiophores and Hülle cells. In our study we were not able to observe ascospores by crossing the two mating strains but these are described by Raper and Fennell (1965: 502-503).
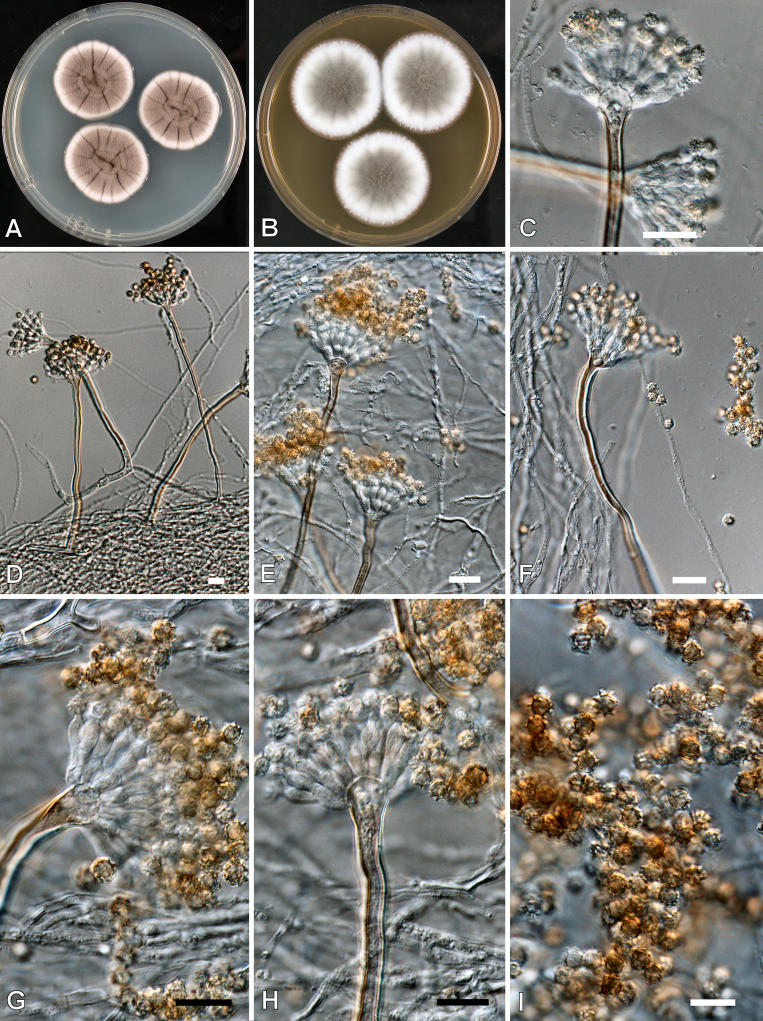
Aspergillus calidoustus Varga et al. Eukaryotic Cell submitted. Fig. 5.
Fig. 5.
Aspergillus calidoustus. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-E, G-H Conidiophores. F. Hülle cells. I. Conidia. Scale bars = 10 μm, except F = 30 μm
Type: CBS 121604 from human, Netherlands
Other no. of the type: strain 677
Description strain
Colony diam, 7 d, in mm: CYA25 27-32; CYA37 20-35; MEA25 35-48; YES 36-41
Colony colour on CYA: blond/greyish yellow, brownish grey or greyish brown
Conidiation on CYA: abundant
Reverse colour (CYA): yellow with beige or olive brown centre
Colony texture: floccose
Conidial heads: loosely columnar
Stipe: 150-300 × 4-7 μm, smooth, brown
Vesicle diam/shape: 9-15 μm, pyriform to broadly spathulate
Conidium size/shape/surface texture: 2.7-3.5 × μm, globose, very rough ornamentation (0.5-0.8 μm high), inner and outer wall visible
Hülle cells: sparsely produced, irregularly elongated, in scattered groups
Ehrlich reaction: violet
Growth on creatine: weak to moderate growth with hyaline mycelium, no acid production
Diagnostic features: good growth at 37 °C, violet Ehrlich reaction, coarsely roughened to echinulate conidia
Cultures examined: CBS 121589, 121601-121616
Similar species: A. pseudodeflectus
Distribution: U.S.A., Turkey, Finland, Germany, Netherlands
Ecology and habitats: indoor air, rubber, construction material, human
Extrolites: Drimans, ophiobolins G and H, austins
Pathogenicity: pathogenic to humans (Verweij et al. 1999; Weiss & Thiemke 1983; Pavie et al. 2005; Panackal et al. 2006; Yildiran et al. 2006; Iwen et al. 1998)
Aspergillus granulosus Raper & Thom, Mycologia 36: 565. 1944. Fig. 6.
Fig. 6.
Aspergillus granulosus. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-H Conidiophores. I. Conidia. Scale bars = 10 μm, except C = 30 μm.
Type: CBS 588.65, from soil, Fayetteville, Arkansas, U.S.A.
Other no. of the type: ATCC 16837, NRRL 1932, WB 1932, CBS 452.93
Description
Colony diam, 7 d, in mm: CYA25 30-48; CYA37 30-51; MEA25 25-37; YES25 35-45; CZA25 17-25
Colony colour: buff to dull brown
Conidiation: moderate
Reverse colour (CYA): dull yellow to red brown
Colony texture: floccose, plane or irregularly furrowed
Conidial head: hemispherical to radiate
Stipe: 100-600 × 5.5-8 μm, thin-walled, smooth, straight, tan to light brown
Vesicle diam/shape: 15-25 × 12-18 μm, ovoid to elliptical
Conidium size/shape/surface texture: (3.3-)4-4.5(-5.5) μm, globose, delicately echinulate
Hülle cells: irregularly globose, ovoid to elongate, 12-30 μm, in colourless clusters at colony margins
Ehrlich reaction: violet
Growth on creatine: poor growth with inconspicuous mycelium, no acid production
Cultures examined: CBS 119.58, CBS 588.65, IBT 23478
Diagnostic features: small colourless clusters of irregularly globose Hülle cells, giving the colony a characteristic granular appearance, good growth at 37 °C and violet Ehrlich reaction
Similar species: -
Distribution: U.S.A.
Ecology and habitats: soil
Extrolites: Ustic acids, a compound resembling sterigmatocystin, nidulol, drimans
Pathogenicity: pathogenic to humans (Fakih et al. 1995)
Aspergillus insuetus (Bainier) Thom & Church, Manual of the aspergilli: 153. 1929. Fig. 7.
Fig. 7.
Aspergillus insuetus. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-H Conidiophores. I. Conidia. Scale bars = 10 μm, except C = 30 μm.
= Sterigmatocystis insueta Bainier (1908)
Type: CBS 107.25, from South Africa, Sartory
Other no. of the type: ATCC 1033; IFO 4128; NRRL 279; NRRL 1726; Thom No. 4658.245
Description
Colony diam, 7 d, in mm: CYA 28-32; CYA37 no growth; MEA25 36-41; YES 23-30
Colony colour: almost black in center, shading through gray to white sterile floccose marginal areas
Conidiation on CYA: moderate to good
Reverse colour (CYA): yellow olive to blackish brown with age
Colony texture: floccose
Conidial head: radiate to hemispherical
Stipe: 300 × 4-8 μm, smooth, brown
Vesicle diam/shape: 11-16 μm, hemispherical to subglobose
Conidium size/shape/surface texture: 3.2-4 μm, globose, distinct roughened and inner and outer wall visible, fuligeneous, the colour mostly aggregated into echinulations of the cell-wall, and even forming bars and tubercules at times
Hülle cells: variously coiled or curved, in scattered groups
Ehrlich reaction: violet
Growth on creatine: good growth with hyaline mycelium, no acid production
Cultures examined: CBS 107.25, CBS 119.27, CBS 102278
Similar species: A. keveii
Distribution: South Africa, Spain
Diagnostic features: no growth at 37 °C, violet Ehrlich reaction, restricted growth on YES, coarsely roughened to echinulate conidia
Ecology and habitats: soil (?), human
Extrolites: Drimans, ophiobolins G and H, pergillin-like
Pathogenicity: caused subcutaneous infection (Gené et al. 2001)
Aspergillus keveii sp. nov. Varga, Frisvad & Samson - MycoBank MB505570. Fig. 8.
Fig. 8.
Aspergillus kerveii. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-H Conidiophores. I. Conidia. Scale bars = 10 μm.
Holotype of Aspergillus keveii, here designated as CBS 209.92T (dried culture) isolated from soil, Las Palmas, Spain.
Coloniae in 7 dieibus et 25 °C in agaro MEA 36-41 mm, in CYA 30-39 mm, in YES 40-46 mm, in CREA 25-32 mm diam; auctus in 7 dieibus et 37 °C in agaro CYA nullus. Sporulatio in CYA abundans; colonia brunneogrisea vel subroseobrunnea; textura coloniae floccosa; colonia reversa flavide olivaceobrunnea vel atrobrunnea. Capitula conidialia laxe columnaria; stipites 150-300 × 4-6 μm, pariete laevi, brunneo; vesciculae pyriformes, 9-13 μm in lat., biseriatae; metulae 4.7-6.7 × 2.8-3.6 μm; phialides 5.7-7 × 2-3 μm; conidia globosa, 2.4-2.8 μm diam., ornamento exasperato vel echinulato. Cellulae “hülle” irregulariter elongatae, (10-) 25-40(-65) μm in long., in cumulis dispersis.
Colonies on MEA 36-41 mm, on CYA 30-39 mm, on YES 40-46 mm, on CREA 25-32 mm in diam. after 7 d at 25 °C, no growth on CYA after 7 d at 37 °C. Conidial heads abundant on CYA, colony colour brownish grey to pinkish brown, colony texture floccose, reverse yellow olive brown to dark brown. Conidial heads loosely columnar; stipes 150-300 × 4-6 μm, smooth walled, brown in colour; vesicles 9-13 μm wide, pyriform, biseriate; metulae covering the upper half to three-fourths of the vesicle, measuring 4.7-6.7 × 2.8-3.6 μm μm; phialides 5.7-7 × 2-3 μm; conidia globose 2.4-2.8 μm, coarsely roughened to echinulate. Hülle cells (10-)25-40(-65) μm, irregularly elongated, produced in scattered groups.
Etymology: named after Prof. Ferenc Kevei, eminent mycologist devoting his life to Aspergillus research.
Type: CBS 209.92
Ehrlich reaction: violet, with exception of CBS 113227
Growth on creatine: good growth with hyaline mycelium, no or weak acid production
Diagnostic features: no growth at 37 °C, good growth on CREA and YES, coarsely roughened to echinulate conidia; Hülle cells in scattered groups, violet Ehrlich reaction
Cultures examined: CBS 561.65, CBS 209.92 and CBS 113227
Similar species: A. insuetus
Distribution: U.S.A., Turkey, Finland, Germany, Netherlands
Ecology and habitats: indoor air, rubber, construction material, human
Extrolites: Drimans, ophiobolins G and H, nidulol
Pathogenicity: not reported
Notes: CBS 113227 is deviating in having larger conidial heads and small (2.6 μm), finely roughened pinkish brown coloured conidia
Aspergillus pseudodeflectus Samson & Mouchacca, Antonie van Leeuwenhoek 41(3): 325. 1975. Fig. 9.
Fig. 9.
Aspergillus pseudodeflectus. A-C. Colonies at 25 °C after 7 d. A. MEA + 40 % sucrose. B. CYA + 20 % sucrose. C. MEA. D-I. Conidiophores. H. Conidia. Scale bars = 10 μm.
Type: CBS 756.74, from desert soil, Western Desert, Egypt
Other no. of the type: IMI 278381
Description
Colony diam, 7 d, in mm: CYA25 43-49; CYA37 15-20; MEA25 35-45; YES 20-30; CZA25 25-26
Colony colour: white mycelial felt intermixed with brown conidiogenous structures
Conidiation: sparse
Reverse colour (CZA): yellow
Colony texture: velvety appearance, no sporulation
Conidial head: radiate, brown
Stipe: 35-200 × 2.5-3.5 μm, rough-walled with warty protuberances, brown
Vesicle diam/shape: 4-12 μm, globose to clavate
Conidium size/shape/surface texture: 3.5-5 μm, globose to ellipsoidal, brown, ornamented with small warts and colour bars
Hülle cells: absent
Ehrlich reaction: none
Growth on creatine: weak to moderate growth with hyaline mycelium, no acid production
Diagnostic features: Growth at 37 °C, curved brown conidiophores and the ornamented conidia, absence of Hülle cells
Cultures examined: CBS 756.74, CBS 596.65
Similar species: A. calidoustus
Distribution: Egypt, U.S.A.
Ecology and habitats: soil
Extrolites: Drimans (Hayes et al. 1996), unknown compounds
Pathogenicity: not reported
Aspergillus puniceus Kwon and Fennell, The genus Aspergillus: 547. 1965. Fig. 10.
Fig. 10.
Aspergillus puniceus. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-H Conidiophores. I. Sclerotia. J. Conidia. Scale bars = 10 μm, except D = 30 μm.
= A. ustus var. laevis Blochwitz (1945)
Type: CBS 495.65, from soil, Zarcero, Costa Rica
Other no. of the type: ATCC 16800; IMI 126692; WB 5077
Description
Colony diam, 7 d, in mm: CYA 40-50; CYA37 no growth; MEA25 40-45; YES 48-53; CZA25: 40-50 mm
Colony colour: pinkish orange near vinaceous pink, with wine red exudate droplets
Conidiation: moderate
Reverse colour (CYA): dark yellow brown or crème brown
Colony texture: floccose
Conidial head: radiate to short columnar, dull green becoming light drab with age
Stipe: 150-250(-300) × 5.5-6(-8) μm, aerially borne stipes up to 135 × 3-4 μm, straight, smooth
Vesicle diam/shape: 8-16 μm (subglobose), 15-18 × 13-15 μm (elliptical)
Conidium size/shape/surface texture: 2.5-3.3 μm, globose, roughened
Hülle cells: elongate, crescent shaped or irregularly twisted, often aggregated into yellowish masses
Ehrlich reaction: no reaction
Growth on creatine: moderate to good growth with bright yellow mycelium, no acid production (in some isolates weak acid production under colony)
Cultures examined: CBS 495.65, CBS 122.33, CBS 128.62, 9377, V41-02, NRRL 29173
Diagnostic features: No growth at 37 °C, good growth on creatine with brightly pigmented yellow mycelium, Hülle cells aggregated into yellowish masses
Similar species: A. ustus
Distribution: Costa Rica, U.S.A., Canada, Netherlands
Ecology and habitats: soil, indoor air, human
Extrolites: ustic acids, austocystins, nidulol, versicolorins, phenylahistin, sterigmatocystin-related compound (in CBS 128.62)
Pathogenicity: isolated from mouth wash and faeces
Aspergillus ustus (Bainier) Thom & Church, The aspergilli: 152. 1924. Fig. 11.
Fig 11.
Aspergillus ustus. A-B. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C-E. G-H Conidiophores. F. Hülle cells. I. Conidia. Scale bars = 10 μm, except F = 30μm.
= Sterigmatocystis usta Bainier (1881)
= Aspergillus humus Abbott (1926)
Type: CBS 261.67, culture contaminant, U.S.A.
Other no. of the type: ATCC 1041; ATCC 16818; IMI 211805; NRRL 275; QM 7477; WB 275; Thom 3556
Description
Colony diam, 7 d, in mm: CYA 36-43; CYA37 no growth; MEA25 39-46; YES 42-50
Colony colour: greyish brown to dark brown
Conidiation on CYA: moderate
Reverse colour (CZA): yellow-olive edge with olive brown centre
Colony texture: floccose, plane, sulcate or umbonate
Conidial head: radiate to hemispherical
Stipe: 400 × 3-6 μm, aerially borne stipes up to 125 × 2-5 μm, smooth, brownish
Vesicle diam/shape: 7-15 μm, hemispherical to subglobose
Conidium size/shape/surface texture: 3.2-4.5 μm, globose, roughened, greenish to dark yellow brown
Hülle cells: irregularly ovoid or elongate, usually scattered
Ehrlich reaction: no reaction
Growth on creatine: good growth with faint yellow mycelium, no acid production
Cultures examined: CBS 116057, CBS 114901, CBS 261.67, CBS 133.55, CBS 239.90, CBS 113233, CBS 113232, NRRL 285, NRRL 280, NRRL 1609, NRRL 29172
Diagnostic features: No growth at 37 °C; good growth on creatine with faint yellow pigmented mycelium; Hülle cells typically scattered or form irregular masses and not associated with pigmented mycelium
Similar species: A. puniceus
Distribution: U.S.A., Poland, Netherlands, Canada
Ecology and habitats: soil, indoor air, bat dung
Extrolites: Ustic acids, austocystins, versicolorins, austalides, a compound related to sterigmatocystin, nidulol
Pathogenicity: isolated from biopsy of man with brain tumour (CBS 239.90). However, this isolate does not grow at 37 °C on normal agar media and might therefore be a culture contamination.
Emericella heterothallica (Kwon-Chung, Fennell & Raper) Malloch & Cain [anamorph: A. compatibilis Samson & Gams], Can. J. Bot. 50: 62. 1972. Fig. 12.
Fig. 12.
Emericella heterothallica. A-C. Colonies at 25 °C after 7 d. A. CYA. B. MEA. C. Crossing of mating strains. D-E, F-H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Type: CBS 489.65, from soil, Costa Rica
Other no. of the type: ATCC 16824; IHEM 2064; IMI 139278; RV 34434; WB 5097; IBT 22604
Description
Colony diam, 7 d, in mm: CYA25 35-39; CYA37 5-8; MEA25 40-42; YES25 38-42
Colony colour: cream to yellow to orange
Conidiation: limited
Reverse colour (CYA): yellow to orange to pink becoming dark reddish brown
Colony texture: floccose
Conidial head: hemispherical to short columnar
Stipe: 185-410 × 5-11 μm, generally sinuous, brownish with age, smooth
Vesicle diam/shape: 13-20 μm
Conidium size/shape/surface texture: 2.5-4 μm, globose, echinulate, yellow green
Hülle cells: 600-700(-1000) μm, pyriform to oval to elongate to twisted, in globose to subglobose masses
Cleistothecia: produced in a heterothallic manner, 270-510 μm, cinnamon to dark purple, surrounded by Hülle cells
Ascospores: 4-4.5 × 3.5-4 μm, lenticular, orange brown in colour, with two pleated equatorial crests (1.5-2 μm), with convex smooth
Ehrlich reaction: none
Growth on creatine: weak growth with yellow coloured mycelium, no acid production
Diagnostic features: heterothallic species, weak growth at 37 °C
Cultures examined: CBS 489.65, CBS 488.65 = IBT 22607
Similar species: -
Distribution: Costa Rica
Ecology and habitats: soil
Extrolites: Found in this study: Sterigmatocystin, versicolorins, Mer-NF8054X. Literature data: emethallicins A-F (Kawahara et al. 1989, 1990a), 5”-hydroxyaveranthin (Yabe et al. 1991), emeheterone (Kawahara et al. 1988), emesterones A & B (Hosoe et al. 1998), 5”-hydroxyaveranthin (Yabe et al. 1991), Mer-NF8054X (Mizuno et al. 1995).
Pathogenicity: not reported
Acknowledgments
We would like to thank the Danish Technical Research Council for support to Center for Microbial Biotechnology and Ellen Kirstine Lyhne for technical support. Dr Emory Simmons kindly provided a Latin diagnosis for the new species.
Taxonomic novelties: Aspergillus insuetus revived, Aspergillus keveii sp. nov.
References
- Chexal KK, Spinger JP, Clardy J, Cole RJ, Kirksey JW, Dorner JW, Cutler HG, Strawter BJ (1976). Austin, a novel polyisoprenoid mycotoxin from Aspergillus ustus. Journal of the American Chemical Society 98: 6748. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Cox RH (1981). Handbook of toxic fungal metabolites. New York: Academic Press.
- Cole RJ, Schweikert MA (2003). Handbook of secondary fungal metabolites. Vols. 1-3. Amsterdam: Elsevier Academic Press.
- Cutler HG, Crumley FG, Springer JP, Cox RH (1981). Dihydropergillin: a fungal metabolite with moderate plant growth inhibiting properties from Aspergillus ustus. Journal of Agricultural and Food Chemistry 29: 981-983. [DOI] [PubMed] [Google Scholar]
- Cutler HG, Crumley FG, Springer JP, Cox RH, Cole RJ, Dorner JW, Thean JE (1980). Pergillin: a nontoxic fungal metabolite with moderate plant growth inhibiting properties from Aspergillus ustus. Journal of Agricultural and Food Chemistry 28: 989-991. [Google Scholar]
- Cutler HG, Crumley FG, Cox RH, Springer JP, Arrendale RF, Cole RJ, Cole PD (1984). Ophiobolins G and H: new fungal metabolites from a novel source, Aspergillus ustus. Journal of Agricultural and Food Chemistry 32: 778-782. [Google Scholar]
- Fakih MG, Barden GE, Oakes CA, Berenson CS (1995). First reported case of Aspergillus granulosus infection in a cardiac transplant patient. Journal of Clinical Microbiology 33: 471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Skoube P, Samson RA (2005). Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Systematic and Applied Microbiology 28: 442-453. [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U (1987). Standardized high performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). Journal of Chromatography 404: 195-214. [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U (1993). Liquid chromatography of mycotoxins. Journal of Chromatography Library 54: 253-372. [Google Scholar]
- Gams W, Christensen M, Onions AH, Pitt JI, Samson RA (1985). Infrageneric taxa of Aspergillus. In: Advances in Penicillium and Aspergillus Systematics. (Samson RA, Pitt JI, eds). New York: Plenum Press: 55-62.
- Garcia-Martos P, Garcia-Agudo L, Gutierrez-Calzada J, Ruiz-Aragon J, Saldarreaga A, Marin P (2005). In vitro activity of amphotericin B, itraconazole and voriconazole against 20 species of Aspergillus using the Sensititre microdilution method. Enfermedades Infecciosas y Microbiología Clínica 23: 15-18 [in Spanish] [DOI] [PubMed] [Google Scholar]
- Gene J, Azon-Masoliver A, Guarro J, De Febrer G, Martinez A, Grau C, Ortoneda M, Ballester F (2001). Cutaneous infection caused by Aspergillus ustus, an emerging opportunistic fungus in immunosuppressed patients. Journal of Clinical Microbiology 39: 1134-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MA, Wrigley SK, Chetland I, Reynolds EE, Ainsworth AM, Renno DV, Latif MA, Cheng XM, Hupe DJ, Charlton P, Doherty AM (1996). Novel drimane sesquiterpene esters from Aspergillus ustus var. pseudodeflectus with endothelin receptor binding activity. Journal of Antibiotics 49: 505-512. [DOI] [PubMed] [Google Scholar]
- Henry T, Iwen PC, Hinrichs SH (2000). Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. Journal of Clinical Microbiology 38: 1510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis DM, Bull JJ (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182-192. [Google Scholar]
- Hinrikson HP, Hurst SF, Lott TJ, Warnock DW, Morrison CJ (2005). Assessment of ribosomal large-subunit D1-D2, internal transcribed spacer 1, and internal transcribed spacer 2 regions as targets for molecular identification of medically important Aspergillus species. Journal of Clinical Microbiology 43: 2092-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA (2005). Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97: 1316-1329. [DOI] [PubMed] [Google Scholar]
- Hosoe T, Sameshima T, Dobashi K, Kawai KI (1998). Structures of two new 18,22-cyclosterols, emesterones A and B, from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 46: 850-852. [Google Scholar]
- Iwen PC, Rupp ME, Bishop MR, Rinaldi MG, Sutton DA, Tarantolo S, Hinrichs SH (1998). Disseminated aspergillosis caused by Aspergillus ustus in a patient following allogeneic peripheral stem cell transplantation. Journal of Clinical Microbiology 36: 3713-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SS, Dorr TE, Biberstein EL, Wong A (1986). Aspergillus deflectus infection in four dogs. Journal of Medical and Veterinary Mycology 24: 95-104. [PubMed] [Google Scholar]
- Jesus AE de, Horak RM, Steyn PS, Vleggaar R (1987). Metabolites of Aspergillus ustus. Part 4. Stable-isotope labelling studies on the biosynthesis of the austalides. Journal of the Chemical Society Perkin Transactions 1 1987: 2253-2257. [Google Scholar]
- Kahler JS, Leach MW, Jang S, Wong A (1990). Disseminated aspergillosis attributable to Aspergillus deflectus in a springer spaniel. Journal of the American Veterinary Medical Association 197: 871-874. [PubMed] [Google Scholar]
- Kanoh K, Kohno S, Asari T, Harada T, Katada J, Muramatsu M, Kawashima H, Sekiya H, Uno I (1997). (-)-Phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorganical and Medicinal Chemistry Letters 7: 2847-2852. [Google Scholar]
- Kawahara N, Nakajima S, Yamazaki M, Kawai K (1989). Structure of a novel epidithiodioxopiperazine, emethallicin A, a potent inhibitor of histamine release from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 37: 2592-2595. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Nozawa K, Nakajima S, Kawai K (1988). Emeheterone, a pyrazinone derivative from Emericella heterothallica. Phytochemistry 27: 3022-3024. [Google Scholar]
- Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai K (1990b). Structures of novel epipolythiodioxopiperazines, emethallicins B, C, and D, potent inhibitors of histamine release, from Emericella heterothallica. Chemical and Pharmaceutical Bulletin 38: 73-78. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai KI (1990a). Novel epidithiodioxopiperazines, emethallicins E and F, from Emericella heterothallica. Heterocycles 30: 507-515. [Google Scholar]
- Kfir R, Johannsen E, Vleggaar R (1986). Mutagenic activity of austocystins - secondary metabolites of Aspergillus ustus. Bulletin of Environmental Contaminants and Toxicology 37: 643-650. [DOI] [PubMed] [Google Scholar]
- Klich MA (1993). Morphological studies of Aspergillus section Versicolores and related species. Mycologia 85: 100-107. [Google Scholar]
- Kozakakiewicz Z (1989). Aspergillus species in stored products. Mycological Papers 161: 1-188. [Google Scholar]
- Kumar S, Tamura K, Nei M (2004). MEGA3: Integrated Software for Molecular Evolutionary Genetics Analysis and Sequence Alignment. Briefings in Bioinformatics 5: 150-163. [DOI] [PubMed] [Google Scholar]
- Malani AN, Kauffman CA (2007). Changing epidemiology of rare mould infections: implications for therapy. Drugs 67: 1803-1812. [DOI] [PubMed] [Google Scholar]
- Mizuno R, Kawahara N, Nozawa K, Yamazaki M, Nakajima S, Kawai K (1995). Stereochemistry of an 18,22-Cyclosterol, Mer-NF8054X, from Emericella heterothallica and Aspergillus ustus. Chemical and Pharmaceutical Bulletin 43: 9-11. [Google Scholar]
- Nakai K, Kanda Y, Mineishi S, Hori A, Chizuka A, Niiya H, Tanimoto T, Ohnishi M, Kami M, Makimoto A, Tanosaki R, Matsuno Y, Yamazaki N, Tobinai K, Takaue Y (2002). Primary cutaneous aspergillosis caused by Aspergillus ustus following reduced-intensity stem cell transplantation. Annals of Hematology 81: 593-596. [DOI] [PubMed] [Google Scholar]
- Panackal AA, Imhof A, Hanley EW, Marr KA (2006). Aspergillus ustus infections among transplant recipients. Emerging Infectious Diseases 12: 403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavie J, Lacroix C, Hermoso DG, Robin M, Ferry C, Bergeron A, Feuilhade M, Dromer F, Gluckman E, Molina JM, Ribaud P (2005). Breakthrough disseminated Aspergillus ustus infection in allogeneic hematopoietic stem cell transplant recipients receiving voriconazole or caspofungin prophylaxis. Journal of Clinical Microbiology 43: 4902-4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SW (2000). Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Samson RA, Pitt JI, eds. Amsterdam: Harwood Academic Publishers: 323-355.
- Rabie CJ, Steyn M, van Schalkwyk GC (1977). New species of Aspergillus producing sterigmatocystin. Applied and Environmental Microbiology 33: 1023-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raistrick H, Stickings CE (1951). Studies in the biochemistry of micro-organisms; ustic acid, a metabolic product of Aspergillus ustus (Bainier) Thom & Church. Biochemical Journal 48: 53-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper KB, Fennell DI (1965). The genus Aspergillus. Baltimore: Williams & Wilkins.
- Rath PM, Petermeier K, Verweij PE, Ansorg R (2002). Differentiation of Aspergillus ustus strains by random amplification of polymorphic DNA. Journal of Clinical Microbiology 40: 2231-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WF, Connole MD, King TJ, Pitt JI, Moss SM (2000). Systemic mycosis due to Aspergillus deflectus in a dog. Australian Veterinary Journal 78: 600-602. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC (eds) (2004). Introduction to food and airborne fungi. 7th ed. Utrecht: Centraal Bureau voor Schimmelcultures.
- Samson RA, Mouchacca J (1975). Additional notes on species of Aspergillus, Eurotium and Emericella from Egyptian desert soil. Antonie van Leeuwenhoek 41: 343-451. [DOI] [PubMed] [Google Scholar]
- Scott FE, Simpson TJ, Trimble LA, Vederas JC (1986). Biosynthesis of meroterpenoid austin by Aspergillus ustus: synthesis and incorporation of 13C, 18O-labelled ethyl 3,5 dimethylorsellinate. Journal of the Chemical Society. Chemical Communications 1986: 214-215. [Google Scholar]
- Singh SB, Smith JL, Sabnis GS, Dombrowski AW, Schaeffer JM, Goetz MA, Bills GF (1991). Structure and conformation of ophiobolin K y 6-epiophiobolin K from Aspergillus ustus as a nematocidal agent. Tetrahedron 47: 6931-6938. [Google Scholar]
- Smedsgaard J (1997). Micro-scale extraction procedure for standardized screening of fungla metabolite production in cultures. Journal of Chromatography A 760: 264-270. [DOI] [PubMed] [Google Scholar]
- Steyn PS (1971). Austamide, a new toxic metabolite from Aspergillus ustus. Tetrahedron Letters 1971: 3331-3334. [Google Scholar]
- Steyn PS (1973). The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 29: 107-120. [Google Scholar]
- Steyn PS, Vleggaar R (1974). Austocystins. Six novel dihydrofuro (3”,2”:4,5) furo(3,2-b)xanthenones from Aspergillus ustus. Journal of the Chemical Society Perkin Transactions 1 1974: 2250-2256. [PubMed] [Google Scholar]
- Stiller MJ, Teperman L, Rosenthal SA, Riordan A, Potter J, Shupack JL, Gordon MA (1994). Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. Journal of the American Academy of Dermatology 31: 344-347. [DOI] [PubMed] [Google Scholar]
- Swofford T (2000). PAUP: Phylogenetic analysis using parsimony. v. 4.0. Sunderland: Sinauer Associates.
- Tamura K, Nei M (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512-526. [DOI] [PubMed] [Google Scholar]
- Thom C, Church MB (1926). The aspergilli. Baltimore: Williams and Wilkins.
- Thom C, Raper KB (1945). Manual of the aspergilli. Baltimore: Williams and Wilkins.
- Tuomi T, Reijula K, Johnsson T, Hemminki K, Hintikka EL, Lindroos O, Kalso S, Koukila-Kahkola P, Mussalo-Rauhamaa H, Haahtela T (2000). Mycotoxins in crude building materials from water-damaged buildings. Applied and Environmental Microbiology 66: 1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilenko OV, Bezmelnitsyn NV, Keselman SA, Shulutko EM, Pivnik AV (2002). Fungemia caused by Aspergillus ustus in a patient with acute myeloblastic leukemia: case report Unpublished: See Entrez “AJ316611” at NCBI: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=nucleotide&list_uids=15706255&dopt=GenBank
- Verweij PE, van den Bergh MF, Rath PM, de Pauw BE, Voss A, Meis JF (1999). Invasive aspergillosis caused by Aspergillus ustus: case report and review. Journal of Clinical Microbiology 37: 1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleggaar R, Steyn PS, Nagel DW (1974). Constitution and absolute configuration of austdiol, the main toxic metabolite from Aspergillus ustus. Journal of the Chemical Society Perkin Transactions 1 1974: 45-49. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Thiemke WA (1983). Disseminated Aspergillus ustus infection following cardiac surgery. American Journal of Clinical Pathology 80: 408-411. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). New York: Academic Press: 315-322.
- Yabe K, Nakamura Y, Nakajima H, Ando Y, Hamasaki T (1991). Enzymatic conv. of norsolorinic acid to averufin in aflatoxin biosynthesis. Applied and Environmental Microbiology 57: 1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiran ST, Mutlu FM, Saracli MA, Uysal Y, Gonlum A, Sobaci G, Sutton DA (2006). Fungal endophthalmitis caused by Aspergillus ustus in a patient following cataract surgery. Medical Mycology 44: 665-669. [DOI] [PubMed] [Google Scholar]