Abstract
BmrI (ACTGGG N5/N4) is one of the few metal-independent restriction endonucleases (REases) found in bacteria. The BmrI restriction-modification system was cloned by the methylase selection method, inverse PCR, and PCR. BmrI REase shows significant amino acid sequence identity to BfiI and a putative endonuclease MspBNCORF3798 from the sequenced Mesorhizobium sp. BNC1 genome. The EDTA-resistant BmrI REase was successfully over-expressed in a pre-modified E. coli strain from pET21a or pBAC-expIQ vectors. The recombinant BmrI REase shows strong promiscuous activity (star activity) in NEB buffers 1, 4, and an EDTA buffer. Star activity was diminished in buffers with 100–150 mM NaCl and 10 mM MgCl2. His-tagged BmrI192, the N-terminal cleavage domain of BmrI, was expressed in E. coli and purified from inclusion bodies. The refolded BmrI192 protein possesses non-specific endonuclease activity. BmrI192 variants with a single Ser to Cys substitution (S76C or S90C) and BmrI200 (T200C) with a single Cys at the C-terminal end were also constructed and purified. BmrI200 digests both single-strand (ss) and double-strand (ds) DNA and the nuclease activity on ss DNA is at least 5-fold higher than that on ds DNA. The Cys-containing BmrI192 and BmrI200 nuclease variants may be useful for coupling to other DNA binding elements such as synthetic zinc fingers, thio-containing locked nucleic acids (LNA) or peptide nucleic acids (PNA).
Keywords: BmrI, EDTA-resistant nuclease, cleavage domain, chimeric endonuclease
INTRODUCTION
Type II restriction endonucleases (REases) are indispensable tools in creating recombinant DNA molecules. Over 240 unique specificities have been discovered from bacteria and viruses [1]. Most REases discovered to date require the divalent cation Mg++ for catalysis. Only two REases, BfiI and BmrI (5′-ACTGGG N5/N4-3′), have been shown to be EDTA resistant and do not require Mg++ for catalytic activity [2] (NEB 2007–08 catalog, page 29). The N-terminal domain of BfiI is structurally similar to Nuc, an EDTA-resistant nuclease from Salmonella typhimurium that belongs to the phospholipase D (PLD) family [3, 4]. The enzymes in the PLD family include phophodiesterases, bacterial nucleases, toxins, and phospholipases [5].
The catalytic mechanism of the Type IIS restriction endonuclease (REase) FokI has been studied in some detail [6, 7]. FokI forms a transient dimer involving two catalytic domains binding at the cognate DNA recognition sites, which activates the catalytic center and leads to a double strand (ds) break [7, 8]. The FokI catalytic domain (nuclease domain) can be physically separated from the DNA binding domain and coupled to other DNA binding elements such as zinc finger proteins. The resulting zinc finger nucleases (ZFNs) can be used to introduce ds breaks and facilitate homologous recombination in gene targeting [9–12].
Artificial endonucleases have also been constructed by fusing DNA binding protein with peptides that can bind to various divalent transition metal ions or organic complexes by formation of a hydrolytic or redox active site. These artificial endonucleases are capable of nicking or creating ds breaks in DNA (US patent #7,091,026) [13, 14]. However, the oxidative cleavage creates 5′ and 3′ nucleotides that are not suitable substrates for T4 or E. coli DNA ligases, which limits their application in molecular biology. A single Cys-containing Staphylococcal nuclease was constructed and successfully coupled to thio-containing oligos to generate a semisynthetic nuclease that was delivered to its target site by triple-helix formation [15]. About 75% of the plasmid DNA can be cleaved by this nuclease although multiple cut sites were detected on both strands. Our goal is to extend the possibility of using modular nucleases that can be readily coupled to other DNA binding elements.
In this work, we cloned and expressed the BmrI R-M system in E. coli. Although divalent cations are not required for the catalytic activity of BmrI REase, inclusion of Mg++ and NaCl in the reaction buffer increases BmrI substrate specificity. BmrI C-terminal deletion variants BmrI192, BmrI192-S76C, BmrI192-S90C, and BmrI200 (T200C) were also constructed, partially purified from inclusion bodies, refolded, and their non-specific endonuclease activity characterized. The purpose for the construction and purification of the single Cys-containing cleavage domains is to provide a platform where thio-containing LNA, PNA and DNA binding peptides can be coupled to the nuclease domains using simple chemistry.
MATERIALS AND METHODS
Bacterial strains and plasmid substrates
The T7 expression strain ER2566 (T7 Express) was provided by M. Sibley and E. Raleigh (NEB). The in vivo DNA damage indicator strain ER1992 with a dinD::lacZ fusion was used as described [16]. The native BmrI-producing strain was obtained from NEB strain collection (the original Bacillus megaterium strain was provided by T. Le). The T7 expression vector pET21a was purchased from Novagen.
Methylase selection procedure, inverse PCR, and DNA sequencing
Genomic DNA library construction and the methylase selection procedure were as described [17]. Inverse PCR was carried out to obtain the DNA sequence adjacent to the M gene. DNA sequencing was conducted using the AmpliTaq dideoxy terminator kit (Applied Biosystems) and an ABI Prism™ 377 sequencer.
Expression of the BmrI R-M system
A BamHI/SphI PCR fragment containing the two methylase genes, bmrIM1M2, was cloned into pACYC184 to generate a pre-modified strain ER2566 [pACYC-bmrIM1M2]. The constitutive expression of BmrI M1 and M2 was driven by the Tc resistance gene promoter. An NdeI/BamHI PCR fragment containing the entire bmrIR gene was cloned into pET21a and BmrI expression was induced by addition of IPTG to 0.5 mM (final concentration) to late-log phase cells. IPTG induction was carried out for 3–6 h. The single-copy-number vector pBAC-expIQ, whose copy number can be modulated by addition of IPTG was also used for BmrI expression as previously described [18].
BmrI restriction endonuclease purification
Recombinant BmrI was purified from cell extracts by a series of FPLC steps consisting of Heparin Sepharose, Source 15Q, Heparin Tsk and Mono S. The final eluted BmrI REase was dialyzed against a storage buffer of 250 mM NaCl, 10 mM Tris-HCl (pH 7.4), 0.1 mM EDTA, 1mM DTT, 50% glycerol, and stored at −20°C.
Restriction buffer compositions: NEB Buffer 1 (10 mM Bis Tris Propane-HCl, 10 mM MgCl2, 1 mM DTT, pH 7.0), Buffer 2 (10 mM Tris-HCl, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, pH 7.9), Buffer 3 (50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, 1 mM DTT, pH 7.9), Buffer 4 (20 mM Tris-acetate, 10 mM magnesium acetate, 50 mM potassium acetate, 1 mM DTT, pH 7.9), high salt buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM MgCl2, 1 mM DTT, pH 7.9). EDTA buffer (50 mM Tris-HCl, 100 mM NaCl, 10 mM EDTA, 1 mM DTT, pH 7.9). MgCl2 is absent in the EDTA buffer. Both Buffer 3 and the EDTA buffer contain 100 mM NaCl. One unit of BmrI REase was defined as the amount of enzyme required for complete digestion of 1 μg of λ DNA at 37°C for 1 h in a total volume of 50 μl in buffer 2.
BmrI192 construction, purification, and refolding
An NdeI/BamHI DNA fragment encoding the N-terminal domain with amino acid residues 1 to 192 (BmrI192) was amplified by PCR and cloned into pET21a. To facilitate mutagenesis and purification, 6xHis-tagged BmrI192 was also constructed in pUC19. Trial refolding of the BmrI192 protein was first carried out in buffers provided in a FOLDit protein refolding kit (Hamilton Research). The protein pellet (inclusion bodies) was resuspended in a buffer containing 7 M Guanidine-HCl, 20 mM NaH2PO4/Na2HPO4, pH 6.8, 0.5 M NaCl, 1 mM EDTA, 0.1% Triton X-100. Four ml of the solubilized protein was mixed with 80 ml of refolding buffer (50 mM Tris-HCl, pH 8.2, 200 mM NaCl, 10 mM MgCl2, 2 mM CaCl2, 400 mM sucrose, 500 mM L-Arg, 1 mM DTT) at 4°C for 30 min. The mixture was then dialyzed against 2 L of dialysis buffer (20 mM Tris-HCl, pH 8.2, 0.2 mM NaCl, 10 mM KCl, 1 mM DTT) at 4°C overnight. The refolded protein was further purified by chromatography through Heparin Sepharose. After extensive washing with a low salt buffer, proteins were eluted by a salt gradient of 0.2 to 1 M NaCl. Protein fractions were assayed for nuclease activity on pUC19 or λ DNA substrates. One unit of BmrI nuclease (non-specific) was defined as the amount of enzyme required for digestion of 1 μg of λ DNA into small fragments of less than 500 bp at 37°C for 1 h in a total volume of 50 μl in the EDTA buffer.
Ser76 and Ser90 were chosen for substitution to Cys because these two residues are located on the surface of the protein based on structural modeling onto the BfiI structure (PDB entry 2C1L) [3]. Site-directed mutagenesis was carried out using mutagenic primers by 2-step overlapping PCR on pUC-BmrI192 template. To monitor in vivo DNA damage activity by SOS induction, pUC-BmrI192, pUC-BmrI192-S76C, pUC-BmrI192-S90C, and pET21a-BmrI200 plasmids were introduced into the DNA damage indicator strain ER1992 by transformation. Transformants were plated on Amp/X-gal rich agar plates. The formation of medium to dark blue colonies indicates the presence of in vivo DNA damage (induction of dinD::lacZ fusion and elevated β–galactosidase activity). After mutagenesis and sequence confirmation, the BmrI192–Cys variants with a C-terminal 6xHis tag were subcloned into pET21a for protein production. The 6xHis-tagged BmrI192 variants S76C and S90C were purified and refolded in a procedure described below for the 6xHis-tagged BmrI200.
BmrI nuclease variants were also purified from soluble fractions (cell extract supernatant). In such cases, cells were cultured at 30°C to late log phase and IPTG induction was carried out at 16°C overnight. 6xHis-tagged proteins were purified by chromatography through Ni-NTA columns (Qiagen). Protein elution was carried out by applying a gradient of 100–250 mM imidazole. The purified protein was dialyzed in a storage buffer (50 mM Tris-HCl, pH 7.9, 50 mM NaCl, 1 mM DTT, 50% glycerol) and stored at −20°C.
BmrI200 construction, purification and refolding
The coding sequence for the N-terminal domain of BmrI, 6xHis-BmrI200 was amplified by PCR and cloned into the NdeI/BamHI sites of pET21a. In BmrI200, the Thr200 codon was changed to a Cys codon. A 6xHis-tag was introduced into the N-terminus of the protein to facilitate protein purification.
To improve the protein refolding procedure, a trial refolding of BmrI nuclease was carried out using a protein refolding kit (US Biological). After the appropriate refolding condition was found, the refolding procedure was scaled up. One liter of cells was cultured at 37°C to late log phase and IPTG induction of BmrI nuclease synthesis was carried out for 3 h. The cell pellet (~ 5 g) was resuspended in 60 ml of lysis buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 1 mg/ml lysozyme). The lysate was kept on ice for 30 min and then centrifuged at 15,000 g for 20 min at 4°C. The BmrI200 nuclease was mainly found in the insoluble fraction (inclusion bodies). The inclusion bodies were resuspended in 30 ml of washing buffer (2 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 8.0) and kept on ice for 30 min. After centrifugation at 15,000 g for 20 min at 4°C, the inclusion bodies were resuspended in 30 ml of denaturing buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 8.0) and kept on ice for 30 min. The denatured protein solution was centrifuged at 15,000 g for 20 minutes at 4°C. The supernatant was loaded onto four 1 ml Ni-NTA columns (Qiagen). After washing with wash buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 6.3), the bound protein was eluted using an elution buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 4.5). The eluted protein (~ 8 ml) was then dialyzed in 500 ml of refolding buffer (50 mM Tris-HCl, pH 8.5, 10 mM NaCl, 0.4 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 0.4 M sucrose, 0.5 % Triton X-100, 0.05% PEG 3350, 1 mM GSH, 0.1 mM GSSH) at 4°C overnight. The refolded protein was dialyzed against the storage buffer (10 mM Tris-HCl, 250 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 0.5 mg/ml BSA, pH 7.4) and stored at 4°C. For long-term storage at −20°C, 50% glycerol was included in the storage buffer.
RESULTS
Cloning and expression of the BmrI R-M system
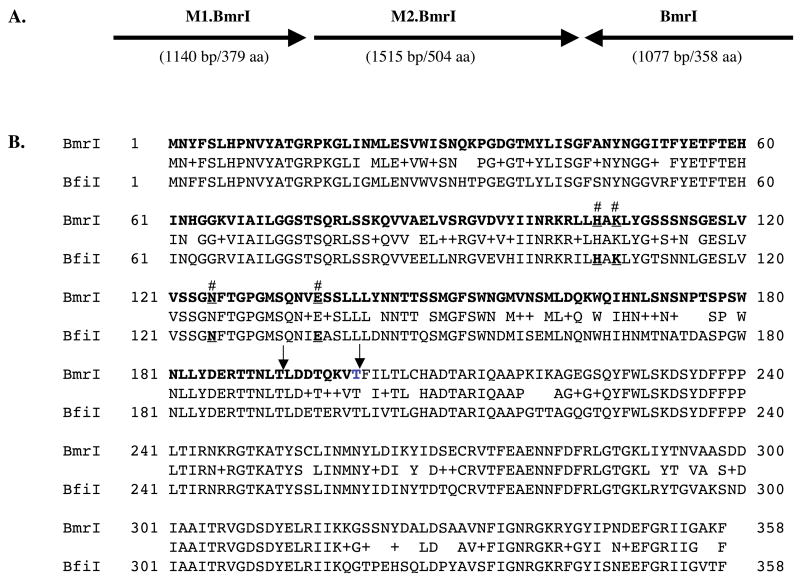
BmrI is an EDTA-resistant REase discovered in Bacillus megaterium. The native strain has a low enzyme yield (S.S. unpublished result). In order to fully characterize BmrI, we first attempted to clone the BmrI R-M system. The bmrIM1 gene was first cloned by the methylase selection method from a partial HindIII genomic DNA library following a published procedure [17, Lunnen, 1988 #1067]. Four rounds of chromosomal walking by inverse PCR and sequencing identified bmrIM2 and bmrIR coding sequences adjacent to the M1 genes. The bmrIM1M2 genes and bmrIR gene are transcribed in opposite directions, analogous to the BfiI R-M system (Figure 1A) [19]. The BmrI and BfiI REases are highly homologous, sharing 78% amino acid sequence identity and 89% amino acid sequence similarity (Figure 1B). The critical residues required for BfiI catalytic activity are all conserved in BmrI (His105, Lys107, Asn125, and Glu136). Substitution of each of these four catalytic residues in BmrI with alanine eliminated the BmrI restriction activity (L.H., unpublished results). Similarly, M1.BmrI and M2.BmrI show 77% and 76% amino acid sequence identity to M1.BfiI and M2.BfiI, respectively (data not shown). The BmrI REase also shares 60% amino acid sequence identity to a putative endonuclease MspBNCORF3798 identified from a sequenced bacterial genome of Mesorhizobium sp. BNC1 (GenBank: CP000390). The putative BmrI isoschizomer was accompanied by two amino methyltransferase genes transcribed in the same direction.
Figure 1.
A. Schematic diagram of BmrI R-M gene organization. B. Amino acid sequence alignment of BmrI and BfiI REases. Active site residues H, K, N, and E are underlined and indicated by #. Plus (+) indicates amino acid residues with similar properties. The N-terminus nuclease domain (residues 1 to 200) is shown in bold. The arrow indicates the end point of BmrI192 and BmrI200 cleavage domain.
The over-expression of the BmrI REase was carried out in a T7 expression vector and in a single-copy expression vector pBAC-expIQ. BmrI restriction activity was detected in IPTG-induced cell extracts of ER2566 [pACYC-bmrIM1M2, pET21a-bmrIR] on λ (HindIII-precut) DNA substrate (data not shown). The BmrI yield from the T7 expression clone was estimated at 80,000 units/gram of wet cells. Compared to the native BmrI-producing strain with 2,500 units of BmrI/gram of wet cells, the recombinant clone over-produced the enzyme approximately 32-fold. The bmrIR gene was also cloned into a single-copy-vector pBAC-expIQ and expressed in a pre-modified strain ER2683 [pACYC-bmrIM1M2]. Under IPTG-induced condition, the copy number of pBAC-expIQ can be increased over 100-fold [18]. The enzyme yield was estimated at 60,000 units/gram of wet cells. The recombinant BmrI activity was detected in IPTG-induced cell extracts (Figure 2, lanes 1 to 15). The low-copy-number feature of the pBAC-expIQ plasmid contributed to the stability of the expression clone and the BmrI enzyme yield was reasonably reproducible.
Figure 2.
Recombinant BmrI restriction activity in cell extracts. IPTG-induction was carried out for 4 h (lanes 1 to 7) or 6 h (lane 8 to 15). Restriction activity assays were carried out in NEB buffer 2. Lanes 1 to 7, 4 μl, 2 μl, 1 μl of cell extract, and 1 μl of 2-fold serial dilutions at 1/2, 1/4, 1/8, and 1/16, respectively. Lanes 8–15, 4 μl, 2 μl, 1 μl of cell extract, and 1 μl of 2-fold serial dilutions at 1/2, 1/4, 1/8, 1/16, 1/32, respectively. Lane 16, 4 μl E. coli cell extract without BmrI. Lane 17, uncut λ DNA. Lanes 18 and 19, λ DNA digested by native BmrI endonuclease. Lane 20, 1 kb DNA ladder.
Purification of the BmrI restriction endonuclease and activity assays
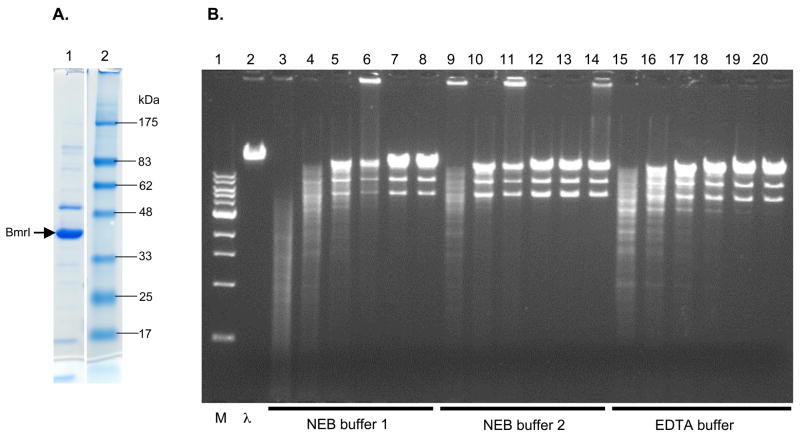
The BmrI REase was partially purified by multiple column chromatography. Figure 3A shows the purified protein as analyzed on SDS-PAGE. There is a major protein band corresponding to the predicted size of the BmrI endonuclease at ~40 kDa. The enzyme is fully active in digestion of λ and plasmid DNA in an EDTA buffer (see results below). The specific activity for recombinant BmrI REase was 50,000 units per mg of protein on λ DNA, which was similar to many Type IIS REases with low turn over numbers.
Figure 3.
A. Purified recombinant BmrI REase. BmrI endonuclease (~40 kDa) was indicated by an arrow (lane 1). Lane 2, pre-stained protein size marker. B. BmrI digestion of λ DNA in NEB buffer 1, buffer 2, and EDTA buffer. Lane 1, 1 kb DNA size marker; lane 2, uncut λ DNA. Lanes 3, 9, 15 = digestion with 128 units of BmrI; lanes 4–8, 10–14, 16–20, digestion of λ DNA with diluted enzyme (by 2-fold serial dilutions). The BmrI unit definition was carried out in buffer 2 as the enzyme required to completely digest 1 μg of λ DNA at 37°C for 1 h in a 50 μl reaction volume. C. BmrI digestion of λ DNA in NEB buffer 3, buffer 4, and high salt (150 mM NaCl) buffer. Lane 1, 1 kb
We next examined the BmrI restriction activity on various buffers to determine whether it showed promiscuous endonuclease activity or star activity (star activity was defined as relaxed specificity and cleavage on miscongate or non-cognate sites). BmrI displays strong non-specific endonuclease activity (relaxed sequence specificity) in low salt buffer (NEB buffer 1, Figure 3B, lanes 3–6) with 5% glycerol. The cleavage specificity could be totally relaxed as no specific bands were detected after BmrI digestion (Figure 3B, lane 3). Similarly, BmrI also displays strong star activity in EDTA buffer with 5% glycerol (Figure 3B, lanes 15–18). BmrI star activity was significantly inhibited in buffer 2 containing 50 mM NaCl and 10 mM Mg++ (Figure 3B, lanes 10–14). BmrI star activity was further diminished in 100 mM NaCl and 10 mM Mg++ (Figure 3C, lanes 3–8). BmrI also shows strong star activity in buffer 4 (Figure 3C, lanes 9–12). BmrI star activity was low in a high salt buffer with 150 mM NaCl and 10 mM Mg++, but BmrI restriction activity was also reduced approximately 4-fold in comparison to the activity in buffer 2 (Figure 3C, lanes 15–20). Divalent cations seem to inhibit the BmrI non-specific endonuclease activity. This is not due to a drastic reduction in the BmrI restriction activity since enzyme activity was comparable in NEB buffer 3 and EDTA buffer through exhaustive serial dilution of the enzyme and activity assays on λ and pBR322 (no more than 2-fold difference in restriction activity on both substrates, data not shown). In summary, BmrI digestion can be carried out in all four NEB buffers and in the EDTA buffer. However, over-digestion (more than 8-fold) should be carried out in buffer 2 or 3 to minimize star activity (promiscuous activity/relaxed specificity). The specific sequences of BmrI star sites remain to be characterized by cloning and sequencing of a large number of cleaved star fragments. Previous studies of restriction enzyme star sites have shown that star sites are usually 1–2 bases deviation from the cognate site [20, Izsvak, 1989 #767, Robinson, 1993 #3399]. In addition, we found that star site sequence variations are quite different in the presence of high concentration of glycerol or 20% of DMSO (I. Murray and SYX, unpublished results).
Temperature profile of recombinant BmrI REase
BmrI is active at a temperature range of 30°C to 42°C. It is partially active at 50°C (~40% activity). BmrI activity was inactivated at 65°C (data not shown).
Expression and purification of the BmrI N-terminal nuclease domain
The N-terminus of BfiI was identified as a non-specific nuclease based on biochemical, genetic, and structural studies [21–23]. Based on the BfiI/BmrI amino acid sequence alignment, two versions of the N-terminal domains, BmrI192 (residues 1 to 192), and BmrI200 (residues 1–200, residue Thr200 substituted for Cys) were independently expressed in E. coli. The immediate goal of this experiment was to purify the N-terminus cleavage domain of the BmrI REase (with the ultimate goal of employing BmrI cleavage domain for coupling to other DNA binding elements). BmrI192 nuclease contains most of the natural linker sequence (aa 171–198) that connects the nuclease domain and specificity domain in the native enzyme. BmrI200 nuclease carries the entire linker sequence. The BmrI192 protein was predominantly found in inclusion bodies when IPTG-induction was carried out at 37°C (induction at 16°C improved the solubility with lower protein yield, see below). After protein refolding, and Heparin Sepharose column chromatography, BmrI192 nuclease protein was partially purified (Figure 4A, lane 1). Figure 4B (lanes 3–10) shows the non-specific nuclease activity of BmrI192 in EDTA buffer on pUC19 DNA. It was noted that the supercoiled DNA was converted to nicked-circular and linear forms before digesting into smaller fragments due to its non-specific endonuclease activity (lanes 3,4,8, and 9). How randomly the nicking and ds cleavage occur has not been studied in details. E. coli cell extracts of the expression host showed non-detectable nuclease activity in the EDTA buffer (data not shown). In addition, ER2566 is deficient in DNAseI which requires divalent cations for optimal activity.
Figure 4.
A longer version of the N-terminal nuclease domain of BmrI (BmrI 200) was also constructed and partially purified. After affinity purification under denatured condition and refolding, the non-specific BmrI nuclease activity was re-established. Figure 5A shows the purified protein after affinity purification through a Ni-NTA column (lanes 2 to 5). The non-specific nuclease activity was assayed on λ DNA (Figure 5B, lanes 1–6, and 8). λ DNA was digested into fragments in less than 500 bp with increasing amounts of BmrI200 (Figure 5B, lanes 4–6). We then compared the BmrI nuclease activity in EDTA buffer (10 mM EDTA, 100 mM NaCl) and Buffer 3 (10 mM MgCl2, 100 mM NaCl). Surprisingly, the BmrI non-specific nuclease activity was inhibited by the presence of 10 mM Mg++ (lane 8). The BmrI200 enzyme input was the same in lanes 4 and 8 except the difference in the buffer compositions. Comparing the digestion patterns of lanes 1 and 8, the relative nuclease activities in EDTA buffer and buffer 3 are estimated at 50 to 1. The inhibition of nuclease activity by Mg++ in buffer 3 could be partially alleviated by adding 20 mM EDTA to the reaction buffer (data not shown).
Figure 5.
Attempts to purify BmrI192 and BmrI200 nucleases from soluble fractions derived from 16°C IPTG-induced cell extracts produced highly active enzymes with lower yield (data not shown). A scale-up procedure remains to be optimized.
We also tested DNA nicking/cleavage activity of BmrI200 on pUC19 dsDNA and M13 ssDNA. Under limited digestion conditions, BmrI200 converted ~80% of supercoiled DNA into linear and nicked circular DNA (Figure 6, lane 3) before digesting the DNA into smaller fragments (Figure 6, lanes 4–6). Thus, BmrI192 and BmrI200 nucleases are most likely DNA nicking endonucleases that predominantly nick pUC19 dsDNA into nicked circular and linear DNA (two nicks introduced on opposite strands nearby result in generating linear DNA). Extensive nicking of dsDNA leads to the collapse of the large DNA into small fragments. BmrI200 is fully active on M13 ssDNA. 0.2 unit of BmrI200 is sufficient to digest 1 μg of M13 ssDNA into small oligos (Figure 6, lane 10). BmrI200 displays at least 5-fold higher nuclease activity on ssDNA than dsDNA (comparing digestion patterns in lane 5 on dsDNA substrate and lane 10 on ssDNA substrate, both lanes showed a complete break down of DNA, and there was a difference of 5-fold in the amount of enzyme used).
Figure 6.

BmrI200 nuclease activity in buffers with high NaCl concentration
BmrI200 nuclease is active in EDTA buffers with 50 mM-0.2 M NaCl concentrations (Figure 7, lanes 2 and 3; data not shown). In EDTA buffers with 0.3–0.6 M NaCl concentration (Figure 7, lanes 4–7), BmrI200 nuclease displays reduced activity and predominantly nicks DNA to generate nicked circular DNA and small amount of linear DNA.
Figure 7.
SOS induction by the BmrI nuclease domain
To confirm the in vivo DNA damage activity and SOS induction, plasmids carrying the BmrI192 and BmrI200 nuclease variants were transformed into the dinD::lacZ indicator strain ER1992 and transformants were plated at 30°C on X-gal plates. Transformants carrying BmrI192, BmrI192-S76C, BmrI192-S90C, and BmrI200 alleles formed dark blue colonies under non-induced conditions, indicating DNA damage and strong SOS induction in vivo. A slightly longer version, BmrI204, also induced SOS response in vivo as judged by the formation of medium blue colonies on X-gal indicator plates. The negative control with vectors formed light blue colonies (data not shown). Presumably, the leaky expression from the lac promoter of pUC19 or cryptic promoter of the pET21a vector produced sufficient nuclease to generate the in vivo DNA damage under non-induced conditions.
DISCUSSION
BmrI cleaves N5/N4 downstream of its recognition sequence ACTGGG. Similarly to its extensively studied isoschizomer BfiI, BmrI is an EDTA-resistant endonuclease that contains two distinct functional domains. The amino acid residues His105, Lys107, Asn125, and Glu136 are conserved in both BfiI and BmrI REases and are located in the same position in the amino acid sequence alignment with Salmonella typhimurium Nuc nuclease [3]. The recombinant BmrI REase shows star activity in low salt buffer or EDTA buffer with 5% glycerol. The BmrI star activity can be inhibited by addition of 50 mM to 150 mM NaCl in the presence of 10 mM Mg++. The addition of Mg++ may enhance the DNA binding specificity or inhibit the non-specific nuclease activity. It was demonstrated before that addition of divalent cations increased EcoRV specificity by over a thousand fold in a DNA mobility shift assay [24]. Under high salt conditions, the hydration status of the DNA/nuclease complex and water-mediated hydrogen bond network could be significantly altered. It was shown recently that KpnI REase displays strong promiscuous activity in the presence of Mg++ or Mn++. The substrate specificity of KpnI can be greatly increased by addition of Ca++. A single amino acid change in KpnI (H149A) also abolished KpnI promiscuous activity [25].
The BmrI REase was over-expressed approximately 32-fold in the T7 expression vector pET21a (bmrIM1M2 genes were constitutively expressed from pACYC184). Another expression strategy was to clone the bmrIR gene in a single-copy-number plasmid pBACexpIQ [18]. The pBACexpIQ-bmrIR plasmid copy number (gene dosage) was elevated by addition of IPTG for enzyme production. This expression method can be applied to other endonucleases and toxic proteins that are difficult to express. The use of a single-copy vector to express toxic gene products had been reported previously [26]. Although the final enzyme yield is slightly lower in the pBACexpIQ plasmid than pET21a, the clone stability seems to improve with the single-copy expression vector. In large-scale fermentation, it is important to have a reproducible and stable production clone.
Majority of REases utilizes the catalytic motif PDX10–30 D/EXK for Mg++-dependent hydrolysis of DNA. A few restriction endonucleases such as KpnI, MnlI, and HphI belong to the HNH superfamily REases with a His residue acting as a general base to polarize a water molecule for breaking the scissile phosphate bond [25, Kriukiene, 2005 #5742, Cymerman, 2006 #5807]. BmrI and BfiI REases are grouped into the phospholipase D (PLD) superfamily because of their conserved catalytic residues H-K-NE and the metal-independent endonuclease activity. The enzymes in the PLD superfamily include phophodiesterases, bacterial nucleases, toxins, and phospholipases. The structure of BfiI REase has been solved recently [3]. The N-terminal domain of BfiI shows extensive similarity to the Nuc nuclease found in Salmonella typhimurium. The BfiI C-terminal domain is structurally similar to the N-terminal domain of the Type IIE enzyme EcoRII and the DNA binding domain of plant transcription factor RAV1. The BfiI nuclease domain and the specificity domain are held together by a 28-aa linker, which may serve as an auto-inhibitor of endonuclease activity on non-cognate DNA sites [3]. BfiI also forms a covalent intermediate with substrate during hydrolysis of dsDNA [27].
It has been demonstrated previously that Tf nuclease, BfiI nuclease domain, restriction endonucleases and their mutants induced SOS response in vivo and formed blue colonies in the dinD::lacZ indicator strain [28, Fomenkov, 1995 #5699, Heitman, 1991 #2242, Sapranauskas, 2005 #5785]. In most cases, the blue color indicator correlates well with the endonuclease activity in vitro. However, in a few cases, blue colonies were isolated due to the over-expression of transposases from insertion elements (SYX, unpublished result). The use of plasmid vectors with low to medium copy numbers also reduced the background blue color.
The construction and purification of active BmrI200 with a C-terminal Cys, BmrI192-S76C, and BmrI192-S90C make it possible for the enzyme to be coupled to DNA binding peptides, Cys-LNA (locked nucleic acids), Lys-LNA, Cys-PNA (peptide nucleic acids) or Lys-PNA by disulfide bond formation, expressed protein ligation, or by bi-functional chemical cross-linkers [29, Takeda, 2004 #5809, Evans, 1998 #5256]. The BmrI cleavage domain (BmrI192 or Bmr200) is a “slow” non-specific endonuclease that may be used to generate small DNA fragments (50 bp – 500 bp) from genomic DNA for “shot-gun” DNA sequencing.
Coupling of a single-chain PvuII endonuclease to an LNA oligo by a chemical cross-linker to generate artificial endonuclease has been demonstrated before. The chimeric endonuclease can be introduced to the target site by triple helix formation [30]. The modular property of the BmrI cleavage domain has been utilized in construction of fusion endonucleases. Fusion of the BmrI cleavage domain (BmrI198) to DNA binding proteins such as C.BclI protein and a cleavage-deficient NotI variant in vivo have been demonstrated previously. In such experiments, the chimeric endonucleases cleave outside of C-box sequence or NotI site (S.-h. Chan et al, Nucl. Acids Res. doi:10.1093/nar/gkm665; P. Zhang et al. Protein Engineering, Design, and Selection, doi:10.1093/protein/gzm049). Endonucleases that recognize more than 12 bp are useful reagents to introduce ds DNA breaks to facilitate homologous recombination in gene targeting. The availability of modular cleavage domains that can be easily coupled to other DNA binding elements should provide more tools to genetic engineers and complement the use of zinc finger nucleases and redesigned homing endonucleases.
Supplementary Material
Acknowledgments
We thank Rich Roberts for critical reading of the manuscript, Jim Samuelson, Mern Sibley, and Elisabeth Raleigh for providing strains and plasmid, the NEB Organic Synthesis Division for providing oligos, and DNA sequencing lab for DNA sequencing, and Vernissia Tam for help with BmrI nuclease purification. This work was partially supported by an SBIR grant (1R43 GM073345-01, NIH) to SYX. We thank Don Comb, Jim Ellard and Rich Roberts for support. The sequence of the BmrI R-M system has been deposited in Genbank with the accession number EF143916.
Abbreviations
- BmrI192
N-terminus nuclease domain including amino acids 1 to 192
- BmrI200
N-terminus nuclease domain including amino acids 1 to 200 (with Thr200 to Cys substitution, T200C)
- ds
double-strand
- REase
restriction endonuclease
- R-M
restriction modification system
Footnotes
Individual contributions to this work: Y. Bao over-expressed BmrI in pBACexpIQ and was responsible for the purification and refolding of 6xHis-tagged BmrI192, BmrI192 Ser-to-Cys variants, BmrI200, and preliminary star activity assays for wt BmrI REase. L. Higgins cloned the bmrIM2 and bmrIR genes and expressed bmrIR under the T7 promoter. P. Zhang analyzed the star activity of recombinant BmrI in various buffers and the temperature profile of BmrI activity. S.-h. Chan constructed pET21a-BmrI192, refolding of BmrI192, homologous structure modeling of BmrI, and examined the E. coli background nuclease activity in the EDTA buffer. S. Laget constructed BmrI192-S76C, -S90C, and BmrI204 variants. K. Lunnen constructed genomic DNA libraries and cloned the bmrIM1 gene, S. Sweeney purified the native and recombinant BmrI REase. S.-y. Xu constructed BmrI200, assayed nuclease activities on ds and ssDNA substrates, and at high salt concentration, designed experiments, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts R, Vincze T, Posfai J, Macelis D. REBASE--enzymes and genes for DNA restriction and modification. Nucl Acids Res. 2007;35:269–270. doi: 10.1093/nar/gkl891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sapranauskas R, Sasnauskas G, Lagunavicius A, Vilkaitis G, Lubys A, Siksnys V. Novel subtype of type IIs restriction enzymes. BfiI endonuclease exhibits similarities to the EDTA-resistant nuclease Nuc of Salmonella typhimurium. J Biol Chem. 2000;275:30878–30885. doi: 10.1074/jbc.M003350200. [DOI] [PubMed] [Google Scholar]
- 3.Grazulis S, Manakova E, Roessle M, Bochtler M, Tamulaitiene G, Huber R, Siksnys V. Structure of the metal-independent restriction enzyme BfiI reveals fusion of a specific DNA-binding domain with a nonspecific nuclease. Proc Natl Acad Sci U S A. 2005;102:15797–15802. doi: 10.1073/pnas.0507949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuckey JA, Dixon JE. Crystal structure of a phospholipase D family member. Nat Struct Biol. 1999;6:278–284. doi: 10.1038/6716. [DOI] [PubMed] [Google Scholar]
- 5.Ponting CP, Kerr ID. A novel family of phospholipase D homologues that includes phospholipid synthases and putative endonucleases: identification of duplicated repeats and potential active site residues. Protein Sci. 1996;5:914–922. doi: 10.1002/pro.5560050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wah DA, Bitinaite J, Schildkraut I, Aggarwal AK. Structure of FokI has implications for DNA cleavage. Proc Natl Acad Sci U S A. 1998;95:10564–10569. doi: 10.1073/pnas.95.18.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc Natl Acad Sci U S A. 1998;95:10570–10575. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanamee ES, Santagata S, Aggarwal AK. FokI requires two specific DNA sites for cleavage. J Mol Biol. 2001;309:69–78. doi: 10.1006/jmbi.2001.4635. [DOI] [PubMed] [Google Scholar]
- 9.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: Zinc finger fusions to FokI cleavage domain. Proc Natl Acad Sci USA. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanasekaran M, Negi S, Sugiura Y. Designer zinc finger proteins: tools for creating artificial DNA-binding functional proteins. Acc Chem Res. 2006;39:45–52. doi: 10.1021/ar050158u. [DOI] [PubMed] [Google Scholar]
- 11.Moehle EA, Rock JM, Lee YL, Jouvenot Y, DeKelver RC, Gregory PD, Urnov FD, Holmes MC. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci U S A. 2007;104:3055–3060. doi: 10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 13.Ebright RH, Ebright YW, Pendergrast PS, Gunasekera A. Conversion of a helix-turn-helix motif sequence-specific DNA binding protein into a site-specific DNA cleavage agent. Proc Natl Acad Sci U S A. 1990;87:2882–2886. doi: 10.1073/pnas.87.8.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatsukasa T, Shiraishi Y, Negi S, Imanishi M, Futaki S, Sugiura Y. Site-specific DNA cleavage by artificial zinc finger-type nuclease with cerium-binding peptide. Biochem Biophys Res Commun. 2005;330:247–252. doi: 10.1016/j.bbrc.2005.02.164. [DOI] [PubMed] [Google Scholar]
- 15.Pei D, Corey DR, Schultz PG. Site-specific cleavage of duplex DNA by a semisynthetic nuclease via triple-helix formation. Proc Natl Acad Sci U S A. 1990;87:9858–9862. doi: 10.1073/pnas.87.24.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fomenkov A, Xu SY. Cloning of a gene from Thermus filiformis and characterization of the thermostable nuclease. Gene. 1995;163:109–113. doi: 10.1016/0378-1119(95)00426-7. [DOI] [PubMed] [Google Scholar]
- 17.Szomolanyi I, Kiss A, Venetianer P. Cloning the modification methylase gene of Bacillus sphaericus R in Escherichia coli. Gene. 1980;10:219–225. doi: 10.1016/0378-1119(80)90051-7. [DOI] [PubMed] [Google Scholar]
- 18.Samuelson JC, Morgan RD, Benner JS, Claus TE, Packard SL, Xu SY. Engineering a rare-cutting restriction enzyme: genetic screening and selection of NotI variants. Nucleic Acids Res. 2006;34:796–805. doi: 10.1093/nar/gkj483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitkute J, Maneliene Z, Petrusyte M, Janulaitis A. BfiI, a restriction endonuclease from Bacillus firmus S8120, which recognizes the novel non-palindromic sequence 5′-ACTGGG(N)5/4-3′. Nucleic Acids Res. 1998;26:3348–3349. doi: 10.1093/nar/26.14.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner RC, Howarth AJ, Messing J, Shepherd RJ. Cloning and sequencing of restriction fragments generated by EcoRI*. DNA. 1982;1:109–115. doi: 10.1089/dna.1.1982.1.109. [DOI] [PubMed] [Google Scholar]
- 21.Sasnauskas G, Halford SE, Siksnys V. How the BfiI restriction enzyme uses one active site to cut two DNA strands. Proc Natl Acad Sci U S A. 2003;100:6410–6415. doi: 10.1073/pnas.1131003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapranauskas R, Lubys A. Random gene dissection: a tool for the investigation of protein structural organization. Biotechniques. 2005;39:395–402. doi: 10.2144/05393RR01. [DOI] [PubMed] [Google Scholar]
- 23.Zaremba M, Urbanke C, Halford SE, Siksnys V. Generation of the BfiI restriction endonuclease from the fusion of a DNA recognition domain to a non-specific nuclease from the phospholipase D superfamily. J Mol Biol. 2004;336:81–92. doi: 10.1016/j.jmb.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Thielking V, Selent U, Kohler E, Landgraf Z, Wolfes H, Alves J, Pingoud A. Mg2+ confers DNA binding specificity to the EcoRV restriction endonuclease. Biochemistry. 1992;31:3727–3732. doi: 10.1021/bi00130a001. [DOI] [PubMed] [Google Scholar]
- 25.Saravanan M, Vasu K, Kanakaraj R, Rao DN, Nagaraja VR. KpnI, an HNH superfamily REase, exhibits differential discrimination at non-canonical sequences in the presence of Ca2+ and Mg2+ Nucleic Acids Res. 2007;35:2777–2786. doi: 10.1093/nar/gkm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild J, Szybalski W. Copy-control pBAC/oriV vectors for genomic cloning. Methods Mol Biol. 2004;267:145–154. doi: 10.1385/1-59259-774-2:145. [DOI] [PubMed] [Google Scholar]
- 27.Sasnauskas G, Connolly BA, Halford SE, Siksnys V. Site-specific DNA transesterification catalyzed by a restriction enzyme. Proc Natl Acad Sci U S A. 2007;104:2115–2120. doi: 10.1073/pnas.0608689104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fomenkov A, Xiao JP, Dila D, Raleigh E, Xu SY. The ‘endo-blue method’ for direct cloning of restriction endonuclease genes in E. coli. Nucleic Acids Res. 1994;22:2399–2403. doi: 10.1093/nar/22.12.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovrinovic M, Seidel R, Wacker R, Schroeder H, Seitz O, Engelhard M, Goody RS, Niemeyer CM. Synthesis of protein-nucleic acid conjugates by expressed protein ligation. Chem Commun (Camb) 2003;7:822–823. doi: 10.1039/b212294d. [DOI] [PubMed] [Google Scholar]
- 30.Eisenschmidt K, Lanio T, Simoncsits A, Jeltsch A, Pingoud V, Wende W, Pingoud A. Developing a programmed restriction endonuclease for highly specific DNA cleavage. Nucleic Acids Res. 2005;33:7039–7047. doi: 10.1093/nar/gki1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.