Abstract
Purpose
Activation of polyol pathway due to increased aldose reductase (ALR2) activity has been implicated in the development of diabetic complications including diabetic retinopathy (DR), a leading cause of blindness. However, the relationship between hyperglycemia-induced activation of polyol pathway in retina and DR is still uncertain. We investigated the relationship between ALR2 levels and human DR by measuring ALR2 activity and its product, sorbitol, in erythrocytes.
Methods
We enrolled 362 type 2 diabetic subjects (T2D) with and without DR and 66 normal subjects in this clinical case-control study. Clinical evaluation of DR in T2D patients was done by fundus examination. ALR2 activity and sorbitol levels along with glucose and glycosylated hemoglobin (HbA1C) levels in erythrocytes were determined.
Results
T2D patients with DR showed significantly higher specific activity of ALR2 as compared to T2D patients without DR. Elevated levels of sorbitol in T2D patients with DR, as compared to T2D patients without DR, corroborated the increased ALR2 activity in erythrocytes of DR patients. However, the increased ALR2 activity was not significantly associated with diabetes duration, age, and HbA1C in both the DR group and total T2D subjects.
Conclusions
Levels of ALR2 activity as well as sorbitol in erythrocytes may have value as a quantitative trait to be included among other markers to establish a risk profile for development of DR.
Introduction
About 200 million people across the globe are estimated to have diabetes of which Southeast Asia alone is home to 46.9 million diabetics and India has 41 million diabetics [1,2]. Type 2 diabetes (T2D) accounts for roughly 90 percent of all diagnosed cases of diabetes [2]. The prevalence of diabetes in India is estimated to be between 5.9%–24.2% (average of 12.1%) [3,4]. It is higher in developed countries compared to developing countries. While in developing countries the average age of people with diabetes is between 45 and 64 years of age, in developed nations it is 65 years and older. These statistics indicate that the world, particularly India, is facing a growing diabetes epidemic of potentially devastating proportions. Prolonged exposure to chronic hyperglycemia, without proper management, can lead to various short-term and long-term secondary complications, both of macro and microvascular nature, which represent the main cause of morbidity and mortality in diabetic patients [5]. Hyperglycemia is the major determinant of microvascular complications in diabetes [6,7].
Diabetic retinopathy (DR), a vascular disorder affecting the microvasculature of the retina, is a leading cause of adult blindness and is the most common complication of diabetes [8]. It is estimated that DR develops in more than 75% of diabetics who have had diabetes for 15–20 years. It is projected that by 2005, diabetes will affect 300 million people worldwide, of whom 10% will develop visual impairment secondary to DR [9]. While a study reported the prevalence of DR among diabetic subjects (both rural and urban) in India was 10% [10], another study reported the prevalence of DR about 17% among urban diabetic subjects in India [11]. In a clinical study the prevalence of DR was 34% among T2D patients [12]. The prevalence of DR was 0.5% in the general rural populations of Southern India (this in total population but not among diabetics) and 10.5% among diabetic patients [13].
Although the exact mechanism involved in the pathogenesis is not known, many biochemical pathways associated with hyperglycemia have been implicated in the development of diabetic complications including DR. These include glucose autoxidation, polyol pathway, prostanoid synthesis, protein glycation, protein kinase C activation, and the hexosamine pathway [5]. Among these, the polyol pathway has been extensively studied. Aldose reductase (ALR2; EC: 1.1.1.21), the first and rate-limiting enzyme in the polyol pathway, reduces glucose to sorbitol using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. Sorbitol is then metabolized to fructose by sorbitol dehydrogenase [14]. Studies on animal models of diabetes and galactosemia suggest increased polyol pathway activity in the pathogenesis of DR [15]. Further, several studies based on specific inhibitors of ALR2 support the role of polyol pathway in the pathology of DR [16-18]. Retinal capillary pericytes contain ALR2, and the accumulation of polyols in pericytes has been linked to their degeneration and selective death [19]. Pericyte loss, the major event of early DR, has been observed in galactose-fed dogs that developed retinopathy [20]. In addition, the involvement of ALR2 in DR has been recently supported by the findings that ALR2 inhibitor prevented a spectrum of neural, glial, and vascular abnormalities associated with development of DR in rat and humans [17,18]. Furthermore, evidence for the involvement of ALR2 as a risk factor for DR and other diabetic complications comes from genetic polymorphism studies [21,22]. Several studies indicate that the Z-2 allele and a putative protective allele, Z+2, of ALR2 are significantly associated with DR [21-24]. Although, most animal studies with ALR2 inhibitors (ARI) have yielded encouraging results (considering some inconsistent data), on the whole, clinical trials of ARI have failed to shown efficacy against various diabetic complications. This may be due, in part, to differences in tissue levels of ALR2 in rodents as compared with humans. In addition, development of diabetic complications in humans may be influenced by metabolic and signaling pathways that have a less significant impact on the pathogenesis of complications in animal models. In principle, all diabetic patients might be expected to develop diabetic microvascular complications if hyperglycemia alone were the triggering factor for activation of the polyol pathway. Multiple factors are likely to be involved in predisposing diabetic subjects to DR, as evidenced by the fact that many, but not all, diabetic patients develop one or more microvascular complications. It has been reported that the prevalence of DR is associated with increased erythrocyte ALR2 protein levels [25]. This observation is based on previous studies that revealed that erythrocyte ALR2 protein levels correlates with ALR2 protein levels in retinal cells, particularly pericytes [25,26]. However, it was shown that ALR2 in human erythrocytes exists in activated and unactivated forms, and in hyperglycemia the total activity of ALR2 increases [15,27]. Hence, correlating total ALR2 activity with DR prevalence may provide an important link between an easily measurable marker in peripheral blood and risk of progression toward eye disease. In this study, we examined the activity of ALR2 in erythrocytes obtained from diabetic patients with and without retinopathy. Further, we also measured the levels of sorbitol as a surrogate marker for ALR2 activity levels in erythrocytes. These data demonstrate that erythrocyte ALR2 activity and sorbitol levels are significantly elevated in diabetic patients with retinopathy as compared with diabetics without retinopathy or patients without diabetes.
Methods
Subjects and study design
A hospital-based prospective case control study was conducted. The study protocols were approved by the Institutional Ethics Committees of the institutes involved. Subjects were recruited from the patients who visited the Sarojini Devi Eye Hospitals and Institute of Ophthalmology, Hyderabad, India and Department of Endocrinology, Osmania General Hospital, Hyderabad, India. A total of 362 T2D subjects (198 with DR, 164 without ocular complications) and 66 normal subjects were investigated. Written consent was obtained from the participants after they were given an explanation of the study details. A complete history of each participant, with respect to age, gender, clinical symptoms, diabetes type and duration, medication, and socioeconomic background, was collected using a well designed questionnaire. None of the diabetics in this study were on insulin treatment. The fundus of each subject was evaluated by both direct and indirect ophthalmoscopy, and DR was defined and classified according to Viswanath and McGavin [28]. The presence of retinal microaneurysm, dot and slot hemorrhages, intraretinal microvascular abnormalities, and cotton wool spots were defined as nonproliferative DR (NPDR), which was then categorized as mild, moderate, severe, and diabetic maculopathy. Formation of new vessels with and without bleeding and production of vitreous hemorrhage was defined as proliferative DR (PDR).
Sample collection and processing
Blood was drawn from the subjects into anticoagulant tubes and immediately transported to the laboratory on ice. Red blood cells (RBC) were separated by centrifugation, washed thrice with saline, and stored at –85 °C until further analysis.
Glucose estimation
Glucose was estimated in plasma by the GOD-POD method using a kit (BioSystems, Barcelona, Spain). Ten µl of serum or standard (100 mg/dl glucose) was added to reagent A (100 mM phosphate buffer, pH 7.5 with 5 mM phenol, 10 U/ml glucose oxidase, 1 U/ml peroxidase, and 0.4 mM 4-aminoantipyrine) and incubated for 5 min at 37 oC. Absorbance was measured at 505 nm in a spectrophotometer.
Glycosylated hemoglobin
HbA1C was estimated by ion-exchange chromatography using a kit (BioSystems). Fifty µl of blood was added to reagent 1 (50 mM potassium phosphate, pH 5.0 with 5 g/l detergent and 0.95g/l sodium azide) and mixed thoroughly to prepare the hemolysate. From this 50 µl of hemolysate was added to the ion-exchange resin and washed with 2.2 ml of reagent 2 (30 mM potassium phosphate, pH 6.5 containing 0.95 g/l sodium azide). HbA1C was eluted using reagent 3 (72 mM potassium phosphate, pH 6.5 containing 0.95 g/l sodium azide). Absorbance of the eluted HbA1C was read at 415 nm in a spectrophotometer.
Aldose reductase activity
A 10% erythrocyte suspension was made by adding 50 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl. The suspension was lysed by repeated freezing and thawing (three cycles) and centrifuged to remove insoluble material, if any. ALR2 activity was measured spectrophotometrically using an appropriately diluted hemolysate according to a previously described method [29] using a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, CA). One unit was defined as micromoles NADPH oxidized/g Hb/ min. The assay mixture in 1 ml contained 50 µmol potassium phosphate buffer pH 6.2, 0.4 mmol lithium sulfate, 5 µmol 2-mercapto ethanol, 10 µmol DL-glyceraldehyde, 0.1 µmol NADPH and enzyme preparation (hemolysate). The assay mixture was incubated at 37 oC and initiated by the addition of NADPH at 37 oC. The change in the absorbance at 340 nm due to NADPH oxidation was followed.
Estimation of sorbitol
Sorbitol was extracted by homogenizing RBC in nine volumes of 0.8 M perchloric acid. The homogenate was centrifuged at 5,000g at 4 °C for 10 min, and the pH of the supernatant was adjusted to 3.5 with 0.5 M potassium carbonate. The sorbitol content of the supernatant was measured by fluorometric method as described previously [30] using a fluorometer (Jasco-FP-6500, Tokyo, Japan). One ml reaction mixture, consisted of 50 µmol glycine buffer, pH 9.4, 2 µmol magnesium chloride, 0.2 µmol nicotinamide adenine dinucleotide (NAD) and protein-free supernatant, was incubated for 5 min at 37 oC and reaction was initiated by the addition of 0.6 U of sorbitol dehydrogenase. The relative fluorescence due to NADH formation was measured in a fluorometer with an excitation wavelength at 360 nm and an emission wavelength of 452 nm. Sorbitol standards, ranging from 0.2-9.0 µg/ml, were analyzed by the same to generate a standard curve.
Statistical analysis
The data were expressed as mean ± standard deviation. Mean values were compared by one-way ANOVA with post hoc tests of least significant difference method. Differences between comparison groups were considered to be significant where p<0.05. Correlations were calculated to study relationship of ALR2 and sorbitol with other variables. P values were also calculated for ALR2 in these groups.
Results
Data on mean age, duration of diabetes, levels of glucose, glycosylated hemoglobin, ALR2 activity, and sorbitol with respect to gender distribution for nondiabetic control, diabetics without retinopathy (DNR) and diabetics with retinopathy (DR) groups are summarized in Table 1. There was no significant difference (p>0.05) between male and female subjects in all three groups with respect to the measured parameters. Therefore, the pooled data for men and women in respective groups were considered for subsequent analysis.
Table 1. Clinical and biochemical features of the study subjects.
|
Group/
parameter |
Nondiabetic | Diabetes without retinopathy | Diabetic retinopathy | |||
|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
Male |
Female |
|
|
Age (Years)
Mean
n
S.D. |
54.37
43
12.925 |
56.00
23
12.803 |
50.91
64
11.394 |
49.09
100
9.242 |
53.09
122
10.188 |
54.49
76
7.431 |
|
Glucose (mg/dL)
Mean
n
S.D. |
110.50
43
25.060 |
104.22
23
17.9 |
210.92
64
111.06 |
217.91
100
101.50 |
244.15
115
97.70 |
251.22
76
106.73 |
|
Duration (Years)
Mean
n
S.D. |
0.00
43
0.000 |
0.00
23
0.000 |
5.26
64
4.200 |
6.79
100
4.850 |
9.43
115
6.073 |
11.04
76
6.673 |
|
HbA1C (%)
Mean
n
S.D. |
5.20
20
1.77 |
5.11
13
1.27 |
7.90
14
1.95 |
7.48
26
2.26 |
8.40
42
2.64 |
8.24
24
1.68 |
|
ALR2 (units/g Hb)
Mean
n
S.D. |
2.49
43
1.60 |
3.6
23
2.3 |
3.60
64
2.23 |
3.5
100
2.36 |
4.62
122
3.05 |
4.67
76
2.69 |
| Sorbitol (μg/mL) Mean n S.D. | 2.9 14 0.99 | 3.0 10 1.4 | 3.4 10 1.4 | 3.8 27 1.0 | 4.5 26 1.7 | 5.3 17 2.3 |
The distribution of age, random glucose, duration of diabetes, glycosylated hemoglobin (HbA1C), ALR2 activity and sorbitol levels between male and females in nondiabetic control, diabetes without retinopathy (DNR) and diabetes with retinopathy (DR) groups. The data (mean ± standard deviation (SD)) indicate no significant difference between the genders. (n = number).
As can be seen from Figure 1, erythrocyte ALR2 activity in the DNR group was not significantly different from nondiabetic control (p>0.05). Interestingly, ALR2 activity in the DR group was significantly different not only from the control group but also from the DNR group (p<0.05) (Figure 1). However, the ALR2 activity ranged from 0.2 units/g Hb in the control group to 18.6 units/g Hb in the DR group with considerable overlap between the groups. Therefore, we examined the data after distribution of individuals into one of four subgroups defined according to level of ALR2 activity (Figure 2). Percentage distributions of ALR2 activity indicated that most subjects in the control group had <3.0 units (58%), about 33% had 3–6 units, and only 9% had activity in the category of 6–9 units. Approximately 43% of the subjects in DNR group had <3.0 units, 35% had 3–6 units, and about 18% had ALR2 activity in the category 6–9 units. Whereas most of the DR subjects (46%) had 3–6 units of ALR2 activity, a substantial proportion (20%) had 6–9 units activity, and those with >9.0 units of ALR2 activity were found predominantly in this group (6%). These results suggest that prevalence of DR is associated with higher ALR2 activity. However, there was no significant difference in ALR2 activity between NPDR and PDR (4.36 units; n=114 vs 4.78; n=84).
Figure 1.
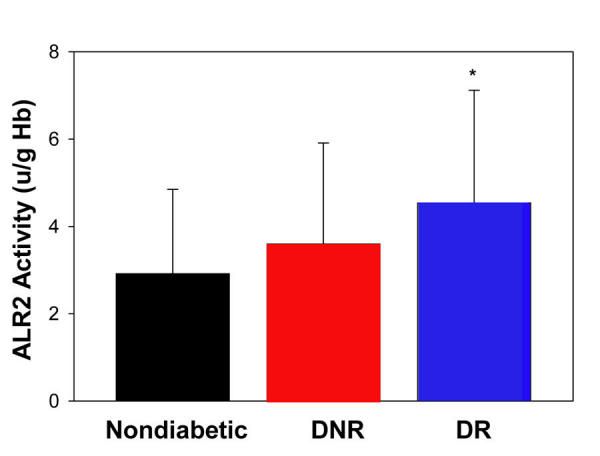
Erythrocyte aldose reductase activity. Data represent mean ± standard deviation of aldose reducatase (ALR2) activity in nondiabetic control (n=66) and diabetics without diabetic retinopathy (DNR; n=164) and those with diabetic retinopathy (DR; n=182). Asterisk (*) designates statistical significance (p<0.05) in comparison to the other groups.
Figure 2.
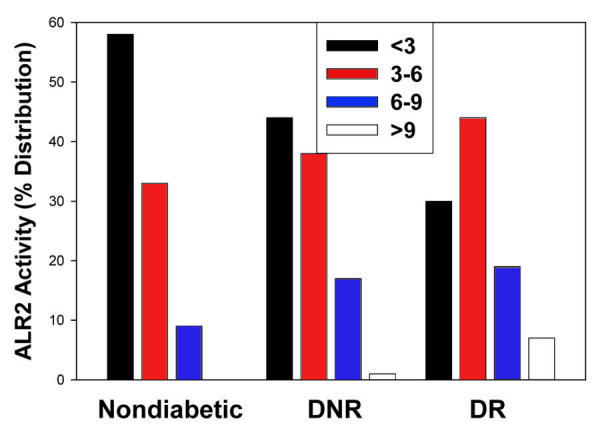
Percentage distribution of aldose reductase activity levels. Aldose reductase (ALR2) activity is distributed into <3, 3–6, 6–9, and >9 units/g Hb subgroups in nondiabetic control, and diabetics without diabetic retinopathy (DNR), and diabetics with diabetic retinopathy (DR). Percentage distribution of ARL2 activity with <3 units is significantly (p<0.05) different between the groups. Percentage distribution of ARL2 activity with >9 units is significantly (p<0.05) different between DNR and DR groups.
We further measured the levels of sorbitol, the product of ALR2-mediated reduction of glucose, in a subset of subjects in all three groups. While the levels of sorbitol were found to be higher in the DNR group as compared to control group, the difference was not statistically significant (p>0.05). However, sorbitol levels were significantly (p<0.05) higher in the DR group as compared to both the DNR and the control groups (Figure 3). Increased levels of sorbitol in DR patients were consistent with the higher ALR2 activity in DR patients. Activity of ALR2 was not correlated with age, glucose, diabetes duration, and HbA1C levels in all three groups (control, DNR, and DR; see Table 2) as well as with pooled data. Similarly, levels of sorbitol did not correlate with age, glucose, diabetes duration, and HbA1C levels in all three groups (Table 3) as well as with pooled data. However, as Figure 4 demonstrates, ALR2 activity was correlated with sorbitol levels (r=0.188; p<0.05) . As with ALR2 activity, there was no significant difference in sorbitol levels between NPDR and PDR subjects (4.80 mg/mL; n=41 vs 5.02; n=27).
Figure 3.

Erythrocyte sorbitol levels. Data represent mean ± standard deviation in nondiabetic control (n=31) and diabetics without diabetic retinopathy (DNR; n=44) and those with diabetic retinopathy (DR; n=52). Asterisk (*) designates statistical significance (p<0.05) in comparison to the other groups.
Table 2. Correlation of aldose reductase activity with other clinical variables.
| Variables |
Correlation coefficient values | ||
|---|---|---|---|
| Nondiabetic |
Diabetes without retinopathy |
Diabetic retinopathy |
|
| Age |
−0.181 |
−0.015 |
0.047 |
| Glucose |
0.030 |
−0.021 |
0.001 |
| Duration |
— |
−0.049 |
0.016 |
| HbA1C | 0.092 | −0.177 | 0.051 |
Correlation of aldose reductase (ALR2) activity with age, glucose, diabetes duration, and glycosylated hemoglobin (HbA1C) in different groups indicate all these correlates are not significant with ALR2 activity at p>0.05.
Table 3. Correlation of sorbitol levels with other clinical variables.
| Variables |
Correlation coefficient values | ||
|---|---|---|---|
| Nondiabetic |
Diabetes without retinopathy |
Diabetic retinopathy |
|
| Age |
0.067 |
−0.033 |
−0.146 |
| Glucose |
0.087 |
0.012 |
−0.047 |
| Duration |
— |
−0.208 |
−0.015 |
| HbA1C | 0.047 | 0.297 | 0.130 |
Correlation of sorbitol levels with age, glucose, diabetes duration, and glycosylated hemoglobin (HbA1C) in different groups indicate that all these correlates are not significant with sorbitol levels at p>0.05.
Figure 4.
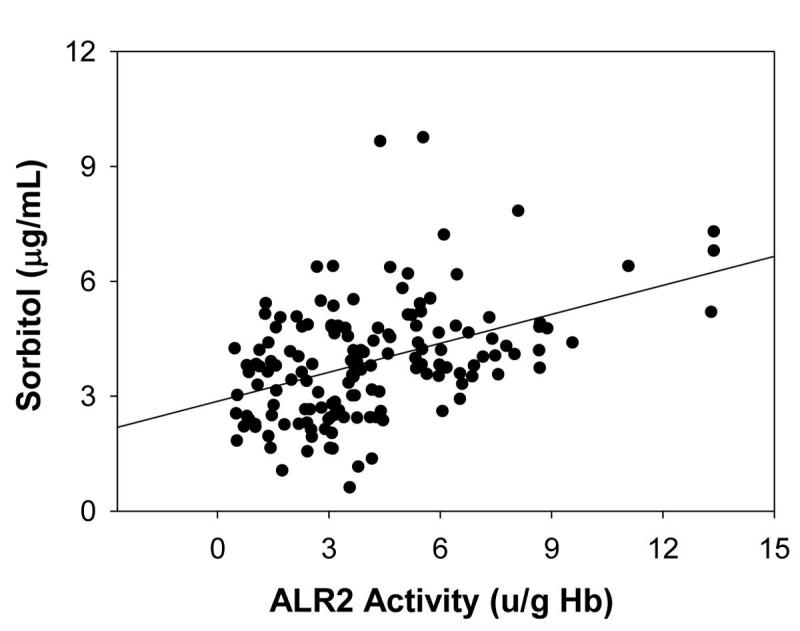
Correlation between erythrocyte sorbitol levels and aldose reductase activity. Correlation (r=0.188) between erythrocyte sorbitol levels and aldose reductase (ALR2) activity in control, diabetics without diabetic retinopathy (DNR), and diabetics with retinopathy (DR) was found to be significant at p<0.05. Correlation was done for those samples in which sorbitol was determined.
Discussion
Although uncontrolled hyperglycemia is the major factor, the link between diabetes and susceptibility to various secondary complications has not been unraveled. The polyol pathway of glucose metabolism is activated when intracellular glucose levels are high [31]. The activation is immediately linked to hyperglycemia and occurs prominently in tissues that develop complications [31,32]. In addition, polymorphisms associated with regions flanking the ALR2 gene have been implicated in human susceptibility to DR and other diabetic complications [21,22]. There is also a strong evidence to show that diabetic complications including DR are associated with increased oxidative stress [5], and activation of polyol pathway is known to contribute to oxidative stress [33]. Evidence for the involvement of ALR2 in DR comes from studies that demonstrated ALR2 was present in different cell types of retina [17-20]. In addition, disruption of the ALR2 gene leads to a reduction in lesions associated with DR in a diabetic mouse model [34]. However, studies with ARI have yielded inconsistent results against DR or diabetic-like retinopathy in experimental animals [14,35] and in clinical trials to assess efficacy against various diabetic complications [36]. Nevertheless, ALR2 remains an intriguing candidate/target for the treatment of secondary complications. Hence, the role of the polyol pathway vis á vis ALR2 in the pathogenesis of diabetic complications, particularly DR, requires further investigations from various angles.
The present study investigated the functional state of erythrocyte ALR2 in DR patients in comparison with DNR. The results demonstrated that the activity of ALR2 is significantly higher in DR patients as compared to DNR patients. Earlier studies had focused on enzyme activity and protein levels of ALR2 in diabetic complications in humans [reviewed in 22]. In type 1 diabetes, patients with the highest ALR2 activity were found to be four times more likely to develop diabetic microvascular complications than those whose activity was similar to normal. Oishi et al. suggested that increased prevalence of DR is correlated with increased erythrocyte AR protein levels, particularly the prevalence of DR in patients who have diabetes for fewer than 10 years [25]. A correlation between erythrocyte ALR2 protein levels and diabetic cataract, particularly posterior subcapsular cataract, has also been reported [37]. However, in these studies ALR2 levels were determined by ELISA and not by catalytic activity. The underlying assumption behind these studies was that erythrocyte ALR2 might reflect ALR2 in pericytes and lens, the vulnerable cell types in DR and cataract, respectively. The best way to assess the functional role of ALR2 in DR is to determine ALR2 in retinal pericytes and capillaries. However, a noninvasive procedure is not currently available to make such measurements. Therefore, we and others used erythrocytes as a surrogate tissue for enzyme measurements.
Increased oxidative stress has been linked to the development of diabetic complications [5] and altered redox homeostasis is known to affect ALR2 activity. For example, oxidation of cysteine residue under oxidative conditions modulates ALR2 activity [38]. Thus the specific activity of the protein may also be critical for the development of diabetic complications. The results of the present study show that higher activity of ALR2 is associated with prevalence of DR. On the other hand, we found no significant difference in ALR2 activity between NPDR and PDR, indicating higher ALR2 might be involved in the initiation of disease, but not in the progression, which needs further investigation. However, there was a large variation in ALR2 activity, and there was considerable overlap of activity between nondiabetic control, DNR, and DR groups. Nevertheless, percentage distributions of ALR2 activity indicate that a substantial number of subjects in the DR group had activity in the category of 6–9 units, and, most important, >9.0 units of ALR2 activity was found almost exclusively in this group.
Development of DR and other microvascular complications are generally linked to diabetes duration and patient age [25,39-41]. It is estimated that DR develops in more than 75% of diabetics who have had diabetes for 15–20 years. Interestingly, the higher ALR2 activity in the DR group in the present study was not associated with diabetes duration and patient age. This observation suggests that ALR2 activity might serve as an independent risk identification factor for DR, irrespective of duration of diabetes and age of the patient. In this study, we found a substantial number of DR patients whose duration of diabetes was less than 15 years: Of 198 DR patients in our study, 28% had been diabetic for <5 years, 35% for <10 years, and 35% for >15 years. This may have implications in the development of DR in Indian context. Even though the prevalence of microvascular complications of diabetes-like retinopathy is comparatively lower in the Indian populations, the age of onset of diabetes in this part of the world is much earlier [2,42]. The impact of early onset diabetes and development of complications with shorter duration of diabetes needs to be addressed. We also note that, in contrast to studies [41,43,44], we did not observe an association between HbA1C and prevalence of DR in our study population. Furthermore, the increase in ALR2 activity in DR group was not associated with HbA1C levels. A previous study also reported that while the protein level of ALR2 in erythrocyte was associated with DR, there was no correlation between enzyme levels and age, duration of diabetes, fasting blood glucose, and HbA1C in patients with T2D [45]. The interindividual variability of ALR2 protein content has been shown to be associated with variation in polyol pathway metabolites [46], which could be associated, at least in part, to the prevalence of diabetic complications. A significant proportion (30%) of DR patients in our study were shown to have >6 units of ALR2 activity, whereas most of the DNR patients and nondiabetics had <6.0 units activity. Although relatively few of our study participants had ALR2 activity above 9.0 units, the strong association of these high enzyme levels with the DR group suggests that elevated erythrocyte ALR2 activity may represent a significant risk factor for the susceptibility of diabetic subjects to develop DR. Further, the possibility that patients in DNR group with 6–9 units activity might eventually develop DR needs further investigation.
Measurement of ALR2 activity by the spectrophotometric method using DL-glyceraldehyde as the aldehyde substrate could lead to confusing results because other aldo-keto reductases are active with this substrate [47]. Thus, we measured sorbitol level in a subset of samples as an index of ALR2 activity since ALR2 is unique among human aldo-kedo reductases in its ability to catalyze the NADPH-dependent conversion of glucose to sorbitol [48]. As with ALR2 activity, the sorbitol levels were significantly higher in the DR group as compared to the control and DNR groups (Figure 3). It should be noted that there was linear correlation between sorbitol levels and ALR2 activity irrespective of the group to which they belonged (Figure 4). Moreover, the contribution of other aldo-keto reductases to the apparent ALR2 activity is likely to be minimal because of the good correlation between apparent ALR2 activity levels and sorbitol levels in RBC. In addition, sorbitol levels were not associated with diabetes duration, age, glucose, and HbA1c levels of the subjects. As with ALR2 activity, we observed that sorbitol levels were also not related to the severity of DR, as there was no significant difference between NPDR and PDR subjects.
Based on the results of the current study, it is reasonable to hypothesize that some diabetics with ALR2 activity levels above a certain “threshold” level might be predisposed to develop DR. However, validation of this hypothesis will require additional longitudinal studies. Additional studies are needed to determine whether ALR2 activity and sorbitol levels in erythrocytes may have value as quantitative traits that need to be considered, along with other known risk factors (including genetic susceptibility), to assess risk for development of DR.
Although the beneficial impact of strict glycemic control on prevention of diabetic complications has been well established, most individuals with diabetes rarely achieve consistent euglycemia. Hence, agents that can substantially delay or prevent the onset and development of diabetic complications, irrespective of glycemic control, would offer many advantages. In principle, ARI can be included in this category. Although clinical trials of ARI have failed to demonstrate efficacy against various diabetic complications, trials of other compounds such a protein kinase Cβ inhibitor have also failed to show efficacy against progression of DR [49]. Thus, intensive research continues to identify and test both synthetic as well as natural products for their therapeutic value to prevent the onset as well as progression of diabetic complications [29,50-52].
Acknowledgments
The authors are grateful to all the participants for their cooperation in conduct of this study. We thank Dr. P. Suryanarayana and P. Yadagiri Reddy for assistance with biochemical analysis. We also thank Drs. G. Chandrasekhar Reddy, H. Anupama, and K. Neelaveni, Department of Endocrinology, Osmania General Hospital, Hyderabad for their assistance in the selection and diagnosis of study patients. This work was supported in part by the Indian Council of Medical Research and Department of Science and Technology, New Delhi and NIH grants EY05856, EY02687 as well as grants from Research to Prevent Blindness, Inc. and the Pearle Vision Foundation.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Baskar AD, Mary S, Kumar CK, Selvam S, Catherine S, Vijay V. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–5. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Shyamala P, Vijay V, Viswanathan M. High prevalence of NIDDM and IGT in an elderly south Indian population with low rates of obesity. Diabetes Care. 1994;17:1190–2. doi: 10.2337/diacare.17.10.1190. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study Group Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 8.Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF, Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Fact Sheet N 138: Geneva, Switzerland, WHO, 2002. [Google Scholar]
- 10.Agarwal S, Raman R, Kumari RP, Deshmukh H, Paul PG, Gnanamoorthy P, Kumaramanickavel G, Sharma T. Diabetic retinopathy in type II diabetics detected by targeted screening versus newly diagnosed in general practice. Ann Acad Med Singapore. 2006;35:531–5. [PubMed] [Google Scholar]
- 11.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 12.Rema M, Ponnaiya M, Mohan M. Prevalence of retinopathy in non-insulin dependent diabetes mellitus at a diabetes center in southern India. Diabetes Res Clin Pract. 1996;34:29–36. doi: 10.1016/s0168-8227(96)01327-7. [DOI] [PubMed] [Google Scholar]
- 13.Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, Kim R, Narendran V, Ramakrishnan R, Krishnadas R, Thulasiraj RD, Suan E. Prevalence of vitreoretinal disorders in a rural population of southern India: the Aravind Comprehensive Eye Study. Arch Ophthalmol. 2004;122:581–6. doi: 10.1001/archopht.122.4.581. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita JH. A thirty-year journey in the polyol pathway. Exp Eye Res. 1990;50:567–73. doi: 10.1016/0014-4835(90)90096-d. [DOI] [PubMed] [Google Scholar]
- 15.Anil Kumar P, Bhanuprakash Reddy G.Focus on molecules: aldose reductase. Exp Eye Res 200785739–40.Epub 2006 Sep 25 [DOI] [PubMed] [Google Scholar]
- 16.Kador PF, Akagi Y, Takahashi Y, Ikebe H, Wyman M, Kinoshit JH. Prevention of retinal vessel changes associated with diabetic retinopathy in galactose-fed dogs by aldose reductase inhibitors. Arch Ophthalmol. 1990;108:1301–9. doi: 10.1001/archopht.1990.01070110117035. [DOI] [PubMed] [Google Scholar]
- 17.Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. studies on rat and human retinas predict a role for the polyol path way in human diabetic retinopathy. Diabetes. 2004;53:2404–11. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- 18.Asnaghi V, Gerhardinger C, Hoen T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in rat. Diabetes. 2003;52:506–11. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- 19.Hohman TC, Nishimura C, Robison WJ. Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp Eye Res. 1989;48:55–60. doi: 10.1016/0014-4835(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 20.Kador PF, Akagi Y, Terubayashi H, Wyman M, Kinoshita JH. Prevention of pericyte ghost formation in retinal capillaries of galactose-fed dogs by aldose reductase inhibitors. Arch Ophthalmol. 1988;106:1099–102. doi: 10.1001/archopht.1988.01060140255036. [DOI] [PubMed] [Google Scholar]
- 21.Chung SS, Chung SK. Genetic analysis of aldose reductase in diabetic complications. Curr Med Chem. 2003;10:1375–87. doi: 10.2174/0929867033457322. [DOI] [PubMed] [Google Scholar]
- 22.Demaine AG. Polymorphisms of the aldose reductase gene and susceptibility to diabetic microvascular complications. Curr Med Chem. 2003;10:1389–98. doi: 10.2174/0929867033457359. [DOI] [PubMed] [Google Scholar]
- 23.Kao YL, Donaghue K, Chan A, Knight J, Silink M. A novel polymorphism in the aldose reductase gene promoter region is strongly associated with diabetic retinopathy in adolescents with type 1 daibetes. Diabetes. 1999;48:1338–40. doi: 10.2337/diabetes.48.6.1338. [DOI] [PubMed] [Google Scholar]
- 24.Kumaramanickavel G, Sripriya S, Ramprasad VL, Upadayay NK, Paul PG, Sharma T. Z-2 aldose reductase allele and diabetic retinopathy in India. Ophthalmic Genet. 2003;24:41–8. doi: 10.1076/opge.24.1.41.13889. [DOI] [PubMed] [Google Scholar]
- 25.Oishi N, Kubo E, Takamura Y, Maekawa K, Tanimoto T, Akagi Y. Correlation between erythrocyte aldose reductase level and human diabetic retinopathy. Br J Ophthalmol. 2002;86:1363–6. doi: 10.1136/bjo.86.12.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura C, Hamada Y, Tachikawa T, Ishikawa T, Gui T, Tsubouchi J, Hotta N, Tanimoto T, Urakami T. Enzyme immunoassay for erythrocyte aldose reductase. Clin Chem. 1994;40:889–94. [PubMed] [Google Scholar]
- 27.Srivastava SK, Hair GA, Das B. Activated and unactivated forms of human erythrocyte aldose reductase. Proc Natl Acad Sci USA. 1985;82:7222–6. doi: 10.1073/pnas.82.21.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanath K. Murray McGavi DD: Diabetic Retinopathy: Clinical findings and management. Community Eye Health. 2003;16:21–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB. Inhibition of aldose reductase by tannoid principles of Emblica officinalis: implications for the prevention of sugar cataract. Mol Vis. 2004;10:148–54. [PubMed] [Google Scholar]
- 30.Malone JI, Knox G, Benford S, Tedesco TA. Red Cell Sorbitol an indicator of diabetic control. Diabetes. 1980;29:861–4. doi: 10.2337/diab.29.11.861. [DOI] [PubMed] [Google Scholar]
- 31.Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831–6. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- 32.Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, Van Den Eden M, Kilo C, Tilton RG. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–13. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 33.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233–6. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 34.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–25. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 35.Engerman RL, Kern TS. Aldose reductase inhibition fails to prevent retinopathy in diabetic and galactosemic dogs. Diabetes. 1993;42:820–5. doi: 10.2337/diab.42.6.820. [DOI] [PubMed] [Google Scholar]
- 36.Oates PJ, Mylari BL. Aldose reductase inhibitors: therapeutic implications for diabetic complications. Expert Opin Investig Drugs. 1999;8:2095–119. doi: 10.1517/13543784.8.12.2095. [DOI] [PubMed] [Google Scholar]
- 37.Oishi N, Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Tanimoto T, Akagi Y. Correlation between adult diabetic cataracts and red blood cell aldose reductase levels. Invest Ophthalmol Vis Sci. 2006;47:2061–4. doi: 10.1167/iovs.05-1042. [DOI] [PubMed] [Google Scholar]
- 38.Kaiserova K, Srivastava S, Hoetker JD, Awe SO, Tang XL, Cai J, Bhatnagar A. Redox activation of aldose reductase in the ischemic heart. J Biol Chem. 2006;281:15110–20. doi: 10.1074/jbc.M600837200. [DOI] [PubMed] [Google Scholar]
- 39.Cai XL, Wang F, Ji LN. Risk factors of diabetic retinopathy in type 2 diabetic patients. Chin Med J (Engl) 2006;119:822–6. [PubMed] [Google Scholar]
- 40.Cugati S, Kifley A, Mitchell P, Wang JJ. Temporal trends in the age-specific prevalence of diabetes and diabetic retinopathy in older persons: Population-based survey findings. Diabetes Res Clin Pract. 2006;74:301–8. doi: 10.1016/j.diabres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The wisconsin epidemiologic study of diabetic retinopathy. ii. prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 42.Rema M, Pradeepa R. Diabetic retinopathy: An Indian perspective. Indian J Med Res. 2007;125:297–310. [PubMed] [Google Scholar]
- 43.The relationships between risk factors and the distribution of retinopathy lesions in type 2 diabetes. Acta Ophthalmol Scand. 2006;84:619–23. doi: 10.1111/j.1600-0420.2006.00710.x. Hove MN, Kristensen JK, Lauritzen T, Bek T [DOI] [PubMed] [Google Scholar]
- 44.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260:2864–71. [PubMed] [Google Scholar]
- 45.Nishimura C, Saito T, Ito T, Omori Y, Tanimoto T. High levels of erythrocyte aldose reductase and diabetic retinopathy in NIDDM patients. Diabetologia. 1994;37:328–30. doi: 10.1007/BF00398062. [DOI] [PubMed] [Google Scholar]
- 46.Hamada Y, Nishimura C, Koh N, Sakakibara F, Nakamura J, Tanimoto T, Hotta N. Influence of interindividual variability of aldose reductase protein content on polyol pathway metabolites and redox state in erythrocytes in diabetic patients. Diabetes Care. 1998;21:1014–8. doi: 10.2337/diacare.21.6.1014. [DOI] [PubMed] [Google Scholar]
- 47.Spite M, Baba SP, Ahmed Y, Barski OA, Nijhawan K, Petrash JM, Bhatnagar A, Srivastava S. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. Biochem J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crosas B, Hyndman DJ, Gallego O, Martras S, Pares X, Flynn TG, Farres J. Human aldose reductase and human small intestine aldose reductase are efficient retinal reductases: consequences for retinoid metabolism. Biochem J. 2003;373:973–9. doi: 10.1042/BJ20021818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–97. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 50.Suryanarayana P, Krishnaswamy K, Reddy GB. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol Vis. 2003;9:223–30. [PubMed] [Google Scholar]
- 51.Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–29. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 52.Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol Vis. 2007;13:1291–7. [PubMed] [Google Scholar]


