Abstract
Uterine natural killer (uNK) cells are the most abundant lymphocyte in the uterus during early pregnancy that plays a role in spiral arteriole modifications. In this study, we determined if uNK cell populations differed between mouse decidua and deciduoma. Histochemical staining using Dolichos biflorous agglutinin (DBA) lectin was used to identify uNK cells and classify their stage of maturation. We found a difference in the pattern of localization and density of uNK cells between the decidua and deciduoma at Days 2–4 after the onset of decidualization. Cells were more distributed and the densities were significantly greater in the mesometrial region of the decidua compared to that of the deciduoma. Using double labeling for both DBA lectin binding and bromodeoxyuridine incorporation, we found that the greater number of uNK cells in the decidua were not due to an increase in uNK cell proliferation. Western blot analyses revealed that the increase in uNK cell number was accompanied by a significant increase in both the levels of interferon-gamma (IFNG) and pro-interleukin-18 when a conceptus was present. Vascular morphometry revealed that modifications of the spiral arterioles occurred in the mesometrial decidua but not the deciduoma, which could be attributed to the differences observed in uNK cell number and IFNG production. The present study demonstrates that differences exist in uNK cell populations between decidua and deciduoma, providing evidence that the conceptus provides signals which regulate uNK cell number and function in the uterus during implantation.
INTRODUCTION
Two key processes necessary for the advancement of mammalian pregnancy are implantation and formation of the placenta. Implantation begins with the attachment of the embryo to the uterine wall and ends in the formation of the definitive placenta. One of the first major events that begins to occur in the uterus during this time is the proliferation then differentiation of the endometrial fibroblast-like cells into large polyploid decidual cells [1, 2]. This process, called decidualization, results in the formation of tissue that is referred to as the decidua and occurs in response to the implanting conceptus in rodents. However, due to an observation first reported almost a century ago [3], molecular signals from the conceptus do not appear to be required for decidualization to occur. This is because the uterus can undergo decidualization in response to an artificial stimulus such as an intraluminal injection of sesame oil or transfer of beads into ovariectomized hormonally-sensitized or pseudopregnant animals, respectively [4]. In order to discern it from the decidua that forms in pregnant animals, the tissue that develops in response to an artificial stimulus is termed a deciduoma [5]. In the present study, both pregnant uteri (conceptus present) and those undergoing artificially-induced decidualization (conceptus absent) were used to determine if the conceptus plays a role in regulating natural killer (NK) cell populations in the mesometrial region of the uterus during decidualization.
Natural killer (NK) cells are a part of the innate immune system and defend against allogenic cells and cells under stress such as a virally infected or tumor cell [6]. Although the conceptus is a “semi-allogenic” graft which expresses both maternal and paternal antigens, it is not rejected by the maternal immune system under normal conditions [7]. It is well known that a special set of NK cells are present in the uterus during implantation and later in pregnancy [8–11]. However, instead of being harmful to the conceptus, recent evidence shows these cells play a key role in maintaining decidual integrity and evoking changes in the spiral arterioles in the mesometrial region of the mouse uterus during pregnancy [12]. These NK cells, therefore, seem to play a role in the process of normal implantation.
Natural killer cells are derived from pluripotent hematopoeitic stem cells which differentiate into common lymphoid progenitor cells in the bone marrow [13]. These cells then become progenitor NK (pre-NK) cells in the secondary lymphoid tissues and then migrate to the various tissues of the body to further differentiate [14]. Once these small agranular pre-NK cells home to and are within the uterus, they become uterine natural killer (uNK) cells and begin their maturation process into large granulated cells [15]. The process of pre-uNK cell recruitment into the uterus and uNK cell maturation within the uterus has been shown to involve interleukin-15 (IL15) [16, 17]. This was demonstrated by the establishment of mice deficient for IL15, which completely lack NK cells including uNK cells [18, 19].
Uterine NK cells are the most abundant lymphocyte and are a major source of cytokines in the uterus during early pregnancy [20]. They secrete both interferon-gamma (IFNG) and interleukin-18 (IL18) [21], which appear to play roles in the uterus during implantation. Paracrine IL18 signaling is responsible, at least in part, for stimulating IFNG synthesis by uNK cells. In turn, the uNK cell derived IFNG plays a key role in the spiral arteriole modifications [12]. Although maternal hormones have been suggested to play a key role in regulating uNK cell populations in the uterus during pregnancy [22], it has never been proven if the conceptus plays a role or not. In fact, since uNK cells are present in the deciduoma [23], one could argue that the conceptus is not required for the presence of all the uNK cells found in the uterus during implantation. In the present study, we set out to determine if the conceptus has an effect on uNK cell numbers, localization and maturation in the mouse uterus over the first 4 days after the onset of decidualization. The results provide evidence that indeed the conceptus does have an influence on uNK cell populations and their function.
MATERIALS AND METHODS
Animals
For each experiment carried out in this study, treatment and time-point data were from 3 to 6 independent animals (N=3–6). All procedures involving mice were approved by the Southern Illinois University Institutional Animal Care and Use Committee. CD1 mice (6–8 wk old), purchased from Charles River Breeding Laboratories (Wilmington, MA), were kept under controlled light conditions (lights on from 07:00–19:00 h) and allowed free access to food and water. Females were placed with fertile males and the morning a vaginal plug was detected was considered to be Day 0.5 of pregnancy. Mice were killed at 9:00 h on Days 3.5 to 9.5 of pregnancy which approximately corresponds to -1 to 5 days after the onset of decidualization, respectively (see Fig. 1 A).
Figure 1.

Time-lines for uteri collected on days after the onset decidualization. A. Pregnant uteri were collected on Days -1 to 5 corresponding to days of pregnancy (DOP) 3.5–9.5. B. Uteri undergoing artificially-induced decidualization in ovariectomized mice injected with estradiol-17β (E2) and progesterone (P4) to sensitize the uterus for a deciduogenic stimulus. Pseudopregnant females received a transfer of C. Concavalin A coated agarose beads or D. injection of sesame oil into the uterine lumen to artificially induce decidualization. Uteri were collected at Days 1–4 after the onset of artificially-induced decidualization (B–D).
Three models of artificially-induced decidualization were utilized in this study. In the first model (Fig. 1B), animals were ovariectomized, allowed 1 wk recovery, then injected subcutaneously with estradiol and/or progesterone in order to adequately sensitize the uterus for an artificial deciduogenic stimulus [24]. The ovariectomized mice were injected with estradiol and/or progesterone at 09:00 h and an intra-luminal injection of 10–15 μl of sesame oil was used as an artificial deciduogenic stimulus between 11:00–13:00 h. Mice were killed at exactly 24, 48, 72 or 96 h after artificially-inducing decidualization, corresponding to Days 1 to 4 after the onset of decidualization. The tissue collected was referred to as ovariectomized oil-induced (Ovx-OID) deciduoma. Samples for Day 5 onwards were not collected because the deciduoma undergoes a massive regression at this time [25]. The second and third models of artificially-induced decidualization utilized pseudopregnant females generated by mating to vasectomized males (day a vaginal plug was detected was considered Day 0.5 pseudopregnancy). Uterine horns then received either transfer of 12 concavalin A-coated blastocyst sized agarose beads (Pseudo-BID, Fig. 1C) at 13:00–15:00 h [26] or an intraluminal injection 15 μl of sesame oil (Pseudo-OID, Fig. 1D) at 11:00–13:00 h on days 2.5 and 3.5, respectively. In both cases, mice were killed on Days 6.5–9.5 of pseudopregnancy at 09:00, corresponding to Days 1–4 after the onset of decidualization.
Tissue Processing For Paraffin Section Analyses
For samples collected for histochemical and immunohistochemical analyses, mice were anesthetized with an interperitoneal injection of ketamine hydrochloride (200 mg/kg) plus xylazine (20 mg/kg) (Henry Schein, Melville, NY) mixture. They were then perfused with 10 ml of phosphate buffered saline (PBS) containing 1% sodium nitrate followed by 50–100 ml of 4% paraformaldehyde (Fisher, Pittsburg, PA) (w/v) in PBS (PFA-PBS) at a pressure of 120 mmHg. After dissection, uterine tissues were placed in 4% PFA-PBS for 24 h then 70% ethanol for 24 h at 4° C. Tissues were then dehydrated, cleared in xylene, then embedded in paraffin using routine histological procedures. Cross sections (5 μm) of the uteri were prepared and mounted onto silanized glass slides.
Uterine NK Cell Staining Using Dolichos Biflorus Agglutinin (DBA) Lectin Histochemistry
DBA lectin positive histochemistry was used to identify uNK cells in uterine cross sections essentially as described previously by Paffaro et al. [15]. Briefly, the cross sections were deparaffinized in xylene, re-hydrated then treated with 1% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Sections were then washed with PBS, blocked with 1% (w/v) Bovine Serum Albumin (BSA) (Fisher) in PBS (BSA-PBS) for 1 h followed by an overnight incubation with 2 μg/ml biotinylated-DBA lectin (ICN Biomedicals Inc., Aurora, OH) in 1% BSA-PBS at 4°C. After washing in PBS, the sections were incubated with 10 μg/ml ExtrAvidin™ Peroxidase conjugate (Sigma, St. Louis, MO) in 1% BSA-PBS for 30 min at room temperature. After washing with PBS, the slides were incubated with the peroxidase substrate 3,3′-diaminobenzidine tetrahydrochloride (ICN Biomedicals Inc.) yielding a brown stain. In order to visualize the nuclei, sections were then counter-stained with Harris hematoxylin (Statlab Medical Products, Lewisville, TX). Control sections were stained as above with the addition of 0.1 M N-acetyl-D-galactosamine (Sigma) to the DBA lectin incubation. In every case, this displaced all of the DBA lectin binding to the sections verifying the specificity (data not shown). All microscopy was conducted using a Leica MZFLIII stereomicroscope (North Central Instruments, Maryland Heights, MO) and Nikon microscope (Hitschtel Instruments Inc., St. Louis, MO), each equipped with a Retiga digital camera (QImaging, Burnaby, Cananda).
DBA-lectin positive immunohistochemical staining pattern within the uNK cells was used for classification of their stage of maturation, essentially as that of Paffaro et al. [15]. The stages of uNK cell maturation include subtypes I (immature), II (intermediate), III (fully mature) and IV (senescent) uNK cells (Fig. 2A) that showed the characteristics summarized in Table 1. The three experiments below were conducted to determine if the conceptus has an effect on uNK cells in the uterus on Days 2–4 after the onset of decidualization.
Figure 2.

Uterine NK cell maturation in the mouse. A. Uterine NK cells found in the mesometrial region of the decidua and deciduoma by DBA lectin histochemistry were classified as subtypes I–IV (scale bar = 10 μm). B. Day 4 after the onset of decidualization, uterine cross section illustrating subregions 1–3 (central axis toward conceptus from the myometrium) and 4 (lateral region). AM, antimesometrial region; C, conceptus; M, mesometrial region; MLAp, mesometrial lymphoid aggregate of pregnancy.
Table 1.
Uterine natural killer subtypes within the uterus during decidualization. Uterine NK cells were classified into subtypes I–IV based on several characteristics including: cell shape, nuclear morphology, amount of granules present (−agranular, + few granules, +++ many granules), cell size and location of DBA-lectin reactivity (+ = positive staining, − = negative staining).
| Subtype | Morphology
|
Size
(microns) |
DBA-lectin Stain
|
|||
|---|---|---|---|---|---|---|
| Cell Shape | Nucleus | Granules | Cell Surface | Granules | ||
| I | Round | Round | − | 6–12 | + | − |
| II | Round | Round | + | 11–15 | + | + |
| III | Round | Round euchromatic | +++ | 19–33 | + | + |
| IV | Irregular | Apoptotic-like | +++ | 26–34 | + | + |
Total Mesometrial uNK Cell Density: Total number of uNK cells in the mesometrial region of uteri were counted and normalized to mesometrial tissue area using Image J [27]. A two-way ANOVA was conducted to detect an effect between type of decidual tissue and time after the onset of decidualization on uNK cell density. This was followed by Duncan’s multiple range tests to determine differences between mean on each day after the onset of decidualization.
Regional uNK cell density: Uterine NK cells were classified as subtypes I–IV (Fig. 2A) in subregions 1–4 (Fig. 2B) of the mesometrial area of the decidua and Ovx-OID deciduoma, essentially as described by Paffaro et al (2003). Briefly, subregion 1 is the area which later becomes the mesometrial lymphoid aggregate of pregnancy. Subregion 2 is located in the central area at the midpoint between the myometrium and conceptus. Finally, subregions 3 and 4 are found in areas adjacent to the conceptus centrally and laterally, respectively. Uterine NK cell subtypes I–IV were counted in subregions 1–4 of the decidua and the Ovx-OID deciduoma in order to determine the density of each uNK cell subtype per test area (25,600 μm2). A minimum of 9 random test areas and 800–4000 cells were counted in each of the mesometrial subregions of decidua and Ovx-OID deciduoma of each independent sample. A two-way ANOVA was performed to detect overall differences in uNK cell subtype density for each individual day and subregion. This was followed by the use of Duncan’s multiple range tests to determine differences between means.
Regional uNK Cell Maturation: The percentage of each uNK cell subtype in mesometrial subregions 1–4 on Days 2, 3 and 4 after the onset of decidualization was determined. Chi-square tests were conducted to determine if there was a significant difference in the uNK cell subtype profile between the decidua and Ovx-OID deciduoma.
Measuring uNK Cell Proliferation Index
In order to visualize those uNK cells undergoing proliferation, mice were injected intraperitoneally with 1 mg bromodeoxyuridine (BrdU) dissolved in 0.1 ml Dulbecco phosphate buffered saline (Invitrogen Corp, Grand Island, NY) at exactly 4 h prior to perfusion and uterine collection [28]. Brdu was incorporated to those cells in the S phase of the cell cycle for 4 h prior to tissue collection. Paraffin sections of these samples were then subjected to histochemical and immunohistochemical staining for DBA lectin and BrdU, respectively. All incubations were carried out at room temperature except where noted. Briefly, after deparaffinization and hydration, sections were digested with 0.2% trypsin in PBS for 10 min at 37°C for antigen retrieval. After washing in PBS, sections were incubated with 1.5M HCL for 15 min at 37°C, then borate buffer (0.1M Boric acid pH 8.5) for 10 min with a single wash of PBS in between. Next, sections were blocked with 1% BSA-PBS for 1 hr prior to incubation in the primary antibody (sheep anti-BrdU, Biodesign, Sako, ME) at a concentration of 8 μg/ml IgG in BSA-PBS for 1 h. Control sections were incubated with 8 μg/ml normal IgG (Sigma) in 1% BSA-PBS. After washing with PBS, sections were incubated with the secondary antibody (biotinylated donkey anti-sheep IgG, Jackson Immunoresearch, West Grove, PA) at a concentration of 4 μg/ml in BSA-PBS for 1 h. Next, sections were washed in PBS and covered with 2.5 μg/ml of alkaline phosphatase streptavidin conjugate (Vector Laboratories, Burlingame, CA) in BSA-PBS for 15 min. After washing sections with Tris-buffered saline containing 0.6 mg/ml levamisole (Acros Organics, Morris Plains, NJ), sections were incubated with Vector Blue chromagen kit (Vector Laboratories) containing 1 mg/ml levamisole. Sections were then washed with water, then PBS and covered with 3% hydrogen peroxide diluted in PBS for 10 min to block endogenous peroxidase activity. Next, sections were washed with PBS then blocked in 1% BSA-PBS for 10 min prior to incubation with 2 μg/ml biotinylated DBA lectin in BSA-PBS for 1 h. After washing with PBS, sections were covered with a horseradish peroxidase streptavidin conjugate (2.5 μg/ml) (Vector Laboratories) in BSA-PBS for 15 min. After washing with PBS, sections were then incubated with aminoethyl carbozole substrate (ZyMed Laboratories Inc., South San Francisco, CA) and washed in water. Finally, sections were covered with 4′,6-diamidino-2-phenylindole, dihydrochloride (Pierce Biotechnology, Rockford, IL), washed in PBS and mounted with Fluoromount-G™ (Southern Biotechnology Associates, Inc., Birmingham, CA). Proliferation index was measured as the proportion of uNK cells (brown color) which stained positive for BrdU (blue color) and a minimum of 500 cells were assessed from 2-3 sections from 3 or more independent samples. A two-way ANOVA was performed to detect overall differences in proliferation index followed by the use of Duncan’s multiple range tests to determine the significance of differences between means.
Western Blot Analyses
Protein extracts were collected from decidua and Ovx-OID plus Pseudo-BID deciduomas from four independent samples. Briefly, uteri were collected then homogenized in Tissue Protein Extraction Reagent (TPER™, Pierce Biotechnology). After the homogenate was centrifuged (12,000 g for 20 min at 4°C), the supernatants were collected and the concentration of protein was determined using a BCA™ Protein Assay Kit (Pierce Biotechnology) according to manufacturer’s instructions. Protein samples (18 μg per lane) were then subjected to reducing SDS-PAGE using the method of Laemmli [29] with 15% Tris-Glycine PAGEr® Gold Precast gels (Pierce Biotechnology). The proteins were then transferred onto Immunobilon-FL membrane (Millipore, Billerica, MA) using the method of Towbin et al. [30]. The remaining steps below were carried out at room temperature with gentle agitation. Membranes were washed in PBS and blocked in ODYSSEY® Blocking Buffer (LI-COR Biosciences, Lincoln, NE) diluted 1:1 with PBS for 1 h. Next, membranes were incubated with primary antibodies in blocking buffer against IL15, IL18, IFNG or beta actin (ACTB). Primary antibody concentrations used were 0.4 μg/ml goat anti-IL15 IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 0.1 μg/ml goat anti-IFNG IgG (Abcam, Inc., Cambridge, MA), 2 μg/ml rabbit anti-IL18 IgG (Rockland, Inc, Gilbertsville, PA) or rabbit anti-ACTB antiserum at a dilution recommended by manufacturer (Biolegend, San Diego, CA) for 60 min. For controls, purified IgG and normal serum at the same concentrations and dilution were used in place of the primary antibody. Membranes were then washed in 1% PBS containing 0.05% Tween-20 (PBST) then incubated with the appropriate secondary antibody at a concentration of 0.06 μg/ml (IRDye™700DX conjugated donkey-anti-goat IgG or IRDye™800DX conjugated donkey-anti-rabbit IgG, Rockland Inc., Gilbertsville, PA) for 60 min followed by washing with PBST then PBS. Finally, infrared fluorescent signals were then measured using the Odyssey Infared Imaging System and software (LI-COR Biosciences). All fluorescent intensities were normalized to ACTB. Where possible, a two-way ANOVA was performed to determine overall differences in relative fluorescent intensities followed by the use of t-tests to determine differences between means on a given day after the onset of decidualization.
Vascular Morphometry
Previous work has shown that uNK cells play a key role in the spiral arteriole modifications that normally occur during implantation, which includes thinning of their wall and dilation [12]. Therefore, an experiment was conducted to determine if such a modification occurs by Day 4 after the onset of decidualization in the decidua and Ovx-OID plus Pseudo-BID deciduomas. Cross sections adjacent to those used for DBA-lectin staining were stained with Harris hematoxylin and eosin in order to visualize the spiral arterioles in the mesometrial region. Photomicrographs were then taken and calibrated using QCapture Pro (QImaging) and a stage micrometer, respectively. At the narrowest point, vessel (V) and lumen (L) diameters of the spiral arterioles were measured and the vessel wall thickness was determined using the formula (V-L)/2. Vessels were considered spiral arterioles based on size (10–100 μm in diameter) and presence of smooth muscle layer. Spiral arteriole wall thickness and lumen diameter were collected not only from implantation sites or areas undergoing artificially-induced decidualization from each animal, but also from adjacent non-implantation sites or non-stimulated areas (controls), respectively. Data was collected from a total of 50–200 vessels per tissue type (from 6 independent samples) and a two-way repeated measures ANOVA was used to analyze the data.
Statistical Analyses
All statistical analyses described in the sections above were carried out using either SAS (SAS Institute Inc., Cary, NC) or Sigmastat (Systat Software Inc., Point Richmond, CA) software.
RESULTS
Total Mesometrial uNK Cell Density
In our initial studies of DBA lectin-stained cross sections, it appeared that uNK cell numbers in the mesometrial region of decidua differed dramatically to those found in all types of deciduomas on the same days after onset of decidualization. On Day 3 after the onset of decidualization, it was most apparent that there was a dramatic difference in the pattern of localization of uNK cells in the cross sections of all types of deciduoma examined as compared to the decidua (Fig. 3A). Uterine NK cells were distributed more diffusely throughout the mesometrial subregions of the decidua, while those in the deciduoma were found more centrally located in the mesometrial region of the deciduoma.
Figure 3.

Uterine NK cell distribution and density. A. Photomicrographs of DBA lectin-stained cross sections of the mouse decidua and deciduomas on Day 3 after the onset of decidualization. Brown color indicates DBA lectin binding to uNK cells, Scale bar = 500 μm. .B. Graph showing density of uNK cells normalized to total area of mesometrial subregion in the mouse decidua and deciduomas at various days after the onset of decidualization. Bars represent the mean (±SEM; N= 6) and those with different letters on a given day are significantly different (P <0.01). An asterisk (*) denotes only determined for decidua.
Very few DBA lectin positive uNK cells were found in the uterine sections of the decidua and deciduoma on the first day after the onset of decidualization (Fig. 3B). No significant differences in total uNK cell density between the decidua and deciduomas were detected on this day. In contrast, uNK cells were plentiful and readily seen in the mesometrial region in the days thereafter during decidualization (Fig. 3A). As shown in Figure 3B, the total density of uNK cells within the mesometrial decidua was significantly greater (P<0.01) compared to that of all types of deciduomas on Days 2, 3 and 4 after the onset of decidualization. Notably, total uNK cell density between all three deciduoma models on each day after the onset of decidualization were not significantly (P>0.05) different (Fig. 3B). Since there were no differences between all types of deciduomas examined, in subsequent studies we decided to only compare the decidua to that of Ovx-OID and Pseudo-BID deciduomas.
Regional Cell Density
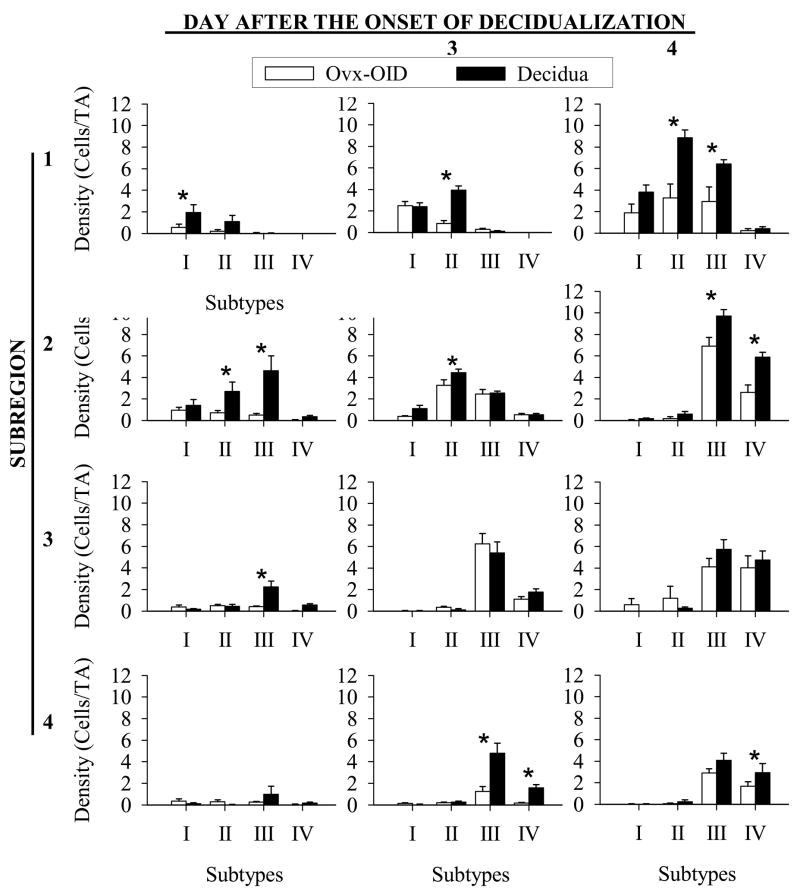
Since total uNK cell densities were dramatically different between the decidua and Ovx-OID deciduoma, we looked at the density of each of the uNK cell subtypes (Fig. 2A) in subregions 1–4 (Fig. 2B) of the mesometrial area of the uterus (Fig. 4). On Day 2 after the onset of decidualization, there was significantly (P < 0.05) greater density of subtypes II and III in subregion 2, and subtype III in subregion 3 in the decidua compared to the Ovx-OID. A similar difference in density was also seen on Day 3 after the onset of decidualization for subtype II cells in subregion 1 and also for subtypes III plus IV in subregion 4. Finally, on Day 4 the density of subtypes II and III in subregion 1, subtypes III and IV in subregion 2, and subtype IV in subregion 4 were significantly (P < 0.05) greater in the decidua compared to the Ovx-OID deciduoma. Finally, small, but significantly greater uNK cell densities were seen on Day 2 in subregion 1 subtype I and on Day 3 subregion 2 subtype II in the decidua compared to the Ovx-OID deciduoma. The lower uNK cell densities found in subregions 1 and 4 of the deciduoma in Fig. 4 are correlated to the more central distribution of cells compared to that of the decidua where they are more diffusely spread out in the mesometrial region. Similar to the Ovx-OID deciduomas, decreased uNK cell subtype densities were also seen in several subregions of the Pseudo-BID deciduomas on days 2, 3 and 4 after the onset of decidualization relative to that of the deciduas (data not shown)
Figure 4.
Graphs summarizing regional uNK cell subtype (I–IV) density per test area in subregions 1–4 of the mouse Ovx-OID deciduoma and decidua on Days 2, 3 and 4 after the onset of decidualiztion. An asterisk (*) indicates a significant difference (P <0.05) between Ovx-OID deciduoma and decidua for a given subtype. Bars represent the mean and error bars denote standard error of the mean (N=6).
Regional uNK Cell Maturation
To further investigate the difference in uNK cell populations between the decidua and Ovx-OID deciduoma, we next measured regional uNK cell maturation. We determined the percentage of each uNK cell subtype (Fig. 2A) in the four subregions (Fig. 2B) of the mesometrial region of uteri. On Day 2 after the onset of decidualization, there was a significant (P < 0.05) difference in the percentage of uNK cell subtypes in subregions 2 and 3 of the deciduas compared to the Ovx-OID deciduoma (Fig. 5). In subregion 2, this was due to a greater percentage of subtype III with less subtype I uNK cells in the decidua compared to that of the Ovx-OID deciduoma. In subregion 3, this was due to a greater percentage of subtypes III and IV with less subtypes I and II uNK cells in the decidua compared to that of the Ovx-OID deciduoma. A significant difference (P < 0.05) was also seen on Day 3 in subregion 1 with the majority being subtypes II in the decidua and I in the Ovx-OID deciduoma, respectively. By Day 4, however, there were no significant (P < 0.05) differences in the percentages of uNK cell subtypes found in the decidua compared to that of the Ovx-OID deciduoma. Similar to the Ovx-OID deciduomas, some differences of percentage of uNK cell subtypes were also seen in subregions of the Pseudo-BID deciduomas relative to that of the deciduas (data not shown)
Figure 5.
Graphs summarizing the percentage of uNK cells subtype (I–IV) in mesometrial subregions 1–4 of the mouse Ovx-OID deciduoma and decidua on Days 2, 3 and 4 after the onset of decidualization. Bars represent the mean and error bars denote standard error of the mean (N=6).
Uterine NK Cell Proliferation
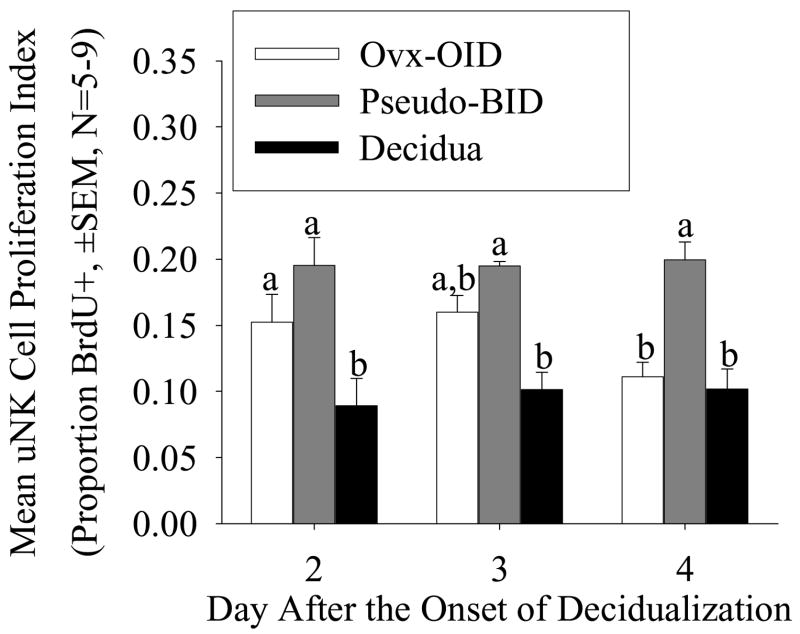
In order to determine if the increase in uNK cell numbers in the decidua was a result of uNK cell proliferation, we compared the proliferation index (the proportion of uNK cells staining positive for BrdU) between the decidua and the Ovx-OID plus Pseudo-BID deciduomas on Days 2, 3 and 4 after the onset of decidualization (Fig. 6). No significant increase in uNK cell proliferation index was seen in the decidua as compared to deciduomas.
Figure 6.
Graph showing the mean (± SEM, N=5–6) uNK cell proliferation index (proportion of uNK cells staining positive for BrdU) in the mouse Ovx-OID plus Pseudo-BID deciduomas and decidua on Days 2, 3 and 4 after the onset of decidualization. Bars with different are significantly different (P <0.01).
Western Blot Analyses of IL15, IFNG and IL18
Previous work has indicated certain cytokines to be important in the modulation of uNK cell function [12]. Therefore, Western blot analyses were performed to determine if the levels of some of these cytokines differed between the decidua and deciduoma (Fig. 7A). IL15 has been shown to be important in the maturation of uNK cells [12]. Our results revealed that IL15 levels did not significantly (P > 0.05) differ between decidua and Ovx-OID plus Pseudo-BID deciduomas on each day after the onset of decidualization examined (Fig. 7B). Both IFNG and IL18 have been shown to be produced by uNK cells [21]. IFNG exists in both glycosylated and non-glycosylated forms [31] and as shown in Figures 7A and C, the level of glycosylated-IFNG is significantly (P<0.05) increased in decidua compared to Ovx-OID and Pseudo-BID deciduomas on Days 2–4 after the onset of decidualization. This increase was 80-fold at Day 3 and 30- fold at Day 4 after the onset of decidualization. IL18 exists in an unprocessed pro-form and a processed active form [32], and as shown in Figure 7A and D, pro-IL18 was detected in the decidua but not Ovx-OID plus Pseudo-BID deciduomas on Days 2–4 after the onset of decidualization. Finally, no fluorescent bands were seen in the negative control Western blots where the primary antibodies were replaced with rabbit or goat IgG (Fig. 7E and F).
Figure 7.
Western Blot Analysis of IL15, IFNG and IL18 in the mouse Ovx-OID plus Pseudo-BID deciduomas and decidua on Days 1–4 after the onset of decidualization. A. Representative Western blot analysis. ACTB was used as a control for equal protein loading. Graph bars represent (mean ± SEM, N=4) relative fluorescence normalized to ACTB for B. IL15, C. IFNG and D. IL18. An asterisk (*) indicates a significant difference (P <0.05) between deciduomas and decidua. N indicates no fluorescence signal detected above background. E. Rabbit IgG (2 μg/ml) negative control. F. Goat IgG (0.4 μg/ml) negative control.
Vascular Morphometry
Previous work indicates uNK cells and the IFNG secreted by them are involved in the thinning and dilation of the spiral arterioles by midpregnancy [12]. Since we found significantly more uNK cells and IFNG in the decidua compared to the Ovx-OID plus Pseudo-BID deciduomas, we next examined the spiral arterioles in the mesometrial region of these tissues. As shown in Figure 8A, on Day 4 after the onset of decidualization, spiral arterioles in the mesometrial region of the decidua appear to be both thinner and more dilated compared to those present in the mesometrial region of the deciduomas. Using a more quantitative approach, we confirmed that the spiral arteriole wall thickness is significantly (P<0.05) decreased in areas of an implantation stimulus (implantation site) relative to control areas (non-implantation site) of the decidua with no similar change in the deciduomas (Fig. 8B). Finally, the lumen diameters of the spiral arterioles were significantly (P<0.05) increased in areas of an implantation stimulus of the decidua relative to control areas but again with no similar change in the deciduomas (Fig. 8C).
Figure 8.

Mouse spiral arteriole morphometry on Day 4 after the onset of decidualization. A. Photomicrographs of representative spiral arterioles in the Ovx-OID plus Pseudo-BID deciduomas and decidua. Scale bar equals 25 μm. B. Mean (± SEM, N=4–6) spiral arteriole thickness and C. lumen diameter in non-stimulated (control) plus stimulated (implantation stimulus) areas and non-implantation (control) plus implantation (implantation stimulus) sites of the deciduomas and decidua, respectively. V, vessel thickness; L, lumen diameter. Star denotes a significant (P<0.050 difference between the control and areas undergoing decidualization in response to an implantation stimulus.
DISCUSSION
Uterine NK cells play a role in the vascular changes and maintaining decidual integrity during pregnancy, however very little is known about what controls their presence and maturation [20, 33, 34]. Previous work, has shown that uNK cells appear in the rodent decidua and increase in number during decidualization [11, 35]. It has been known for some time that the presence of uNK cells occurs in artificially-induced deciduoma [23]. Further, in the human uterus there is evidence that the presence of uNK cells is associated with endometrial decidualization [36]. Therefore, current data suggests that regardless of if an embryo is present, uNK cells appear in the uterus during decidualization [37]. One could conclude from this that signals from the conceptus are not required for the presence and changes which occur in the uNK cell populations during decidualization. However, this conclusion might be premature given the limited data. Therefore, in the present study, we closely examined the uNK cell populations in the decidua and compared them to those in the deciduomas during the process of decidualization. Although many uNK cell changes that occur in the response to decidualization are independent of the conceptus, for the first time we detected a conceptus-dependent increase in uNK cell numbers and cytokine production in uteri undergoing decidualization.
A focal decidualization response is observed in response to the implanting blastocyst. However, a decidualization response is seen along the entire length of the uterine horn in an oil-induced deciduoma. Therefore, it is possible that there is the same total number of uNK cells present in both types of uteri, but the uNK cells are spread out along the entire length of the uterine horn in the deciduoma compared to focally in decidua. This would suggest that the results of these experiments are due to effects of the model used for induction of the deciduomas and not the conceptus. In order to rule out this possibility, one model of artificially-induced decidualization where the decidual response is a response to a focal deciduogenic stimulus was incorporated into the present study. Indeed, when uNK cells were enumerated in this deciduoma, uNK cell number remained similar to that of oil-induced deciduoma rather than that of the decidua. This indicated that the decreased number of uNK cells in the deciduoma is not related to the nature of the artificial deciduogenic stimulus.
Uterine NK cells proliferate in the uterus during implantation, but little is known about how this is controlled in the rodent. Previous work indicates that the proliferation of uNK cells in the uterus might require the presence of progesterone [38–40]. In the current study, we hypothesized that the conceptus-dependent increase in uNK cell numbers seen in the present study may be due to increased proliferation. However, an increase in the proliferation of uNK cells in the decidua compared to the deciduoma could not be found. Therefore, the effect of the conceptus on uNK cell number is not through proliferation. An alternative explanation is that it is due to a conceptus-dependent effect on the recruitment of uNK cells into uteri undergoing decidualization. This is consistent with previous data that the increasing number of uNK cells in the mouse uterus is mainly due to that of recruitment of uNK cell precursors from secondary lymphoid tissues and not from self-renewal of an existing uNK cell population [14]. Recently there has been great interest in examining factors associated with leukocyte recruitment into areas of uteri undergoing decidualization [41, 42] and clearly more work is needed to determine the effect of the conceptus on these factors.
IL15 has been suggested to play a role in the regulation of uNK cell proliferation and is present in decidual macrophages and uNK cells [43]. In the present study, there were no differences in the level of IL15 in the decidua compared to the deciduomas. This suggests that the conceptus-dependent increase in uNK cell numbers might not involve IL15 signaling.
A more important and highly researched function of IL15 is its involvement in the maturation of uNK cells from immature subtype I to senescent subtype IV uNK cells [16, 17]. This was demonstrated by use of IL15 knockout mice, which completely lack uNK cells [18, 19]. In addition, when bone marrow from IL15-deficient mice is injected into alymphoid Rag 2−/−yc−/− recipients, that do express IL15, the uNK cell populations are completely rescued [44]. When mice lacking IL15 are given exogenous IL15, uNK cell populations are restored confirming the role of this cytokine in the maturation of uNK cells [18]. In this study, we found that there were no differences in IL15 levels in the decidua compared to deciduomas. In addition, very little difference was observed in the maturation of uNK cells between the decidua and deciduomas. These two pieces of evidence support the hypothesis that signals from the conceptus do not play a role in the maturation of uNK cells. This is consistent with previous work that suggests that uNK cell maturation is directed by maternal- but not fetal-derived factors associated with pregnancy [35, 45].
Uterine NK cells modify spiral arterioles by controlling both the dilation and thinning of the wall in spiral arterioles present in the mesometrial region of the uterus during decidualization [12]. IFNG is a major product of uNK cells and it has been established that it is responsible for these changes in the uterine vasculature associated with pregnancy [20, 46]. By Day 6 of pregnancy IL18 is produced exclusively by uNK cells [21]. Recently, it has been demonstrated that IL18 is at least partially responsible for the induction of uNK cell IFNG synthesis leading to the pregnancy-induced spiral arteriole modifications [21]. In the present study, we found that when a conceptus is present there are significantly higher levels of both glycosylated IFNG and pro-IL18, and these higher levels correlate with the observed increase in uNK cell number. Therefore, although uNK cells are present in the deciduomas, there are fewer uNK cells that secrete glycosylated IFNG and pro-IL18 than are found in the decidua. The increased levels of glycosylated IFNG may explain, at least in part, why spiral arteriole modifications were seen only in the decidua. However, since only the biologically inactive pro-IL18 but not active IL18 levels differed between the decidua and deciduomas, it seems unlikely that IL18 is regulating the increased glycosylated IFNG levels. Further work is necessary to determine if the conceptus has an effect on uNK cell Ifng and Il18 gene expression.
This study strongly supports the hypothesis that the conceptus plays a critical role in regulating uNK cell populations in the uterus undergoing decidualization. These effects on uNK cell number and production of cytokines could be modulated either directly or indirectly. Currently, it is unknown what the signal could be from the conceptus. Notably, although prolactin-like protein A is believed to be a uNK cell regulatory signal from the conceptus [47, 48], mice that are deficient do not show differences in the distribution of uNK cells [49]. Therefore, it is unlikely that this is the signal and clearly more research is needed to determine what signals are involved.
In conclusion, the conceptus likely has an effect on uNK cell recruitment. It has been shown that the conceptus is not involved during the initial recruitment process which precedes implantation in rodents [37], however past researchers have shown that the increase in uNK cell number during pregnancy in the mouse is mainly due to recruitment of uNK cell precursors from secondary lymphoid tissues [14]. Therefore, we are currently investigating the role of the conceptus in the process of recruitment of uNK cells during mouse pregnancy.
Acknowledgments
This study was supported by NIH Grant HD049010. The Division of Statistics and Research Consulting, Southern Illinois University School of Medicine, provided recommendations in the use of the chi-square analyses of our data.
Footnotes
Supported by NIH grant HD049010
Conceptus effects on uNK cell number and function.
References
- 1.Das RM, Martin L. Uterine DNA synthesis and cell proliferation during early decidualization induced by oil in mice. J Reprod Fertil. 1978;53:125–128. doi: 10.1530/jrf.0.0530125. [DOI] [PubMed] [Google Scholar]
- 2.Lejeune B, Van Hoeck J, Leroy F. Satellite versus total DNA replication in relation to endopolyploidy of decidual cells in the mouse. Chromosoma. 1982;84:511–516. doi: 10.1007/BF00292852. [DOI] [PubMed] [Google Scholar]
- 3.Loeb L. The production of deciduomata and the relation between the ovaries and the formation of the decidua. J Am Med Assoc. 1908;50:1897–1901. [Google Scholar]
- 4.Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39:195–206. doi: 10.1530/jrf.0.0390195. [DOI] [PubMed] [Google Scholar]
- 5.Krehbiel RH. Cytological studies of the decidual reaction in the rat during early pregnancy and in the production of the deciduomata. Physiological Zoology. 1937;X:212–233. [Google Scholar]
- 6.Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immuno Biology: the immune system in health and disease. New York: Garland Science; 2005. [Google Scholar]
- 7.Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol. 2003;60:1–11. doi: 10.1016/s0165-0378(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 8.Linnemeyer PA, Pollack SB. Murine granulated metrial gland cells at uterine implantation sites are natural killer lineage cells. J Immunol. 1991;147:2530–2535. [PubMed] [Google Scholar]
- 9.Mukhtar DD, Stewart IJ, Croy BA. Leucocyte membrane antigens on mouse granulated metrial gland cells. J Reprod Immunol. 1989;15:269–279. doi: 10.1016/0165-0378(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 10.Parr EL, Parr MB, Zheng LM, Young JD. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod. 1991;44:834–841. doi: 10.1095/biolreprod44.5.834. [DOI] [PubMed] [Google Scholar]
- 11.Stewart I, Peel S. The differentiation of the decidua and the distribution of metrial gland cells in the pregnant mouse uterus. Cell Tissue Res. 1978;187:167–179. doi: 10.1007/BF00220629. [DOI] [PubMed] [Google Scholar]
- 12.Ashkar AA, Di Santo JP, Croy BA. Interferon γ contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peel S, Stewart IJ, Bulmer D. Experimental evidence for the bone marrow origin of granulated metrial gland cells of the mouse uterus. Cell Tissue Res. 1983;233:647–656. doi: 10.1007/BF00212232. [DOI] [PubMed] [Google Scholar]
- 14.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 15.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- 16.Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: key regulators of placental development (a review) J Reprod Immunol. 2002;57:151–168. doi: 10.1016/s0165-0378(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 17.Zheng LM, Liu CC, Ojcius DM, Young JD. Expression of lymphocyte perforin in the mouse uterus during pregnancy. J Cell Sci. 1991;99(Pt 2):317–323. doi: 10.1242/jcs.99.2.317. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye W, Zheng LM, Young JD, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med. 1996;184:2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkar AA, Croy BA. Interferon-γ contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA. Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod. 2003;69:404–411. doi: 10.1095/biolreprod.103.015529. [DOI] [PubMed] [Google Scholar]
- 22.Stewart I. Differentiation of granulated metrial gland cells in ovariectomized mice given ovarian hormones. J Endocrinol. 1987;112:23–26. doi: 10.1677/joe.0.1120023. [DOI] [PubMed] [Google Scholar]
- 23.Peel S, Stewart I, Bulmer D. Metrial gland cells in deciduomata of pseudopregnancy. J Anat. 1979;129:21–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Bany BM, Harvey MB, Schultz GA. Expression of matrix metalloproteinases 2 and 9 in the mouse uterus during implantation and oil-induced decidualization. J Reprod Fertil. 2000;120:125–134. doi: 10.1530/jrf.0.1200125. [DOI] [PubMed] [Google Scholar]
- 25.Hall K. Uterine mitosis, alkaline phosphatase and adenosine triphosphatase during development and regression of deciduomata in pseudopregnant mice. J Endocrinol. 1969;44:91–100. doi: 10.1677/joe.0.0440091. [DOI] [PubMed] [Google Scholar]
- 26.Sakoff JA, Murdoch RN. Alterations in uterine calcium ions during induction of the decidual cell reaction in pseudopregnant mice. J Reprod Fertil. 1994;101:97–102. doi: 10.1530/jrf.0.1010097. [DOI] [PubMed] [Google Scholar]
- 27.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 28.Heryanto B, Girling JE, Rogers PA. Intravascular neutrophils partially mediate the endometrial endothelial cell proliferative response to oestrogen in ovariectomised mice. Reproduction. 2004;127:613–620. doi: 10.1530/rep.1.00161. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinderknecht E, O’Connor BH, Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984;259:6790–6797. [PubMed] [Google Scholar]
- 32.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 34.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 36.King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 37.Redline RW. Role of uterine natural killer cells and interferon gamma in placental development. J Exp Med. 2000;192:F1–4. doi: 10.1084/jem.192.2.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peel S, Bulmer D. The effects of late ovariectomy on the proliferation and differentiation of the uterus of the pregnant rat. J Anat. 1975;119:569–578. [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma R, Bulmer D, Peel S. Effects of exogenous progesterone following ovariectomy on the metrial glands of pregnant mice. J Anat. 1986;144:189–199. [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart I, Peel S. Granulated metrial gland cells at implantation sites of the pregnant mouse uterus. Anat Embryol (Berl) 1980;160:227–238. doi: 10.1007/BF00301863. [DOI] [PubMed] [Google Scholar]
- 41.Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS. Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J Immunol. 2003;171:4011–4019. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–1126. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 44.Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 45.Zheng LM, Joag SV, Parr MB, Parr EL, Young JD. Perforin-expressing granulated metrial gland cells in murine deciduoma. J Exp Med. 1991;174:1221–1226. doi: 10.1084/jem.174.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platt JS, Hunt JS. Interferon-gamma gene expression in cycling and pregnant mouse uterus: temporal aspects and cellular localization. J Leukoc Biol. 1998;64:393–400. doi: 10.1002/jlb.64.3.393. [DOI] [PubMed] [Google Scholar]
- 47.Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003;204:65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 48.Muller H, Liu B, Croy BA, Head JR, Hunt JS, Dai G, Soares MJ. Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology. 1999;140:2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- 49.Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci U S A. 2004;101:16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]