Abstract
CD4+ T helper cells may play a critical role in the induction and maintenance of a therapeutic immune response to cancer. To evaluate the efficacy with which a recombinant tumor-associated protein can induce antigen-reactive CD4+ T cells, we stimulated peripheral blood lymphocytes from patients with melanoma in vitro with the purified melanoma antigen gp100 produced in Escherichia coli. In preliminary experiments, we observed that peripheral blood mono-nuclear cells could process and present known HLA-DRβ1*0401 and HLA-DRβ1*0701 restricted epitopes to gp100-reactive CD4+ T cell lines after being loaded exogenously with protein. Therefore, we used autologous protein-loaded peripheral blood mononuclear cells as antigen presenting cells. From four of nine patients who expressed both HLA-DRβ1*0401 and HLA-DRβ1*0701, we raised five gp100-reactive CD4+ T cell populations that secreted TH1 type cytokines in response to exogenously loaded protein as well as target cells that endogenously expressed gp100 and MHC class II molecules, including transfectants and melanoma cells. Four of the five cultures specifically recognized the known HLA-DRβ1*0401 and HLA-DRβ1*0701 restricted epitopes gp100:44–59 and gp100:170–190, respectively. The fifth culture, and 30 T cell clones derived from it, specifically recognized a new peptide, gp100:420–435, in the context of HLA-DRβ1*0701. These results suggest that recombinant tumor-associated proteins may be clinically applicable for the generation of CD4+ T helper cells in active vaccination strategies or adoptive cellular immunotherapies.
Keywords: T lymphocytes, Th1/Th2 cells, MHC, tumor immunity
Data from preclinical murine models and human clinical trials suggest that CD4+ T lymphocytes are required for the initiation and persistence of T cell-mediated immune responses to cancer.1–6 CD4+ T helper cells produce cytokines such as (interleukin) IL-2 and (interferon) INF-γ that stimulate the proliferation and differentiation of CD8+ cytotoxic T lymphocytes (CTLs). Furthermore, they promote efficient antigen presentation, costimulation, and IL-12 production by dendritic cells (DCs).7 Therefore, vaccination strategies that induce T helper cell responses are currently being developed for the immunotherapy of patients with cancer.
MHC class II restricted epitopes that are recognized by CD4+ T lymphocytes have been identified from several melanoma differentiation antigens, including MART-1,8 gp100,9–12 tyrosinase,13,14 TRP-1,15 and TRP-2,16 as well as the “cancer testis” antigens MAGE3,17–19 MAGE1,20 and NY-ESO-1,21–24 HLA class II restricted epitopes have also been identified from other tumor-associated antigens, including the catalytic subunit of telomerase,25 the bcr-abl fusion protein,26 and HER2/neu.27 Despite this information, evidence of immunization against specific HLA class II restricted tumor-associated epitopes has not been consistently observed in human vaccination trials. In a study conducted in the Surgery Branch of the National Cancer Institute, patients with meta-static melanoma vaccinated the HLA-DRβ1*0401 restricted peptide gp100:44–5910–12 developed no consistent reactivity against this epitope.
An alternative strategy to active immunization is the adoptive transfer of tumor-reactive T cell populations. We have recently reported a high incidence of objective clinical responses and long-term persistence of transferred T cells in patients with metastatic melanoma receiving the adoptive transfer of autologous tumor-infiltrating lymphocytes containing both CD8+ and CD4+ cells after non-myeloablative chemotherapy.1 In contrast, no objective clinical responses were observed in an earlier study in which patients were similarly treated with adoptively transferred CD8+ tumor-reactive T cell clones. Furthermore, no peripheral persistence of these clones was observed beyond a few days.28 We hypothesized that CD4+ T helper cells in tumor-infiltrating lymphocytes enabled the proliferation, activation, and persistence of melanoma-reactive CTLs concomitantly present in the adoptively transferred cell population. Therefore, much effort is currently being directed toward developing techniques for raising tumor antigen-reactive CD4+ T cells from peripheral blood mononuclear cells (PBMCs) ex vivo to evaluate the efficacy of co-adoptively transferring these cells along with tumor-reactive CTLs.
Recombinant proteins have previously been used to stimulate antigen-reactive CD4+ T lymphocytes in vitro that specifically recognized the “cancer-testis” antigens MAGE120 and MAGE3.17 These CD4+ T cells recognized synthetic peptides derived from these proteins as well as lysates of cells expressing MAGE1 or MAGE3 when pulsed onto HLA-DR matched antigen-presenting cells (APCs). However, they did not recognize MHC class II positive tumor cells that endogenously expressed these proteins. This phenomenon was probably due to a lack of MHC class II presentation pathway targeting sequences in “cancer-testis” antigens.17,20 In contrast, melanocyte differentiation proteins are known to contain specific lysosomal targeting sequences that enable presentation of epitopes in the context of MHC class II molecules.29 In one study, recombinant gp100 protein was used to stimulate CD4+ T cells in vitro.30 However, no data were presented to evaluate whether the resulting T cells were capable of recognizing MHC class II positive melanoma cells, and the possibility exists that these T cells may have been responding to contaminants in the protein preparation. In most studies, DCs were used as APCs to process and present relevant epitopes from the recombinant proteins. We have previously observed that repeated stimulation of peripheral blood lymphocytes (PBL) with autologous DCs can result in the induction of T cells that are strongly stimulated by irrelevant target cells, presumably due to the presence of allogeneic or xenogeneic proteins in sera or from bacterial or viral proteins in the antigen preparation. In contrast, we have not generally observed similar phenomena when autologous PBMCs were used as APCs.
In the current investigation, we first evaluated whether PBMCs could process exogenously loaded gp100 protein and present relevant MHC class II restricted epitopes to defined gp100-reactive CD4+ T cell lines. Based on positive results in these preliminary experiments, we stimulated PBL in vitro with autologous PBMCs loaded with recombinant gp100 protein. Using this methodology, we isolated gp100-reactive CD4+ T cell cultures from patients with metastatic melanoma that specifically recognized the known HLA- DRβ1*0401 and HLA-DRβ1*0701 restricted epitopes gp100:44–59 and gp100:170–190, respectively. In addition, we identified a new HLA-DRβ1*0701 restricted epitope gp100:420–435. All of these T cell populations secreted TH1 type cytokines in response to recombinant protein, individual peptides, and MHC class II positive target cells that endogenously expressed gp100, including transfectants and melanoma cells.
MATERIALS AND METHODS
Media and Cell Culture
Human melanoma cell lines and EBV-transformed B cells (EBV-B) were routinely cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and 2 mmol/L L-glutamine (Invitrogen, Carlsbad, CA); 293 cells expressing HLA-DRβ1*0701 (293-DR7) or HLA-DRβ1*0401 (293-DR4) were previously engineered by transfection with essential components of the MHC class II presentation pathway31 and were maintained in DMEM (Invitrogen) containing 10% heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, and 10 mmol/L HEPES. Human lymphocytes were cultured in complete medium (CM) consisting of RPMI 1640, 2 mmol/L L-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin (Invitrogen) and 10% heat-inactivated human AB serum (Gemini Bio-Products, Woodland, CA; Valley Biomedical, Winchester, VA). Two gp100-reactive CD4+ T cell populations were used to evaluate the presentation of MHC class II restricted epitopes by PBMCs pulsed with recombinant gp100 protein as follows: 1) B104 was a CD4+ T cell clone that recognized gp100:170–190 in the context of HLA-DRβ1*07019; and 2) BR-B8 was a CD4+ T cell line that recognized gp100:44–59 in the context of HLA-DRβ1*040111 and was generated by repeated in vitro stimulation with synthetic peptide.
Expression of gp100 in melanoma cell lines was assessed by FACS using an anti-melanoma monoclonal antibody (HMB45; Enzo Diagnostics, Farmingdale, NY). The F002R melanoma cell line (F002Rmel) did not naturally express gp100, but it had previously been transduced with a vesicular stomatitis virus-pseudotyped retroviral vector encoding either gp100 or green fluorescence protein (GFP) as previously described.9 The DNA fragment encoding gp100 in this retroviral vector contained a single base pair mutation such that the resulting translated protein contained a V residue at amino acid position 184 instead of the naturally encoded M residue at this position. Expression of HLA class II molecules on the surfaces of melanoma cells was up-regulated by transduction with a retroviral contstruct encoding the HLA class II transactivator gene (CIITA) as previously described.32 The expression of cell surface HLA class II molecules on melanoma cells was confirmed by FACS using monoclonal antibodies against HLA-DR (L243; Becton Dickinson Biosciences, Franklin Lakes, NJ), HLA-DR4 (Accurate Chemical and Scientific Corp., Westbury, NY), and HLA-DR7 (Pel-Freez Biologicals, Rogers, AR). In addition, HLA haplotypes of cell lines were determined by DNA sequencing in the HLA Laboratory at the National Institutes of Health. The expression of gp100, HLA-DRβ1*0401, and HLA-DRβ1*0701 in melanoma cell lines was as follows: F002Rmel-gp100 CIITA (HLA-DRβ1*0401−, HLA-DRβ1*0701+, gp100+), F002Rmel-GFP CIITA (HLA-DRβ1*0401−, HLA-DRβ1*0701+, gp100−), 1978mel CIITA (HLA-DRβ1*0401−, HLA-DRβ1*0701+, gp100+), 1087mel CIITA (HLA-DRβ1*0401−, HLA-DRβ1*0701+, gp100+), 1909mel (HLA-DRβ1*0401−, HLA-DRβ1*0701+, gp100+), 624mel CIITA (HLA-DRβ1*0401+, HLA-DRβ1*0701+, gp100+), 526mel CIITA (HLA-DRβ1*0401+, HLA-DRβ1*0701−, gp100+), and 1861mel CIITA (HLA-DRβ1*0401+, HLA-DRβ1*0701−, gp100+).
Protein and Peptide Synthesis
Recombinant human gp100 and NY-ESO-1 proteins were produced in Escherichia coli using the pET-28(+) pro-karyotic expression vector (Novagen, Madison, WI) and were purified to >95% (Novavax, Inc., Columbia, MD). Since it was not possible to produce large quantities of full-length gp100, a truncated version (gp100:24–594) was made in which the amino-terminal signal sequence (amino acids 1–23) and car-boxy-terminal transmembrane domain (amino acids 595–661) were removed. The recombinant NY-ESO-1 protein was full-length encompassing amino acids 1–180. As was the case for the vesicular stomatitis virus-pseudotyped retrovirus, the gp100 prokaryotic expression vector contained a single base pair mutation such that the resulting recombinant protein contained a V residue at amino acid position 184 instead of the naturally encoded M residue.
gp 100 peptides, including gp 100:170 – 190 (LSIGTGRAMLGTHTMEVTVYH) and a negative control peptide with high binding affinity to HLA-DRβ1*0701 (Ig kappa chain:188–201 KHKVYACEVTHQGL), were synthesized using a solid-phase method based on fluorenyl-methoxycarbonyl (Fmoc) chemistry on a multiple peptide synthesizer (model AMS 422; Gilson Co., Worthington, OH), and molecular weights were verified by laser desorption mass spectrometry (Bio-Synthesis, Inc., Lewisville, TX; Tufts University Core Facility, Boston, MA). Gp100:44–59 (WNRQLYPEWTEAQRLD) and a negative control peptide with high binding affinity to HLA-DRβ1*0401 (Influenza A HLA:306–324 PKYVKQNTLKLATGMRNVP) were commercially synthesized using Fmoc chemistry and purified (>95%) by reversed-phase HPLC (Macromolecular Resources, Fort Collins, CO). A stock solution of each peptide was prepared by solubilization in DMSO at a concentration of 50 mmol/L.
Stimulation of T Lymphocytes with Recombinant gp100 Protein In Vitro
PBMCs from patients with metastatic melanoma who expressed both HLA-DRβ1*0401 and HLA-DRβ1*0701 were stimulated in vitro with recombinant human gp100 using protein-pulsed autologous irradiated PBMCs as APCs. Microcultures were established by initially plating PBMCs in 96-well plates (2 × 105 cells/well; 106 cells/mL; 200 μL/well) in CM containing 10 μg/mL gp100 protein. On day 6, protein-loaded autologous PBMCs were prepared to restimulate responder T lymphocytes. In particular, autologous PBMCs were irradiated (4000 rad) and subsequently incubated with 10 μg/mL gp100 protein overnight at 37°C in 96-well plates (5 × 105 cells/well; 5 × 106 cells/mL; 100 μL/well). On day 7, responder T lymphocytes were transferred into the new 96-well plates containing protein-loaded PBMCs. One day after the re-stimulation, 300 IU/mL rIL-2 was added; and generally, cultures were split 1:1 into new 96-well plates 3 to 4 days later with CM containing 300 IU/mL rIL-2. On day 19, each micro-culture was evaluated for specific recognition of gp100 based on specific secretion of INF-γ. In some experiments, cytokine release was measured in response to gp100 protein-loaded allogeneic PBMCs expressing both HLA-DRβ1*0401 and HLA-DRβ1*0701 in comparison to similarly produced NY-ESO-1 protein. In other experiments, INF-γ secretion was measured in response to autologous DCs transduced with an adenoviral vector encoding gp100 in comparison to a similar virus encoding MART-1 or GFP. gp100-reactive microcultures with INF-γ secretion of >100 pg/mL and at least twice background with a negative control were restimulated individually in 24 well plates with approximately 5 × 106 protein-pulsed autologous irradiated PBMCs per well. Approximately 12 days later, after two restimulations, INF-γ secretion was measured in response to gp100 protein, melanoma cells expressing HLA-DRβ1*0401 and/or HLA-DRβ1*0701, and 293-DR4 and 293-DR7 cells transfected with gp100 cDNA. In addition, phenotypes of T cell microcultures were evaluated using monoclonal antibodies against CD3, CD4, and CD8 (Becton Dickinson Biosciences Inc., San Diego, CA). CD4+ T cell cultures that demonstrated specific recognition of gp100 in the context of either HLA-DRβ1*0401 or HLA-DRβ1*0701 were expanded with PHA-L or anti-CD3 (OKT-3). For PHA-L stimulation, 5 × 105 responder lymphocytes were mixed with 5 × 106 allogeneic irradiated PBMCs from at least 3 different donors as feeder cells in CM containing 1 μg/mL PHA-L (Sigma-Aldrich, St. Louis, MO) in one well of a 24-well plate (2 mL/well). Alternatively, 5 × 105 responder lymphocytes were mixed with 25 × 106 allogeneic irradiated PBMCs in CM containing 30 ng/mL OKT-3 (Ortho Biotech Inc., Raritan, NJ) in an upright 25 cm2 tissue culture flask (25 mL/flask); 300 IU/mL rIL-2 was added 1 day later, and cultures were maintained as needed by splitting with media containing 300 IU/mL r IL-2. Seven to 14 days later, recognition of gp100 protein, HLA-DRβ1*0401+ and/or HLA-DRβ1*0701+ melanoma cells, and 293-DR4 and/or 293-DR7 transfectants, was once again evaluated on the basis of specific INF-γ secretion.
T Lymphocyte Cloning and TCR-β Chain Analysis
A CD4+ T cell population that specifically recognized gp100:420–435 in the context of HLA-DRβ1*0701 was cloned by limiting dilution in the presence of allogeneic irradiated PBMCs from at least 3 different donors as feeder cells in CM containing 1 μg/mL PHA-L and 300 IU/mL rIL-2. Approximately 2 weeks later, growth-positive wells were assayed for specific peptide recognition. Peptide-reactive “clones” were expanded once again with PHA-L and IL-2, and 7 to 14 days later, recognition of gp100 protein and HLA-DRβ1*0701+ melanoma cells was evaluated on the basis of specific INF-γ secretion.
To assess the clonality of selected tumor-reactive CD4+ T cell populations, analyses of TCR-β chain variable regions (BV) were conducted. Initially, T cells were stained with BV family specific monoclonal antibodies (Beckman Coulter, Fullerton, CA; Pierce-Endogen) and were analyzed by FACS as previously described.1 However, commercially available antibodies only encompassed approximately two thirds of the known BV genes. Therefore, if no specific BV families were identified on CD3+ T cells by FACS, TCR expression was determined by 5′ rapid amplification of cDNA ends (5′ RACE) (Invitrogen) using nested beta chain constant region primers (BC). Total RNA was isolated from T cell samples using RNeasy Mini Columns (Qiagen Inc., Valencia, CA) approximately 4 weeks after exposure to feeder cells to decrease the likelihood that TCR-β chain sequences could be derived from allogeneic PBMCs. RNA was reverse transcribed into cDNA using a BC derived primer (BC1 bp 501–518; CTCTTGACCATGGCCATC). After purification and TdT tailing of the cDNA, PCR was performed using a nested BC primer (BC1 bp 379–402; CGAGGTAAGCCACAGTCTGCTCTA). Subsequently, an additional PCR was performed using a second nested BC primer (BC1 bp 202–225; CAGGCAGTATCTGGAGTCATTGAG). Bands of approximately 600 bp were extracted from agarose gels, and after purification, DNA was sequenced using ABI PRISM Big Dye Terminator Cycle Sequencing reagents (Perkin Elmer, Foster City, CA) and an additional nested BC primer (BC1 bp 38–58; TCTCTGCTTCTGATGGCT CAA) on an ABI PRISM 3100 Genetic Analyzer (ABI, Foster City, CA).
Cytokine Release Assays
To determine if PBMCs loaded with recombinant gp100 protein could process and present relevant HLA class II restricted epitopes, INF-γ secretion by two gp100-reactive CD4+ T cell populations was measured. Irradiated (4000 rad) allogeneic PBMCs expressing both HLA-DRβ1*0401 and HLA-DRβ1*0701 were incubated with 10 μg/mL gp100 protein (or NY-ESO-1 as a negative control) overnight at 37°C in 96-well plates (5 × 105 cells/well for PBMC; 105 cells/well; 100 μL/well). The next day, 105 BR-B8 or B104 T cells in 150 μL CM were added to each well containing protein-loaded PBMC. Cocultures were incubated ~20 hours at 37°C, and the concentration of human INF-γ in supernatants was measured by ELISA (Pierce-Endogen, Cambridge, MA). As positive controls for T cell function, cytokine secretion was also measured in response to specific gp100 peptides, melanoma cells expressing HLA-DRβ1*0701 and/or HLA-DRβ1*0401, and 293-DR4 and 293-DR7 transfectants. One day prior to the assay, 293-DR4 and 293-DR7 cells were transfected with cDNAs encoding gp100 or GFP directly in 96-well plates using Lipofectamine 2000 (Invitrogen) (1.2 × 105 cells/well; 200 ng cDNA/well; 1 μL Lipofectamine 2000/well; 150 μL/well). As was the case for the vesicular stomatitis virus-pseudotyped retrovirus and the pET-28(+) prokaryotic expression vector, the mammalian expression vector pcDNA3 (Invitrogen) encoding gp100 contained a single base pair mutation, resulting in an M to V amino acid substitution at position 184. On the day of the assay, EBV-B cells were preincubated with 50 μmol/L peptide 1 to 3 hours at 37°C. Peptide-loaded EBV-B cells and melanoma cells were plated in 96-well plates in CM (105 cells/well); 105 responder T cells were coincubated with stimulator cells (250 μL total) ~20 hours at 37°C, and the concentration of human INF-γ in coculture supernatants was measured by ELISA (Pierce-Endogen).
In some experiments, recognition of gp100 by individual T cell microcultures was preliminarily evaluated 1 week after restimulation on the basis of INF-γ secretion in response to allogeneic PBMCs expressing both HLA-DRβ1*0701 and HLA-DRβ1*0401 preloaded overnight with recombinant protein. Irradiated (4000 rad) PBMCs were incubated with 10 μg/mL gp100 protein (or NY-ESO-1 as a negative control) overnight at 37°C in 96-well plates (5 × 105 cells/well; 100 μL/well). The next day, one fourth of each T cell microculture was plated in a well containing PBMCs with gp100 protein. Another fourth of each microculture was incubated with PBMCs preloaded with NY-ESO-1, and the remaining half of each microculture was retained for future restimulation. Alternatively, T cell microcultures were initially evaluated for specific recognition of autologous DCs transduced with an adenoviral vector encoding gp100. In contrast to the retrovirus, procaryotic, and eukaryotic expression vectors described above, the recombinant adenovirus encoding gp100 did not contain any mutations. Immature DCs were prepared as previously described.33 Briefly, adherent PBMCs were cultured in CM containing 1000 U/mL GM-CSF and 1000 U/mL IL-4 (Peprotech, Rocky Hill, NJ) for 5 to 7 days. DCs were then transduced with recombinant adenoviruses encoding gp100 (or MART-1 or GFP as negative controls) (Genzyme Corporation, Cambridge, MA) as previously described34 and were incubated 24 to 48 hours at 37°C in 96-well plates (0.1–1 × 105 cells/well; 100 μL/well). One fourth of each T cell microculture was plated in a well containing autologous DCs transduced with the adenoviral vector encoding gp100. Another fourth of each microculture was incubated with DCs expressing MART-1 or GFP after adenoviral transduction, and the remaining half of each microculture was retained for future restimulation. Responder cells were coincubated with protein-loaded PBMCs or adenovirally transduced DCs ~20 hours at 37°C, and the concentration of human INF-γ in coculture supernatants was measured by ELISA (Pierce-Endogen).
Recognition of gp100 protein as well as individual gp100 peptides, melanoma cells expressing HLA-DRβ1*0701 and/or HLA-DRβ1*0401, and 293-DR4 and 293-DR7 transfectants, was evaluated 7 to 12 days after the second restimulation on the basis of INF-γ secretion. As described above, 1 day prior to the assay, 293-DR4 and 293-DR7 cells were transfected with cDNAs, and irradiated PBMCs were loaded with recombinant proteins. On the day of the assay, EBV-B cells were preincubated with peptide 1 to 3 hours at 37°C, and melanoma cell lines were harvested. In titration experiments, EBV-B cells were incubated with peptides at various concentrations and were subsequently washed to remove exogenous peptide. Responder T cells were coincubated with stimulator cells ~20 hours at 37°C, and the concentration of multiple TH1 and TH2 type cytokines in coculture supernatants was measured by ELISA (Pierce-Endogen) including INF-γ, GM-CSF, TNF-α, IL-2, IL-4, and IL-10.
RESULTS
Presentation of MHC Class II Restricted Epitopes by PBMCs Loaded with Recombinant gp100 Protein
We first investigated whether PBMCs could process and present relevant MHC class II restricted epitopes from exogenously loaded recombinant gp100 protein. INF-γ secretion by two previously established gp100-reactive CD4+ T cell lines was measured in response to irradiated PBMCs expressing appropriate HLA-DR molecules after overnight incubation with recombinant gp100 protein or NY-ESO-1 protein as a negative control (Table 1). BR-B8, which recognizes gp100:44–59 in the context of HLA-DRβ1*0401, specifically secreted cytokine in response to three different protein-loaded PBMCs expressing this HLA molecule. In addition, the gp100:170–190-reactive CD4+ T cell clone, B104, specifically released INF-γ in response to three different protein-loaded PBMCs expressing HLA-DRβ1*0701, although background cytokine secretion was significantly higher in response to NY-ESO-1 than to CM alone. These results suggested that PBMCs could efficiently engulf and process exogenously loaded gp100 protein and present at least two different MHC class II restricted epitopes.
TABLE 1.
Recognition of PBMC Pulsed with Recombinant gp 100 Protein by HLA-DRβ1*0701 and -DRβ1*0401 Restricted, gp 100-Reactive CD4+ T Cell Populations*
| Target Cells | HLA-DR Expression | Preloaded Protein† | Introduced Gene‡ | BR-B8§ | B104|| |
|---|---|---|---|---|---|
| Media | None | — | None | 0 | 0 |
| 293-DR4 | DR4+ | — | MART-1 | 52 | nt |
| 293-DR4 | DR4+ | — | gp100 | 1,908 | nt |
| 293-DR7 | DR7+ | — | MART-1 | 0 | 1 |
| 293-DR7 | DR7+ | — | gp100 | 20 | 4,349 |
| F002Rmel CIITA | DR7+ | — | GFP | 16 | 12 |
| F002Rmel CIITA | DR7+ | — | gp100 | 11 | 13,686 |
| 624mel CIITA | DR4+ DR7+ | — | — | 2,278 | 354 |
| 1861mel CIITA | DR4+ | — | — | 5,746 | 5 |
| Donor 1 PBMC | DR4+ DR7+ | — | — | 16 | 0 |
| Donor 1 PBMC | DR4+ DR7+ | NY-ESO-1 | — | 25 | 1,107 |
| Donor 1 PBMC | DR4+ DR7+ | gp100 | — | 6,151 | 15,123 |
| Donor 2 PBMC | DR4+ DR7+ | — | — | 27 | 12 |
| Donor 2 PBMC | DR4+ DR7+ | NY-ESO-1 | — | 37 | 428 |
| Donor 2 PBMC | DR4+ DR7+ | gp100 | — | 5,956 | 12,500 |
| Donor 3 PBMC | DR4+ DR7+ | — | — | 37 | 23 |
| Donor 3 PBMC | DR4+ DR7+ | NY-ESO-1 | — | 13 | 225 |
| Donor 3 PBMC | DR4+ DR7+ | gp100 | — | 2,818 | 6,850 |
INF-γ secretion (pg/mL) in 20-hour coculture supernatants of target cells with T lymphocytes.
Recombinant proteins were preloaded onto PBMC at 10 νg/mL overnight at 37°C.
The indicated genes were transfected into 293 cells or retrovirally transduced into F002mel CIITA cells.
BR-B8 is a CD4+ T cell line that specifically recognizes gp100:44–59 in the context of HLA-DRβ1*0401.
B104 is a CD4+ T cell clone that specifically recognizes gp100:170–190 in the context of HLA-DRβ1*0701.
Note. Underlined values indicate that INF-γ secretion in response to target cells expressing gp100 and either HLA-DRβ1*0701 or HLA-DRβ1*0401 was ≥ 100 pg/mL and at least twice background with relevant control target cells.
Stimulation of MHC Class II Restricted gp100 Reactive CD4+ T Lymphocytes in Vitro
PBMCs from nine different patients with metastatic melanoma, each of whom expressed both HLA-DRβ1*0401 and HLA-DRβ1*0701, were stimulated in vitro with recombinant gp100 protein. PBMCs microcultures from four of these patients were initially evaluated for recognition of gp100 on the basis of specific INF-γ secretion in response to autologous DCs transduced with an adenoviral vector encoding gp100. Multiple microcultures from two of these four patients secreted significant amounts of INF-γ (>100 pg/mL) in response to DCs transduced with gp100 in comparison to MART-1 (at least twice background; data not shown). These microcultures were restimulated with protein-loaded PBMCs in 24-well bulk cultures. Approximately 12 days later, they were evaluated for recognition of gp100 protein, individual MHC class II restricted gp100 peptides, melanoma cells expressing HLA-DRβ1*0401 and/or HLA-DRβ1*0701, and 293-DR4 and 293-DR7 cells transfected with gp100 cDNA. Only two of the original 96 cultures from one patient (patient 1) exhibited specific MHC class II restricted recognition of gp100 on multiple target cells (Table 2). One culture recognized the known HLA-DRβ1*0401 restricted epitope gp100:44–59, and a second culture was reactive with the HLA-DRβ1*0701 restricted epitope gp100:170–190. Although both of these cultures specifically recognized PBMCs loaded with gp100 protein, they also secreted significant, but far lesser, amounts of INF-γ in response to NY-ESO-1 in comparison to CM alone. This finding suggested that some T lymphocytes were present in these cultures that recognized a contaminant common to both protein preparations and that cloning of these T cell populations might be necessary to eliminate such reactivities.
TABLE 2.
Recognition of Known HLA-DRβ1*0401 and HLA-DRβ1*0701 Restricted gp100 Peptides, Transfectants, and CIITA Transduced Melanoma Cells by T Lymphocytes From Patients Expressing Both of These MHC Molecules Stimulated With Recombinant gp100 Protein*
| Target Cells | HLA-DR Expression | Peptide or Protein† | Introduced Gene† | Patient 1 (66% CD4+) | Patient 1 (77% CD4+) | Patient 2 (86% CD4+) | Patient 3 (100% CD4+) | Patient 4 (97% CD4+) | Targets Only |
|---|---|---|---|---|---|---|---|---|---|
| Media | None | — | — | 44 | 0 | 0 | 14 | 0 | 0 |
| 293-DR4 | DR4+ | — | MART-1 | 32 | 4 | 12 | 16 | 10 | 26 |
| 293-DR4 | DR4+ | — | gp100 | 2,986 | 0 | 21 | 11 | 68 | 10 |
| 293-DR7 | DR7+ | — | MART-1 | 33 | 0 | 15 | 5 | 12 | 0 |
| 293-DR7 | DR7+ | — | gp100 | 36 | 10,041 | 1,138 | 1,674 | 7 | 16 |
| 1088 EBV-B | DR4+ | HA:306–324 | — | 73 | 22 | 24 | 145 | 6 | 15 |
| 1088 EBV-B | DR4+ | gp100:44–59 | — | 864 | 23 | 26 | 124 | 1,546 | 24 |
| 1978 EBV-B | DR7+ | IgK:188–201 | — | 81 | 21 | 29 | 27 | 23 | 32 |
| 1978 EBV-B | DR7+ | gp100:170–190 | — | 65 | 8,062 | 306 | 35 | 14 | 21 |
| F002Rmel CIITA | DR7+ | — | GFP | 39 | 4 | 17 | 36 | 23 | 9 |
| F002Rmel CIITA | DR7+ | — | gp100 | 132 | 59,647 | 4,670 | 56,194 | 26 | 18 |
| 1909mel CIITA | DR7+ | — | — | 98 | 51,344 | 1,752 | 577 | 16 | 17 |
| 624mel CIITA | DR4+ DR7+ | — | — | 4,308 | 5,847 | 266 | 1,557 | 112 | 11 |
| 526mel CIITA | DR4+ | — | — | 5,838 | 19 | 16 | 24 | 203 | 41 |
| 1861mel CIITA | DR4+ | — | — | 4,860 | 13 | 33 | 46 | 72 | 40 |
| Patient 3 PBMC | DR4+ DR7+ | — | — | 71 | 50 | 31 | 27 | 23 | 15 |
| Patient 3 PBMC | DR4+ DR7+ | NY-ESO-1 | — | 2,128 | 8,421 | 85 | 1,146 | 47 | 32 |
| Patient 3 PBMC | DR4+ DR7+ | gp100 | — | 15,133 | 69,269 | 3,761 | 20,183 | 1,630 | 10 |
IFN-γ secretion (pg/mL) in 20-hour coculture supernatants of target cells with T lymphocytes.
Synthetic peptides were preloaded onto EBV-B cells at 50 μmol/L ~3 hours at 37°C, and recombinant proteins were preloaded onto PBMC at 10 μg/mL overnight at 37°C.
The indicated genes were transformed into 293 cells or retrovirally transduced into F002Rmel CIITA cells.
Note. Underlined values indicate that IFN-γ secretion in response to target cells expressing gp100 and either HLA-DRβ1*0701 or HLA-DRβ1*0401 was ≥50 pg/mL and at least twice background with relevant control target cells.
PBMC microcultures from five other patients expressing both HLA-DRβ1*0401 and HLA-DRβ1*0701 were initially assayed for specific INF-γ secretion in response to protein-loaded allogeneic PBMCs matched for both HLA-DRβ1 alleles. Multiple microcultures from each of these five patients secreted significant amounts of INF-γ (>100 pg/mL) in response to PBMCs loaded with recombinant gp100 protein in comparison to NY-ESO-1 protein (at least twice background; data not shown). These microcultures were restimulated with autologous protein-loaded PBMCs in 24-well bulk cultures and were evaluated for specific MHC class II restricted recognition of gp100 on multiple target cells approximately 12 days later. Only one of the original 48 cultures from each of three patients specifically released INF-γ in response to gp100+ target cells with clearly distinguishable MHC class II restriction patterns (patients 2, 3, and 4 in Table 2). The culture from patient 2 specifically recognized the known HLA-DRβ1*0701 restricted epitope gp100:170–190, and the culture from patient 4 specifically recognized gp100:44–59 in context of HLA-DRβ1*0401, although the avidity of this culture seemed weak since comparatively low amounts of INF-γ were secreted in response to HLA-DRβ1*0401+ melanoma cells and 293-DR4 transfectants. The T cell culture from patient 3 clearly demonstrated HLA-DRβ1*0701 restricted recognition of gp100 but did not recognize the known epitope gp100:170–190.
Identification of a New HLA-DRβ1*0701 Restricted Epitope from gp100
To attempt to identify a new HLA-DRβ1*0701 restricted epitope from gp100, the T cell culture from patient 3 was used to screen a previously synthesized overlapping gp100 peptide library.9 Only one peptide from this library, gp100:419–439, was specifically recognized in multiple experiments (data not shown). To specify the minimal determinant of this new epitope, additional overlapping peptides of 20 amino acids in length were synthesized between position 413 and 445 and were evaluated for recognition by the culture from patient 3 (Fig. 1). In this analysis, we avoided peptides containing a P residue at the carboxy-terminus since we were unable to synthesize these peptides with ~>50% purity (including the originally identified gp100:419–439). An amino-terminal truncation from position 421 to 422 significantly abrogated INF-γ secretion, as did a carboxy-terminal truncation from position 435 to 433. These results suggested that the minimal determinant lay somewhere between amino acid (aa) positions 421 and 435.
FIGURE 1.
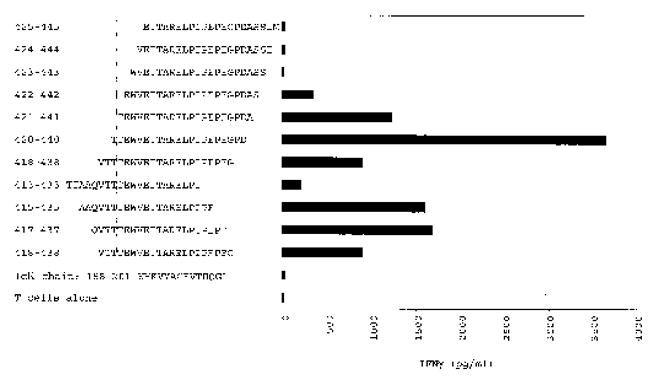
Optimization of the new HLA-DRβ1*0701 restricted gp100 epitope. PBMCs from patient 3 were stimulated three times with recombinant human gp100 protein loaded on autologous irradiated PBMCs and were subsequently expanded with PHA-L and allogeneic feeder cells. INF-γ secretion (pg/mL) by one of the resulting CD4+ T cell cultures was measured in response to HLA-DRβ1*0701+ 1978 EBV-B cells preincubated with overlapping 20 amino acid peptides derived from gp100:413–445 at a concentration of 50 μmol/L. The dotted lines indicate the predicted locations of the critical amino- and carboxy-termini required for peptide recognition.
In the experiment presented in Figure 1, the T cell culture from patient 3 secreted the highest amount of INF-γ in response to gp100:420–440. Therefore, to identify an optimal peptide associated with specific T cell recognition, we synthesized two carboxy-terminally truncated peptides, gp100:420–437 and gp100:420–435. In one experiment, the T cell culture from patient 3 secreted 2043 pg/mL INF-γ in response to HLA-DRβ1*0701+ 1978 EBV-B cells pulsed with the 50 μmol/L gp100:420–440. In the same experiment, gp100:420–437 stimulated the release of 2897 pg/mL INF-γ, and the response to gp100:420–435 was 3591 pg/mL INF-γ. These findings were consistent in replicate experiments suggesting that the three peptides were comparably recognized.
To assess the clonality of the T cell culture from patient 3, FACS analyses were employed using TCR BV family specific monoclonal antibodies. Although 99.5% of the T cells in the population expressed CD4, more than six different TCR BV families were discovered, including BV2, BV5a, BV8, BV17, BV18, and BV22 families (data not shown). To determine if a single TCR was responsible for recognition of both peptide and HLA-DRβ1*0701+ melanomas, we cloned the culture from patient 3 by limiting dilution and screened the clones for peptide recognition. Multiple clones specifically released INF-γ in response to gp100:420–435, and those were expanded with PHA-L or OKT-3 for further evaluation. Thirty peptide-reactive “clones” were isolated, all of which specifically recognized three different melanoma cell lines that expressed HLA-DRβ1*0701 and gp100. Representative cytokine secretion data for two of these “clones” are presented in Table 3. TCR BV family analyses were conducted by FACS for seven of the clones that secreted the highest amounts of INF-γ in response to melanoma cell lines. However, none of the BV families that were identified in the original T cell culture from patient 3 were found in any of these “clones.” Therefore, 5′ rapid amplification of cDNA ends (5′ RACE) was employed using nested beta chain constant region primers (CB) to amplify TCR-β chain cDNAs from two separate T cell “clones” (A1 and H2) that recognized both gp100:420–435 and HLA-DRβ1*0701+ melanomas (Table 3). cDNAs of approximately 600 bp were isolated from each of the two “clones,” and each cDNA consisted of a single well-defined sequence. The cDNAs of the two clones were identical throughout the V, D, and J regions and into the small segment of the C region encompassed by the BC primer used for sequencing (Fig. 2). The BV region sequence was identical to that of BV18S1, and analysis of DNA and amino acid sequences indicated that each cDNA was productively rearranged since the V and C regions were in frame with each other. Nonetheless, it is unclear why the commercially available antibody against the BV18S1 family did not stain either of these T cell clones. These results strongly suggested that the same TCR was responsible for the recognition of both gp100:420–435 and HLA-DRβ1*0701+ tumor cells. Furthermore, in subsequent experiments using recombinant gp100 protein, we raised melanoma-reactive CD4+ T lymphocytes from two additional patients that specifically recognized gp100:420–435 in the context of HLA-DRβ1*0701 (data not shown).
TABLE 3.
Recognition of gp100:420–435 and HLA-DRβ1*0701+ Melanoma Cells by Clones A1 and H2 Derived From PBMC From Patient 3 After Stimulation With Recombinant gp100 Protein*
| Target Cells | HLA-DR Expression | Preloaded Peptide† | Transduced Gene | Patient 3 (Clone A1) | Patient 3 (Clone H2) |
|---|---|---|---|---|---|
| Media | None | — | — | 22 | 22 |
| 1978 EBV-B | DR7+ | IgK:188–201 | — | 28 | 20 |
| 1978 EBV-B | DR7+ | gp100:420–435 | — | 1978 | 5917 |
| F002mel CIITA | DR7+ | — | GFP | 20 | 24 |
| F002mel CIITA | DR7+ | — | gp100 | >10,000 | >10,000 |
| 1978mel CIITA | DR7+ | — | — | >10,000 | >10,000 |
| 624mel CIITA | DR7+ | — | — | >10,000 | >10,000 |
| 1087mel CIITA | DR7+ | — | — | 870 | 869 |
IFN-γ secretion (pg/mL) in 20-hour coculture supernatants of target cells with T lymphocytes.
Synthetic peptides were preloaded onto EBV-B cells at 5 μmol/L ~3 hours at 37°C.
Note. Underlined values indicate that IFN-γ secretion in response to HLA-DRβ1*0701+ EBV-B cells preloaded with 5 μmol/L gp100:420–435 or melanoma cell lies expressing HLA-DRβ1*0701 and gp100 was ≥100 pg/mL and at least twice background with any gp100− control target cells.
FIGURE 2.

TCR-β chain sequence isolated from T cell clones A1 and H2 from patient 3 using 5′ RACE. cDNAs from both T cell clones were identical, and sequence analysis indicated that the TCR-β chain was productively rearranged as BV18S1/BD1/BJ1S1/BC1. The genomic portion of the 3′ end of the BV gene and the 5′ end of the BC region are presented in bold text, and the genomic portion of the BJ segment is underlined.
Peptide titration experiments were performed to assess the avidity of the bulk T cell culture from patient 3 as well as three T cell clones derived from this culture. Similar results were observed with all four T cell populations, and representative data from one clone are presented in Figure 3. In particular, gp100:420–440, gp100:420–437, and gp100:420–435 were comparably well recognized when pulsed onto allogeneic HLA-DRβ1*0701+ 1978 EBV-B cells down to a concentration of 5 μmol/L. However, INF-γ secretion dramatically decreased at lower peptide concentrations. Other epitopes recognized by CD4+ T cell clones in the context of HLA class II molecules have, in some cases, been well recognized at significantly lower concentrations. For example, half-maximal stimulation of a T cell clone by MAGE3:114–127 occurred at 10 nmol/L,17 demonstrating that the gp100:420–435-reactive T cell clones we isolated from patient 3 were not highly avid for the peptide. However, these results also suggested that at least some melanomas processed and presented large amounts of peptide on their surfaces in the context of HLA-DRβ1*0701 since low avidity T cells were capable of recognizing these tumor cells.
FIGURE 3.
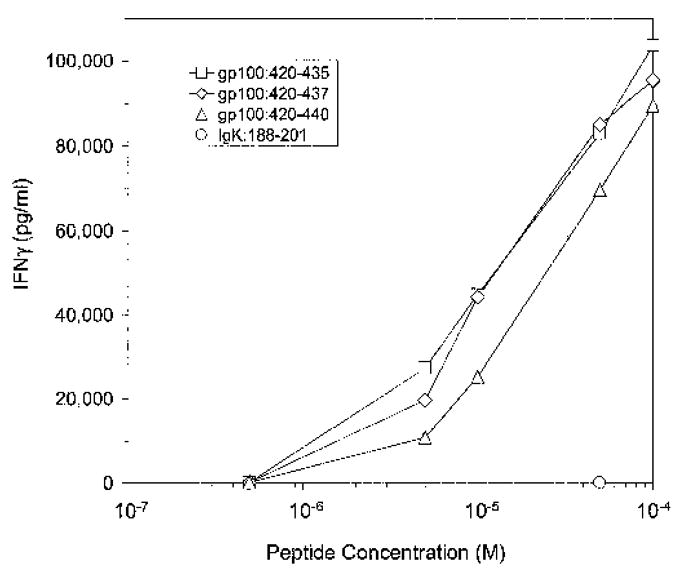
Recognition of truncated gp100:420–440 peptides. PBMCs from patient 3 were stimulated three times with recombinant human gp100 protein and were subsequently cloned by limiting dilution. INF-γ secretion (pg/mL) by one CD4+ T cell clone was measured in response to various concentrations of the parent 20-mer as well as two truncated forms of the peptide, gp100:420–437 and gp100:420–435. INF-γ secretion (pg/mL) was also measured in response to 50 μmol/L Ig kappa chain:188–201 as a negative control.
TH1 Versus TH2 Type Cytokine Secretion by gp100 Reactive T Helper Cells
T cell microcultures were specifically screened for the ability to secrete the primary TH1 type cytokine INF-γ. To determine if selected gp100-reactive populations secreted other TH1 or TH2 cytokines, we evaluated secretion of the additional TH1 cytokines GM-CSF, TNF-α, and IL-2 and the TH2 cytokines IL-4 and IL-10 (Table 4). Each of the five previously identified MHC class II restricted INF-γ secreting, tumor-reactive T cell cultures also specifically released GM-CSF in response to relevant target cells. However, specific TNF-α secretion was limited to cultures from patients 1, 3, and 4. Only the gp100:170–190-reactive cultures from patients 1 and 3 demonstrated consistent IL-2 secretion, although this assay was not conducted in the presence of a CD25 blocking antibody so that any IL-2 produced by the T cells may have been consumed during the course of the experiment. None of the T cell populations secreted the primary TH2 cytokine IL-4. However, three of the five produced some IL-10 in response to peptide-pulsed targets but not to cells that endogenously expressed gp100 protein.
TABLE 4.
Thl Versus Th2 Type Cytokine Secretion by T Lymphocytes From Patients Expressing HLA-DRβ1*0401 and HLA-DRβ1*0701 Stimulated with Recombinant gp100 Protein*
| Peptide | Transfected Gene | Patient 1 (DR4)‡ | Patient 1 (DR7) | Patient 2 (DR7) | Patient 3 (DR7) | Patient 4 (DR4) | |
|---|---|---|---|---|---|---|---|
| GM-CSF | |||||||
| Media | — | — | 319 | 11 | 15 | 13 | 16 |
| 293-DR4/DR7§ | — | MART-1 | 555 | 17 | 30 | 30 | 53 |
| 293-DR4/DR7§ | gp100 | 1,973 | 1,979 | 865 | 1,893 | 2,601 | |
| EBV-B cells|| | Control | — | 672 | 35 | 53 | 52 | 81 |
| EBV-B cells|| | Relevant | — | 2,273 | 4,414 | σ959 | 33,520 | 24,332 |
| Control melanoma** | — | — | 541 | 25 | 38 | 26 | 35 |
| Relevant melanoma†† | — | — | 2,372 | 21,412 | 3,053 | 33,165 | 3,513 |
| TNF-α | |||||||
| Media | — | — | 114 | 4 | 16 | 20 | 6 |
| 293-DR4/DR7§ | — | MART-1 | 51 | 3 | 5 | 15 | 8 |
| 293-DR4/DR7§ | — | gp100 | 738 | 904 | 18 | 434 | 146 |
| EBV-B cells|| | Control¶ | — | 90 | 12 | 18 | 12 | 20 |
| EBV-B cells|| | Relevant# | — | 472 | 820 | 28 | 6,019 | 973 |
| Control melanoma** | — | — | 35 | 4 | 11 | 8 | 0 |
| Relevant melanoma†† | — | — | 845 | 3,510 | 44 | 8,498 | 185 |
| IL-2 | |||||||
| Media | — | — | 62 | 105 | 78 | 58 | 58 |
| 293-DR4/DR7§ | — | MART-1 | 111 | 107 | 86 | 52 | 74 |
| 293-DR4/DR7§ | — | gp100 | 277 | 263 | 77 | 112 | 92 |
| EBV-B cells|| | Control¶ | — | 30 | 58 | 30 | 48 | 85 |
| EBV-B cells|| | Relevant# | — | 16 | 121 | 17 | 168 | 308 |
| Control melanoma** | — | — | 99 | 48 | 73 | 58 | 139 |
| Relevant melanoma†† | — | — | 1,436 | 3,125 | 139 | 2,999 | 113 |
| IL-4 | |||||||
| Media | — | — | 0 | 0 | 0 | 0 | |
| 293-DR4/DR7§ | — | MART-1 | 0 | 0 | 0 | 0 | 0 |
| 293-DR4/DR7§ | — | gp100 | 0 | 1 | 0 | 1 | 18 |
| EBV-B cells|| | Control¶ | — | 0 | 0 | 0 | 0 | 0 |
| EBV-B cells|| | Relevant# | — | 0 | 4 | 0 | 3 | 36 |
| Control melanoma** | — | — | 0 | 0 | 0 | 0 | 0 |
| Relevant melanoma†† | — | — | 0 | 0 | 0 | 9 | 24 |
| IL-10 | |||||||
| Media | — | — | 1 | 3 | 5 | 6 | 0 |
| 293-DR4/DR7§ | — | MART-1 | 5 | 0 | 2 | 0 | 3 |
| 293-DR4/DR7§ | — | gp100 | 5 | 35 | 3 | 8 | 10 |
| EBV-B cells|| | Control¶ | — | 96 | 82 | 98 | 80 | 65 |
| EBV-B cells|| | Relevant# | — | 126 | 805 | 130 | 249 | 218 |
| Control melanoma** | — | — | 7 | 0 | 9 | 0 | 0 |
| Relevant melanoma†† | — | — | 5 | 6 | 3 | 10 | 18 |
GM-CSF, TNF-α, IL-2, IL-4, and IL-10 secretion (pg/mL) in 20-hour coculture supernatants of target cells with T lymphocytes.
Synthetic peptides were preloaded onto EBV-B cells at 50 μmol/L ~3 hours at 37°C.
The HLA-DR restriction element associated with gp100 recognition by the indicated T cell population.
293-DR4 transfectants were used as target cells for HLA-DRβ1*0401 restricted T cells, and 293-DR7 transfectants were used as target cells for HLA-DRβ1*0701 restricted T cells.
Peptide loaded 1088 EBV-B cells (DR4+) were used as target cells for HLA-DRβ1*0401 restricted T cells, and 1978 EBV-B cells (DR7+) were used as target cells for HLA-DRβ1*0701 restricted T cells.
Influenza A HA:306–324 was the negative control peptide for HLA-DRβ1*0401 restricted T cells, and IgKappa chain:188–201 was the negative control peptide for HLA-DRβ1*0701 restricted T cells.
gp100:44–59 was the relevant peptide for HLA-DRβ1*0401 restricted T cells. gp100:170–190 was the relevant peptide for HLA-DRβ1*0701 restricted T cells from patients 1 and 2, and gp100:420–435 was the relevant peptide for HLA-DRβ1*0701 restricted T cells from patient 3.
F002mel CIITA retrovirally transduced with GFP (DR7+ gp100−) was the negative control melanoma cell line for HLA-DRβ1*0701 restricted T cells, and F002mel CIITA retrovirally transduced with gp100 (DR4− gp100+) was the negative control melanoma cell line for HLA-DRβ1*0401 restricted T cells.
F002mel CIITA retrovirally transduced with gp100 (DR7+ gp100+) was the relevant melanoma cell line for HLA-DRβ1*0701 restricted T cells, and 526mel CIITA (DR4+ gp100+) was the relevant melanoma cell line for HLA-DRβ1*0401 restricted T cells.
Note: Underlined values indicate that cytokine secretion in resposne to target cells expressing gp100 and either HLA-DRβ1*0701 or HLA-DRβ1*0401 was ≥50 pg/mL and at least twice background with relevant control target cells. CIITA (DR4+ gp100−)
DISCUSSION
The work described here demonstrates that populations of gp100-reactive CD4+ T cells can be raised in vitro using PBMCs loaded with recombinant protein as APCs. Some of these populations recognized known HLA- DRβ1*0401 and HLA- DRβ1*0701 restricted peptides from gp100, and one recognized a new HLA-DRβ1*0701 restricted epitope, gp100:420–435. Furthermore, all of these T cells specifically released cytokine in response to MHC class II+ target cells that endogenously expressed gp100, including melanoma cells and 293 transfectants. CD4+ T lymphocytes from patients 1 and 2 secreted significantly more INF-γ in response to melanoma cells expressing MHC class II molecules and protein-loaded PBMCs than to EBV-B cells loaded with high concentrations of synthetic peptides. This may be due to rapid dissociation of peptides from MHC class II molecules on the surfaces of exogenously loaded APCs in comparison to cells that endogenously process the protein and continually replenish the supply of relevant epitopes. Alternatively, since these cell populations were not clonal, they may have contained additional gp100-reactive CD4+ T lymphocytes with unknown epitope specificities.
The use of full-length recombinant proteins to stimulate CD4+ T cells is more attractive than the use of individual peptides because it circumvents the limitations to particular MHC class II molecules and allows the development of reactivities to previously unknown epitopes. In addition, although we have developed effective techniques for stimulating tumor-reactive MHC class I restricted CTLs using synthetic peptides, we have not optimized a similar system for the consistent stimulation of antigen-reactive CD4+ T cells in vitro. In particular, we have consistently been able to generate peptide-reactive CD4+ T cells using known MHC class II restricted epitopes from tumor-associated antigens. However, in only very rare instances have those T cells recognized MHC class II positive tumor cells, transfectants, or APCs loaded with whole protein (data not shown).
In previous investigations, DCs were used as APCs for the presentation of epitopes from recombinant MAGE1,20 MAGE3,17 and gp10030 proteins. However, we have previously observed that repeated in vitro stimulation of PBL with DCs often induced the expansion of T lymphocytes that secreted cytokines in response to irrelevant stimulator cells. Presumably, this was due to allogeneic or xenogeneic proteins in sera or to pathogen-derived contaminants in the antigen preparation. To avoid these complications, in the earlier studies with MAGE and gp100 proteins, autologous plasma or serum was used in DC preparations. In contrast, we have not generally observed such erratic T cell stimulation when peptide-pulsed PBMCs were used as APCs. Therefore, in the current investigation, we first evaluated the capacity of PBMCs to act as APCs by determining if these cells could engulf exogenously loaded gp100 protein and subsequently process and present relevant MHC class II restricted epitopes. Based on the results presented in Table 1, we opted to use protein-loaded PBMCs as APCs. However, since DCs are known to be significantly more potent APCs, both in terms of antigen-processing capacity and costimulation, we conducted several experiments in which PBL from several patients were stimulated either with autologous protein-loaded DCs or PBMCs (data not shown). In these experiments, we found no consistent advantage to using DCs as APCs for the generation of antigen-reactive CD4+ T cells. Indeed, when protein-loaded DCs were used to stimulate PBL from patient 1, none of the original 96 microcultures demonstrated gp100 reactivity. In contrast, in the same experiment, two gp100-reactive CD4+ T cell populations were raised using protein-loaded PBMCs as APCs (Table 2).
Upon stimulation of PBL from patient 3 with gp100 protein-loaded autologous PBMC, we isolated one microculture containing CD4+ T lymphocytes that specifically recognized a new epitope, gp100:420–435 (TTEWVETTARELPIPE) in the context of HLA-DRβ1*0701. More recently, we have isolated CD4+ T lymphocytes with similar reactivities from two additional patients. This peptide contains a 9 amino acid sequence that is reasonably consistent with the known HLA-DRβ1*0701 binding motif, which suggests that for optimal binding, the minimal determinant peptide should contain a residue with an aromatic ring in its side chain at the amino-terminus (F, Y, or W at P1), D or E at P4, N at P6, and a small aliphatic residue at the carboxy-terminus (V, I, L at P9).35 In the peptide gp100:420–435, W at aa 423 may represent P1, and L at aa 431 may represent P9 such that the minimal determinant would be gp100:423–431 (WVETTAREL). However, we have not synthesized this peptide and evaluated recognition because optimal MHC class II restricted peptides tend to be amino- and carboxy-terminally extended from the core 9 amino acid sequence.
We have attempted to raise gp100-reactive CD4+ T cells in vitro with gp100:420–435 (data not shown). However, as has often been our experience with synthetic peptides, we consistently raised peptide-reactive CD4+ T cell cultures that did not recognize endogenously produced or exogenously loaded gp100 protein. One possible explanation may be that this peptide does not represent the native epitope that is processed and presented on the surfaces of APCs that have been loaded with whole protein or tumor cells expressing class II MHC molecules. Alternatively, this peptide may only be capable of stimulating T cells with comparatively lower avidity for the naturally processed epitope than APCs loaded with whole protein. In addition, previous studies have suggested that longer peptides (~20 amino acids in length) may be more immunogenic than truncated versions of the same core sequence for the induction of CD4+ T cells.11,15 Therefore, perhaps the 15 amino acid peptide we esed in these experiments was not optimal for T cell induction.
As observed in the work presented here and in previous studies with MAGE1 and MAGE3 proteins, a primary complication with the use of recombinant proteins has been the induction of T cells that recognized contaminants in the protein preparation. As previously suggested,17,20 one means for circumventing this problem may be to stimulate T cells with protein made in one organism such as E. coli and screen for specific recognition of protein produced by another cell type such as insect cells infected with a recombinant baculovirus. Alternatively, as was done here for microcultures from patient 1, protein-stimulated T cells could be initially screened for recognition of autologous cells transfected or transduced with a gene-based vector such as DCs transduced with recombinant adenovirus. Still another possibility presented here is that T cells could be stimulated with protein and subsequently screened for recognition of the same protein in comparison to a negative control protein that was identically produced, potentially containing the same contaminants.
Antigens can be presented on the surfaces of cells in the context of MHC class II molecules after processing through one of two different pathways. Exogenously loaded protein can be engulfed by APCs and cleaved into peptides in endosomes for association with MHC class II molecules.36 Alternatively, some endogenous proteins can be targeted to endosomal compartments, allowing processed peptides to be presented on the surfaces of cells in the context of MHC class II molecules.37 In previous studies in which recombinant proteins from the MAGE family were used to stimulate PBL, antigen-reactive CD4+ T cell clones were generated that recognized exogenously loaded protein, either in the form of recombinant purified material or lysates of cell that expressed the protein. However, these T cells did not recognize MHC class II+ cells that endogenously expressed MAGE proteins. Apparently, MAGE proteins lack necessary endosomal targeting sequences such that some epitopes are not efficiently generated through the endogenous MHC class II processing pathway.17,20 In contrast, melanocyte specific proteins are known to contain specific lysosomal targeting sequences that enable presentation of epitopes in the context of MHC class II molecules.29 The data presented here support those findings since gp100-reactive CD4+ T cells were capable of recognizing endogenously processed protein the context of surface MHC class II molecules.
Many melanoma cells constitutively express MHC class II molecules.38 Therefore, gp100-reactive CD4+ T cells have the potential to impact tumor cells directly in vivo. In addition, DCs may process and present relevant MHC class II restricted epitopes to CD4+ T cells in vivo after engulfing tumor cell debris. Activated CD4+ T cells may up-regulate antigen presentation, costimulation, and IL-12 production by DCs that can subsequently stimulate tumor-reactive CD8+ CTLs. Activated CD4+ T cells may also provide direct cytokine-mediated help to CD8+ CTL. Futhermore, TH1 type cytokines produced by CD4+ T helper cells may enable the recruitment of macrophages capable of lysing tumor cells through the production of reactive oxygen intermediates.4
Several potential clinical applications exist for the use of recombinant tumor-associated proteins. For example, proteins could be employed in active immunization strategies to vaccinate patients with cancer directly. Some exogenously loaded proteins can enter MHC class I processing pathways leading to cross-presentation of relevant epitopes on professional APCs.39 Therefore, these full-length proteins may simultaneously stimulate CD8+ and CD4+ T cell responses. Alternatively, proteins like gp100, that do not seem be naturally cross-presented, could be linked to polymeric beads40 or coupled to antibodies that bind DC surface receptors41 to facilitate internalization and, hence, entry into MHC class I processing pathways. Also, proteins that are not efficiently cross-presented could be used in conjunction with known MHC class I restricted peptides or gene-based constructs in active vaccination therapies to elicit concomitant CTL and T helper cell responses.
Recombinant tumor-associated proteins may also be useful in adoptive therapy protocols. Proteins could be used to expand antigen-reactive CD4+ T cells ex vivo, as described here, and those cells could be co-adoptively transferred to patients with autologous tumor-reactive CD8+ CTLs. However, with the current methodology, we were only able to raise tumor antigen-reactive CD4+ T cells in very rare instances from a limited number of patients. In particular, specific MHC class II restricted recognition of gp100 was only observed in 5 of 624 microcultures initially established, and we were only able to isolate those cultures from 4 of 9 patients. Therefore, much effort is currently being directed toward optimizing the induction of antigen-reactive CD4+ T cells ex vivo.
Acknowledgments
The authors thank Yong Li for DNA sequencing, Dr. Rejean LaPointe for the B104 T cell clone, Arnold Mixon and Shawn Farid for FACS analyses, and Dr. Michael Nishimura and Yong Li for providing useful advice with respect to TCR-β chain sequence analysis.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2− T cells: tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ossendorp F, Mengede E, Camps M, et al. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. CurrOpin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 5.Surman DR, Dudley ME, Overwijk WW, et al. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 7.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarour HM, Kirkwood JM, Kierstead LS, et al. Melan-A/MART-1(51–73) represents an immunogenic HLA-DR4-restricted epitope recognized by melanoma-reactive CD4(+) T cells. Proc Natl Acad SciUSA. 2000;97:400–405. doi: 10.1073/pnas.97.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapointe R, Royal RE, Reeves ME, et al. Retrovirally transduced human dendritic cells can generate T cells recognizing multiple MHC class I and class II epitopes from the melanoma antigen glycoprotein 100. J Immunol. 2001;167:4758–4764. doi: 10.4049/jimmunol.167.8.4758. [DOI] [PubMed] [Google Scholar]
- 10.Li K, Adibzadeh M, Halder T, et al. Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and annexin II eluted from melanoma cells. Cancer Immunol Immunother. 1998;47:32–38. doi: 10.1007/s002620050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touloukian CE, Leitner WW, Topalian SL, et al. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J Immunol. 2000;164:3535–3542. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder T, Pawelec G, Kirkin AF, et al. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238–3244. [PubMed] [Google Scholar]
- 13.Topalian SL, Gonzales MI, Parkhurst M, et al. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Kokubo T, Sato K, et al. CD4+ T cells from peripheral blood of a melanoma patient recognize peptides derived from nonmutated tyrosinase. Cancer Res. 1998;58:296–301. [PubMed] [Google Scholar]
- 15.Touloukian CE, Leitner WW, Robbins PF, et al. Expression of a “self”-antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62:5144–5147. [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins PF, El Gamil M, Li YF, et al. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J Immunol. 2002;169:6036–6047. doi: 10.4049/jimmunol.169.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaux P, Vantomme V, Stroobant V, et al. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. J Exp Med. 1999;189:767–778. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz ES, Lethe B, Cambiaso CL, et al. A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 2000;60:6272–6275. [PubMed] [Google Scholar]
- 19.Manici S, Sturniolo T, Imro MA, et al. Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med. 1999;189:871–876. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaux P, Lethe B, Van Snick J, et al. A MAGE-1 peptide recognized on HLA-DR15 by CD4(+) T cells. Eur J Immunol. 2001;31:1910–1916. doi: 10.1002/1521-4141(200106)31:6<1910::aid-immu1910>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Jager E, Jager D, Karbach J, et al. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101–0103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng G, Wang X, Robbins PF, et al. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY- ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarour HM, Storkus WJ, Brusic V, et al. NY-ESO-1 encodes DRB1*0401-restricted epitopes recognized by melanoma-reactive CD4+ T cells. Cancer Res. 2000;60:4946–4952. [PubMed] [Google Scholar]
- 24.Zeng G, Touloukian CE, Wang X, et al. Identification of CD4+ T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. J Immunol. 2000;165:1153–1159. doi: 10.4049/jimmunol.165.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroers R, Huang XF, Hammer J, et al. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- 26.Bosch GJ, Joosten AM, Kessler JH, et al. Recognition of BCR-ABL positive leukemic blasts by human CD4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood. 1996;88:3522–3527. [PubMed] [Google Scholar]
- 27.Sotiriadou R, Perez SA, Gritzapis AD, et al. Peptide HER2(776–788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer. 2001;85:1527–1534. doi: 10.1054/bjoc.2001.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich JR, Yang JC, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayasaradhi S, Xu Y, Bouchard B, et al. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting of the human brown locus protein, gp75. J Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochlovius B, Linnebacher M, Zewe-Welschof M, et al. Recombinant gp100 protein presented by dendritic cells elicits a T-helper-cell response in vitro and in vivo. Int J Cancer. 1999;83:547–554. doi: 10.1002/(sici)1097-0215(19991112)83:4<547::aid-ijc18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Wang RF, Wang X, Atwood AC, et al. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science. 1999;284:1351–1354. doi: 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Boss JM, Hu SX, et al. Apoptosis-independent retinoblastoma protein rescue of HLA class II messenger RNA INF-gamma inducibility in non-small cell lung carcinoma cells: lack of surface class II expression associated with a specific defect in HLA-DRA induction. J Immunol. 1996;156:2495–2502. [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linette GP, Shankara S, Longerich S, et al. In vitro priming with adenovirus/gp100 antigen-transduced dendritic cells reveals the epitope specificity of HLA-A*0201-restricted CD8+ T cells in patients with melanoma. J Immunol. 2000;164:3402–3412. doi: 10.4049/jimmunol.164.6.3402. [DOI] [PubMed] [Google Scholar]
- 35.Rammensee H, Bachmann J, Emmerich NP, et al. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 36.Geuze HJ. The role of endosomes and lysosomes in MHC class II functioning. Immunol Today. 1998;19:282–287. doi: 10.1016/s0167-5699(98)01269-9. [DOI] [PubMed] [Google Scholar]
- 37.Nuchtern JG, Biddison WE, Klausner RD. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature. 1990;343:74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- 38.Houghton AN, Thomson TM, Gross D, et al. Surface antigens of melanoma and melanocytes: specificity of induction of Ia antigens by human gamma-interferon. J Exp Med. 1984;160:255–269. doi: 10.1084/jem.160.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 40.Shen Z, Reznikoff G, Dranoff G, et al. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 41.Bonifaz L, Bonnyay D, Mahnke K, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8(+) T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]


