Abstract
The authors describe a patient who experienced recurrence of metastatic melanoma after an initial dramatic response to immunotherapy using peptides derived from gp100, MART-1, and tyrosinase emulsified in incomplete Freund’s adjuvant, and present data to support the hypothesis that the progression of disease in this patient was due to in vivo immunoselection for immunoresistant tumor variants. The authors previously demonstrated the existence of T-cell clones in this patient’s peripheral blood and tumor-infiltrating lymphocytes (TILs) reactive against multiple antigens, including gp100, the tyrosinase-related protein (TRP)-2, a novel TRP-2 isoform-TRP-2-6b, SOX10, and the melanoma antigen NY-ESO-1. In addition to the multiple HLA-A2 restricted T-cell clones, the authors have now identified additional HLA-B/C-restricted as well as class II (HLA-DP)-restricted anti-melanoma antigen T-cell clones from this patient’s TIL. One recurrent tumor showed loss of expression of multiple tumor antigens but retention of HLA class I expression. The other recurrent lesion showed total loss of HLA class I expression even though the tumor cells still expressed many melanoma antigens. This paper thus provides evidence for both the effectiveness of the immune destruction of cancer as well as problems associated with antigen-loss tumor escape mechanisms.
Keywords: tumor-infiltrating lymphocytes, vaccination, melanoma antigens, tumor escape, immunoselection
The identification of tumor-associated antigens (TAA) has opened new opportunities for the development of cancer immunotherapies. Most of the human melanoma antigens reported so far belong to two major groups: the melanocyte/melanoma-differentiation antigens (MDAs) and the cancer-testis antigens (CTA), such as MAGE and NY-ESO-1, that are overexpressed in tumors of various histologies but not in normal tissues except for testis. The commonly known MDAs include gp100, MART-1, tyrosinase, and the tyrosinase-related proteins TRP-1 and TRP-2. Many clinical vaccine trials have been conducted based on these antigens, using immunization with peptide epitopes with or without modification or with recombinant DNA vaccines. A recent study has suggested the efficacy of immunotherapy using a modified gp100 antigen together with high-dose interleukin-2 (IL-2).1 Both humoral and cellular immune responses directed against NY-ESO-1 have been reported in patients with NY-ESO-1-expressing tumors.2
Despite recent progress in tumor immunobiology and technical advances in the field of tumor immunotherapy, current cancer vaccine strategies employed in the treatment of patients with cancer have been successful in only rare and sporadic cases. The lack of observed tumor regression is most likely due to inadequacies of current immunotherapy strategies. In some instances where objective clinical responses (i.e., complete and partial responses) have been observed after immunotherapy, tumors often recur or progress. It is not clear to what extent immunoselection for tumor variants that fail to express the appropriate antigens are responsible for these recurrences.
In this paper, we describe a patient who experienced progression and recurrence of melanoma tumors after an initial dramatic response to immunotherapy using peptides derived from gp100, MART-1, and tyrosinase emulsified in incomplete Freund’s adjuvant (IFA), and present data to support the hypothesis that the progression of disease in this patient was due to in vivo immunoselection for immunoresistant tumor variants. We demonstrated the existence of T-cell clones reactive against multiple antigens in this patient’s peripheral blood and tumor-infiltrating lymphocytes (TILs). This patient developed cellular immunity against 12 different MHC-restricted cancer antigens, including the HLA-A*0201 restricted gp100, TRP-2, a novel TRP-2 isoform-TRP-2-6b, the CTA NY-ESO-1, and the newly identified MDA SOX10,3 as well as to HLA-B/C-restricted and class II (HLA-DP)-restricted melanoma antigens. Simultaneous loss of expression of multiple antigens or loss of expression of MHC molecules was seen that might have accounted for this patient’s recurrence after a substantial clinical regression following immunotherapy.
MATERIALS AND METHODS
The patient was a 63-year-old woman who underwent a wide local excision of a primary melanoma on her back in 1981. In May 1997, she developed a subcutaneous metastasis on her left chest wall; it was resected, and she was started on a chemoimmunotherapy regimen comprising cisplatin, vinblastine, dacarbazine, IL-2, and interferon [IFN]-α. Because of disease progression, she was referred to the Surgery Branch, National Cancer Institute (Bethesda, MD) in June 1998. At that time, she had developed metastatic disease in multiple sites including the lungs, liver, intrapelvic area, left abdominal wall, left thigh, and subcutaneous (SQ) areas. She was started on a four-peptide vaccination protocol using 1 mg each of gp100: 209–217 (210M), gp100: 280–288 (288V), MART-1: 27–35, and tyrosinase: 368–376, emulsified in IFA SQ every 3 weeks. Most of her tumors completely regressed after two cycles of treatment (day 45), including complete resolution of a large tumor in her left thigh, an intrapelvic mass, a liver lesion, most of the nodules in her lungs, and all but one SQ lesion. She completed a total of six cycles of vaccinations in October 1998. The remaining SQ lesion was resected at the end of treatment (October 1998). A year later (October 1999), she developed a frontal lobe metastatic brain lesion; it was resected, together with a slow-growing subcutaneous nodule on her right chest wall. TIL 1790 used in this study was grown from the chest wall lesion. In March 2000, she underwent a second right temporal craniotomy for resection of recurrent disease at the prior brain resection site, followed by whole-brain irradiation. She continued to develop metastatic lesions at multiple sites, including the brain, and eventually died of disease in early 2002.
Cell Lines
Melanoma-reactive CTLs were derived from bulk TIL cultures grown in Iscove’s modified Dulbecco medium (Gibco BRL, Gaithersburg, MD) containing 6,000 IU/mL of hrIL-2 (Chiron, Emeryville, CA). CTL clones were derived from bulk TIL cultures by limiting dilution with the addition of anti-CD3 antibody (Ab) (OKT-3, Ortho Pharmaceuticals, Raritan, NJ) as previously described.4 Briefly, 5 × 104 irradiated (3,000 cG) peripheral blood mononuclear cells (PBMCs) from three allogeneic donors were plated in round-bottom 96-well plates with 0.5 to 90 T cells per well. Cells were cultured in RPMI 1640 medium (Gibco BRL) containing 20% heat-inactivated fetal bovine serum (FBS) (Gibco BRL) and 30 ng/mL OKT-3 Ab with 300 IU/ml IL-2. The same dose of IL-2 was added on day 7, and clones were tested for recognition of HLA-A2+ versus HLA-A2− melanoma cell lines 14 days after stimulation. After testing, the remainder of T cells were expanded by plating them in a T25 flask with 2.5 × 107 irradiated PBMCs from three allogeneic donors in 25 mL RPMI 1640 medium containing 10% FBS and 30 ng/mL OKT-3 Ab. Subsequent expansion was similarly done, but with 1 to 2 × 106 CTLs and 2.5 × 108 allogeneic PBMCs plated in an upright T162 flask in 150 mL medium.
All melanoma cell lines were established in the Surgery Branch (NCI) and previously described.4 The autologous melanoma cell lines 1928 and 1973 were established from two recurrent SQ lesions resected in May and October 2001, respectively. T2 cells, deficient in transporter associated protein, were used to test HLA-A2-restricted peptides for CTL activity. All cell lines were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 10 mmol/L HEPES buffer, 100 U/mL penicillin-streptomycin (Biofluids), and 2 mmol/L L-glutamine (Biofluids).
Cytokine Release Assays
Five × 104 CTL cells were plated with 1 × 105 target cells (tumor cells or T2 cells that had been pulsed with peptides at 1 μmol/L for 2 hours at 37°C and washed once) in 96-well round-bottom plates in 200 μL CM. After18 to 24 hours of incubation at 37°C, the supernatant was harvested for detection of IFN-γ release using enzyme-linked immunosorbent assay (ELISA) kits (Endogen, Cambridge, MA).
For the MHC blocking assays, target cells were incubated with the appropriate monoclonal antibody (mAb) at a final concentration of 50 μg/mL for 1 hour at 37°C prior to the addition of CTLs. W6/32 (anti-HLA class I), IVA12 (anti-HLA class II), and HB55 (anti-HLA-DR) were obtained from ATCC. Anti-HLA-DP and DQ (clones B7/21 and 1a3, respectively) were obtained from Leinco (St. Louis, MO).
RT-PCR Assays for Tumor Antigen Expression
Total RNA of melanoma samples was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was prepared from total RNA using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ). Primers used for PCR were as follows: gp100 (forward) 5′-GCTTGGTGTCTCAAGGCAACT-3′ and (reverse) 5′-CTCCAGGTAAGTATGAGTGAC-3′, PCR product size: 751 bp5; MART-1 (forward) 5′-ATGCCAAGAGAAGATGCTCAC-3′ and (reverse) 5′-AGCATGTCTCAGGTGTCTCG-3′, PCR product size: 384 bp5; tyrosinase (forward) 5′-TTGGCAGATTGTCTGTAGCC-3′ and (reverse) 5′-GCTATCCCAGTAAGTGGACT-3′, PCR product size: 252 bp5; NY-ESO-1 (forward) 5′ AGCCGCCTGCTTGAGTTCTACCTC 3′ and (reverse) 5′ AGGGAAAGCTGCTGGAGACAG 3′ PCR product size: 221bp6; SOX10 (forward) 5′-CTTCGGCAACGTGGACATT-3′ and (reverse) 5′-TCAGCCACATCAAAGGTCTCC-3′, PCR product size: 72 bp; TRP-2 and TRP-2-6b (forward) 5′-ACTGCGAGCGGAAGAAACCA-3′ and (reverse) 5′-GGCATCTGCAGGAGGATTAA-3′ and 5′-ATGCAGGGAAGGGAGTTCCT-3′, respectively, PCR product size: 998 bp and 923 bp, respectively; β-actin (forward) 5′-ACACTGTGCCCATCTACGAGG-3′ and (reverse) 5′-AGGGGCCGGACTCGTCATACT-3′, PCR product size: 621 bp7; β2-m (forward) 5′-ATT CGGGCCGAGATGTC-3′ and (reverse) 5′-ACCTCCATGATGCTGCTTACA-3′, PCR product size: 388 bp. Cycling conditions were 94°C for 1 minute, 60°C for 30 seconds, and 72°C for 2 minutes for 35 cycles, except for β-actin (31 cycles).
cDNA Sequencing to Detect Mutations in β2-m
Sequencing of β2-m cDNA derived from 1973 melanoma cells was performed with an ABI Prism 3100 Avant Genetic Analyzer (Applied Biosystems, Foster City, CA) using the Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer). Mutations were searched against the Gen-Bank database using the BLAST algorithm and Global Alignment of Two Sequences.
RESULTS
Activities of TIL Clones/Cloids Derived From Bulk TIL 1790
TIL line 1790 was isolated from a slow-growing SQ lesion resected from the patient’s right chest wall in November 1999. In an attempt to identify the antigen(s) recognized by TIL 1790, a number of TIL clones/cloids were generated from this TIL line by limiting dilution. The activities of the HLA-A2-restricted TIL clones and cloids against melanoma cell lines and multiple peptide antigens are shown in Table 1. Two clones with separate HLA-B- or C-restricted activities were also isolated from the bulk TIL (Tables 2 and 3). In addition, the patient’s pretreatment PBMCs also showed strong preexisting immunity to gp100 (epitope 154–162), the two TRP-2 antigens, and NY-ESO-1 (data not shown).
TABLE 1.
HLA-A2-Restricted Activities of Patient’s TIL Clones and Cloids
| T-Cell Clones or Cloids from Patient
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M26 | M-A1 | M18 | M-E5 | M-D2 | M-G5 | M8 | M37 | MR1 | ||
| Targets | HLA-A2 | (pg/mL IFN-γ) | ||||||||
| 624mel | + | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 59 |
| 526mel | + | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 0 | >1000 | 2 |
| F002Rmel | + | 1 | 0 | 24 | 0 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 1102mel | + | >1000 | >1000 | 303 | >1000 | >1000 | 226 | 0 | >1000 | 11 |
| 624-28mel | − | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 7 |
| 397mel | − | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 4 |
| 938mel | − | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| T2 pulsed with | ||||||||||
| gp100:154–162 | + | 0 | 0 | 0 | >1000 | 3 | 0 | 0 | 0 | 0 |
| gp100:209–217 | + | >1000 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| gp100:280–288 | + | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 |
| MART–1:27:35 | + | 0 | >1000 | >1000 | >1000 | 0 | 0 | 0 | 0 | 7 |
| TYR:368–376 | + | 0 | 0 | 761 | 0 | 0 | 0 | 0 | 0 | 11 |
| TRP-2:180–188 | + | 0 | 0 | 4 | 5 | >1000 | 1 | 0 | 0 | 4 |
| TRP-2-6b:403–411 | + | 0 | 2 | 7 | 9 | 5 | >1000 | 1 | 0 | 4 |
| NY-ESO-1:157–165 | + | 0 | 1 | 6 | 6 | 7 | 2 | >1000 | 0 | 5 |
| SOX10:331–340 | + | 0 | 1 | 7 | 8 | 7 | 4 | 0 | >1000 | 0 |
| T cell alone | 0 | 1 | 6 | 7 | 7 | 4 | 0 | 0 | 4 | |
| Antigen recognized | gp100 (209–217) | MART-1 | MART-1; Tyrosinase | gp100 (154–162); MART-1 | TRP-2 | TRP-2-6b | NY-ESO-1 | SOX10 | Unknown | |
TABLE 2.
Activities of Non-HLA-A2-Restricted TIL Clones
| Clones
|
||
|---|---|---|
| M-D5 | M-E10 | |
| Targets | (pg/mL IFN-γ) | |
| 624-28mel | 3 | 42 |
| 624mel | 2 | 132 |
| 888mel | 7 | 58 |
| 888A2mel | 8 | 52 |
| 938mel | 10 | 46 |
| 526mel | 1 | >1000 |
| SK23mel | 5 | 65 |
| 1973mel | 9 | 53 |
| 1928mel | >1000 | 52 |
| 1938mel | 9 | 457 |
| Medium | 1 | 52 |
TABLE 3.
HLA-Blocking Assay of Clones M-E10 and M-D5
| Controls† |
|||||
|---|---|---|---|---|---|
| M-E10 | M-D5 | M-A1 | LB7 | LB11 | |
| Target* Plus | (pg/mL IFN-γ) | ||||
| No mAb | 940 | 1009 | 860 | 484 | 606 |
| Anti-HLA class I | 0 | 1 | 0 | 336 | 13 |
| Anti-HLA A2 | 839 | 765 | 30 | 446 | 536 |
| Anti-HLA B/C | 0 | 34 | 610 | 419 | 8 |
| Anti-HLA class II | 788 | 657 | 659 | 64 | 440 |
| T cell alone | 0 | 0 | 0 | 3 | 4 |
| % reduction | 100 | 97 | 97 | 87 | 99 |
Targets: 526 mel for M-E10 and M-A1; 1928 for M-D5; F002Rmel for LB7 and LB11.
Controls: M-A1 is an HLA-A2-restricted, MART-1-specific CTL clone. LB7 and LB11 are two allogeneic HLA-DR- and HLA-B/C-restricted clones, respectively.
Besides the class I-restricted reactivities, the bulk TILs also contained a CD4+ population that recognized tumors in a class II HLA-DP-restricted fashion (data not shown).
Loss of Recognition by Autologous TIL Clones of Autologous Melanoma Cell Lines Established From Recurrent Lesions
Recurrent subcutaneous tumors on the right upper abdomen and right lower back lesions were resected in May and October 2001, respectively. Melanoma lines, 1928mel and 1973mel, respectively, were established in vitro from the two lesions and were used as targets for recognition by autologous TIL clones. Neither cell line was recognized by autologous HLA-A2-restricted, antigen reactive TIL clones that recognized gp100, MART-1, TRP-2, TRP-2-6b, or NY-ESO-1 epitopes (Table 4).
TABLE 4.
No Recognition of Autologous Cell Lines, 1928mel and 1973mel, by Autologous HLA-A2-Restricted TIL Clones
| TIL Clones from Patient
|
|||||
|---|---|---|---|---|---|
| MB4 | M6 | MR7 | M8 | M26 | |
| Targets | (pg/mL IFN-γ) | ||||
| 938mel* | 7 | 8 | 11 | 40 | 36 |
| 624mel† | >1000 | >1000 | >1000 | >1000 | >1000 |
| 1928mel | 32 | 41 | 41 | 11 | 11 |
| 1973mel | 41 | 43 | 43 | 16 | 7 |
| Medium | 42 | 23 | 14 | 15 | 4 |
| Antigen recognition | TRP-2-6b | MART-1 | TRP-2 | NY-ESO-1 | gp100 |
HLA-A2 positive control melanoma cell line.
HLA-A2 negative control melanoma cell line.
Characterization of Autologous Melanoma Lines 1928mel and 1973mel
To elucidate the mechanisms responsible for the lack of recognition of melanoma lines 1928mel and 1973mel by autologous HLA-A2-restricted TIL clones, RT-PCR analysis was performed to check for expression of known melanoma antigens. In addition, expression of HLA class I and HLA-A2 antigens on the two cell lines was analyzed by FACS. 1928mel expressed both HLA class I and HLA-A2 (Fig. 1) but did not express any known melanoma antigens tested except for weak expression of the MDA SOX10 (Fig. 2).
FIGURE 1.
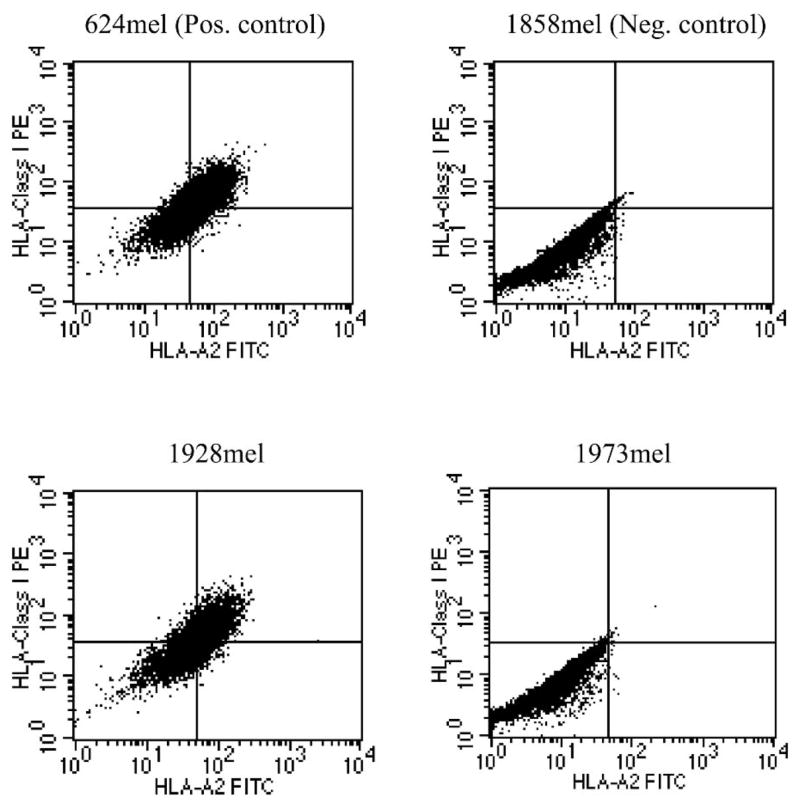
HLA class I and HLA-A2 expression of the two autologous cell lines, 1928mel and 1973mel.
FIGURE 2.

Melanoma antigen expression patterns in the two autologous cell lines, 1928mel and 1973mel, derived from two recurrent lesions. The allogeneic cell line 624mel, which expressed all antigens of interest, was used as a positive control. Lanes 1 and 2: 1928mel; lanes 3 and 4: 1973mel; lanes 5 and 6: 624mel; lane 7: no template control (H2O control)
In contrast, 1973mel expressed all known melanoma antigens tested except for NY-ESO-1 (see Fig. 2) but did not express HLA class I antigens (see Fig. 1). A functional peptide-pulsing assay was performed to confirm the findings. 1928mel, but not 1973mel, when pulsed with an HLA-A2 peptide (MART-1: 27–35) was recognized by a MART-1-specific TIL clone (Table 5), further demonstrating the lack of HLA-A2 expression by 1973mel.
TABLE 5.
Functional Assay to Test for HLA-A2 Expression by Autologous Cell Lines 1928mel and 1973mel
| M6* | LB7.4.2† | ||
|---|---|---|---|
| Targets | Pulsed with | (pg/mL IFN-γ) | |
| 1928mel | None | 10 | 969 |
| 1928mel | gp100:280–288 | 14 | 931 |
| 1928mel | MART-1:27–35 | 687 | 953 |
| 1973mel | None | 5 | 728 |
| 1973mel | gp100:280–288 | 10 | 723 |
| 1973mel | MART-1:27–35 | 6 | 507 |
| T2 | None | 6 | 12 |
| T2 | gp100:280–288 | 7 | 11 |
| T2 | MART-1:27–35 | 923 | 11 |
| Medium | 9 | 8 | |
M6 is an HLA-A2-restricted, MART-1-specific CTL clone.
LB7.4.2 is a control allogeneic CD4+ clone that recognized 1928mel and 1973mel.
Mutation of β2m in 1973mel Resulted in Loss of MHC Class I Expression
Since IFN-γ-treated 1973mel was not recognized by autologous HLA-A2-restricted TIL clones (data not shown), the mechanism underlying total loss of MHC class I antigens in 1973mel was more likely a result of structural rather than functional alterations. From our previous studies8 and recent unpublished observations, loss of β2m (due to somatic mutations) was demonstrated in some melanoma cell lines that exhibited total loss of MHC class I expression. Therefore, the β2m cDNA derived from 1973mel was sequenced and found to have a 2 base-pair (TT) deletion at positions 331–332, resulting in a truncated protein secondary to an early stop codon (Fig. 3). To confirm that the mutated β2m was the underlying cause of total loss of MHC class I antigens, 1973mel was electroporated with a plasmid encoding the wild-type (WT) β2m cDNA and tested for recognition by autologous HLA-A2-restricted TIL clones. Only 1973mel transfected with WT β2m but not the control GFP plasmid was recognized (Table 6).
FIGURE 3.
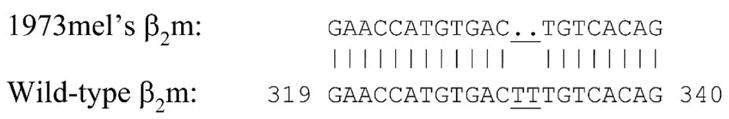
Double base-pair deletion (underlined) detected in the β2m cDNA derived from melanoma cell line 1973mel. Wild-type β2m was from GenBank sequence NM_004048.1.
TABLE 6.
Recognition of 1973 Melanoma Cells Transfected with Wild-Type β2m by Autologous HLA-A2-Restricted TIL Clones
| M8 | M-A1 | M28 | M37 | ||
|---|---|---|---|---|---|
| Targets | HLA-A2 | (pg/mL IFN-γ) | |||
| 1973mel/GFP | 0 | 15 | 1 | 0 | |
| 1973mel/32m | 0 | 438 | >1000 | 137 | |
| 624-28mel | − | 0 | 0 | 0 | 0 |
| 624mel | + | 943 | >1000 | >1000 | >1000 |
| Medium | 3 | 7 | 1 | 4 | |
| Antigen recognized | NY-ESO-1 | MART-1 | TRP-2-6b | SOX10 | |
| Antigen expressed in 1973mel | − | + | + | + | |
Earlier Tumor Recognized by Autologous HLA-A2-Restricted TIL Clones
To test whether the loss of recognition of the two recurrent tumors, 1928mel and 1973mel, was a later event during the patient’s disease progression, an earlier tumor (FrTu 1790), resected approximately 1.5 years before lesions 1928 and 1973, was used to test for recognition by autologous HLA-A2-restricted TIL clones. Since we could not establish a cell line from this lesion, a fresh tumor cell suspension was used as a target. FrTu 1790 was recognized by multiple TIL clones (Table 7). This functional assay demonstrated that many of the cells in FrTu 1790 expressed both HLA-A2 antigen and multiple melanoma-associated antigens.
TABLE 7.
Recognition of an Earlier Tumor (FrTu 1790) by Autologous HLA-A2-Restricted TIL Clones
| TIL Clones from Patient
|
|||||||
|---|---|---|---|---|---|---|---|
| M26 | M-A1 | M-D2 | M-G5 | M8 | M37 | ||
| Targets | HLA-A2 | (pg/mL IFN-γ) | |||||
| FrTu 1790 | 261 | 751 | 847 | 803 | >1000 | 318 | |
| 1928mel | + | 0 | 45 | 10 | 24 | 5 | 369 |
| 1973mel | − | 0 | 0 | 14 | 21 | 6 | 0 |
| 624mel | + | >1000 | >1000 | >1000 | 494 | 770 | >1000 |
| 624-28mel | − | 0 | 2 | 12 | 5 | 4 | 0 |
| Medium | 0 | 0 | 35 | 0 | 0 | 0 | |
| Antigen recognized | gp100 | MART-1 | TRP-2 | TRP-2-6b | NY-ESO-1 | SOX10 | |
DISCUSSION
Current cancer vaccine strategies have been only sporadically successful in eradicating tumors, probably mainly due to the inadequacies of current immunization regimens. A few patients have experienced dramatic clinical responses following immunization with cancer antigens, although tumors recur in many of these patients. Partial or total loss of HLA class I expression or downregulation of tumor antigens in the residual or recurrent tumors has been demonstrated in some cases, thus accounting for the escape of tumor from immune destruction.9–11
This article describes immune studies of a unique patient who exhibited a dramatic regression of cancer at multiple visceral and soft tissue sites following immunization with four HLA-A2 peptides from three MDAs—gp100, MART-1, and tyrosinase—emulsified in IFA. Most of her tumors completely regressed after two cycles of treatment (day 45), including complete resolution of a large tumor in her left thigh, an intra-pelvic mass, a liver lesion, most of the nodules in her lungs, and all but one SQ lesion. We demonstrate here that tumor recurrence was associated with loss of either melanoma antigens or MHC molecules.
Evidence is presented here for in vivo immunoselection of NY-ESO-1-negative tumor variants. Tumor 1790 (year resected, 1999) was recognized by an NY-ESO-1-specific TIL clone (M8). However, both recurrent tumors in 2001–tumors 1928 and 1973–were negative for NY-ESO-1 expression by RT-PCR, and neither was recognized by clone M8. The patient had strong preexisting immunity to NY-ESO-1 as detected in her pretreatment PBMCs,4 even though she was not vaccinated against NY-ESO-1 antigen. In addition, NY-ESO-1-specific lymphocytes have also been cloned from her TILs.
In addition, the patient’s TILs and peripheral blood contained reactivities directed against multiple melanoma antigens, including gp100: 209–217, gp100: 154–162, MART-1, tyrosinase, TRP-2, TRP-2-6b, NY-ESO-1, SOX10, an as-yet-known HLA-A2-restricted antigen, two HLA-B- or C-restricted antigens, as well as an HLA-DP-restricted antigen. These multiple immune reactivities may account for the emergence of immunoresistant tumor variant 1928, which lost expression of most of the known antigens mentioned previously. Even though cell line 1928mel expressed SOX10, the level of expression was minimal, as demonstrated by a weaker band on a highly sensitive RT-PCR assay (see Fig. 2), and by real-time quantitative RT-PCR.3 Even though downregulation of individual antigens due to separate mutational events is a possibility, regulatory mechanisms such as cytokine-driven antigen silencing12 seem to be a more plausible explanation. Since SOX10 is a potent transactivator of the microphthalmia-associated transcription factor (MITF) gene,13 which in turn regulates the expression of multiple MDAs,14,15 downregulation of SOX10 through regulatory mechanisms may interfere with the expression of multiple MDAs. Therefore, we hypothesize that the loss of antigen expression was a result of two separate events: one involved the loss of the CTA NY-ESO-1, most likely by a mutation, and the other involved SOX10 and other MDAs, most likely through a regulatory mechanism.
Another immunoresistant phenotype that emerged from this patient’s recurrent tumors was a total HLA class I loss variant. Tumor 1973, which recurred in October 2001, expressed multiple melanoma antigens but did not express HLA class I. The mechanism underlying this phenotype was a double base-pair deletion in the β-2m molecule. This accounts for the lack of recognition of 1973mel by HLA-A2-restricted autologous TIL clones and helps explain the recurrence and progression of tumor 1973.
The fact that autologous HLA-A2-restricted TIL clones specific for multiple melanoma antigens recognized an earlier tumor (FrTu 1790) resected close to 2 years before the resection of the two recurrent lesions is an indication that FrTu 1790 expressed both HLA-A2 antigen and multiple melanoma antigens. Therefore, it seems that in vivo immunoselection of immunoresistant tumor variants that exhibited total loss of HLA-class I antigens or multiple melanoma antigens indeed occurred in this patient, who experienced a dramatic response but later died of recurrent disease. The appearance of FrTu 1790 recurrent lesion in the presence of multiple tumor-specific T-cell clones 1 year after treatment may reflect the suboptimal activation state of these T cells without help from exogenous sources such as the peptide/IFA vaccination used in the previous treatment. Recurrent tumors 1928 and 1973 most likely originated from a few immunoresistant tumor cell clones that escaped immune destruction during the peptide/IFA treatment. It is likely that these tumor cell clones might have taken a few years to become clinically detectable, as seen in 2001 when they were resected.
In conclusion, this paper illustrates a situation that may become more prevalent as immunotherapy-based treatments for cancer become more effective: the destruction of immunosensitive tumor cells but the eventual progression of immunoresistant tumor variants.
References
- 1.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jager E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khong HT, Rosenberg SA. The Waardenburg syndrome type 4 gene, SOX10, is a novel tumor-associated antigen identified in a patient with a dramatic response to immunotherapy. Cancer Res. 2002;62:3020–3023. [PMC free article] [PubMed] [Google Scholar]
- 4.Khong HT, Rosenberg SA. Pre-existing immunity to tyrosinase-related protein (TRP)-2, a new TRP-2 isoform, and the NY-ESO-1 melanoma antigen in a patient with a dramatic response to immunotherapy. J Immunol. 2002;168:951–956. doi: 10.4049/jimmunol.168.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries TJ, Fourkour A, Wobbes T, et al. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997;57:3223–3229. [PubMed] [Google Scholar]
- 6.Mashino K, Sadanaga N, Tanaka F, et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85:713–720. doi: 10.1054/bjoc.2001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, Guan Y, Li L, et al. Expression in normal human tissues of five nucleotide excision repair genes measured simultaneously by multiplex reverse transcription-polymerase chain reaction. Cancer Epidemiol Bio-markers Prev. 1999;8:801–807. [PubMed] [Google Scholar]
- 8.Restifo NP, Marincola FM, Kawakami Y, et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulie PG, Ikeda H, Baurain JF, Chiari R. Antitumor immunity at work in a melanoma patient. Adv Cancer Res. 1999;76:213–242. doi: 10.1016/s0065-230x(08)60778-2. [DOI] [PubMed] [Google Scholar]
- 10.Maeurer MJ, Gollin SM, Martin D, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with down-regulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–1641. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Robbins PF, et al. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–393. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durda PJ, Dunn IS, Rose LB, et al. Induction of “antigen silencing” in melanomas by oncostatin M: down-modulation of melanocyte antigen expression. Mol Cancer Res. 2003;1:411–419. [PubMed] [Google Scholar]
- 13.Potterf SB, Furumura M, Dunn KJ, et al. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 14.Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22:3035–3041. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Miller AJ, Widlund HR, et al. MLANA/MART1 and SILV/MEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333–343. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


