Abstract
Administration of ghrelin, a key peptide in the regulation of energy homeostasis, has been shown to decrease LH pulse frequency while concomitantly elevating cortisol levels. Because increased endogenous CRH release in stress is associated with an inhibition of reproductive function, we have tested here whether the pulsatile LH decrease after ghrelin may reflect an activated hypothalamic-pituitary-adrenal axis and be prevented by a CRH antagonist. After a 3-h baseline LH pulse frequency monitoring, five adult ovariectomized rhesus monkeys received a 5-h saline (protocol 1) or ghrelin (100-μg bolus followed by 100 μg/h, protocol 2) infusion. In protocols 3 and 4, animals were given astressin B, a nonspecific CRH receptor antagonist (0.45 mg/kg im) 90 min before ghrelin or saline infusion. Blood samples were taken every 15 min for LH measurements, whereas cortisol and GH were measured every 45 min. Mean LH pulse frequency during the 5-h ghrelin infusion was significantly lower than in all other treatments (P < 0.05) and when compared with the baseline period (P < 0.05). Pretreatment with astressin B prevented the decrease. Ghrelin stimulated cortisol and GH secretion, whereas astressin B pretreatment prevented the cortisol, but not the GH, release. Our data indicate that CRH release mediates the inhibitory effect of ghrelin on LH pulse frequency and suggest that the inhibitory impact of an insufficient energy balance on reproductive function may in part be mediated by the hypothalamic-pituitary-adrenal axis.
GHRELIN, A 28-AMINO-ACID peptide, is the endogenous ligand of the GH secretagogue receptor (GHS-R) and is predominantly secreted by the stomach (1). Injection of ghrelin not only induces a potent GH release (2,3) but also strongly stimulates appetite in rodents and humans (4,5,6,7). Ghrelin levels may increase during negative energy balance, a condition also known to inhibit reproductive axis activity (8). For example, high total ghrelin levels (about 1.4- to 2.6-fold of control levels) are reported in anorexia nervosa (9,10,11), a syndrome characterized by decreased food intake and amenorrhea (12). Central to energy deficiency-related reproductive dysfunction is a decreased LH pulse frequency (13,14,15). This is not surprising because a proper GnRH/LH pulse frequency is mandatory for the normal activity of the reproductive axis (16). Recent data suggest that ghrelin may play a role in mediating the inhibitory effect of a negative energy balance on the reproductive axis. Indeed, we have previously reported that a short-term peripheral ghrelin infusion in the ovariectomized (OVX) monkey significantly decreases LH pulse frequency (17). An acute inhibitory effect of ghrelin on LH pulse frequency has also been observed in the OVX rat (18), whereas a delay and a decrease in the amplitude of LH pulses have been reported in the male human (19).
Peripheral infusion of ghrelin has been shown to stimulate cortisol release, suggesting an activation of the hypothalamic-pituitary-adrenal (HPA) axis by this peptide (17). Intriguingly, significant increases in cortisol levels are also observed in women showing decreases in LH pulsatility induced by a 5-d food restriction (15), and elevated ghrelin levels in patients with anorexia nervosa are accompanied by elevated cortisol levels (10,11). These observations suggest a potential linkage between activation of the HPA axis and the inhibition of reproductive function in a negative energy balance environment. Because a primary inhibitory role of central HPA pathways in the control of the reproductive axis during stress in the primate is well demonstrated (20,21,22), we have postulated that enhanced central HPA activity is causal to the inhibition of LH pulse frequency that follows ghrelin infusion. To verify this hypothesis, we have tested whether blocking endogenous CRH activity, using astressin B (a nonspecific CRH receptor antagonist) (23), can prevent this inhibitory effect of ghrelin.
Materials and Methods
Animals
Five adult long-term OVX rhesus monkeys (Macaca mulatta) (body weight 5.0–8.5 kg) were used in this study. The animals were kept in individual cages in a temperature- and light-controlled room (lights on 0800–2000 h) and fed twice a day with a high-protein Purina monkey chow (Purina Mills, St. Louis, MO) supplemented with fresh fruit or vegetables. All animals participated in an active enrichment program provided by the staff of Veterinary Medicine. All procedures were approved by the Institutional Animal Care and Use Committee of Columbia University, and the research was conducted in accord with the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
Experimental protocols
Monkeys were briefly sedated with ketamine (5–7 mg/kg; Ketaset, Ford Dodge, IA) in the early morning, and catheters were inserted into both saphenous veins for blood sampling and infusions. The animals were then seated in a primate chair (to which they had previously been habituated), and the experiment was initiated about 1.5 h later, at which time they were fully awake. Each experiment lasted 8 h and included first a 3-h baseline control period followed by a 5-h treatment period. Blood samples (1.2 ml) were taken at 15-min intervals for hormone measurements. During the experiment, animals received fruits and snacks. They were returned to their home cages after the end of each experimental protocol and fed with their daily amount of food.
To investigate the role of CRH in mediating the inhibitory effect of ghrelin on LH pulse frequency, four experimental protocols were performed in each monkey; after a 3-h control period to determine the baseline pulsatile LH release pattern, animals received a 5-h saline iv infusion (1 ml/h, protocol 1), a 5-h iv infusion of ghrelin (100 μg/h bolus, followed by 100 μg/h, protocol 2), ghrelin together with astressin B, a nonspecific CRH receptor antagonist (24) (0.45 mg/kg in one injection im 90 min before the initiation of the ghrelin infusion, protocol 3), or astressin B injected 90 min before a saline infusion (protocol 4). Each animal underwent all experimental protocols in random order (except one monkey not tested under protocol 4), and at least 2 wk elapsed between two protocols. Human acylated ghrelin and astressin B were synthesized in the laboratory of J.R. Doses of ghrelin and astressin B used were shown in previous studies to be effective in the rhesus monkey (17,25).
Assays and data analysis
Blood samples were centrifuged, and sera were kept at −20 C until assay. LH was measured in all samples by a recombinant homologous RIA (reagents provided by Dr. A. F. Parlow, Pituitary Hormones and Antisera Center, Torrance, CA) in duplicate, as described previously (26). Assay sensitivity was 0.06 ng/ml. Intra- and interassay coefficients of variation (CV) were 4.5 and 12.6%, respectively. Cortisol and GH were measured in every fourth sample. Cortisol levels were assayed by RIA (Diagnostic Systems Laboratories/Beckham, Webster, TX) in duplicate. Intra- and interassay CV were 4.0 and 7.5%, respectively. All samples from individual animals were measured in the same assay. GH was measured by a chemiluminescent immunoassay (Immulite System; Diagnostic Products Corp./Siemens, Los Angeles, CA). Intraassay CV was 3.4%. Total ghrelin levels during infusion were measured in duplicate by RIA (Millipore Corp., St. Charles, MO) in hourly pooled samples. All samples were measured in one assay, with an intraassay CV of 3.3%.
Mean LH pulse frequency, LH pulse amplitude, and LH concentrations were calculated. LH pulse frequency during the treatment periods in the four protocols was analyzed by one-way ANOVA with repeated measures followed by the Newman-Keuls multiple comparison test. The differences between baseline and the treatment periods were compared by Student’s t test. Three criteria were used for LH pulsatility analysis, as described previously (27): 1) the LH peak occurs within 30 min from the previous nadir; 2) the peak level from the previous nadir must be 3-fold greater than the intraassay CV; and 3) the LH increase must be followed by declining levels in accord with LH half-life. This approach to identify LH pulses was previously verified by the Cluster pulse-detection algorithm program (17). LH peaks that occurred at time 0 were included as pulses in the baseline period, because the treatments were initiated after the time 0 sample. Mean cortisol and GH levels, their percent changes from baseline at each time point, and areas under the curves in response to treatment were calculated. Differences between baseline and treatment and between treatments in the four protocols were analyzed by one-way ANOVA (Kruskal-Wallis nonparametric test) followed by the Dunn’s test. Mean hourly ghrelin changes during ghrelin infusion were calculated and compared by ANOVA. All statistical analyses were performed using PRISM (GraphPad, San Diego, CA).
Results
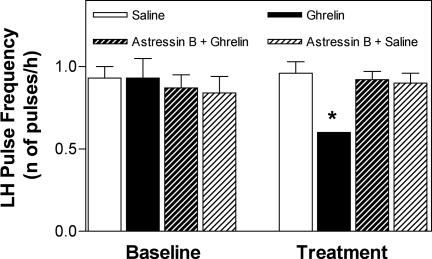
Mean LH pulse frequency during the 5-h ghrelin treatment was significantly lower than that in the other three treatments: 0.60 ± 0/h (P < 0.05 vs. 0.96 ± 0.07/h in saline and 0.92 ± 0.05/h and 0.87 ± 0.07/h in ghrelin and saline with pretreatment of astressin B, respectively) (Fig. 1 and Table 1). When compared with the 3-h baseline period, ghrelin infusion significantly decreased LH pulse frequency (P < 0.05); this decrease was prevented by astressin B administration. LH pulse frequency remained unchanged during saline infusion (with or without astressin B pretreatment). There were no significant changes in LH pulse amplitude or LH concentrations in any of the treatments (Table 1).
Figure 1.
The decrease in LH pulse frequency induced by a 5-h ghrelin infusion (100-μg bolus followed by 100 μg/h) is prevented by astressin B (a CRH receptor antagonist) pretreatment. LH pulse frequency during ghrelin treatment was significantly lower (*, P < 0.05) than during all other treatments and during the 3-h baseline.
Table 1.
Comparison of LH pulse parameters (mean ± se) during the 3-h baseline and 5-h treatment periods
| Pulse frequency (n/h)
|
Pulse amplitude (ng/ml)
|
Mean concentrations (ng/ml)
|
||||
|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | Baseline | Treatment | |
| Saline alone | 0.93 ± 0.07 | 0.96 ± 0.07 | 2.60 ± 0.58 | 2.92 ± 0.35 | 9.92 ± 1.93 | 10.0 ± 1.72 |
| Ghrelin | 0.93 ± 0.12 | 0.60 ± 0.00a,b | 3.16 ± 0.64 | 3.22 ± 0.53 | 11.24 ± 1.90 | 9.54 ± 1.60 |
| Astressin B + ghrelin | 0.87 ± 0.08 | 0.92 ± 0.05 | 3.14 ± 0.45 | 3.10 ± 0.50 | 10.40 ± 1.99 | 9.40 ± 1.90 |
| Astressin B + saline | 0.84 ± 0.10 | 0.90 ± 0.06 | 2.20 ± 0.09 | 2.42 ± 0.18 | 8.63 ± 1.80 | 7.85 ± 1.43 |
P< 0.05 vs. treatment with saline, astressin + ghrelin, and astressin B + saline.
P < 0.05 vs. baseline.
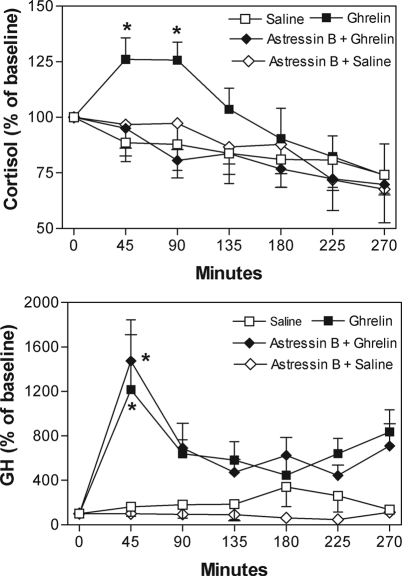
Cortisol levels significantly increased 45 and 90 min after initiation of the ghrelin treatment (126.0 ± 10.8% and 125.7 ± 8.1% of baseline, respectively, P < 0.05). Pretreatment with astressin B prevented ghrelin stimulation of cortisol release (Fig. 2, upper graph). Mean area under the cortisol curve after astressin pretreatment was significantly lower than that after ghrelin alone and similar to that after saline or saline plus astressin B (Table 2).
Figure 2.
Upper graph, The increase in cortisol (percent over baseline) during ghrelin infusion is prevented by astressin B (*, P < 0.05). Overall mean baseline cortisol (100%) was 26.8 ± 1.0 μg/dl. Lower graph, The GH increase after ghrelin is not prevented by astressin B; responses in the two groups are not statistically different but are significantly higher than in the saline alone or saline plus astressin groups (*, P < 0.05). Overall mean baseline GH (100%) was 2.6 ± 0.71 ng/ml.
Table 2.
Comparison of the areas under the cortisol and GH curves (mean ± se) during the 3-h baseline and 5-h treatment periods
| Cortisol (μg/dl·min) | GH (ng/ml·min) | |
|---|---|---|
| Saline alone | 5987 ± 584.3 | 1048 ± 467.6 |
| Ghrelin | 7520 ± 474.4a | 4829 ± 405.2a |
| Astressin B + ghrelin | 6027 ± 557.6 | 4391 ± 651.0a |
| Astressin B + saline | 6034 ± 498.5 | 685 ± 359.4 |
P < 0.05 vs. other treatments.
GH levels significantly increased 45 min after ghrelin injection (2126 ± 628.2% of baseline, P < 0.05; n = 4). In contrast to cortisol, astressin B did not prevent the stimulatory effect of ghrelin on GH release (Fig. 2, lower graph). Mean area under the GH curves in response to ghrelin infusions (with or without astressin B) were significantly higher than saline infusion. Astressin B itself did not show any effect on GH release (Table 2).
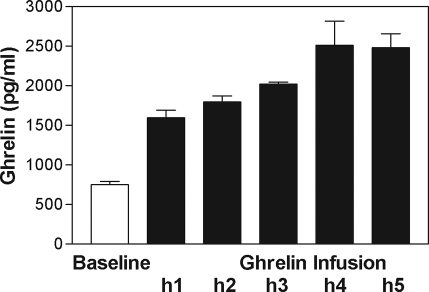
Total ghrelin levels remained stable during the 3-h baseline period (754.2 ± 38.8 pg/ml). Ghrelin infusion significantly increased ghrelin levels: by 5 h, mean ghrelin had increased to 3.29-fold of baseline, whereas overall increase over the 5-h infusion period was 1.76-fold of baseline (Fig. 3).
Figure 3.
Increase in total ghrelin levels during ghrelin infusion.
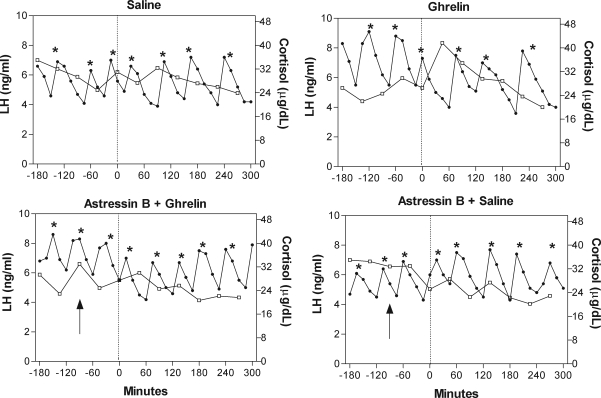
Figure 4 illustrates the LH and cortisol responses in the four protocols in an individual animal. No ill effects were observed upon administration of either ghrelin or astressin B.
Figure 4.
Pulsatile LH release and cortisol concentrations in response to infusions of saline, ghrelin, ghrelin plus astressin B, and saline plus astressin B in an individual monkey (no. 89D361). The experiment was divided into two periods, a 3-h baseline period (i.e.13 samples) and a 5-h treatment period. A dotted vertical line denotes the time of the ghrelin bolus and the start of the 5-h ghrelin or saline infusions. Arrows indicate the time of injection of astressin B. Note the reversal of the decrease in LH pulse frequency that occurs during ghrelin infusion by the CRH receptor antagonist. *, Identified LH pulse.
Discussion
The data confirm our previous observations that a short-term ghrelin infusion, elevating total ghrelin levels to 1.76-fold of baseline over a 5-h period, significantly decreases LH pulse frequency in the OVX nonhuman primate. We also confirm that ghrelin activates the HPA axis as shown by the increase in cortisol. Most importantly, we report for the first time that pretreatment with astressin B, a nonspecific CRH antagonist, prevents this inhibitory effect of ghrelin on LH pulse frequency and concomitantly suppresses the cortisol increase.
The pivotal observation in this study is that blockage of endogenous CRH activity by a CRH receptor antagonist entirely prevents the suppressive effects of a short-term elevation of ghrelin levels on LH pulse frequency. Because the cortisol response to ghrelin is also prevented, it is likely that there exists a causal relationship between an activation of the HPA axis and the decrease in LH pulsatile activity. Because our antagonist is specific to CRH (28) and because other investigators have shown that ghrelin releases CRH from rat hypothalamic explants (29), the data suggest a central role for CRH in mediating inhibitory effects of ghrelin on pulsatile LH release. A large body of evidence has already demonstrated a primary inhibitory role of CRH on the GnRH pulse generator and on the reproductive axis in several species (30,31,32,33). In the monkey, antagonism of endogenous CRH activity also readily prevents the decrease in pulsatile LH release after an acute stress challenge in OVX animals (21,28) and accelerates the return to normal cyclic activity after a more chronic stress (25). The results from the present study suggest, for the first time, a mediatory role of CRH in the disruption of an essential element in reproductive function, i.e. the GnRH pulse generator, under conditions other than under stress challenges, such as in a negative energy balance condition.
The overall increase in total ghrelin levels during our ghrelin infusions was 1.76-fold of baseline, within the reported range of total ghrelin levels in patients with anorexia nervosa (9,10,11), a syndrome that includes malnutrition as well as elevated cortisol levels. These patients also show a decreased activity of the GnRH pulse generator (12,34,35). An 85% increase in ghrelin level was reported in amenorrheic athletes and uniquely discriminated these subjects from those with less severe cyclic disturbances of exercising women (36). In the present study in the nonhuman primate, LH pulse frequency decreased from one pulse/63 min during the control saline infusion to one pulse/100 min during the 5-h ghrelin infusion, whereas CRH antagonist pretreatment restored it to one pulse/65 min. This effect might at first view appear modest. However, experiments in the monkey have clearly shown that small changes in GnRH pulse frequency in the range of those induced here by the 5-h ghrelin infusion do over a more chronic timeline significantly interfere with the normal reproductive cycle (16). In this regard, we believe that restoration to a normal LH pulse frequency by astressin B is a physiologically relevant observation.
Like the modest suppression of LH pulse frequency induced by ghrelin infusions in the present study, cortisol increases were moderate compared with much steeper elevations observed in previous experiments in the monkey that included acute inflammatory stress challenges in which mean cortisol levels increased by 60% from baseline. However, this more potent activation of the HPA axis was accompanied by a remarkably deeper suppression of LH pulsatility (21,37). Significant in this aspect is the demonstration by Loucks and Thuma (15) that LH pulse frequency inhibition after graded energy deficits is proportional to the increase in cortisol levels. The smaller inhibitory effect of ghrelin on LH pulse frequency in the present experiment most probably reflects the lower degree of activation of the HPA axis and of central CRH release and is presumably more representative of the finer tuning process exerted by the HPA axis on the reproductive system.
The pathways by which peripherally secreted ghrelin stimulates the HPA axis and whether ghrelin directly activates centrally located CRH neurons remain to be fully investigated in both the acute and chronic condition. However, there is good evidence suggesting that hypothalamic neuropeptide Y (NPY)/agouti-related peptide (AGRP) neurons mediate the effects of ghrelin. We know, for instance, that NPY/AGRP neurons express GHS-R (38), that ghrelin administration in the rodent increases the synthesis of both NPY and AGRP mRNA levels (39), and that synthesis of these two peptides is increased in a negative energy balance environment (40,41). Like ghrelin, both AGRP and NPY exert a powerful orexigenic effect in the rodent and the monkey (42,43,44,45), and the orexigenic action of ghrelin is abolished in double-knockout NPY and AGRP mice (46). Data in the OVX monkey have also shown that NPY or AGRP infusions into the third ventricle readily inhibit pulsatile LH (47,48). These two neuropeptides have also been shown to stimulate the HPA axis (48,49). Finally, in the rat, NPY/AGRP neurons located in the arcuate nucleus of the hypothalamus densely innervate paraventricular CRH-containing neurons (50,51). Overall, the data suggest that activation of the HPA axis by elevated ghrelin is mediated by a NPY/AGRP-induced CRH release, which is in turn responsible for the decline in LH pulse frequency.
Ghrelin, an acylated peptide, was first discovered as the endogenous ligand of the GHS-R (1), and as expected, our data show that there is a brief but significant increase in GH release during ghrelin infusion, as shown by others (2,3). In contrast to its effect on the cortisol increase that it abolishes, the CRH antagonist has no effect on the GH response to ghrelin, indicating that astressin B does not interfere with the bioactivity of ghrelin. Recently, a study has shown that chronic administration of unacylated ghrelin, initially thought of as an inert form of the hormone because it failed to modify GH secretion, can fully mimic the inhibitory effect of acylated ghrelin on LH and FSH release in the rat (52). These data highlight the fact that the effects of ghrelin on the somatotropic and gonadotropic axes are mediated by different pathways.
In conclusion, we have demonstrated that pretreatment with astressin B, a nonspecific CRH antagonist, prevents the inhibitory effect of ghrelin on LH pulse frequency. These data demonstrate that the deleterious impact of ghrelin and possibly of a negative energy balance on the GnRH pulse generator and the reproductive axis is at least partially mediated by the central HPA axis. It should be pointed out, however, that the present data were obtained in an acute study and that a potential role of ghrelin and of the HPA axis in long-term situations remains to be explored. The data also suggest that the use of a CRH antagonist may provide a novel treatment approach in patients with reproductive dysfunction caused by an activated HPA axis. What has not been fully appreciated until recently in the human is the frequent association of elevated cortisol and reproductive dysfunction. Increased circulating and central cortisol levels are reported in most women with the functional chronic hypothalamic anovulation syndrome, a main cause of infertility classically associated with lifestyle changes that include not only energy deficiency but also psychogenic stress and strenuous exercise (53,54,55). Not only is an activated HPA axis common to functional chronic hypothalamic anovulation syndrome patients, but metabolic aberrations have also been frequently observed (56,57,58). In these patients, the role of the HPA axis in integrating a metabolic signal, such as that from ghrelin, in the fine-tuning process of the reproductive system remains to be fully investigated.
Footnotes
This work was supported by National Institutes of Health Grants RO1-HD-46715 (M.F.) and PO1-DK-26741 (J.R.). J.R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor.
Disclosure Statement: N.R.V., E.X., L.X.-Z., and M.F. have nothing to disclose. J.R. is founder of Sentis Medical Sciences.
First Published Online December 6, 2007
See editorial p. 867.
Abbreviations: CV, Coefficients of variation; GHS-R, GH secretagogue receptor; HPA, hypothalamic-pituitary-adrenal; OVX, ovariectomized.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR 2000 The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR 2001 Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992 [DOI] [PubMed] [Google Scholar]
- Schneider LF, Warren MP 2006 Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil Steril 86:1744–1749 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tatebe Y, Nakahara T, Yasuhara D, Sagiyama K, Muranaga T, Ueno H, Nakazato M, Nozoe S, Naruo T 2003 Eating pattern and the effect of oral glucose on ghrelin and insulin secretion in patients with anorexia nervosa. Clin Endocrinol (Oxf) 59:574–579 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A 2005 Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289:E373–E381 [DOI] [PubMed] [Google Scholar]
- Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B 2007 Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr 85:967–971 [DOI] [PubMed] [Google Scholar]
- Katz MG, Vollenhoven B 2000 The reproductive endocrine consequences of anorexia nervosa. BJOG 107:707–713 [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Cameron JL 1992 Suppression of luteinizing hormone secretion during food restriction in male rhesus monkeys (Macaca mulatta): failure of naloxone to restore normal pulsatility. Neuroendocrinology 56:464–473 [DOI] [PubMed] [Google Scholar]
- Whisnant CS, Harrell RJ 2002 Effect of short-term feed restriction and refeeding on serum concentrations of leptin, luteinizing hormone and insulin in ovariectomized gilts. Domest Anim Endocrinol 22:73–80 [DOI] [PubMed] [Google Scholar]
- Loucks AB, Thuma JR 2003 Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88:297–311 [DOI] [PubMed] [Google Scholar]
- Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E 1983 Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 112:2076–2080 [DOI] [PubMed] [Google Scholar]
- Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M 2004 Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 89:5718–5723 [DOI] [PubMed] [Google Scholar]
- Furuta M, Funabashi T, Kimura F 2001 Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun 288:780–785 [DOI] [PubMed] [Google Scholar]
- Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A 2007 Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab 92:3202–3205 [DOI] [PubMed] [Google Scholar]
- Ferin M 2006 Stress and the reproductive System. In: Neill J, ed. Physiology of reproduction. 3rd ed. San Diego: Elsevier; 2627–2696 [Google Scholar]
- Feng YJ, Shalts E, Xia LN, Rivier J, Rivier C, Vale W, Ferin M 1991 An inhibitory effects of interleukin-1a on basal gonadotropin release in the ovariectomized rhesus monkey: reversal by a corticotropin-releasing factor antagonist. Endocrinology 128:2077–2082 [DOI] [PubMed] [Google Scholar]
- Shalts E, Feng YJ, Ferin M 1992 Vasopressin mediates the interleukin-1α-induced decrease in luteinizing hormone secretion in the ovariectomized rhesus monkey. Endocrinology 131:153–158 [DOI] [PubMed] [Google Scholar]
- Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL 1999 Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem 42:3175–3182 [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH 2004 Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology 29:1112–1121 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Vulliemoz N, Rivier J, Ferin M 2007 Astressin B, a corticotropin-releasing hormone receptor antagonist, accelerates the return to normal luteal function after an inflammatory-like stress challenge in the rhesus monkey. Endocrinology 148:841–848 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia L, Shanen D, Khabele D, Ferin M 1994 Stimulatory effects of interleukin-induced activation of the hypothalamo-pituitary-adrenal axis on gonadotropin secretion in ovariectomized monkeys replaced with estradiol. Endocrinology 135:2093–2098 [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Diefenbach WD, Alston E, Ferin M 1985 Gonadotropin-releasing hormone pulses in third ventricular cerebrospinal fluid of ovariectomized rhesus monkeys: correlation with luteinizing hormone pulses. Endocrinology 117:1550–1558 [DOI] [PubMed] [Google Scholar]
- Rivier CL, Grigoriadis DE, Rivier JE 2003 Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 144:2396–2403 [DOI] [PubMed] [Google Scholar]
- Mozid AM, Tringali G, Forsling ML, Hendricks MS, Ajodha S, Edwards R, Navarra P, Grossman AB, Korbonits M 2003 Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm Metab Res 35:455–459 [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W 1984 Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology 114:914–921 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sutton S, Vale W, Plotsky P 1987 Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology 120:1083–1088 [DOI] [PubMed] [Google Scholar]
- Olster DH, Ferin M 1987 Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 65:262–267 [DOI] [PubMed] [Google Scholar]
- Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E 1990 Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology 52:133–137 [DOI] [PubMed] [Google Scholar]
- Armeanu MC, Berkhout GM, Schoemaker J 1992 Pulsatile luteinizing hormone secretion in hypothalamic amenorrhea, anorexia nervosa, and polycystic ovarian disease during naltrexone treatment. Fertil Steril 57:762–770 [DOI] [PubMed] [Google Scholar]
- Berga SL 1996 Functional hypothalamic chronic anovulation. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive endocrinology, surgery and technology. Philadelphia: Lippincott-Raven; 1061–1075 [Google Scholar]
- De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI 2004 Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab 89:3536–3542 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M 2000 Inhibitory effects of endotoxin on LH secretion in the ovariectomized monkey are prevented by naloxone but not by an interleukin-1 receptor antagonist. Neuroimmunomodulation 7:6–15 [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J 1999 Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 70:306–316 [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I 2001 Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes 50:2438–2443 [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW 1998 Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV 1999 Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140:4551–4557 [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP 1984 Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115:427–429 [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Stidsen CE, Madsen K, Smith MS, Cameron JL 1999 Activation of central neuropeptide Y Y1 receptors potently stimulates food intake in male rhesus monkeys. J Clin Endocrinol Metab 84:3781–3791 [DOI] [PubMed] [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR 2001 Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes 50:248–254 [DOI] [PubMed] [Google Scholar]
- Koegler FH, Grove KL, Schiffmacher A, Smith MS, Cameron JL 2001 Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 142:2586–2592 [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S 2004 Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145:2607–2612 [DOI] [PubMed] [Google Scholar]
- Kaynard AH, Pau KY, Hess DL, Spies HG 1990 Third-ventricular infusion of neuropeptide Y suppresses luteinizing hormone secretion in ovariectomized rhesus macaques. Endocrinology 127:2437–2444 [DOI] [PubMed] [Google Scholar]
- Vulliemoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M 2005 Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology 146:784–789 [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH 2007 Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology 148:3666–3673 [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD 1999 Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140:1408–1415 [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS 2000 Corticotropin releasing hormone neurons in the paraventricular nucleus are direct targets for neuropeptide Y neurons in the arcuate nucleus: an anterograde tracing study. Brain Res 854:122–129 [DOI] [PubMed] [Google Scholar]
- Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M 2006 Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 147:2374–2382 [DOI] [PubMed] [Google Scholar]
- Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL 2006 Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 91:1561–1565 [DOI] [PubMed] [Google Scholar]
- Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, Yen SS 1989 Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 68:301–308 [DOI] [PubMed] [Google Scholar]
- Biller BM, Federoff HJ, Koenig JI, Klibanski A 1990 Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70:311–317 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Dominguez CE, Yen SS 1998 Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 83:25–32 [DOI] [PubMed] [Google Scholar]
- Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M 2001 Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145:669–673 [PubMed] [Google Scholar]
- Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL 2001 Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86:5184–5193 [DOI] [PubMed] [Google Scholar]