Abstract
The forkhead transcription factor forkhead box protein O1 (FoxO1), a downstream target of phosphatidylinositol 3-kinase/Akt signaling, has been reported to suppress skeletal myocyte differentiation, but the mechanism by which FoxO1 regulates myogenesis is not fully understood. We have previously demonstrated that a nutrient-sensing mammalian target of rapamycin (mTOR) pathway controls the autocrine production of IGF-II and the subsequent phosphatidylinositol 3-kinase/Akt signaling downstream of IGF-II in myogenesis. Here we report a regulatory loop connecting FoxO1 to the mTOR pathway. Inducible activation of a FoxO1 active mutant in the C2C12 mouse myoblasts blocks myogenic differentiation at an early stage and meanwhile leads to proteasome-dependent degradation of a specific subset of components in the mTOR signaling network, including mTOR, raptor, tuberous sclerosis complex 2, and S6 protein kinase 1. This function of FoxO1 requires new protein synthesis, consistent with the idea that a transcriptional target of FoxO1 may be responsible for the degradation of mTOR. We further show that active FoxO1 inhibits IGF-II expression at the transcriptional activation level, through the modulation of mTOR protein levels. Moreover, the addition of exogenous IGF-II fully rescues myocyte differentiation from FoxO inhibition. Taken together, we propose that the mTOR-IGF-II pathway is a major mediator of FoxO’s inhibitory function in skeletal myogenesis.
SKELETAL MUSCLE development requires the progression of a highly ordered cascade of events comprising myogenic lineage commitment, myoblast proliferation, and terminal differentiation. Coordination of these events is achieved through endocrine, paracrine, and autocrine actions via distinct signaling pathways, which results in the activation of a myogenic differentiation-specific gene expression program (1,2). The IGFs (IGF-I and IGF-II) are critically involved in skeletal muscle development as well as adult muscle regeneration (3,4,5). In various myoblast cultures, the autocrine/paracrine actions of IGFs, induced in response to growth factor deprivation, are instrumental in the initiation of differentiation (6,7,8,9). Pharmacological and genetic evidence has indicated the phosphatidylinositol 3-kinase (PI3K)/Akt pathway as a major mediator of myogenic signaling downstream of IGFs (10,11,12,13).
The targets of Akt responsible for its myogenic function had remained elusive until a report identified forkhead box protein O1 (FoxO1), a forkhead transcription factor, as a strong candidate for that role (14). The FoxO proteins are key regulators of a wide range of cellular functions, such as proliferation, survival, differentiation, and metabolism (15,16). Many signaling pathways converge on FoxO, but inactivation by Akt phosphorylation appears to be a prevalent mechanism in a variety of cellular contexts (17) and through a variety of mechanisms (18). FoxO1 negatively regulates myogenesis, as evidenced by the ability of a constitutively nuclear FoxO1 mutant to inhibit C2C12 cell differentiation and a transcriptionally inactive mutant to partially rescue differentiation from PI3K inhibition (14). It has also been reported that FoxO1 is required for myotube formation in primary mouse myoblasts after the initiation of differentiation (19), implying that FoxO may play dual roles in different stages of myogenesis. Studies have also revealed FoxO’s role in muscle atrophy; two muscle-specific ubiquitin E3 ligases responsible for muscle wasting, atrogin-1 and muscle specific RING finger 1 (MuRF1), are transcriptional targets of FoxO in mature myofibers/myotubes (20,21). However, direct or indirect targets of FoxO in the regulation of myoblast differentiation are currently unknown.
As a master regulator of cellular processes from growth and proliferation to survival and differentiation, the mammalian target of rapamycin (mTOR) (22) has been found to be indispensable for the differentiation of C2C12 myoblasts (23,24,25,26). Our previous studies have led to the revelation that mTOR regulates distinct stages of myogenesis by assembling different pathways. At the initiation stage, the well-established downstream target of mTOR in the regulation of cell growth and proliferation, S6 protein kinase 1 (S6K1), is not required for differentiation, nor is the Ser/Thr kinase activity of mTOR previously thought to be essential for all mTOR functions (25,27). On the other hand, the catalytic activity of mTOR is required for a second-stage fusion that results in mature myotubes (28).
mTOR controls the autocrine production of IGF-II at the transcriptional level to regulate the initiation of differentiation (27). Although the PI3K and mTOR pathways cross-talk extensively in the regulation of cell growth and proliferation (22,29), our previous observations suggest that the two pathways act independently in myogenesis, with mTOR regulating IGF-II production and PI3K/Akt mediating IGF-II signaling (27). Here we report that activation of FoxO1 induces proteasome-dependent degradation of a specific subset of components in the mTOR signaling network during differentiation and subsequently impairs IGF-II expression. The mTOR-IGF-II axis appears to be a major mediator of FoxO’s negative role in myogenesis.
Materials and Methods
Antibodies and other reagents
All antibodies were obtained from commercial sources as follows: anti-estrogen receptor-α (anti-ERα), -Ras homolog enriched in brain (-Rheb), and -tuberous sclerosis complex 2 (-TSC2) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-sarcomeric myosin heavy chain (MHC) (MF20) and -myogenin (F50) from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development, National Institutes of Health, and maintained by The University of Iowa, Department of Biological Sciences; anti-tubulin antibody from Abcam Inc. (Cambridge, UK); all secondary antibodies from Jackson Immunological Labs; and all other antibodies from Cell Signaling Technology (Beverly, MA). IGF-II, gelatin, and 4-hydroxytamoxifen (4-HT) were from Sigma Chemical Co. (St. Louis, MO). Rapamycin and MG-132 were from Calbiochem (La Jolla, CA). All other cell culture reagents were from Invitrogen (Carlsbad, CA).
Cell culture
Parental and stable C2C12 myoblasts were maintained in DMEM with 10% fetal bovine serum in 7.5% CO2 at 37 C. To initiate differentiation, confluent cells were switched to DMEM containing 2% horse serum. Myotubes were fully formed 72 h after induction of differentiation. Whenever applicable, 4-HT was added to the differentiation medium at 1 μm. The C2C12 cells stably expressing FoxO1–3A-ER were described previously (30).
Immunocytochemistry
Cells were fixed, stained with anti-MHC or anti-ERα as indicated in the figure legends, and examined by microscopy as described previously (25). Microscopy was performed with a Leica DMIL microscope with CPLAN ×10/0.22 NA lenses. The images were captured using a monochrome SPOT RT charge-couple device camera (Diagnostic Instruments, Sterling Heights, MI), and processed as 8-bit images using Adobe Photoshop CS2.
Western analysis
Cells were lysed in 20 mm Tris-Cl (pH 7.5), 0.1 mm Na3VO4, 25 mm NaF, 25 mm glycerophosphate, 2 mm EGTA, 2 mm EDTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, and 0.3% Triton X-100. The lysates were mixed with SDS sample buffer and boiled before loading on SDS-PAGE. Western blot analysis was carried out following procedures recommended by the manufacturers of the antibodies. Results were developed on x-ray films and scanned with an Epson scanner (Perfection 2400) in Photoshop CS2.
Quantitative RT-PCR
RNA isolation, real-time RT-PCR, and data analysis were all described previously (28). β-Actin (ACTB) was used as the reference to obtain the relative fold change for target samples using the comparative cycle threshold method. The PCR primers used in the study have been validated, and the sequences are as follows: ACTB+, 5′-TTGCTGACAGGAT GCAGAAG; ACTB−, 5′-ATCCACATCTGCTGG AAGGT-3′; raptor+, 5′-CATCTTTGTCTACGACT GTT-3′; raptor−, 5′-AGTGGATGGTTTGGGTTAA T-3′; atrogin-1+, 5′-GCAACAAGGAGGTATACA GTAAGG-3′; atrogin-1−, 5′-TCCTTCGTACTTCCT TTGTGAAC-3′; MuRF1+, 5′-TCCTGGACGAGAA GAAGAGC-3′; MuRF1−, 5′-TGCTCCCTGTACTG GAGGAT-3′; Znf216+, 5′-GCCTTGCCTGTAACT CAACA-3′; Znf216−, 5′-TTCTTTGGTTTGGGCAA CTC-3′.
IGF-II ELISA
Conditioned media from cells differentiating in the presence or absence of 4-HT at various time points were collected and directly subjected to ELISA using the mouse IGF-II DuoSet ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer’s protocols.
IGF-II mesenchymal enhancer (ME) reporter assays
FoxO1–3A-ER cells were cotransfected with H19-luc-ME (27) and pCDNA3 (to introduce neomycin resistance) at a 1:1 ratio using FuGene6 (Roche, Indianapolis, IN). After 24 h transfection, the cells were reseeded and selected in 1 mg/ml G418 for 5 d and then plated in 12-well plates for differentiation. The transfected cells were differentiated in the presence or absence of 4-HT or rapamycin, and at various time points, cell lysates were generated and subjected to luciferase assay using the Luciferase Assay Systems kit (Promega, Madison, WI) following the manufacturer’s protocols.
Results
Active FoxO1 inhibits C2C12 cell differentiation
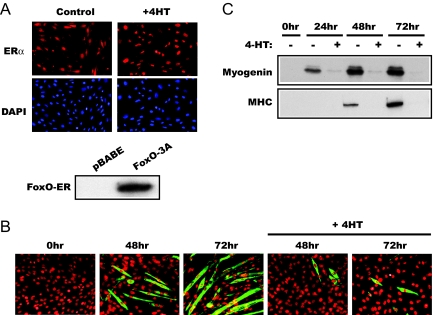
To facilitate investigation into the mechanisms underlying FoxO regulation of myogenesis, we took advantage of a C2C12 cell pool stably expressing a FoxO1 mutant fused to the modified ligand-binding domain of ERα, which selectively interacts with tamoxifen (30). All three Akt phosphorylation sites (Thr24, Ser256, and Ser319) were replaced with alanine in this FoxO1 mutant (designated FoxO1–3A-ER), rendering it resistant to inactivation by PI3K/Akt signaling. The ligand-binding domain of ER retains the fusion protein in the cytoplasm until ligand binding of ER releases the protein and allows its nuclear translocation. As shown in Fig. 1A, the FoxO1–3A-ER protein was distributed throughout the cytoplasm and nucleus, and it became concentrated in the nucleus upon treatment of the cells by 4-HT as previously described (30). Expression of the recombinant protein was also confirmed by Western analysis (Fig. 1A). As we have reported previously, the level of the recombinant protein was similar to that of the endogenous FoxO1 (30).
Figure 1.
Active FoxO1 inhibits C2C12 differentiation. A, C2C12 cells stably expressing FoxO1–3A-ER were immunostained using an anti-ER antibody, accompanied by 4′,6-diamidino-2-phenylindole nuclear staining, before or after 2-h treatment by 1 μm 4-HT. No ER signal was detected in parental C2C12 cells (not shown). The lower panel shows the Western analysis of FoxO1–3A-ER and pBABE cell lysates using the anti-ER antibody. B, FoxO1–3A-ER cells were differentiated in the presence or absence of 4-HT as described in Materials and Methods. At the indicated times, the cells were fixed and immunostained for MHC to reveal myotube formation. C, Lysates of FoxO1–3A-ER cells treated as in B were analyzed by Western blotting for the expression of myogenin and MHC.
The FoxO1–3A-ER cells differentiated normally into myotubes upon serum deprivation for 72 h, and 4-HT treatment drastically blocked the myotube formation (Fig. 1B), consistent with the previously reported negative role of FoxO1 in myogenesis (14). The expression of myogenin, an early marker of myogenesis, as well as that of the contractile protein MHC, was almost completely blocked by 4-HT treatment (Fig. 1C), implying that FoxO1 inhibits muscle differentiation at an early stage, upstream of myogenin induction. As a negative control, the differentiation of C2C12 cells containing the empty vector pBABE was not affected by 4-HT treatment (data not shown).
Activation of FoxO1 results in a dramatic loss of specific proteins in the mTOR pathway
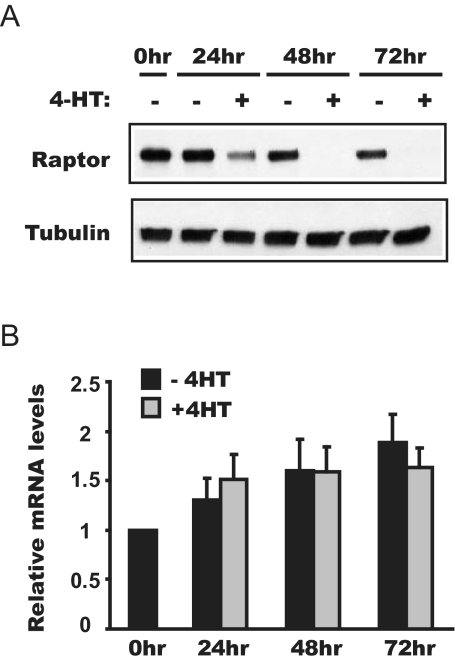
In search for a mechanism underlying the negative regulation of myogenesis by FoxO, we considered the report that FoxO (DAF16) negatively regulates TOR signaling by suppressing the expression of raptor (DAF15), leading to dauer formation in Caenorhabditis elegans (31). Mammalian raptor associates with mTOR (32,33) and serves as a scaffold to recruit substrates for the kinase activity of mTOR (34,35,36). We asked whether a mechanism similar to that in C. elegans existed in mammals. Indeed, the protein level of raptor dropped drastically upon 4-HT treatment; the decrease was apparent by 24 h differentiation in the presence of 4-HT, and by 72 h, the protein was ablated (Fig. 2A). It is suggested that FoxO directly regulates the transcription of raptor in C. elegans (31). However, as assessed by quantitative RT-PCR, the mRNA level of raptor was not significantly affected by 4-HT treatment in the FoxO1–3A-ER cells (Fig. 2B). Thus, the FoxO-induced raptor loss in myocytes likely occurs at the translation or protein stability level.
Figure 2.
Activation of FoxO1 reduces raptor at the protein level. FoxO1–3A-ER cells were differentiated in the presence or absence of 4-HT and lysed at various time points to analyze raptor protein levels by Western blot analysis (A) and mRNA levels by quantitative RT-PCR (B). The average results from three independent experiments are shown, and the error bars represent sd.
Because raptor was known to exist in an mTOR-containing protein complex, we decided to examine the protein levels of mTOR as well as other known components in the rapamycin-sensitive signaling pathway. Strikingly, mTOR was also eliminated at the protein level by d 3 of 4-HT treatment, as were S6K1 and TSC2; on the other hand, 4E-binding protein 1 (4E-BP1), Rheb, Akt, PI3K (p85 subunit), phosphate and tensin homolog, Erk, and tubulin levels were unaffected (Fig. 3A). 4-HT treatment had no effect on raptor or any of the other proteins in cells expressing the empty vector pBABE (Fig. 3B and data not shown), confirming that activation of FoxO1 was fully responsible for the observed 4-HT treatment outcome in the FoxO1–3A-ER cells.
Figure 3.
Activation of FoxO1 leads to selective loss of proteins in the mTOR signaling pathway. A, FoxO1–3A-ER cells were differentiated in the presence or absence of 4-HT and lysed at various time points for Western blot analyses using the antibodies indicated. B, Cells with the empty vector (pBABE) stably integrated were treated as above and subjected to Western blot analysis. C, C2C12 cells were induced to differentiate in the presence or absence of 50 nm wortmannin (Wort) and lysed at various time points for Western blot analyses using the antibodies indicated. Wortmannin was replenished every 24 h as the differentiation medium was changed.
To assess whether endogenous FoxO(s) might be similarly involved in the phenomenon described above, we made use of the specific PI3K inhibitor wortmannin, which presumably activates FoxO. Indeed, when plain C2C12 cells were treated with wortmannin during the induction of differentiation, reduced mTOR and raptor levels were also observed, accompanying dephosphorylation of the endogenous FoxO1 at Thr24, an Akt site (Fig. 3C). Thus, the activation of endogenous FoxO1 may also lead to a loss of mTOR pathway components. The modest, but reproducible, decrease of endogenous FoxO1 protein levels is consistent with activation of Akt during differentiation and reported Akt induction of FoxO1 degradation (37). The effect of wortmannin was not as dramatic as that of expressing the active FoxO1 mutant, which is consistent with the incomplete dephosphorylation of FoxO1 phosphorylation (Fig. 3C) and residual differentiation (data not shown) in the presence of wortmannin, possibly due to the labile nature of this inhibitor. Taken together, our observations suggest that FoxO1 may inhibit myogenesis by targeting the mTOR pathway. It is noted that 24 h after induction of FoxO1, the expression of myogenin was almost completely blocked even though raptor underwent only modest degradation. It is possible that a decrease in raptor concentration below a certain threshold is sufficient to block the differentiation program. Alternatively, this effect of FoxO1 also may be mediated by an additional pathway, such as Notch (see Discussion).
Active FoxO1-induced loss of mTOR pathway components is dependent on the proteasome
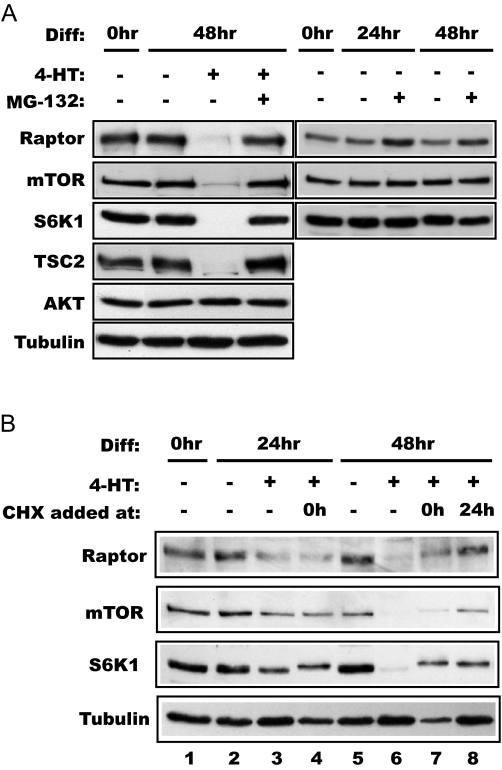
Based on the reports indicating that FoxO proteins may stimulate proteasome-dependent protein turnover in atrophied muscles (20,21), we examined the effect of a proteasome inhibitor, MG132, on FoxO action. Strikingly, the presence of MG132 completely blocked 4-HT-induced protein loss of raptor, mTOR, S6K1, and TSC2, whereas MG132 alone had negligible effect on the levels of raptor, mTOR, and S6K1 (Fig. 4A). Because mTOR appears to be a protein of low turnover rate in a variety of cell types (our unpublished observation), the observation above strongly suggests that active FoxO1 induces proteasome-dependent degradation in the mTOR pathway, although FoxO inhibition of translation cannot be definitively ruled out.
Figure 4.
FoxO1-induced protein degradation is proteasome dependent and requires new protein synthesis. A, FoxO1–3A-ER cells were differentiated (Diff) for 2 d in the presence or absence of 1 μm 4-HT with or without 1 μm MG132 as indicated, followed by Western blot analysis of the lysates. B, The cells were differentiated up to 48 h in the presence of 1 μm 4-HT. Some cells were exposed to 5 μm cycloheximide (CHX) at either 0 or 24 h of differentiation as indicated. The cell lysates were analyzed by Western blotting. Note that the S6K1 band displayed decreased mobility in CHX-treated samples, suggesting phosphorylation of this protein induced by CHX, consistent with reported observations (48).
FoxO1 likely regulates degradation of the mTOR pathway through transcriptional activation
FoxOs function as transcriptional factors by regulating specific gene expression either directly or through modulating other transcriptional factors (16,38). However, a recent report suggests that FoxO1 may also function outside of the nucleus, independent of its transcriptional activity, in the modulation of a TSC-mTOR pathway (39). Although the recombinant FoxO1 protein used in our studies was activated by nuclear translocation, implying a nuclear function for FoxO1, it was necessary to ascertain that the observed phenomenon of selective protein degradation was mediated by FoxO’s effect on gene expression. To this end, we examined the effect of cycloheximide, a protein synthesis inhibitor, on the ability of active FoxO1 to elicit specific protein degradation. Although typically 48 h of 4-HT treatment led to an almost complete loss of raptor, mTOR, and S6K1, when cycloheximide was present during the last 24 h, the proteins were significantly preserved (Fig. 4B, compare lanes 6 and 8). Prolonged cycloheximide treatment (48 h) was highly toxic to the cells and led to a general reduction of cellular proteins, but nevertheless, it partially blocked the degradation of those specific proteins (Fig. 4B, compare lanes 6 and 7). Therefore, new protein synthesis is required for the FoxO1-induced and proteasome-dependent protein degradation.
To our knowledge, the expression of three proteins presumably functioning in the ubiquitin/proteasome pathway has been reported to be induced by FoxO: the ubiquitin E3 ligases atrogin-1 and MuRF1 (20,21) and a polyubiquitin-binding protein, Znf216 (40). However, none of them had previously been demonstrated to be a target of FoxO during myoblast differentiation. We found that levels of atrogin-1 and Znf216 mRNA transcripts (but not MuRF1) were induced by FoxO1 activation during the course of differentiation (data not shown). Whether these proteins contribute to FoxO regulation of the specific protein degradation will require additional investigation.
IGF-II is a critical mediator of FoxO’s inhibitory function in myogenic differentiation
To further ascertain FoxO1’s role in the modulation of myogenic mTOR signaling, we considered our previous finding that mTOR regulates myogenic differentiation by controlling the expression of IGF-II (27). Because active FoxO1 induced the degradation of mTOR, we asked whether IGF-II expression would be suppressed as a consequence. As shown in Fig. 5A, over the course of normal differentiation, the FoxO1–3A-ER cells secreted increasing amounts of IGF-II into the medium as measured by ELISA, and this secretion was significantly blocked by 4-HT treatment, suggesting that active FoxO1 indeed suppresses IGF-II expression.
Figure 5.
Activation of FoxO1 suppresses IGF-II expression. A, IGF-II in conditioned media of differentiating FoxO1–3A-ER cells was analyzed by ELISA, and the relative amounts of IGF-II are shown. The average results from three independent experiments are shown, and the error bars represent sd. B, IGF-II ME luciferase reporter was transfected into FoxO1–3A-ER cells, and the luciferase activity was measured at various time points of differentiation in the presence or absence of 4-HT. Data shown are from a representative experiment with triplicate cell treatment for each condition; four independent experiments were performed with similar results.
We have previously demonstrated that a ME located downstream of the IGF-II gene (41) is largely responsible for the induction of IGF-II transcription during C2C12 differentiation and its activation is regulated by amino acid-sensing mTOR signaling (27). To assess whether FoxO activation affects this ME activity (via down-regulation of the mTOR pathway), we transfected an IGF-II ME luciferase reporter into the FoxO1–3A-ER cells. The ME activity increased during normal differentiation, and this increase was suppressed by rapamycin treatment as previously reported (27). Importantly, the increase in reporter activity was abolished by FoxO activation upon 4-HT treatment (Fig. 5B). Although at present we cannot definitively rule out the possibility that FoxO may directly regulate this enhancer, this scenario seems unlikely considering our previous observation that wortmannin (which presumably activates FoxO; see Fig. 3C) has no effect on the activity of this enhancer (27). Thus, FoxO most likely exerts its inhibitory effect on the IGF-II enhancer through regulation of mTOR stability.
These observations clearly indicate that the mTOR-IGF-II axis is one of the targets of FoxO. To address whether IGF-II is a major target of FoxO in the regulation of myogenesis, we asked whether the addition of exogenous IGF-II could rescue myogenesis from FoxO inhibition. As shown in Fig. 6A, IGF-II at 150 ng/ml, a concentration comparable to those reportedly secreted by C2C12 cells (8), reversed the inhibitory effect of FoxO induction and fully supported formation of myotubes in FoxO1–3A-ER cells in the presence of 4-HT. Thus, FoxO’s antimyogenic function appears to be mediated by the attenuation of IGF-II expression via removal of the critical regulator of IGF-II transcription, mTOR.
Figure 6.
Exogenous IGF-II rescues myogenic differentiation from inhibition by active FoxO1. A, FoxO1–3A-ER cells were differentiated for 3 d in the presence or absence of 4-HT, with or without 150 ng/ml recombinant IGF-II. Myotube formation was assessed by MHC staining. B, A proposed model: active FoxO suppresses mTOR signaling and subsequent IGF-II production in a feedback loop that modulates myogenesis.
Discussion
Our study has uncovered a molecular mechanism by which FoxO1 negatively regulates myoblast differentiation. By suppressing the protein levels of mTOR through a proteasome-dependent mechanism, FoxO1 impedes the nutrient-dependent production of IGF-II in myocytes and consequently impairs the progression of myogenic differentiation that relies on the autocrine actions of IGF-II (Fig. 6B). This unexpected relationship between mTOR and FoxO presents a feedback loop that may serve to fine-tune the regulation of myogenesis. The importance of FoxO regulation in myogenesis is underlined by the fact that dysregulation of FoxO activity due to chromosomal translocation may be associated with the majority cases of alveolar rhabdomyosarcoma (42), a common soft-tissue sarcoma in children and young adults, of myogenic precursor cell origin.
Most recently, FoxO1 has been reported to cooperate with the Notch pathway in the negative regulation of myoblast differentiation. FoxO1 physically interacts with the target of Notch, Csl, at the Hes1 promoter and is required for Notch-induced gene expression (38). Notch signaling has been well established as an inhibitor of myoblast differentiation, possibly through multiple gene targets (43). Our study here suggests that FoxO1’s inhibitory function in myogenesis manifests at yet another level, where it impinges on the nutrient-sensing mTOR pathway that regulates the production of the autocrine factor IGF-II. Interestingly, mTOR has recently been shown to mediate Notch signaling in the survival of neuronal stem cells (44). The relationship between Notch and mTOR signaling has not been examined in other systems. An intricate regulatory network resulting from extensive cross-talks among these signaling pathways is an eminent possibility to be probed in the future.
In contrast to FoxO’s role in muscle atrophy (20,21), where it induces massive loss of cellular protein contents such as the myofibrillar proteins, our findings reveal that FoxO-induced protein degradation during differentiation regulates signaling events. Although a search at the proteomic level will be necessary to definitively evaluate the scale of FoxO-induced protein degradation in myocytes, the results of our limited survey strongly suggest specific targeting of selected components in the mTOR pathway by FoxO. A most recent report also shows decreased mTOR and raptor levels in active FoxO1-expressing myotubes and myofibers after differentiation (45). However, a key observation in that study was an increase of the 4E-BP1 protein and decreased 4E-BP1 phosphorylation (and thus inhibition of protein synthesis) upon FoxO activation (45), whereas we observed that active FoxO1 slightly enhanced 4E-BP1 phosphorylation during differentiation (Fig. 3A), even though 4E-BP1 is not a relevant player in the initial myogenic signaling by mTOR (25,27). Furthermore, we did not observe degradation of any of the proteins shown in Fig. 2 in proliferating myoblasts even in the absence of growth factors (data not shown). Hence, the FoxO1-induced degradation of mTOR pathway components we have observed represents a regulatory mechanism specific to the differentiation program.
FoxO is thought to promote muscle atrophy through its induction of muscle-specific ubiquitin E3 ligases, namely atrogin-1 and MuRF1 (20,21), which presumably target myofibrillar proteins to degradation, although there has been no experimental evidence for the latter part of this model; direct substrates for these E3 ligases have not been identified. We have found that atrogin-1, but not MuRF1, is dramatically up-regulated by the expression of active FoxO1 (data not shown), making it an attractive candidate as the potential regulator of proteasome-dependent degradation of mTOR components. Another protein that is reportedly up-regulated in muscle atrophy and induced by active FoxO is Znf216, a polyubiquitin chain-binding protein that presumably functions in the ubiquitin-dependent proteasome pathway (40). Indeed, expression of this gene is also up-regulated by activation of FoxO1 during C2C12 differentiation (data not shown). The potential role of Znf216 in FoxO-induced specific protein degradation should certainly be probed in the future.
Identification of the specific E3 ligase(s) and/or other regulators in the ubiquitin-proteasome pathway will also help delineate the mechanism underlying the simultaneous degradation of multiple components of the mTOR pathway. It is conceivable that a single E3 ligase may recognize a group of protein targets sharing a common structural element. However, it is also tempting to speculate that these targets may be found in a protein complex, which maintains the stability of the individual components or allows a concerted regulation of the components by the ubiquitination/proteasome machinery. Indeed, several protein-protein interactions are known in the mTOR pathway: mTOR-raptor (32,33), raptor-S6K1 (35), and TOR-TSC2 in Drosophila (46). At least mTOR and raptor have been reported to stabilize each other in the complex (32). However, it is interesting to note that Rheb and 4E-BP1 remained stable upon FoxO1 activation, whereas mTOR, raptor, TSC2, and S6K1 were degraded (Fig. 3A), even though Rheb is known to interact with mTOR (47) and 4E-BP1 with raptor (34,35,36). Obviously, the concerted protein degradation would be influenced by the biochemical nature and dynamics of the mTOR signaling complex, which remains to be fully characterized. It should also be pointed out that the functional consequence of the FoxO1-induced degradation of raptor and TSC2 is unknown. Because mTOR regulates the production of IGF-II and the initiation of myogenesis in a kinase-independent manner (25,27), raptor is unlikely to assume its role as a scaffold for mTOR kinase substrates. The requirement of TSC2 in myogenesis is also unexplored, and no upstream regulator of mTOR myogenic signaling has been identified. Investigation of the components in the cell growth-regulating mTOR pathway in the context of myogenesis will shed light on the unusual assembly of the myogenic mTOR signaling pathway.
Acknowledgments
We thank Drs. Huarong Guo and In Hyun Park for technical assistance.
Footnotes
First Published Online December 13, 2007
Abbreviations: 4E-BP1, 4E-binding protein 1; ERα, estrogen receptor-α; FoxO1, forkhead box protein O1; 4-HT, 4-hydroxytamoxifen; ME, mesenchymal enhancer; MHC, myosin heavy chain; mTOR, mammalian target of rapamycin; MuRF1, muscle specific RING finger 1; PI3K, phosphatidylinositol 3-kinase; Rheb, Ras homolog enriched in brain; S6K1, S6 protein kinase 1; TSC2, tuberous sclerosis complex 2.
This work was supported by National Institutes of Health Grants AR48914 (to J.C.) and DK41430 (to T.G.U.), an American Diabetes Association grant (to J.C.), and a Department of Veterans Affairs Merit Review Program award (to T.G.U.).
Disclosure Statement: The authors have nothing to disclose.
References
- Weintraub H 1993 The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75:1241–1244 [DOI] [PubMed] [Google Scholar]
- Naya FS, Olson E 1999 MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11:683–688 [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA 1991 Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol 53:201–216 [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL 1998 Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95:15603–15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N 2001 Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200 [DOI] [PubMed] [Google Scholar]
- Tollefsen SE, Lajara R, McCusker RH, Clemmons DR, Rotwein P 1989 Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem 264:13810–13817 [PubMed] [Google Scholar]
- Tollefsen SE, Sadow JL, Rotwein P 1989 Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci USA 86:1543–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS 1991 “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem 266:15917–15923 [PubMed] [Google Scholar]
- Musaro A, Rosenthal N 1999 Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol Cell Biol 19:3115–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A 1996 Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem 271:19146–19151 [DOI] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Shepherd PR, Beeton CA, Testar X, Palacin M, Zorzano A 1998 Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol 12:66–77 [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Vogt PK 1998 An essential role of phosphatidylinositol 3-kinase in myogenic differentiation. Proc Natl Acad Sci USA 95:14179–14183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Aoki M, Zheng JZ, Li J, Vogt PK 1999 Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA 96:2077–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribal ML, Nakae J, Kitamura T, Shutter JR, Accili D 2003 Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol 162:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D, Arden KC 2004 FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426 [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG 2005 FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab 16:183–189 [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith EC, Greenberg ME 2003 The many forks in FOXO’s road. Sci STKE 2003:RE5 [DOI] [PubMed] [Google Scholar]
- Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG 2004 Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 378:839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois PR, Grosveld GC 2003 FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. EMBO J 22:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL 2004 Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ 2004 The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403 [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N 2004 Upstream and downstream of mTOR. Genes Dev 18:1926–1945 [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P 1999 Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem 274:4341–4346 [DOI] [PubMed] [Google Scholar]
- Conejo R, Valverde AM, Benito M, Lorenzo M 2001 Insulin produces myogenesis in C2C12 myoblasts by induction of NF-κB and downregulation of AP-1 activities. J Cell Physiol 186:82–94 [DOI] [PubMed] [Google Scholar]
- Erbay E, Chen J 2001 The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem 276:36079–36082 [DOI] [PubMed] [Google Scholar]
- Shu L, Zhang X, Houghton PJ 2002 Myogenic differentiation is dependent on both the kinase function and the N-terminal sequence of mammalian target of rapamycin. J Biol Chem 277:16726–16732 [DOI] [PubMed] [Google Scholar]
- Erbay E, Park IH, Nuzzi PD, Schoenherr CJ, Chen J 2003 IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J Cell Biol 163:931–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Chen J 2005 Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem 280:32009–32017 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM 2005 Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- Bastie CC, Nahle Z, McLoughlin T, Esser K, Zhang W, Unterman T, Abumrad NA 2005 FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem 280:14222–14229 [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL 2004 The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131:3897–3906 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM 2002 mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175 [DOI] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K 2002 Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110:177–189 [DOI] [PubMed] [Google Scholar]
- Schalm SS, Fingar DC, Sabatini DM, Blenis J 2003 TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 13:797–806 [DOI] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K 2003 The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278:15461–15464 [DOI] [PubMed] [Google Scholar]
- Choi KM, McMahon LP, Lawrence Jr JC 2003 Two motifs in the translational repressor PHAS-I required for efficient phosphorylation by mammalian target of rapamycin and for recognition by raptor. J Biol Chem 278:19667–19673 [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A 2003 Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100:11285–11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, Depinho RA, Kitajewski J, Accili D 2007 A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 23:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Kamioka Y, Yokoi N, Kobayashi T, Hino O, Onodera M, Mochizuki N, Nakae J 2006 Interaction of FoxO1 and TSC2 induces insulin resistance through activation of mTOR/p70 S6K pathway. J Biol Chem 281:40242–40251 [DOI] [PubMed] [Google Scholar]
- Hishiya A, Iemura SI, Natsume T, Takayama S, Ikeda K, Watanabe K 2006 A novel ubiquitin-binding protein ZNF216 functioning in muscle atrophy. EMBO J 25:554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffer CR, Grinberg A, Pfeifer K 2001 Regulatory mechanisms at the mouse Igf2/H19 locus. Mol Cell Biol 21:8189–8196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia SJ, Pressey JG, Barr FG 2002 Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther 1:97–104 [DOI] [PubMed] [Google Scholar]
- Luo D, Renault VM, Rando TA 2005 The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol 16:612–622 [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD 2006 Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826 [DOI] [PubMed] [Google Scholar]
- Southgate RJ, Neill B, Prelovsek O, El-Osta A, Kamei Y, Miura S, Ezaki O, McLoughlin TJ, Zhang W, Unterman TG, Febbraio MA 2007 FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282:21176–21186 [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D 2002 Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol 4:699–704 [DOI] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J 2005 Rheb binds and regulates the mTOR kinase. Curr Biol 15:702–713 [DOI] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J 1992 Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69:1227–1236 [DOI] [PubMed] [Google Scholar]