Abstract
Adipose tissue is unique in that it can undergo significant hypertrophy and atrophy, resulting in wide ranges of obesities and lipodystrophies. At the base of this elasticity is the lipid-filled adipocyte, which can either overfill by storing large amounts of triglycerides or shrink to a tiny cell by depleting its lipids and as such is remarkable in sustaining insults. As a major energy reservoir, the adipocyte may hold considerable calories necessary for survival and reproduction, two functions that are essential for the survival of the species. This review will summarize some of the recent studies that have advanced our understanding of the central and peripheral mechanisms that are initiated by adipocyte-secreted factors such as leptin, adiponectin, resistin, and retinol-binding protein 4. The intersection of obesity and lipodystrophy results in insulin resistance, which may be unlocked by elucidating the roles of these factors in pathways that control insulin sensitivity and glucose uptake.
NEITHER OBESITY NOR lipodystrophy is a simple disorder, whether defined by clinical or biological criteria. In fact, they are distinct disorders with, however, a common characteristic in that they both involve adipose tissue insults. These alterations in the adipose tissue mass are in turn influenced by common factors such as genetics, environment, and behavior. A great deal of our present knowledge of obesity and lipodystrophy originates from mouse models and the characterization of mutations in the monogenic form of either disease. Thus, this review will concentrate on the relevant pathways that have emerged from animal models and human studies and are most relevant to human biology.
Obesity: The First Circle
The increasing rate of the obesity epidemic argues against rapid genetic drift, thus reinforcing the notion that although genetics are pivotal in rare forms of obesity, the interaction of genes and the environment remains the most plausible explanation for the alarming steep rise in overweight disorders. The uncovering of multiple genes and their encoded proteins have come to light in recent years and have contributed to the understanding and regulation of central and peripheral pathways that govern energy metabolism. Some of these pathways are reviewed here.
Central pathways
Although positional cloning successes and the Human Genome Project were at the helm of late 20th century research, the tide is currently turning toward functional genomics, aimed at delineating pathways encoded by new genes. In the years that followed cloning of the obese gene from the morbidly obese ob/ob mouse and the discovery of its secreted hormone, leptin (1), the presence and consolidation of a neuronal circuit that regulates food intake came into light (Fig. 1). The role of this pathway in human obesity was justified by undertaking a wide search for mutations in genes governing this pathway among obese individuals. Although only a couple of leptin mutations were uncovered (2,3), they vindicated the ob/ob animal model and suggested that leptin could trigger and orchestrate a series of events leading to body weight regulation. In fact, the central action of leptin is initiated by the binding to its signaling-competent long-form receptor in the hypothalamus to stimulate and inhibit anorexigenic and orexigenic pathways, respectively, leading overall to the suppression of food intake. Mutations of the leptin receptor and inactivation of the leptin pathway in its early steps led to obesity as shown by the obese animal models, the db/db mouse and fa/fa rat, and in a few obese individuals (4), demonstrating the critical roles of leptin and its receptor in the regulation of body weight.
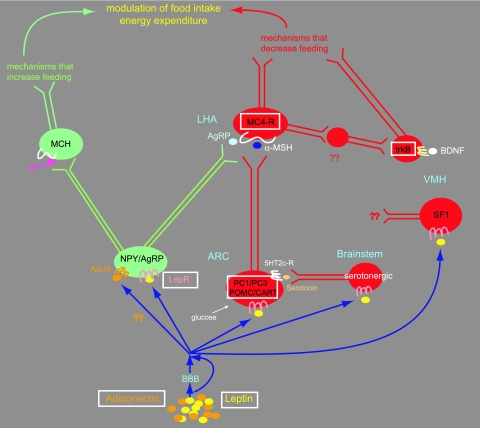
Figure 1.
Diagram showing activation of the neuronal circuit initiated by the binding of leptin and adiponectin to their respective receptors on hypothalamic neurons. Neurons in green and red represent those that act as stimulatory or inhibitory nodes for food intake, respectively. Leptin crosses or bypasses the blood-brain barrier (BBB) to bind neuropeptide Y (NPY)/AgRP-expressing neurons in the arcuate nucleus (ARC) to stimulate pathways that inhibit food intake. Leptin performs this function by suppressing, via STAT3-induced abrogation of FoxO1, the expression of the orexigenic peptide AgRP that would otherwise antagonize α-MSH at the MC4R-receptor. Conversely, the binding of adiponectin to its receptor on leptin-responsive neurons to stimulate food intake (30) is presumed to result from activation of the orexigenic NPY/AgRP neuronal node. Leptin also binds to POMC neurons in the ARC, to serotonergic neurons in the brainstem, and to SF1-expressing neurons in the ventromedial hypothalamus (VMH), which also contains neurons that bind BDNF on trkB receptors. POMC, MC4, serotonergic, SF1, and BDNF neurons activate anorexigenic pathways that inhibit food intake. In the orexigenic arm of this neurocircuit, which is inhibited by leptin but stimulated by adiponectin, the melanin-concentrating hormone (MCH) neurons are located in the lateral hypothalamic area (LHA) and are activated by NPY. The boxes around some factors represent those that are mutated or have been found to be associated with obesity in humans.
Leptin-binding neurons.
In the anorexigenic arm of the central leptin pathway, the binding of leptin to its receptor on proopiomelanocortin (POMC) neurons led to the finding that obesity is associated with rare human mutations in the prohormone convertase PC1/PC3 gene (5,6), which encodes a serine endoprotease that cleaves prohormones such as insulin, glucagon, and POMC into their mature forms. Obesity-causing mutations are also present in the POMC (7,8,9) and cocaine- and amphetamine-regulated transcript (CART) genes (10) substantiating the physiological relevance of the POMC pathway to human obesity. Furthermore, deletion of the leptin receptor from POMC neurons in a mouse model yielded only a mild obesity, alluding to the presence of other neurons that are critical for body weight regulation (11). The importance of POMC neurons in obesity and insulin resistance was even more strengthened by recent data showing that POMC neurons are critical for glucose sensing. In these studies, disruption of glucose sensing on mouse POMC neurons impaired whole-body glucose disposal, demonstrating yet another role for POMC neurons in the control of blood glucose (12) and their involvement in insulin resistance.
Proximal and distal neurons.
Downstream of POMC neurons are the melanocortin 4 receptor (MC4R) neurons, which play an important role in body weight regulation. In humans, various mutations in the MC4R gene have been delineated and account for approximately 6% of severely obese individuals (13,14,15), demonstrating that they represent the most common form of monogenic obesity. The cross-talk between the MC4R and other neurons was further delineated by the finding that a mutation in trkB, a receptor for the brain-derived growth factor (BDNF) was detected in an obese individual (16), adding credence to the previous finding in mice that trkB regulates energy balance downstream of the MC4R (17). Furthermore, conditional deletion of BDNF in mice leads to obesity (18). Thus, trkB neurons, which are located in the ventromedial hypothalamus, an area that has long been known to be associated with body weight regulation, represent a new set of neurons that regulate body weight. Also in the hypothalamus are neurons expressing the steroidogenic factor 1 (SF1), a transcription factor that plays a prominent role on the reproductive axis (19). SF1 knockout mice unexpectedly revealed an obesity, thus interjecting SF1 as a putative factor linking the reproductive axis to obesity (20). Combining the leptin axis with SF1, Dhillon et al. (21) demonstrated that mice lacking the leptin receptor on SF1 neurons have increased body weight and fat stores but normal fertility. Thus, SF1 neurons represent a new class of first-order leptin-responsive neurons, and because SF1 plays a role in reproduction as does leptin (22,23), it was disappointing that SF1 did not turn out to be the long-sought leptin link to the reproductive system, thus leaving this question open-ended.
On another level, pharmacological studies have stressed the importance of the serotonergic system in food intake and energy balance. Even though the serotonin 2c receptor (5HT2c-R) is expressed on POMC neurons (24), no mutations were found in the 5HT2c-R gene among a cohort of human obese patients, making it an unlikely candidate for an obesity gene (25). Consistently, deletion of the 5HT2c-R from lean leptin-overexpressing mice failed to reverse their skinny phenotype, showing that at least in experimental mouse models, the 5HT2c-R is not directly impacted by leptin (26).
New pathways.
The mechanisms that allow leptin to exert its anorexigenic role through the hypothalamus are continuously emerging. Leptin has been known for quite some time to activate signal transducer and activator of transcription 3 (STAT3) signaling in leptin-responsive neurons in the hypothalamus (27). Recently, the forkhead transcription factor 1 (FoxO1), which is known for its peripheral effects, was shown to have a central role in the hypothalamus and to antagonize the effects of leptin on food intake and body weight by preventing leptin via STAT3 from suppressing the expression of the orexigenic peptide agouti-related peptide (AgRP). Thus, the transcription factors FoxO1 and STAT3 compete for activation of the AgRP promoter (28). Consistent with its anorexigenic role, leptin inhibits hypothalamic AMP-activated protein kinase (AMPK), which is stimulated by AgRP (29).
An exciting and recent finding was the demonstration that adiponectin, an adipocyte-secreted factor that acts peripherally, can also bind centrally on leptin-responsive neurons in the hypothalamus to stimulate food intake by activating AMPK (30). Thus, adiponectin antagonizes the effects of leptin on AMPK and food intake. This finding is important not only because it establishes adiponectin as a central regulator of food intake but also because it implies that leptin and adiponectin together modulate the firing of the same set of neurons and must be intimately involved in their differential regulation. New challenges will be aimed at pinpointing the nature and the balance of this modulation in different feeding states.
A recent study in knockout mice established a critical role for AMPK in the hypothalamus by demonstrating that deletion of AMPK from either AgRP or POMC neurons resulted in increased or decreased body weight, respectively, thus establishing AMPK as a leptin and insulin-independent central regulator of energy balance, (31). Thus, AMPK responds to different stimuli and integrates multiple pathways that regulate food intake, energy balance, and adiposity.
Deceptively, the orexigenic arm of the leptin pathway has failed to demonstrate that it is associated with a human mutation causing obesity. In a systematic search, the melanin-concentrating hormone (MCH) receptor was not mutated in a cohort of obese individuals (32). Teleologically, it is conceivable that mutations, which result in reduction of food intake, are detrimental for survival and have been eliminated by natural selection, thus favoring those mutations that convey a selective advantage for the accumulation of energy reserves and ensuring survival of the organism.
Peripheral pathways
New findings regulating energy metabolism in peripheral organs have emerged in the past few years and significantly contributed to our understanding of regulatory mechanisms.
AMPK.
The contribution of AMPK as a regulator of energy balance and a gauge of anabolic and catabolic pathways using ATP, is primordial and well recognized (33,34). AMPK is activated by an increase in AMP/ATP ratio within the cell and acts as an efficient metabolic sensor. Binding of AMP to the γ-subunit activates AMPK allosterically and stimulates the phosphorylation of its α-subunit at threonine residue 172 by the LKB1 kinase (35,36). In addition, phosphorylation of AMPK is sustained by an inhibitory effect of AMP on protein phosphatases (37). Furthermore, and independently of the AMP/ATP ratio, calmodulin-dependent protein kinase kinase (CaMKK) also phosphorylates AMPK in response to an increase in intracellular calcium ions (38,39). Thus, AMPK is intimately implicated in various cellular functions and is activated by exercise, hypoxia, and oxidative stress (40). Most relevant to this review is the fact that AMPK is also activated by peripheral leptin action (41) to stimulate fatty acids oxidation and glucose transport in muscle (42) via glucose transporter 4 (GLUT4) translocation (43) and the myocytes-enhancing factor 2 (MEF2), which transcriptionally activates GLUT4 expression (44). In non-GLUT4-expressing cell types, AMPK stimulates glucose uptake by activating membrane-bound GLUT1 (45) via an undefined mechanism. Overall, due to its wide role, especially in energy metabolism, AMPK activation has been the target of the insulin-sensitizing type II diabetes drugs metformin (46) and the thiazolidinediones (47).
Adipocyte-secreted factors.
The role of factors secreted from adipocytes continues to unveil networks of glucose uptake and insulin signaling regulation (Fig. 2). Although the peripheral role of leptin has so far been unveiled by its activation of AMPK (41), the mechanisms of other factors remain at large. Adiponectin, a highly abundant plasma protein secreted from adipose tissue circulates as low, medium, and high molecular weight forms (48). Its high molecular weight complex appears to be the active form, and its levels are reduced in patients with type II diabetes (49), although earlier reports have shown that the total serum levels were reduced in diabetic individuals (50). Similar to the effect of leptin on skeletal muscle (41), adiponectin stimulates glucose uptake and fatty acid oxidation by activating AMPK (51). Recently, adiponectin overexpression in the leptin-deficient ob/ob mouse was shown to rescue its diabetic phenotype while paradoxically exacerbating its obesity (52), thus offering a mechanism to explain the heterogeneity of diabetes among obese individuals and for its potential use in the treatment of hyperglycemia. Although the mechanisms by which adiponectin acts as an insulin-sensitizing agent are largely unknown, it activates the LKB1-AMPK pathway, which alleviates negative regulation of insulin signaling via p70 S6 kinase (53).
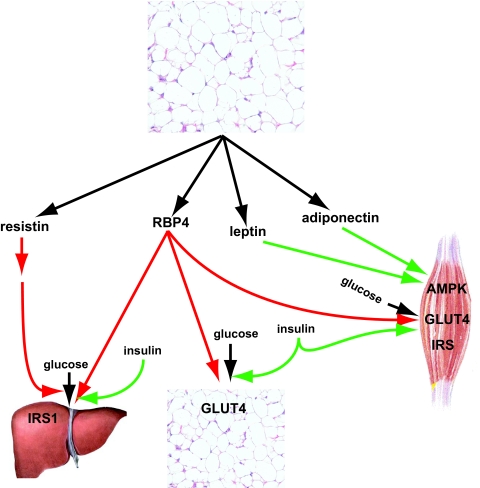
Figure 2.
Peripheral actions of adipocyte-secreted factors leptin, adiponectin, resistin, and RBP4 on the uptake of glucose in muscle, adipose, and liver cells. Green and red arrows denote stimulatory or inhibitory actions. Both leptin and adiponectin activate AMPK in muscle, resulting in increased insulin sensitivity and glucose uptake. RBP4 antagonizes glucose uptake in muscle and liver and performs the same action in adipose tissue via decreased expression of GLUT4 in adipocytes. Resistin also antagonizes the uptake of glucose in liver, either directly and/or via the hypothalamus. The net effect of these targeted actions of leptin, adiponectin, RBP4, and resistin is the modulation of glucose uptake in insulin-sensitive peripheral tissues.
The role of resistin, a 108-amino-acid plasma protein, first identified as being secreted from adipose tissue in rodents (54), was later found to be expressed in humans from macrophages, mononuclear cells, and adipose tissue (55,56,57). The association of resistin with obesity and diabetes in mice is tightly involved with the impairment of glucose homeostasis (58,59). However, such an association in humans is blurred, disparate, and controversial. In some studies, resistin expression levels were elevated in human abdominal adipose tissue (56) and its plasma levels correlated with obesity (60), insulin resistance (61), and atherosclerosis (62). Yet in other studies, circulating resistin levels were associated with neither obesity nor insulin resistance (63,64,65). The discrepancy in the role of resistin between rodent and human studies underscores the differential regulation of energy metabolism between these species, especially in light of the fact that rodents also regulate their energy metabolism via brown fat, a pathway that is largely confined to the neonatal period in humans. Recently, resistin emerged as another hypothalamic factor (66) that when infused into the brain of rats led to hepatic insulin resistance (67). Overall, the contrasting findings linking resistin levels to energy balance in humans suggest that perhaps the heterogeneity found in various studies arises from different genetic backgrounds and environmental factors, both of which could significantly influence resistin expression levels. Hopefully, the latest findings linking central resistin to hepatic glucose production (67) might further bring the picture of resistin into focus.
Retinol (vitamin A)-binding protein 4 (RBP4), a plasma glycoprotein secreted from adipose tissue and liver, was unexpectedly found to be associated with insulin resistance (68). Remarkably, elevated RBP4 levels correlated with body mass index, insulin resistance, and impaired glucose homeostasis and were inversely correlated with the levels of GLUT4 in adipocytes, suggesting that its plasma levels could serve as a marker before the onset of diabetes (69). Although variability in RBP4 associations was noted in different populations and in obese individuals, they reinforce the effects of dietary factors, lifestyle, genetics, and even methodology on circulatory RBP4 levels (70,71,72,73,74). Thus, the correlation of RBP4 with the suppression of glucose uptake in muscle and the stimulation of glucose release from liver, classifies it as an insulin antagonizer. It remains to be determined whether agents that decrease circulatory RBP4 levels could serve as insulin sensitizers and ameliorate hyperglycemia in prediabetic or diabetic states.
Overall it can be concluded that the adipocyte-secreted proteins leptin, adiponectin, resistin, and RBP4 chiefly regulate glucose uptake and insulin signaling via the AMPK pathway. As such, these factors serve a central system that tightly regulates energy intake and responds to fluctuations of energy metabolism.
Impact of adipocyte-secreted factors on insulin signaling.
It is well established that the binding of insulin to its receptor kinase induces the phosphorylation of insulin receptor substrates (IRS), which activate the phosphatidylinositol 3-kinase (PI3K) signaling cascade. Knockout mice for IRS1 or IRS2 revealed that either model exhibited insulin resistance; however, only IRS2 knockout mice developed overt diabetes (75,76,77). Furthermore, double-knockout mice for IRS1 and IRS3 resulted in a lipoatrophy caused by defective adipogenesis and an insulin resistance that could be rescued with leptin overexpression (78). Thus, the complementary roles of IRS1 and IRS3 on glucose homeostasis are in part mediated by leptin. Because a functional IRS3 allele is not found in humans, it is possible that a closer association between the functions of IRS1 and leptin may be even more pronounced in humans than in rodents (78).
A coculture model of myocytes and adipocytes suggested that factors secreted by adipocytes block the insulin-stimulated tyrosine phosphorylation of IRS1 in myocytes (79). Whereas the insulin-sensitizing effects of leptin have been demonstrated in lipoatrophic mouse models (see section below), the role of leptin in the disruption of insulin signaling is less clear (80). Nonetheless, leptin was shown to inhibit insulin signaling by its ability to phosphorylate IRS1 at serine 318 in a protein kinase C manner, which resulted in reduced association of IRS1 with the insulin receptor (81,82) and, thus, decreased insulin signaling. Furthermore, the attenuation of insulin signaling by resistin and RBP4 were recently demonstrated. First, resistin treatment of rat skeletal muscle cells decreased IRS1 levels, suggesting that resistin could mediate a yet unknown mechanism that leads to IRS1 degradation (83). Second, RBP4 blocked the insulin-stimulated phosphorylation of IRS1 at serine 307 and concurrently increased by 4-fold the concentration of insulin that is required for stimulation of IRS1 tyrosine phosphorylation (84). On the other hand, the insulin-sensitizing effects of adiponectin were recently shown to induce IRS1 tyrosine phosphorylation in the presence of active AMPK (53).
Hence, the emerging pathways by which adipocyte-secreted factors have the potential to increase or decrease insulin signaling at the IRS level demonstrate their critical roles in the treatment of diabetes and obesity.
Unknown pathways.
Although the majority of obesity-causing lesions remain at large, it is clear by now that single monogenic disorders are not at the root of human obesity. Complex phenotypes, arising from polygenic disorders that include obesity in their presentation, may provide further clues into additional pathways that have significant impact on energy balance. For example, the association of a missense mutation in the neutrophin receptor trkB in a hyperphagic obese individual resulting in impaired MAPK signaling (16), has opened yet another pathway that regulates energy intake. It is likely that multiple redundant pathways control food intake because these pathways are responsible for one of the most important survival functions of the organism. The complexities and heterogeneities of such pathways are exemplified by the 12 genes that underlie the Bardet-Biedl syndrome, which presents with obesity among most affected individuals. Most of the gene products encoded by the Bardet-Biedl syndrome genes are located in the basal body and cilia of the cell causing cilium dysfunction (85). How these proteins and this pathway affect energy metabolism is enigmatic and remains to be defined.
In another yet different level of complexity are reproductive and imprinted disorders, best exemplified in human by the polycystic ovary and Prader-Willi syndromes, respectively. In polycystic ovary syndrome, reproductive abnormalities in women are frequently accompanied by insulin resistance, which may or may not be manifested with obesity. Thus, another axis that remains to be elucidated is the triumvirate of reproduction-insulin resistance-obesity. Most interestingly, are Prader-Willi syndrome individuals who exhibit a hyperphagic obesity and frequently carry a microdeletion in an actively expressed region of their paternal chromosome 15, thus alluding to the presence of a dominant gene in this region of the genome. The possible involvement of imprinting mechanisms on energy metabolism is still unexplored, and it is conceivable that imprinted human and mouse genes encompassed by the multiple imprinted regions of their respective genomes may play a key role in obesity and lipodystrophy. Although the identity of the genes encompassed by the Prader-Willi microdeletion remain to be defined, it is very likely that their characterization will uncover new pathways regulating food intake. Thus, a continuous search for the underlying molecular lesions in obese individuals with complex and unusual phenotypes will chart the map for additional pathways that are relevant to energy metabolism and food intake regulation.
LipOdystrophy: The Second Circle
Lipodystrophies are characterized by selective loss of adipose tissue, which extends from simple cosmetic problems to severe metabolic complications. Lipodystrophy has gained less attention than obesity, namely because of its lower prevalence, as most forms of genetic lipodystrophies are quite rare. Research into acquired and genetic lipodystrophy is becoming increasingly significant mostly because the incidence of the former is rising and studies of the latter may provide new clues to decipher the biology of adipocytes. Thus, the study of lipodystrophy may be the backdoor to further understanding mechanisms leading to obesity.
A form of localized adipose tissue reduction, known as lipoatrophy, is most prevalent in individuals undergoing HIV treatment. Although quite a bit is to be learned about the mechanisms by which protease inhibitors cause fat reduction, whether through inhibition of adipocyte differentiation (86) or apoptosis (87), this review will not expand on this aspect of lipodystrophy but rather concentrate on its genetic aspects and animal models.
Clinical and molecular basis of human lipodystrophies
The clinical features of inherited lipodystrophies are generally classified as congenital generalized lipodystrophy (CGL) or familial partial lipodystrophy (FPLD), and genetic diseases affecting these conditions are autosomal dominant or recessive (88). Diabetes is a very common trait in CGL and HIV-related lipodystrophy but less common in FPLD. If biology were one-dimensional, then one would hypothesize that the genes associated with lipodystrophy are identical to the ones associated with obesity, with the exception that loss- or gain-of-function mutations might differentiate these two disorders. Although this is obviously not the case, except for the known role of peroxisome proliferator-activated receptor-γ (PPARγ) in adipocyte differentiation and in its mutation in one form of lipodystrophy (89), a new set of genes and associated complex pathways have turned out to be the cause of genetic lipodystrophy.
Generalized lipodystrophy.
Cloning of the genes underlying two forms of CGL (CGL1 and CGL2) have revealed the culprits of these disorders. The molecular basis of CGL1 is caused by mutations in the gene encoding the acyltransferase 1-acylglycerol-3-phosphate-O-acyltransferase 2 (AGPAT2) (90), which catalyzes the formation of lysophosphatidic acid to phosphatidic acid during triacylglycerol and glycerophospholipid synthesis. Although a family of six different AGPAT proteins is known (91), each of which could mediate this biosynthetic step, the selectivity for AGPAT2 and the lack of compensation from other AGPATs remain puzzling, especially in light of the AGPAT6 knockout mice, which exhibit subdermal lipodystrophy (92). In CGL2 or Berardinelli-Seip syndrome, null mutations were found in the BSCL2 gene, which codes for a 398-amino-acid protein termed seipin that is expressed diffusely in many tissues but predominantly in testis and brain (93). The mechanism by which seipin leads to lipodystrophy and insulin resistance remains unknown and does not appear to be connected to AGPAT either. Interestingly, missense heterozygous mutations in seipin were found in an autosomal dominant form of hereditary motor neuropathy (Silver syndrome) (94), suggesting that perhaps the lack of seipin expression (as in Berardinelli-Seip syndrome) leads to lipodystrophy, whereas alterations in its conformation (as in Silver syndrome) could result in a dominant-negative effect, leading to autosomal dominant neurological disorders. Due to the main expression of seipin in brain and testis, the elucidation of its effect on adipose tissue ought to unveil new mechanisms of adipocyte biology, perhaps through the reproductive axis because it is also expressed in reproductive organs.
Partial lipodystrophy.
In the partial forms of lipodystrophy, FPLD1, -2, and -3 that are differentiated from each other by clinical criteria, the molecular basis of FPLD2 and FPLD3 have been reported. In FPLD2, the LMNA gene, which encodes the two isoforms of nuclear lamins A and C, was mutated in multiple families (95,96). The ubiquitous expression of lamin and the fact that it is also mutated in other types of dystrophies, such as Emery-Dreifuss (97) and limb girdle muscular dystrophy type 1B (98) highlights the complexity of this system and our weak understanding of how it could lead not only to lipodystrophy but also to a wide group of disorders referred to as the laminopathies (99). FPLD3 is caused by mutations in PPARγ (89,100). Because PPARγ is a well-known master regulator of adipocyte differentiation (101), its mutation in lipodystrophy did not reveal new pathways but reaffirmed its importance by showing that disruption of its expression via haploinsufficiency (102,103) or of its activity by a dominant-negative effect (104) perturbs adipocyte differentiation. In another vein, protein kinase B/Akt, recognized for its multiple roles in cell signaling, promotes cell survival, regulates the cell cycle, glycogen synthesis, cell growth, and insulin-stimulated glucose transport (105). The critical role of akt2 in insulin-sensitive tissues was physiologically vindicated by the findings that a missense mutation in the human gene abrogates its ability to phosphorylate downstream targets, resulting in insulin resistance and lipodystrophy of an affected individual (106).
Mouse models of lipodystrophy
Consistent with the akt2 mutation described above, homozygous akt2 knockout mice are insulin resistant and have reduced adipose tissue mass (107), adding more weight to the critical role of akt2 in metabolism. Other mouse models of lipodystrophy were also generated in known and novel pathways, leading to clues into lipodystrophy-associated metabolic complications. Foremost was the overexpression of a dominant-negative transcription factor that blocks adipocyte differentiation (108) and that of a constitutive active form of the sterol-regulating binding protein SREBP1c (109). Both of these models revealed a similar phenotype, namely a drastic reduction in adipose tissue mass, hepatic steatosis, and an insulin resistance that could largely be rescued with leptin treatment either exogenously (110), via fat transplantation (111) or transgenic overexpression (112).
More striking are those animal models that have shed light into pathways not previously anticipated. For example, the fld (fatty liver dystrophy) mouse mutation was cloned and found to encode a nuclear protein termed lipin, which is expressed at high levels in testis (reminiscent of seipin expression), liver, and adipocytes (113). Overexpression of lipin in muscle and adipose tissue has yielded the opposite phenotype, namely increased adiposity, suggesting that its absence causes lipodystrophy and its overexpression causes obesity (114). Even though a mutation in human lipin has not yet been described in either lipodystrophic or obese subjects, it appears that lipin plays a role in human metabolism because its adipose tissue gene expression is decreased in obese and insulin-resistant subjects and increases with pioglitazone treatment (115,116). The mechanisms of action of lipin are just starting to emerge, and recent studies in the mouse have shown that its expression is induced in the liver after fasting and that it interacts with the PPARγ coactivator PGC1α to stimulate the expression of genes involved in fatty acid oxidation (117).
Another mouse model of lipodystrophy resulted from adipocyte-induced apoptosis. The inducible activation of caspase-8 in adipocytes resulted in adipose tissue apoptosis and a lipodystrophy that was associated with impaired glucose homeostasis but surprisingly not steatosis (118), raising the question as to the fate and disposal of lipids in nonadipose tissues. Transfer of the caspase-8 transgene onto morbidly obese ob/ob mice resulted in fatless ob/ob mice that exhibited an exacerbation of their metabolic parameters compared with their obese ob/ob counterparts (118). Thus, ablation of fat from an obese mouse does not necessarily ameliorate its diabetic phenotype. Other mouse models with absence or significant reductions of the adipose tissue mass were generated through the overexpression of leptin either from adipose tissue (119) or liver (120). Contrary to lipodystrophic mice but consistent with lipodystrophic mice treated with leptin or fat transplants (110,111,112), leptin-overexpressing mice exhibited a marked insulin sensitivity (119,120), demonstrating that reduction of the fat mass in the presence of leptin has beneficial effects on the glucose-insulin axis.
Intersection of the Circles
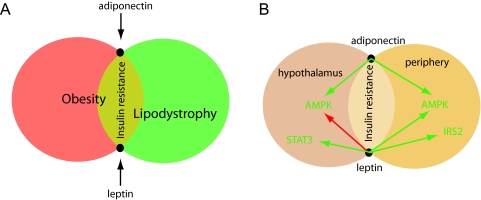
Obesities and lipodystrophies, representing stretches and shrinks of the adipose tissue, find common grounds in insulin resistance (Fig. 3A). Although the molecular basis of lipodystrophy has remarkably revealed a wide heterogeneity with respect to the underlying genes and their biological pathways, a common characteristic in the majority of lipodystrophies is that of insulin resistance. Because the absence or great reduction of adipose mass in the lipodystrophies also signifies that secreted factors from adipocytes are absent or low, the culprit of insulin resistance may be found in the pathways governed by those factors secreted exclusively from adipocytes, namely leptin and adiponectin.
Figure 3.
A, Schematic diagram showing that insulin resistance is the common area that represents the intersection of lipodystrophy and obesity. The adipocyte-secreted factors adiponectin and leptin, represented by the intersection points of the two circles, obesity and lipodystrophy, are depicted as modulators of glucose uptake and insulin sensitivity. B, Mechanisms by which adiponectin and leptin regulate food intake in the hypothalamus and glucose uptake in peripheral tissues via their actions on intracellular signaling proteins that mediate glucose uptake and insulin signaling. Green and red arrows represent stimulatory and inhibitory effects, respectively.
Leptin
In obesity, the dysregulation of leptin and adiponectin leading, respectively, to hyperleptinemia and hypoadiponectinemia, emphasizes again their importance in insulin resistance. Thus, leptin and adiponectin are the common denominators of insulin resistance in lipodystrophy and obesity. Although leptin treatment was largely ineffective in obese individuals (121), who already have a hyperleptinemia and a natural state of leptin resistance, leptin significantly ameliorated insulin resistance in lipodystrophy (122,123) and may even improve the amenorrhea associated with lipodystrophy (124). The mechanisms by which leptin and adiponectin ameliorate insulin resistance and improve our understanding of insulin sensitivity are intertwined because they both act on common targets in the periphery and the central nervous system (Fig. 3B). In lipodystrophic SREBP1c-overexpressing mice, leptin treatment ameliorated diabetes by enhancing insulin signaling transduction via IRS-2 and akt in the liver (125). Other insulin-independent pathways mediated by leptin include the stimulation of mouse fatty acid oxidation in skeletal muscle via the stimulation of AMPK activity, which in turn inhibits acetyl coenzyme A carboxylase activity (42). Fatty acid oxidation via the leptin-AMPK axis also helps in relieving the lipotoxicity induced by the accumulation of lipid droplets in skeletal muscle and liver in lipodystrophy and obesity (126). Centrally, a leptin-mediated decrease in AMPK activity in the hypothalamus of mice resulted in decreased food intake and reduced body weight, both of which are contributory factors for improving insulin resistance (127). Thus, leptin lies at the interphase of lipodystrophy and obesity and, although providing a significant improvement of insulin resistance in lipodystrophy, has the potential of exerting similar effects in obesity provided the leptin resistance block can be overcome. Therefore, unlocking leptin resistance in obesity will allow leptin to perform its insulin-sensitizing function. Although the latter is easier said than done, an area of research that could provide significant and additional input into leptin resistance but that has been relatively underexplored is the transport of leptin through the blood-brain barrier. Studies have shown that the transport of leptin through the blood-brain barrier is saturable (128) and that the physiological defect in the New Zealand obese (NZO) mouse, which exhibits a polygenic obesity associated with hyperinsulinemia and hyperglycemia, appears to be insensitive to peripheral leptin administrations but responsive to its intracerebroventricular infusions. Thus, the phenotype of the NZO mouse results at least in part from decreased leptin transport to the brain (129).
Adiponectin
The role of adiponectin as a peripheral factor and its promising role as a hypothalamic factor parallel those of leptin in terms of sites of action even though leptin, unlike adiponectin, performs its role predominantly in the hypothalamus and to a lesser extent in the periphery. In addition, both leptin and adiponectin find common ground in their targeted actions on AMPK, whether centrally or in peripheral target tissues. Whereas leptin inhibits AMPK in the hypothalamus and activates it in the periphery, adiponectin mimics leptin in the periphery but antagonizes it in the hypothalamus. The interplay of leptin and adiponectin both in the hypothalamus and the periphery, along with the modulation of glucose uptake into peripheral tissues by RBP4 and resistin, should allow us to further our understanding of the control of energy metabolism by providing an adequate armament to unlock the mechanisms underlying insulin resistance in obesity and lipodystrophy.
Acknowledgments
I thank Jun Zhu and Robert Lustig for help and critical reading of the manuscript.
Footnotes
Disclosure Statement: The author has nothing to disclose.
First Published Online January 17, 2008
Abbreviations: AGPAT2, 1-Acylglycerol-3-phosphate-O-acyltransferase 2; AgRP, agouti-related peptide; AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; CGL, congenital generalized lipodystrophy; FoxO1, forkhead transcription factor 1; FPLD, familial partial lipodystrophy; GLUT4, glucose transporter 4; 5HT2C-R, serotonin 2c receptor; IRS, insulin receptor substrate; MC4R, melanocortin 4 receptor; POMC, proopiomelanocortin; PPARγ, peroxisome proliferator-activated receptor-γ; RBP4, retinol (vitamin A)-binding protein 4; SF1, steroidogenic factor 1; STAT3, signal transducer and activator of transcription 3.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–443 [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in human. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD 1998 A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18:213–215 [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401 [DOI] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S 1997 Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16:303–306 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, Keogh J, O’Rahilly S, Creemers JW 2007 Hyperphagia and early onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab 92:3369–3373 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A 1998 Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS 2006 POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 3:135–140 [DOI] [PubMed] [Google Scholar]
- Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H 2006 A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab 3:141–146 [DOI] [PubMed] [Google Scholar]
- Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP 2006 The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology 47:39–43 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–999 [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB 2007 Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228–232 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P 2000 Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S 2003 Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O’Rahilly S 2003 Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12:561–574 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, O’Rahilly S, Farooqi IS 2004 A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 7:1187–1189 [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF 2003 Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R 2001 Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 15:1748–1757 [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen W, Nachtigal MW, Abbud R, Nilson JH, Parker KL 1994 The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–2312 [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P. Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL 2002 Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143:607–614 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME 1997 Early onset of reproductive function in normal female mice treated with leptin. Science 275:88–90 [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R 1996 Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA 2006 Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51:239–249 [DOI] [PubMed] [Google Scholar]
- Gibson WT, Ebersole BJ, Bhattacharyya S, Clayton P, Farooqi IS, Sealfon SC, O’Rahilly S 2004 Mutational analysis of the serotonin receptor 5HT2c in severe early-onset human obesity. Can J Physiol Pharmacol 82:426–429 [DOI] [PubMed] [Google Scholar]
- Wang B, Chehab FF 2006 Deletion of the serotonin 2c receptor from transgenic mice overexpressing leptin does not affect their lipodystrophy but exacerbates their diet-induced obesity. Biochem Biophys Res Commun 351:418–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell Jr JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua Jr SC, Xu AW, Barsh GS, Rossetti L, Accili D 2006 Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12:534–540 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T 2007 Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ 2007 AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WT, Pissios P, Trombly DJ, Luan J, Keogh J, Wareham NJ, Maratos-Flier E, O’Rahilly S, Farooqi IS 2004 Melanin-concentrating hormone receptor mutations and human obesity: functional analysis. Obes Res 12:743–749 [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG 2007 AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2007 AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785 [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D 2003 LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 13:2004–2008 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG 2003 Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2:28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG 1995 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett 377:421–425 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG 2005 Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19 [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D 2005 Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2:21–33 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005 AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB 2002 Leptin stimulates fatty acid oxidation by activation of AMP-activated protein kinase. Nature 415:339–343 [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW 1997 AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 1997 273:E1107–E1112 [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW 1999 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48:1667–1671 [DOI] [PubMed] [Google Scholar]
- Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL 2001 Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol 91:1073–1083 [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA 2002 Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115:2433–2442 [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE 2001 Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E 2006 Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab 291:E175–E181 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Bown M, Scherrer PE 2003 Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity, J Biol Chem 278:9073–9085 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE 2004 Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y 2000 Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T 2002 Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295 [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE 2007 Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Clin Invest 117:2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F 2007 Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 282:7991–7996 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O’Rahilly S 2001 Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes 50:2199–2202 [DOI] [PubMed] [Google Scholar]
- McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, Crocker J, Barnett AH, Kumar S 2002 Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab 87:2407 [DOI] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA 2003 Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun 300:472–476 [DOI] [PubMed] [Google Scholar]
- Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS 2006 Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes 55:3083–3090 [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA 2004 Regulation of fasted blood glucose by resistin. Science 303:1195–1198 [DOI] [PubMed] [Google Scholar]
- Azuma K, Katsukawa F, Oguchi S, Murata M, Yamazaki H, Shimada A, Saruta T 2003 Correlation between serum resistin level and adiposity in obese individuals. Obes Res 11:997–1001 [DOI] [PubMed] [Google Scholar]
- Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ 2003 Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol 149:331–335 [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ 2005 Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 111:932–939 [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS 2003 Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 88:4848–4856 [DOI] [PubMed] [Google Scholar]
- Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA 2004 High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 53:1279–1288 [DOI] [PubMed] [Google Scholar]
- Hasegawa G, Ohta M, Ichida Y, Obayashi H, Shigeta M, Yamasaki M, Fukui M, Yoshikawa T, Nakamura N 2005 Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol 42:104–109 [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Wilkinson D, Wiesner G, Morash B, Ur E 2005 Hypothalamic resistin immunoreactivity is reduced by obesity in the mouse: co-localization with α-melanostimulating hormone. Neuroendocrinology 81:19–30 [DOI] [PubMed] [Google Scholar]
- Muse ED, Lam TK, Scherer PE, Rossetti L 2007 Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest 117:1670–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB 2005 Serum retinol binding protein contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362 [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB 2006 Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354:2552–2563 [DOI] [PubMed] [Google Scholar]
- Takashima N, Tomoike H, Iwai N 2006 Retinol-binding protein 4 and insulin resistance. N Engl J Med 355:1392 [DOI] [PubMed] [Google Scholar]
- Erikstrup C, Mortensen OH, Pedersen BK 2006 Retinol-binding protein 4 and insulin resistance. N Engl J Med 355:1393–1394 [PubMed] [Google Scholar]
- Graham TE, Smith U, Kahn BB 2006 Retinol-binding protein 4 and insulin resistance. N Engl J Med 355:1394–1395 [PubMed] [Google Scholar]
- Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J 2006 Retinol-binding protein 4 in human obesity. Diabetes 55:2805–2810 [DOI] [PubMed] [Google Scholar]
- Graham TE, Wason CJ, Bluher M, Kahn BB 2007 Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 50:814–823 [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa Y, Kasuga M, Yazaki Y, Aizawa S 1994 Insulin and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182–186 [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Brüning JC, Haag 3rd B, Johnson RS, Kahn CR 1994 Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186–190 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- Laustsen PG, Michael MD, Crute BE, Cohen SE, Ueki K, Kulkarni RN, Keller SR, Lienhard GE, Kahn CR 2002 Lipoatrophic diabetes in Irs1(−/−)/Irs3(−/−) double knockout mice. Genes Dev 16:3213–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze D, Koenen M, Röhrig K, Horikoshi H, Hauner H, Eckel J 2002 Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 51:2369–2376 [DOI] [PubMed] [Google Scholar]
- Ceddia RB, Koistinen HA, Zierath JR, Sweeney G 2002 Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J 16:1163–1176 [DOI] [PubMed] [Google Scholar]
- Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R 2004 Protein kinase C-ζ-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem 279:25157–25163 [DOI] [PubMed] [Google Scholar]
- Hennige AM, Stefan N, Kapp K, Lehmann R, Weigert C, Beck A, Moeschel K, Mushack J, Schleicher E, Häring HU 2006 Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. FASEB J 20:1206–1208 [DOI] [PubMed] [Google Scholar]
- Palanivel R, Maida A, Liu Y, Sweeney G 2006 Regulation of insulin signaling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia 49:183–189 [DOI] [PubMed] [Google Scholar]
- Ost A, Danielsson A, Lidén M, Eriksson U, Nystrom FH, Strålfors P 2007 Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J 21:1–9 [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK 2007 A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129:1201–1213 [DOI] [PubMed] [Google Scholar]
- Dowell P, Flexner C, Kwiterovich PO, Lanes MD 2000 Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem 275:41325–41332 [DOI] [PubMed] [Google Scholar]
- Domingo P, Matias-Guiu X, Pujol RM, Francia E, Lagarda E, Sambeat MA, Vazquez G 1999 Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS 13:2261–2267 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Garg A 2006 Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet 7:175–199 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Garg A 2002 A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 87:408–411 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A 2002 AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31:21–23 [DOI] [PubMed] [Google Scholar]
- Coleman RA, Lee DP 2004 Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43:134–176 [DOI] [PubMed] [Google Scholar]
- Vergnes L, Beigneux AP, Davis R, Watkins SM, Young SG, Reue K 2006 Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J Lipid Res 47:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magre J, Delepine M, Khallouf E, Gedde-Dahl Jr T, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, BSCL Working Group, Lathrop M, Capeau J 2001 Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28:365–370 [DOI] [PubMed] [Google Scholar]
- Windpassinger C, Auer-Grumbach M, Irobi J, Patel H, Petek E, Horl G, Malli R, Reed JA, Dierick I, Verpoorten N, Warner TT, Proukakis C, Van den Bergh P, Verellen C, Van Maldergem L, Merlini L, De Jonghe P, Timmerman V, Crosby AH, Wagner K 2004 Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet 36:271–276 [DOI] [PubMed] [Google Scholar]
- Cao H, Hegele RA 2000 Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 9:109–112 [DOI] [PubMed] [Google Scholar]
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O’Rahilly S, Trembath RC 2000 LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24:153–156 [DOI] [PubMed] [Google Scholar]
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K 1999 Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21:285–288 [DOI] [PubMed] [Google Scholar]
- Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K 2000 Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet 9:1453–1459 [DOI] [PubMed] [Google Scholar]
- Capell BC, Collins FS 2006 Human laminopathies: nuclei gone genetically awry. Nat Rev Genet 7:940–952 [DOI] [PubMed] [Google Scholar]
- Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J Bath L, Zalin A, Labib M, Kumar S, Simpon H, Blom D, Marais D, Schwabe J, Barroso I, Thembath R, Wareham N, Nagy L, Gurnell M, O’Rahilly S, Chatterjee K 2006 Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metab 4:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM 1994 mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T 2002 PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes 51:3586–3590 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Ur E, Ransom TP, Cao H 2006 A frameshift mutation in peroxisome-proliferator-activated receptor-γ in familial partial lipodystrophy subtype 3 (FPLD3; MIM 604367). Clin Genet 70:360–362 [DOI] [PubMed] [Google Scholar]
- Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, Dixon AK, Dunne F, Boiani R, Cinti S, Vidal-Puig A, K Chatterjee VK, O’Rahilly S 2003 Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ. Diabetes 52:910–917 [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC 2007 AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O’Rahilly S, Barroso I 2004 A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304:1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG 2003 Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB β. J Clin Invest 112:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C 1998 Life without white fat: a transgenic mouse. Genes Dev 12:3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS 1998 Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12:3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL 1999 Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76 [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML 2000 Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K 2001 Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 50:1440–1448 [DOI] [PubMed] [Google Scholar]
- Peterfy M, Phan J, Xu P, Reue K 2001 Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet 27:121–124 [DOI] [PubMed] [Google Scholar]
- Phan J, Reue K 2005 Lipin, a lipodystrophy and obesity gene. Cell Metab 1:73–83 [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Ryden M, Sjolin E, Hoffstedt J 2007 A role of lipin in human obesity and insulin resistance: relation to adipocyte glucose transport and GLUT4 expression. J Lipid Res 48:201–206 [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN, Phan J, Spencer HJ 3rd, McGehee Jr RE, Reue K, Kern PA 2006 Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor γ activation. Diabetes 55:2811–2818 [DOI] [PubMed] [Google Scholar]
- Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence Jr JC, Kelly DP 2006 Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab 4:199–210 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE 2005 Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 11:797–803 [DOI] [PubMed] [Google Scholar]
- Qiu J, Ogus S, Lu R, Chehab FF 2001 Transgenic mice overexpressing leptin accumulate adipose mass at an older, but not younger, age. Endocrinology 42:348–358 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, Odaka H, Kasuga H, Fujisawa Y, Inoue G, Nishimura H, Yoshimasa Y, Nakao K 1999 Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48:1822–1829 [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M 1999 Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282:1568–1575 [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A 2002 Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P 2007 Long-term efficacy of leptin replacement in patients with Dunning-type familial partial lipodystrophy. Metabolism 56:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM 2004 Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 113:414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH 2003 Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144:5159–5165 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB 2004 AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM 1996 Leptin enters the brain by a saturable system independent of insulin. Peptides 17:305–311 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM 1997 Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94:8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]