Abstract
Obesity, insulin resistance, type 2 diabetes mellitus, and aging are associated with impaired skeletal muscle oxidation capacity, reduced mitochondrial content, and lower rates of oxidative phosphorylation. Several studies have reported ultrastructural abnormalities in mitochondrial morphology and reductions in mitochondrial mass in insulin-resistant individuals. From lower organisms to rodents, mitochondrial membrane structure, function, and programmed cell death are regulated in part by the balance between the opposing forces of mitochondrial fusion and fission, suggesting they may also play an important role in human physiology.
MORE THAN 300 million individuals worldwide are projected to be afflicted with type 2 diabetes mellitus (T2DM) by the year 2025 (1). Other comorbidities that cluster with diabetes include obesity, dyslipidemia, hypertension, and inflammation. Common to all these conditions is insulin resistance (2). Although the primary cause of T2DM is unknown, it is clear that insulin resistance in skeletal muscle and liver plays a primary role in its pathogenesis before the failure of pancreatic β-cells (3). In fact, insulin resistance in the skeletal muscle of those with a family history (FH+) of T2DM has been shown to be the best predictor for the later development of T2DM (4).
T2DM is as much a disease of disordered lipid metabolism as a disease of glucose metabolism (5). Our prospective studies among Pima Indians have clearly identified impaired fat oxidation and low metabolic rate as risk factors for body weight gain and insulin resistance (6). In parallel, a strong relationship exists between elevated intracellular fatty acid metabolism and insulin resistance in the skeletal muscle of FH+ individuals (7). Defects in mitochondrial oxidative capacity have also been shown to result in hepatic steatosis in humans and mice, leading to increased hepatic glucose production and hyperglycemia (8,9,10). In addition, in the pancreatic β-cell, tight coupling of substrate-level synthesis of mitochondrial GTP and ATP is an important event in glucose-stimulated insulin secretion (11). Recent studies into the origins of insulin resistance and T2DM, including our work, revealed a reduced number and impaired function of skeletal muscle mitochondria in diabetes and in FH+ for T2DM as well as in elderly individuals (12,13,14,15). Collectively, these data suggest that early defects in the regulation of mitochondrial mass and oxidative phosphorylation may be a contributing factor to mitochondrial dysfunction in the prediabetic state and the transition to overt T2DM. This review will focus on the role of mitochondrial dysfunction the development of insulin resistance in skeletal muscle with significant discussion on the potential role of the opposing forces of mitochondrial fusion and fission in T2DM and aging.
More than a decade ago, it was shown that low mitochondrial content and impaired lipid oxidation were present in insulin resistance (16,17). Later, this observation was extended, and it was proposed that skeletal muscle inflexibility (impaired basal fat oxidation and blunted switch to carbohydrate oxidation in response to a meal or insulin infusion) plays a major role in the etiology of insulin resistance (18). The impairment in lipid oxidation and lower oxidative metabolism is thought to lead to the accumulation of intrahepatic and intramyocellular lipids including acylcarnitines, long-chain fatty acyl coenzymes A, diacylglycerols, and ceramides. These toxic lipids can activate serine kinases, thus inhibiting insulin signaling at the insulin receptor substrate-1 and therefore impairing glucose transport (19,20,21).
Mitochondrial Biogenesis and Insulin Resistance
Most research into the regulation of mitochondrial number and function has focused on nuclear transcription factors such as nuclear respiratory factors (NRF) 1 and 2 and mitochondrial transcription factor A (TFAM). Peroxisomal proliferator activator receptor γ coactivator 1α (PGC1α) lies upstream of NRF1 and TFAM and serves as a nutrient-sensing system that increases mitochondrial biogenesis and shifts substrate utilization toward fat and away from carbohydrate (22). This family of nuclear receptor coactivators coordinates the transcription of both nuclear and mitochondrial genes involved in mitochondrial biogenesis (23,24), fiber-type determination (25), lipid oxidation (12), and reactive oxygen species scavenging (12,26). Defects in PGC1α content and signaling are found early in the development of insulin resistance. Recent studies using candidate gene analysis reported a decrease in PGC1α mRNA and the expression of genes encoding proteins of mitochondrial oxidative phosphorylation in the skeletal muscle of FH+ subjects (13) and T2DM (27). Overexpression of PGC1α in cultured myotubes results in tighter coupling of β-oxidation to tricarboxylic acid cycle, thus lowering the concentration of lipid intermediates such as acylcarnitines (28). Therefore, decreased skeletal muscle PGC1α activity and the resulting impairments in skeletal muscle and mitochondrial oxidation may lead to the development of insulin resistance in humans (28).
In the 1960s, work began to emerge describing mitochondria isolated from T2DM subjects with decreased oxidative capacity (29). Several decades later, now classical studies described reduced skeletal muscle oxidation capacity in T2DM as central to the pathogenesis of insulin resistance (30,31). Recent studies have shown that rates of basal and insulin-stimulated ATP synthesis, measured in situ by magnetic resonance spectroscopy are reduced in individuals with T2DM (32) and subjects with FH+ for T2DM before the onset of impaired glucose tolerance (33). Such observations are of relevance because they connect the metabolic inflexibility often described in T2DM (impaired fat oxidation and blunted response to insulin) to reduced mitochondrial oxidative phosphorylation. In parallel, in vivo functional studies (34,35) have identified reduced resting rates of ATP synthesis in elderly subjects with insulin resistance, suggesting that mitochondrial dysfunction of aging may be relevant to mitochondrial dysfunction seen in T2DM. In addition, ultrastructural defects in mitochondria of T2DM patients have also been described (36). This suggests that mitochondrial turnover and assembly (mitochondrial fusion and fission; see below) (37,38) may be as important as reduced mitochondrial content. Taken together, these data support the hypothesis that insulin resistance in human muscle arises from defects in mitochondrial content, structure, and function leading to mitochondrial-mediated diabetes.
In rodents, muscle-specific PGC1α deletions impairs glucose tolerance; however, these mice display normal peripheral insulin sensitivity (39). Global PGC1α-null mice have multiple systemic abnormalities, including complete resistance to diet-induced obesity and hyperactivity, secondary to central nervous system abnormalities (40,41). Collectively, these studies support the hypothesis that PGC1α-independent mechanisms may govern the pathogenesis of mitochondrial dysfunction in insulin-resistant states. Supportive of this notion, Morino et al. (42) did not observe decreased content of PGC-1α, PGC-1β, NRF-1, NRF-2, and TFAM proteins despite a 60% increase in intramyocellular lipid content and 38% reduction in mitochondrial density in individuals with a FH+ for T2DM. The reason for the discrepancy with most human studies (12,13,27) may relate to 1) the measured molecular targets (mRNA vs. protein), 2) the age-dependent decrease in muscle gene expression of PGC1α, 3) the ethnicity of the studied populations, 4) environmental triggers (i.e. diet), or 5) PGC1α-independent mechanisms such as the balance between mitochondrial fusion and fission.
Mitochondrial Fusion and Fission, the Cellular Yin-Yang
Mitochondria carry out a vast array of cellular functions including ATP production by oxidative phosphorylation and biosynthesis of amino acids and lipids. Importantly, mitochondria are pivotal for cytosolic calcium transport (43) and play a key role in amplifying apoptotic stimuli (44), an important event in regulating the balance between cellular necrosis and/or programmed cell death. The mitochondrial contribution to cellular homeostasis and thermoregulation is indispensable to the survival of cells. In short, because of their metabolic diversity, mitochondria can be considered as the gatekeeper of cells.
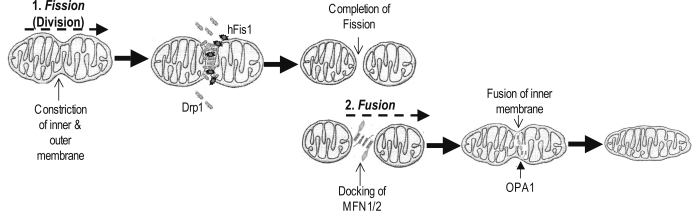
Part of the functional diversity displayed by mitochondria can be attributed to the dynamic and morphological variation within mitochondrial ultrastructure and distribution. Mitochondria are active structures that collide, divide, and fuse with other mitochondria (45). Subsequently, in most mammalian cells, mitochondria exist as branched-chain reticulum networks but can also exist as punctuated structures. Their distribution within the cells is quite diverse and is mediated by the interaction with the cytoskeleton (46) and the balance between mitochondrial fusion and fission. Fusion serves to unify mitochondria and enables mixing of compartments to function in cell development, energy dissipation, and mitochondrial DNA inheritance and complementation of the mitochondrial genome (46,47). Conversely, the opposing force of fission represents mitochondrial division and is required to promote transmission of mitochondria to dividing cells. Mitochondrial biogenesis and division occur in cells undergoing differentiation and growth in response to ATP requirements and the replacement of damaged or aging organelles (43) (Fig. 1).
Figure 1.
Model for mitochondrial fusion/fission in mammalian cells. Mitochondria can undergo constriction and division (1). These events are mediated by the cytosolic Drp1, which bonds and localizes to the constriction site via an interaction with the receptor (anchor)-like protein human Fis1 (hFis1) (2). During fusion, a tether of the MFN-1 or -2 to collateral mitochondrial MFN-1 and -2 conjoins the outer membranes. OPA1, an inner membrane GTPase protein facilitates the fusion of the inner membrane, cristae formation, and unifying of compartments.
Distribution of mitochondria throughout the cell is pivotal for metabolic control, with mitochondria most often localized to sites where ATP demand is the greatest (48) or where they are required to locally regulate Ca2+ signaling (49). This enables cells to cater to different energy requirements and provides means for environmental energy sensing (43). For example, in cardiomyocytes, mitochondrial reticulum conducts mitochondrial membrane potential to distal mitochondrial locations. The conductance of the electrochemical gradient down the mitochondrial network in fibers allows for the distribution of energy from the periphery of cells to the cellular core (50). Tissues with high demand for aerobic respiration, such as skeletal muscle and heart, have the most prominent reticulum network (50,51). In addition, fusion is essential to maintain mitochondrial DNA integrity and regulation of oxidative phosphorylation (50,51).
Mitochondrial Fission
Fission in mammals is mainly controlled by two proteins: dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1). Drp1 is a cytosolic GTPase protein, whereas Fis1 is localized to the outer mitochondrial membrane via a C-terminal domain and is thought to recruit Drp1 to participate in fission (52). In vitro, the overexpression of Drp1 has no phenotype, whereas knockdown of Drp1 blocks fission and results in interconnected mitochondrial tubules (53). On the other hand, perturbations in Fis1 protein content results in opposing phenotypes. For example, overexpression of Fis1 accelerates fission and causes mitochondrial fragmentation (52). Conversely, reduction in Fis1 protein inhibits fission and induces elongation of mitochondrial tubule networks (52). Ultimately, active and viable fission machinery is required to promote transmission of the mitochondrial genome during mitochondrial replication and also participates in the distribution of the organelle throughout the cell.
Mitochondrial Fusion
Mitochondrial fusion events are highly regulated, because fusion involves the recruitment of proteins localized to both the outer and inner mitochondrial membranes that act as adaptor proteins to bind mitochondrial membranes and ensure integrity of mitochondrial compartments. In lower organisms, mitochondrial fusion is regulated in part by three evolutionarily conserved GTPase proteins that form a functional complex consisting of the fuzzy onions (fzo1), mgm1, and ugo1 (54). The human homologs of fzo1 and mgm1 are mitofusin (MFN) 1 and 2 (two isoforms; MFN1/2) and optic atrophia 1 (OPA1), respectively. Mice that are deficient in either Mfn1 or -2 are embryonically lethal (55). Importantly, reductions in MFN2 protein have been shown to lower cellular respiration and glucose oxidation in mammalian fibroblasts (50). In addition, reductions in MFN2 protein are observed in obesity both in obese Zucker rats and in obese humans (50), suggesting that reduction in MFN2 expression may partially explain some of the metabolic perturbations associated with obesity.
Given the accumulating data over the past decade demonstrating impairments in oxidative phosphorylation and decreased mitochondrial mass in insulin-resistant states, it appears logical to assume that an imbalance in mitochondrial fusion and fission in metabolically active tissue such as skeletal muscle can result in defects associated with lipid and glucose metabolism such as that observed in insulin resistance and sarcopenia in aging.
Other than its role in fusion, OPA1 has several other functions, most notably the remodeling of the inner mitochondrial cristae (44) by keeping cristae junctions tight to inhibit cytochrome c release and subsequent apoptosis (38). In budding yeast (56) and in mice (38), mgm1/OPA1 is partially cleaved and processed by pcp1, an intramembrane rhomboid protease. In humans, a mutation in the OPA1 gene (-del TTAG deletion on exon 27) is associated with reduced rates of oxidative phosphorylation and ATP synthesis in skeletal muscle (57). This suggests that changes is OPA1 function resulting from either OPA1 mutations or processing by pcp1 may be associated with mitochondrial dysfunction observed in insulin-resistant states.
Is PARL Involved in the Development of Insulin Resistance and Mitochondrial Dysfunction in Skeletal Muscle?
We have previously shown that presenillin-associated rhomboid-like (PARL) protein, the human homolog of pcp1, is associated with insulin resistance and T2DM (37). PARL mRNA is reduced in the skeletal muscle of obese-diabetic Psammomys obesus (sand rat) and is positively correlated with insulin sensitivity in humans (37). In addition, PARL mRNA is lower in Pima Indians with a family history of T2DM (unpublished data). A Leu262Val polymorphism in the PARL gene is associated with increased plasma insulin, accounting for up to 5% of the variation in fasting plasma insulin in elderly (37). This interaction between the Leu262Val genotype, plasma insulin, and age is consistent with the hypothesis that defects in mitochondria increase throughout life, resulting in a decline in mitochondrial function and insulin resistance. Yeast strains without pcp1 exhibit decreased oxidative capacity, impaired growth, and fragmented mitochondria (45,56). The insertion of the human PARL gene into pcp1-deficient yeast rescued growth and restored mitochondrial morphology in yeast, suggesting that PARL may play a similar role in human physiology (56).
Conclusion
Insulin resistance is associated with lower mitochondrial mass and function. New emerging evidence and concepts suggest that the balance between mitochondrial fusion and fission may be important in the regulation of mammalian mitochondrial energetics. Two studies have described reductions in the proteins involved in mitochondrial fusion (MFN2 and PARL) in insulin-resistant states. There is growing evidence that a reduction in mitochondrial fusion is an important etiological factor in the development of obesity, insulin resistance, T2DM, and age-associated diseases. Such impaired fusion results in lower mitochondrial content and therefore impaired oxidative capacity, leading to a defective energy homeostasis.
Acknowledgments
We thank Dr. Darcy Johansen for her review and critical comments on the manuscript.
Footnotes
First Published Online January 17, 2008
Abbreviations: Drp1, Dynamin-related protein 1; FH+, family history; Fis1, fission protein 1; MFN, mitofusin; NRF, nuclear respiratory factor; OPA1, optic atrophia 1; PARL, presenillin-associated rhomboid-like; PGC1α, peroxisomal proliferator activator receptor γ coactivator 1α; T2DM, type 2 diabetes mellitus; TFAM, mitochondrial transcription factor A.
This review was supported partly by RO1 AG20478 (E.R.) and CNRU P30 DK072476 (E.R.).
Disclosure Summary: The authors have nothing to disclose.
References
- Zimmet P, Alberti KG, Shaw J 2001 Global and societal implications of the diabetes epidemic. Nature 414:782–787 [DOI] [PubMed] [Google Scholar]
- McGarry JD 2002 Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C 1993 Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329:1988–1992 [DOI] [PubMed] [Google Scholar]
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR 1990 Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 15:909–915 [DOI] [PubMed] [Google Scholar]
- McGarry JD 1992 What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258:766–770 [DOI] [PubMed] [Google Scholar]
- Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E 1990 Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 259:E650–E657 [DOI] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI 2007 Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessayre D, Berson A, Fromenty B, Mansouri A 2001 Mitochondria in steatohepatitis. Semin Liver Dis 21:57–69 [DOI] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM 2007 PGC-1β controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104:5223–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI 2007 Mitochondrial dysfunction due to long-chain acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104:17075–17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI 2007 Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 5:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Ukropcova B, Carling S, Hulver M, Defronzo RA, Mandarino L, Ravussin E, Smith SR 2006 Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI 2004 Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR 2007 Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56:720–727 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA 1999 Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE 1997 Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46:1579–1585 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ 2000 Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49:677–683 [DOI] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Summers SA 2003 Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3–L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419:101–109 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM 2003 Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278:26597–26603 [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM 2003 Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM 2006 Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM 2005 Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280:33588–33598 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Schersten T, Fagerberg SE 1967 Respiration and phosphorylation of mitochondria isolated from the skeletal muscle of diabetic and normal subjects. Diabetologia 3:346–352 [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Kelley DE 1997 Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83:166–171 [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE 1999 Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13:2051–2060 [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M 2007 Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 4:e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI 2005 Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2:e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI 2003 Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Amara CE, Jubrias SA, Marcinek DJ 2007 Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp Physiol 92:333–339 [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB 2002 Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- Walder K, Kerr-Bayles L, Civitarese A, Jowett J, Curran J, Elliott K, Trevaskis J, Bishara N, Zimmet P, Mandarino L, Ravussin E, Blangero J, Kissebah A, Collier GR 2005 The mitochondrial rhomboid protease PSARL is a new candidate gene for type 2 diabetes. Diabetologia 48:459–468 [DOI] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B 2006 Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126:163–175 [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM 2007 Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β-cell crosstalk. J Clin Invest 117:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP 2005 PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM 2004 Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI 2005 Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier AE, Kiu C, Stojanovski D, Hoogenraad NJ, Ryan MT 2006 Mitochondrial morphology and distribution in mammalian cells. Biol Chem 387:1551–1558 [DOI] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L 2004 OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101:15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki H, Southard SM, Hobbs AE, Jensen RE 2003 Cells lacking Pcp1p/Ugo2p, a rhomboid-like protease required for Mgm1p processing, lose mtDNA and mitochondrial structure in a Dnm1p-dependent manner, but remain competent for mitochondrial fusion. Biochem Biophys Res Commun 308:276–283 [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM 2005 The axonal transport of mitochondria. J Cell Sci 118:5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Isobe K, Nakada K, Hayashi JI 2001 Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28:272–275 [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M 2004 The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887 [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Scorrano L 2007 A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ 14:1275–1284 [DOI] [PubMed] [Google Scholar]
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A 2003 Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278:17190–17197 [DOI] [PubMed] [Google Scholar]
- Kirkwood SP, Munn EA, Brooks GA 1986 Mitochondrial reticulum in limb skeletal muscle. Am J Physiol 251:C395–C402 [DOI] [PubMed] [Google Scholar]
- Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT 2004 Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci 117:1201–1210 [DOI] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM 1998 A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS 2003 Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J Biol Chem 278:27781–27788 [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT 2001 Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114:867–874 [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Saurya S, Freeman M 2003 Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 423:537–541 [DOI] [PubMed] [Google Scholar]
- Lodi R, Tonon C, Valentino ML, Iotti S, Clementi V, Malucelli E, Barboni P, Longanesi L, Schimpf S, Wissinger B, Baruzzi A, Barbiroli B, Carelli V 2004 Deficit of in vivo mitochondrial ATP production in OPA1-related dominant optic atrophy. Ann Neurol 56:719–723 [DOI] [PubMed] [Google Scholar]