Abstract
Exposure measurement data from several developed countries indicate that human beings are widely exposed to low levels of the synthetic xenoestrogen, bisphenol A. We reported previously that bisphenol A, even at doses below the reference safe daily limit for human exposure, recommended by the U.S. Environmental Protection Agency, impairs the synaptogenic response to 17β-estradiol in the hippocampus of ovariectomized rats. Recent experiments revealed that bisphenol A also interferes with androgen receptor-mediated transcriptional activities. Thus, to investigate whether bisphenol A impairs synaptogenesis in the medial prefrontal cortex (mPFC) and hippocampus of adult male rats, castrated and sham-operated animals were treated with different combinations of bisphenol A (300 μg/kg), testosterone propionate (1.5 mg/kg), and sesame oil vehicle. The brains were processed for electron microscopic stereology, and the number of asymmetric spine synapses in the mPFC and CA1 hippocampal area was estimated. In both regions analyzed, bisphenol A reduced the number of spine synapses in sham-operated, gonadally intact animals, which was accompanied by a compensatory increase in astroglia process density. In addition, bisphenol A prevented both the prefrontal and hippocampal synaptogenic response to testosterone supplementation in castrated males. These results demonstrate that bisphenol A interferes with the synaptogenic response to testosterone in the mPFC and hippocampus of adult male rats. Because the hippocampal synaptogenic action of androgens seems to be independent of androgen and estrogen receptors in males, the potential mechanisms that underlie these negative effects of bisphenol A remain the subject of further investigation.
SINCE THE 1950s, the synthetic xenoestrogen, bisphenol A, has been used in the manufacture of plastics that have a broad range of uses including dental prostheses and sealants (1), the polycarbonate lining of metal cans used to preserve foods (2), and such items as baby bottles (3) and clear plastic cages used in many research institutions to house laboratory animals (4). Bisphenol A is also used as an additive in many products, with a global production rate of more than 6 billion pounds per year. Whereas exposure measurement data from several developed countries, including the United States, consistently indicate that human beings are widely exposed to low levels of bisphenol A, probably on a continuous basis (5), there is considerable debate whether this exposure represents an environmental problem.
The relatively low affinity of bisphenol A for the nuclear estrogen receptors (ERs) and its weak bioactivity in standard tests of estrogenicity (6) initially led to the conclusion that exposure to bisphenol A has negligible biological effects in humans (7). However, recent findings suggest that bisphenol A may interfere with the development, function, and morphology of the brain. We reported earlier that bisphenol A, even at doses below the reference safe daily limit for human exposure, recommended by the U.S. Environmental Protection Agency (EPA), impairs the synaptogenic response to 17β-estradiol in the hippocampus of ovariectomized rats (8,9). Because remodeling of spine synapses on the dendrites of hippocampal pyramidal cells may contribute to the beneficial effects of estrogens on cognition (10), disruption of estrogen-induced spine synapse formation by bisphenol A may result in cognitive impairments, particularly in ages when estrogen levels are naturally low, such as in postmenopausal women.
Whereas earlier studies focused on the estrogenic properties of bisphenol A, recent experiments revealed that bisphenol A antagonizes androgen receptor (AR)-mediated transcriptional activities (11,12,13,14). Because androgens are just as critical in the cognitive functions (15) and synaptogenesis (16,17,18) in males as estrogens are in females, the potential exists that bisphenol A also interferes with the physiology and morphology of the adult male brain. Thus, the following experiments were performed to investigate whether bisphenol A impairs spine synapse formation in the medial prefrontal cortex (mPFC) and hippocampus of adult male rats, brain areas with crucial influence on cognitive functions.
Materials and Methods
Experimental animals
Male Sprague Dawley rats (280–300 g; Charles River Laboratories, Wilmington, MA) were kept under standard laboratory conditions in a 12-h light,12-h dark cycle, with tap water and regular rat chow available ad libitum. Experiments conformed to international guidelines on the ethical use of animals, and experimental protocols were approved by the Institutional Animal Care and Use Committee of Yale University School of Medicine.
Surgery, treatments, and tissue processing
Castration or sham operation was performed on d 1 under deep anesthesia with a ketamine-xylazine mixture (containing 25 mg/ml ketamine, 1.2 mg/ml xylazine, and 0.03 mg/ml acepromazine dissolved in saline; 3 ml/kg, im). One week later (on d 8), treatments were initiated, consisting of daily sc injections of testosterone propionate (TP), bisphenol A, or the sesame oil vehicle. On d 11, rats were killed under deep ether anesthesia by transcardial perfusion of heparinized saline, followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 m phosphate buffer (pH 7.35). Brains were removed and postfixed overnight in the same fixative without glutaraldehyde. Tissue blocks containing the prefrontal region and the hippocampus were dissected out, and 100-μm-thick coronal vibratome sections were cut and sorted into several groups. Different groups of sections were then further processed for electron microscopy or glial fibrillary acidic protein (GFAP) immunostaining.
Electron microscopic stereology
The total number of asymmetric spine synapses in layer II/III of mPFC as well as the stratum radiatum of the CA1 hippocampal subfield was calculated as published previously (19,20). Due to the labor-intensive nature of electron microscopic stereology, the analysis was focused on these particular regions because our earlier studies demonstrated strong synaptogenic response to androgens in these areas of the adult male brain (16,18). First, using embedded sections, the volume of the sampling areas was estimated using the Cavalieri Estimator module of the Stereo Investigator system (MicroBrightField Inc., Villiston, VT) mounted on an Axioplan 2 light microscope (Zeiss, New York, NY). Because the mPFC and its neighboring cortical regions show very limited cytoarchitectonic differences in rodents, the precise anatomical borders of mPFC in rats remain the subject of intensive debate. To address this problem, we determined a sampling area with artificial borders that are related to easily identifiable macroanatomical structures. These borders are described in detail elsewhere (19). Thereafter 20 sampling sites for electron microscopic analysis were localized in both the mPFC and CA1 using a systematic-random approach, as published previously (19,20), and approximately four 75-nm-thick consecutive ultrasections were cut from each of these sampling sites. Digitized electron micrographs (Fig. 1) were taken from neighboring ultrasections for the physical disector by a person, who was blind to the treatment of individual animals. The micrographs were taken in a transmission electron microscope (Tecnai 12; FEI Co., Hillsboro, OR) furnished with an HR/HR-B charge-coupled device camera system (Hamamatsu Photonics, Hamamatsu, Japan); and the pictures were coded for blind analysis. This sampling technique produced 20 dissectors for the mPFC and another 20 dissectors for CA1 from each brain. Asymmetric spine synapses were counted according to the rules of the disector technique (21), and the volumetric density of these synapses (synapse per square micrometer) was determined. Thereafter, the volumetric density was multiplied by the volume of the sampling area to arrive at the total number of spine synapses. The number of spine synapses was determined independently by two different investigators, and the results were cross-checked to preclude systematic analytical errors.
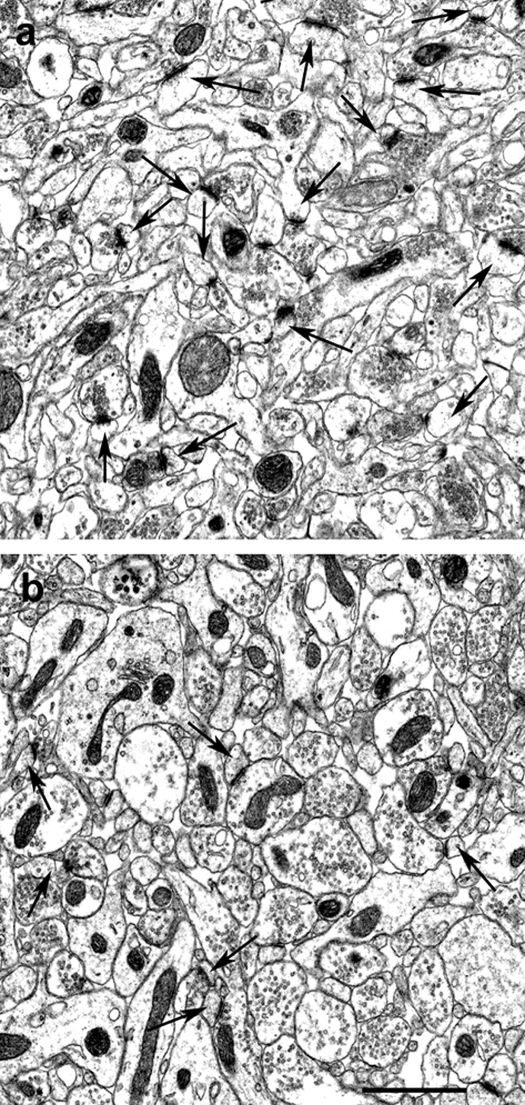
Figure 1.
Representative electron micrographs depicting the stratum radiatum of the CA1 hippocampal subfield of a castrated, testosterone-supplemented (A) and a castrated, testosterone + bisphenol A-treated (B) rat. Arrows indicate spine synapses. Scale bar, 1 μm.
Immunostaining and analysis of astroglia processes
A randomly sampled portion of sections (n = 10 per animal from both the mPFC and hippocampus) was used to analyze astroglia changes. Astroglia was visualized using GFAP immunoperoxidase staining as described earlier (22). Briefly, sections were incubated overnight at room temperature (RT) in monoclonal mouse anti-GFAP (Sigma, St. Louis, MO; 1:4000) dissolved in phosphate buffer containing 2% normal horse serum, followed by biotinylated horse antimouse IgG (Vector Laboratories, Burlingame, CA; 1:250; 2 h at RT), and the ABC Elite kit (Vector Laboratories, 1:500, 2 h at RT). The immunoreaction was visualized using nickel-diaminobenzidine as chromogen [for details see Ref. (22)]. The immunostained sections were mounted onto gelatin-coated slides, air dried, cleared in xylenes, and coverslipped with Permount.
To determine the surface density of GFAP-positive astroglia processes, micrographs were taken with a Zeiss AxioCam digital camera mounted on a BX60 light microscope (Olympus, Tokyo, Japan). Randomly selected sampling sites in mPFC layer II/III or CA1 stratum radiatum of each immunostained section were photographed using the 100× oil immersion lens (Fig. 2). At least 10 pictures were taken from each brain area by an investigator who was blind to the treatment of individual animals (C.L.). The pictures were printed using a laser printer and coded. The code was not broken until the analysis was completed. Astroglia processes were analyzed using a counting grid with gridlines 10 μm apart, covering a total area of 8000 μm2. Intersections of GFAP-positive processes clearly in focus with lines of the test grid were counted (for more details on counting see Ref. 22). Counts from each picture were used to calculate mean astroglia process densities for each rat.
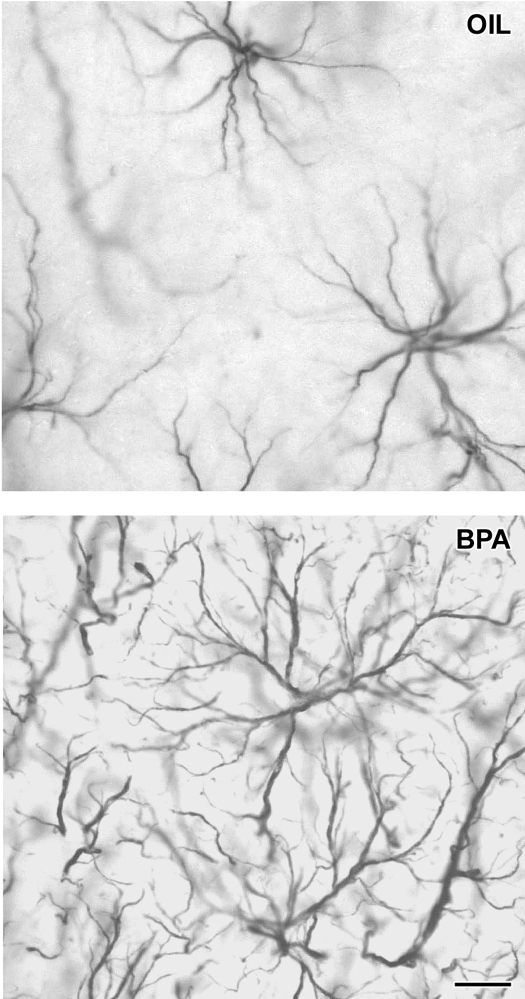
Figure 2.
Light micrographs demonstrating astroglia processes in the medial prefrontal cortex of sham-operated, gonadally intact male rats after vehicle (OIL) or bisphenol A (BPA) administration. Scale bar, 10 μm.
Experiment 1
Six rats were sham castrated on d 1 and sorted into two treatment groups: 1) OIL and 2) bisphenol A (BPA). On d 8–11, animals in the BPA group received 300 μg/kg bisphenol A daily, whereas males in the OIL group were treated with only 200 μl/d sesame oil vehicle. We selected this dose of bisphenol A because it was capable of entirely blocking the synaptogenic effect of 17β-estradiol in our previous study (9). Thirty minutes after the last injections on d 11, rats were killed and the number of asymmetric spine synapses as well as the surface density of astroglia processes was determined as described above. Estimates of the total number of synapses and the glia data obtained from individual animals were used to calculate group means (±sd) for both treatment groups. The two groups were then compared using a t test, with a criterion of statistical confidence of P < 0.05.
Experiment 2
Twelve rats were castrated on d 1 and sorted into four treatment groups: 1) OIL/OIL, 2) OIL/TP, 3) BPA/OIL, and 4) BPA/TP. On d 8–11, animals in the bisphenol A groups received 300 μg/kg bisphenol A daily, whereas males in the non-BPA groups were treated with only 200 μl/d sesame oil vehicle. Thirty minutes after bisphenol A injections on d 8–9, animals in the TP groups received 500 μg/rat TP daily, whereas males in the non-TP groups were treated with only 200 μl/d sesame oil vehicle. This dose of TP is at the very low end of the dose-response curve established for powerful androgens to maintain peripheral androgen-target tissues such as the ventral prostate in castrated male rats (23). When administered to castrated males, this dose of TP is sufficient to reproduce the density of hippocampal spine synapses observed in intact males (16). Thirty minutes after the last injections on d 11, rats were killed and the number of asymmetric spine synapses was determined as described above. Estimates of the total number of synapses obtained from individual animals were used to calculate group means (±sd) for each treatment group. Results were analyzed with one-way ANOVA, followed by Tukey’s multiple-comparison test. A criterion for statistical confidence of P < 0.05 was adopted.
Sample sizes
Typically with these methods, the sd for spine synapse counts was approximately 5% of the mean. With a sd of 5% and sample sizes of three animals per group, a 15% change in the mean number of spine synapses can be detected with α = 0.05 and 80% power. Hence, for purposes of the present study, the minimum treatment group size was set at three animals.
Results
Experiment 1
In sham-operated, gonadally intact animals, exposure to bisphenol A reduced the number of asymmetric spine synapses in layer II/III of mPFC from 4.695 ± 0.589 × 109 to 2.459 ± 0.432 × 109 (a 47.6% loss, t test, P < 0.01, Fig. 3, upper panel). In case of the hippocampus, bisphenol A administration caused a similar decrease in the number of CA1 spine synapses, from 4.152 ± 0.69 × 109 to 1.777 ± 0.258 × 109 (a 57.2% loss, t test, P < 0.02, Fig. 3, lower panel.). Under the light microscope, typical star-shaped astrocytes were visible. In bisphenol A-treated animals, astrocytes gave rise to richly sprouting bundles of processes with many fine fibers (Fig. 2, lower panel), whereas only shorter processes with less branching were observed in control rats (Fig. 2, upper panel). Even the qualitative comparison of glia patterns between bisphenol A-treated and control animals suggested considerably higher process density in rats that received bisphenol A (Fig. 2). The surface density calculation of astroglia processes confirmed this observation: bisphenol A treatment significantly increased astroglia process density in the mPFC by 50.2%, from 73.667 ± 3.512 intersections per 8000 μm2 in control rats to 110.667 ± 4.509 intersections per 8000 μm2 in bisphenol A-treated animals (t test, P < 0.001, Fig. 4, upper panel) and in the CA1 by 237.7%, from 50.333 ± 5.132 intersections per 8000 μm2 in control rats to 170 ± 17.059 intersections per 8000 μm2 in bisphenol A-treated animals (t test, P < 0.01, Fig. 4, lower panel).
Figure 3.
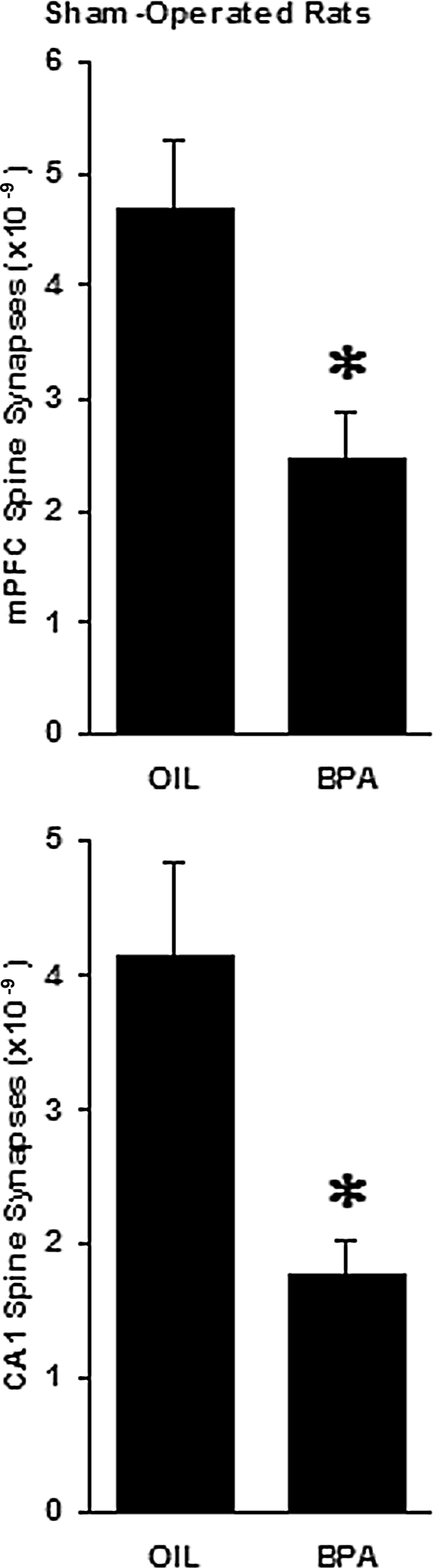
Effect of bisphenol A (BPA) administration on the number of asymmetric spine synapses in layer II/III of the mPFC (upper panel) as well as in the stratum radiatum of the CA1 hippocampal area (lower panel) in sham-operated, gonadally intact male rats. The asterisks indicate significant difference from the oil-treated (OIL) groups (t test, P < 0.01 for mPFC and P < 0.02 for CA1).
Figure 4.
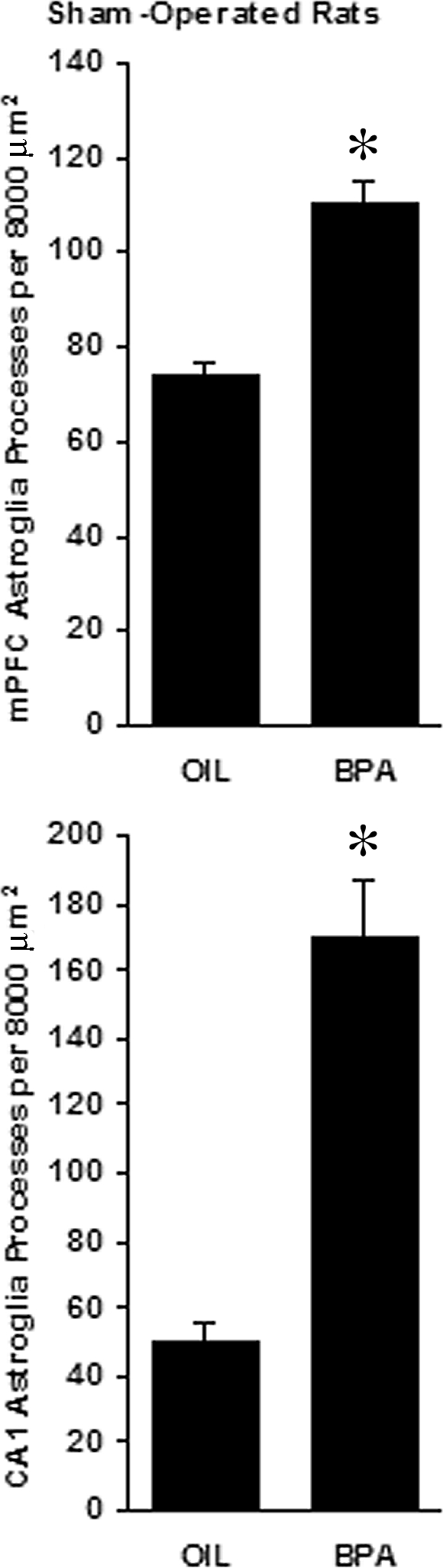
Effect of bisphenol A (BPA) administration on the surface density of astroglia processes in layer II/III of the mPFC (upper panel) as well as the stratum radiatum of the CA1 hippocampal area (lower panel) in sham-operated, gonadally intact male rats. The asterisks indicate significant difference from the oil-treated (OIL) groups (t test, P < 0.001 for mPFC and P < 0.01 for CA1).
Experiment 2
In castrated animals, one-way ANOVA showed a statistically significant difference in the number of spine synapses among treatment groups both in the mPFC (F3,11=48.938, P < 0.001) and the hippocampus (F3,11=26.007, P < 0.001).
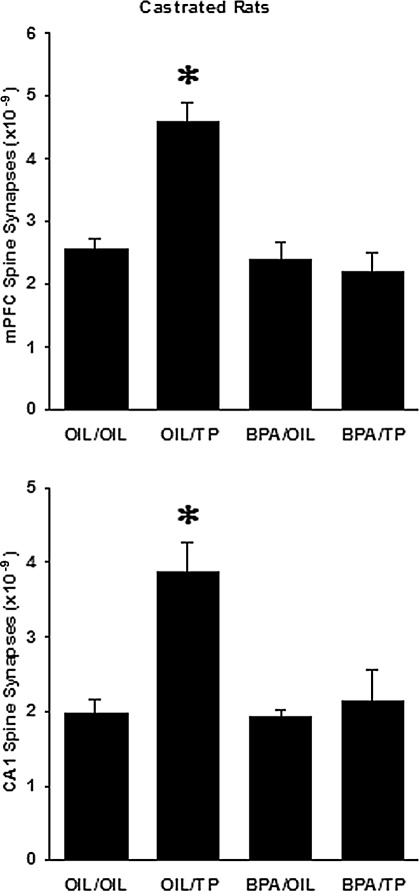
In layer II/III of mPFC, castration reduced the number of asymmetric spine synapses, which was reversed by testosterone supplementation (2.549 ± 0.155 × 109 synapses in the OIL/OIL group vs. 4.58 ± 0.328 × 109 synapses in the OIL/TP group, Tukey test, P < 0.001, Fig. 5, upper panel). Exposure to bisphenol A completely abolished the prefrontal synaptogenic effects of TP because synapse levels in the BPA/TP group were not significantly different from those of the OIL/OIL controls (2.184 ± 0.314 × 109 synapses in the BPA/TP group vs. 4.580 ± 0.328 × 109 synapses in the OIL/TP group, Tukey test, P < 0.001; and 2.184 ± 0.314 × 109 synapses in the BPA/TP group vs. 2.549 ± 0.155 × 109 synapses in the OIL/OIL group, Tukey test, P = 0.417; Fig. 5, upper panel). Administration of bisphenol A alone to castrated rats elicited no significant response (2.404 ± 0.269 × 109 synapses in the BPA/OIL group vs. 2.549 ± 0.155 × 109 synapses in the OIL/OIL group, Tukey test, P = 0.913, Fig. 5, upper panel).
Figure 5.
The number of asymmetric spine synapses in layer II/III of the mPFC (upper panel) as well as the stratum radiatum of the CA1 hippocampal area (lower panel) in castrated male rats after treatments with sesame oil vehicle (OIL/OIL), TP (OIL/TP), bisphenol A (BPA/OIL), and bisphenol A + TP (BPA/TP). The labeling of columns corresponds with the group names in Materials and Methods section. The asterisks indicate that the OIL/TP group is significantly different from all other treatment groups (Tukey’s test, P < 0.001).
In the hippocampus of castrated rats, changes in the number of CA1 spine synapses showed a similar trend in response to treatments. We observed a reduction after castration, which was reversed by TP administration (1.962 ± 0.199 × 109 synapses in the OIL/OIL group vs. 3.866 ± 0.409 × 109 synapses in the OIL/TP group, Tukey’s test, P < 0.001, Fig. 5, lower panel). Bisphenol A prevented the response to TP (2.128 ± 0.432 × 109 synapses in the BPA/TP group vs. 3.866 ± 0.409 × 109 synapses in the OIL/TP group, Tukey test, P < 0.001; and 2.128 ± 0.432 × 109 synapses in the BPA/TP group vs. 1.962 ± 0.199 × 109 synapses in the OIL/OIL group, Tukey test, P = 0.915; Fig. 5, lower panel), whereas bisphenol A alone showed no effect (1.917 ± 0.105 × 109 synapses in the BPA/OIL group vs. 1.962 ± 0.199 × 109 synapses in the OIL/OIL group, Tukey test, P = 0.998, Fig. 5, lower panel).
Discussion
These data provide evidence that exposure to bisphenol A results in a severe loss of spine synapses in both the mPFC and hippocampus of adult male rats. This negative effect appears to be the result of bisphenol A-induced impairment in the synaptogenic response to testosterone. In addition, we observed a compensatory astroglia process proliferation that is likely secondary to the loss of spine synapses. This hypothesis is supported by the fact that we observed similar astroglia process proliferation in association with spine synapse loss a week after ovariectomy (22), but there was no change in astroglia process density 4 h after estradiol administration, although robust spine synapse growth occurred in the CA1 at the same time point (24). The present findings are in line with the results of a recent study from our laboratory indicating that bisphenol A even at doses below the reference safe daily limit for human exposure, recommended by the EPA, inhibits the rapid synaptogenic effect of 17β-estradiol in the hippocampus of ovariectomized rats (8,9), i.e. bisphenol A antagonizes the increase in the number of CA1 spine synapses observed 30 min after the administration of 17β-estradiol.
The dose of bisphenol A required for complete blockade of the synaptogenic effects of both 17β-estradiol in females and testosterone in males falls within the range of 300–400 μg/kg. Although this dose is significantly higher than the EPA-recommended 50 μg/kg reference safe daily limit, earlier studies found bisphenol A being safe, even at these high doses (7,25). These observations indicate that hormonally active chemicals may exhibit radically different potencies in different bioassay systems, making it difficult to assess their potentially harmful effects (26,27). It also has to be noted that our studies analyzed the effects of bisphenol A against nearly maximum hormonal stimulation of spine synapse growth, which usually occurs only during adult life. In periods of life when gonadal steroid levels are naturally low, e.g. in aging, exposure to lower doses of bisphenol A found in the environment may be sufficient to significantly disturb gonadal steroid-induced remodeling of spine synapses.
The neuronal effects of gonadal steroids are not only confined to the control of reproductive functions but also include a wide range of influence on the ability of neurons to develop, adapt, and survive under constantly changing conditions (28,29). The effects are particularly pronounced in the prefrontal cortex and hippocampus, brain areas that are critically involved in cognition and mood. In adulthood, both of these areas retain the potential for considerable plasticity in response to changing levels of circulating gonadal steroids. This was first recognized in studies on the cyclical alterations of hippocampal function that occurs during the female reproductive cycle (30). Subsequent extensive work has demonstrated that both estrogen and androgen administration reverses the loss of CA1 spine synapses observed after gonadectomy in both male and female rodents and nonhuman primates (10,16,17,31,32). Recently similar structural responses to estrogens have been observed in the mPFC of ovariectomized rats (33) and the dorsolateral prefrontal cortex of monkeys (34,35). Our laboratory has also shown asymmetric spine synapse formation induced by both estradiol and androgens in the mPFC of male rats (18). These studies clearly demonstrate that gonadal steroids have a high potential to regulate the remodeling of prefrontal and hippocampal asymmetric spine synapses.
The potential significance of structural synaptic plasticity is derived from the hypothesis that rapid remodeling of dendritic spines and their synapses may represent a morphological substrate of learning and memory (36,37). Thus, it is logical to hypothesize that synapse formation on the dendritic spines of prefrontal and hippocampal pyramidal cells may contribute to the beneficial effects of gonadal steroids on cognition (15,38,39). Our earlier data reinforce the fact that there is an excellent correlation between the positive cognitive and synaptogenic effects of gonadal steroids, even in extreme experimental conditions (8,40). Based on this correlation, the demonstrated interference of bisphenol A with prefrontal and hippocampal spine synapse formation may thus result in disturbed cognitive function. Indeed, several studies have shown that bisphenol A perturbs the development of nonreproductive behaviors, such as play and maze-learning behavior in both female and male rodents (41,42,43,44), the effects being diametrically opposite to what would be predicted for a xenobiotic estrogen but in line with our findings of spine synapse loss. Recently we hypothesized that remodeling of hippocampal spine synapses may play a critical role in not only cognition but also the mechanisms of depression and antidepressant response (45,46). According to this hypothesis, exposure to bisphenol A and the resulting loss of hippocampal spine synapses may elicit depression-like behavior. Although there are limited data available, a couple of studies demonstrated that bisphenol A indeed promotes helpless behavior in the learned helplessness paradigm (47) and increases immobility in the forced swim test (48), signs of depression-like behavior in two widely accepted animal models of depression.
An interesting aspect of the present findings is the potential mechanism that underlies the negative effects of bisphenol A on androgen-induced spine synapse growth. Several studies demonstrated that bisphenol A impairs both ER- and AR-mediated activities (12,13,14,49), suggesting that bisphenol A acts at the level of gonadal steroid receptors. This may explain the interference of bisphenol A with prefrontal synaptogenesis in males because spine synapse remodeling in the male mPFC induced by androgens could be mediated by both the ER (via conversion of the androgens to estrogenic compounds) and the AR (18). However, extensive work from our laboratory indicates that the synaptogenic action of androgens is independent of both the nuclear ER (16) and AR (20,50) in the male hippocampus, raising the possibility that bisphenol A does not interfere with gonadal steroid receptor functions, at least in the male hippocampus. A potential explanation could be that bisphenol A directly targets intracellular mechanisms downstream of the receptors, which are involved in the remodeling of hippocampal spine synapses, such as the ERK and Akt pathways. The ERK1/2 pathway is known to be activated by both estrogens (49,51) and androgens (52,53), and it plays a critical role in both synaptic remodeling (54,55) and cognitive functions (56,57). The phosphatidylinositol 3-kinase/protein kinase B (Akt) pathway is also implicated in downstream signaling of the ER (58). There is evidence that androgens induce Akt phosphorylation in nonneural tissue (59) and that Akt is involved in spine growth (60). Unfortunately, very limited data are currently available about the effects of bisphenol A on intracellular signaling mechanisms (49), and more research is needed to clarify this issue. One of the first steps may be investigating whether the negative synaptogenic effect of bisphenol A is competitively reversible by higher doses of testosterone to reveal the potential role of gonadal hormone receptors. However, due to the labor-intensive nature of electron microscopic stereology, this analysis will be the subject of future studies.
In conclusion, exposure to bisphenol A results in a severe loss of spine synapses in both the mPFC and hippocampus of adult male rats. Previous studies have reported that bisphenol A elicits impaired cognition and disorders of mood, which could be explained, at least partially, by the observed loss of asymmetric spine synapses. However, the potential molecular mechanisms that underlie these negative effects remain the subject of further investigation.
Footnotes
This work was supported by National Institutes of Health Grants MH060858 (to C.L.), NS042644 (to C.L.), and MH074021 (to T.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 29, 2007
Abbreviations: AR, Androgen receptor; ER, estrogen receptor; GFAP, glial fibrillary acidic protein; mPFC, medial prefrontal cortex; RT, room temperature; TP, testosterone propionate.
References
- Suzuki K, Ishikawa K, Sugiyama K, Furuta H, Nishimura F 2000 Content and release of bisphenol A from polycarbonate dental products. Dent Mater J 19:389–395 [DOI] [PubMed] [Google Scholar]
- Kang JH, Kito K, Kondo F 2003 Factors influencing the migration of bisphenol A from cans. J Food Prot 66:1444–1447 [DOI] [PubMed] [Google Scholar]
- Brede C, Fjeldal P, Skjevrak I, Herikstad H 2003 Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam 20:684–689 [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV 2003 Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect 111:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C 2005 An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J 2001 Increasing the sensitivity of the rodent uterotrophic assay to estrogens, with particular reference to bisphenol A. Environ Health Perspect 109:1091–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen GH, Janning P, Wittsiepe J, Upmeier A, Bolt HM 2002 Integration of mechanistic data in the toxicological evaluation of endocrine modulators. Toxicol Lett 127:225–237 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C 2005 The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146:287–293 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C 2005 The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect 113:675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS 1992 Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP 1998 Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339 [DOI] [PubMed] [Google Scholar]
- Satoh K, Ohyama K, Aoki N, Iida M, Nagai F 2004 Study on anti-androgenic effects of bisphenol a diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem Toxicol 42:983–993 [DOI] [PubMed] [Google Scholar]
- Sun H, Xu LC, Chen JF, Song L, Wang XR 2006 Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem Toxicol 44:1916–1921 [DOI] [PubMed] [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR 2005 Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 216:197–203 [DOI] [PubMed] [Google Scholar]
- Janowsky JS 2006 Thinking with your gonads: testosterone and cognition. Trends Cogn Sci 10:77–82 [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ 2003 Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23:1588–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, Horvath TL 2004 Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex 14:503–510 [DOI] [PubMed] [Google Scholar]
- Hajszan T, Maclusky NJ, Johansen JA, Jordan CL, Leranth C 2007 Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148:1963–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Leranth C, Roth RH 2006 Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry 60:639–644 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C 2006 Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology 147:2392–2398 [DOI] [PubMed] [Google Scholar]
- Sterio DC 1984 The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136 [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C 2003 Gonadal hormones act extrinsic to the hippocampus to influence the density of hippocampal astroglial processes. Neuroscience 116:491–498 [DOI] [PubMed] [Google Scholar]
- Dorfman RI 1962 Androgens and anabolic agents. In: Dorfman RI, ed. Methods in hormone research. New York: Academic Press; 275–313 [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C 2006 Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience 138:977–985 [DOI] [PubMed] [Google Scholar]
- Ema M, Fujii S, Furukawa M, Kiguchi M, Ikka T, Harazono A 2001 Rat two-generation reproductive toxicity study of bisphenol A. Reprod Toxicol 15:505–523 [DOI] [PubMed] [Google Scholar]
- Gutendorf B, Westendorf J 2001 Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 166:79–89 [DOI] [PubMed] [Google Scholar]
- Safe SH 2000 Endocrine disruptors and human health—is there a problem? An update. Environ Health Perspect 108:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N 2000 Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry 48:715–720 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG 1997 Ovarian steroids and the brain: implications for cognition and aging. Neurology 48:S8–S15 [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS 1990 Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10:4035–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ 2004 Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci 24:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DEJ 2002 Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol 447:34–42 [DOI] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M 2006 Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126:176–182 [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH 2006 Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci 26:2571–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH 2004 Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223 [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H 2003 Structure-stability-function relationships of dendritic spines. Trends Neurosci 26:360–368 [DOI] [PubMed] [Google Scholar]
- Kandel ER 2001 The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038 [DOI] [PubMed] [Google Scholar]
- Dohanich GP 2002 Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RI, eds. Hormones, brain and behavior. San Diego: Academic Press; 265–327 [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK 2007 Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav 51:183–194 [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ 2003 Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144:2836–2844 [DOI] [PubMed] [Google Scholar]
- Carr R, Bertasi F, Betancourt A, Bowers S, Gandy BS, Ryan P, Willard S 2003 Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J Toxicol Environ Health A 66:2077–2088 [DOI] [PubMed] [Google Scholar]
- Della Seta D, Minder I, Belloni V, Aloisi AM, Dessi-Fulgheri F, Farabollini F 2006 Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm Behav 50:301–307 [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F 2002 Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect 110(Suppl 3):403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F 2002 Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect 110(Suppl 3):409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ 2006 Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology 66:S13–S22 [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C 2005 Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci 21:1299–1303 [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y 2004 Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect 112:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Kubo K, Aou S 2006 Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res 1068:49–55 [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Le HH, Wang HS, Belcher SM 2005 Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 146:5388–5396 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C 2004 Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology 145:4154–4161 [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M 2001 Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA 98:13391–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M 2006 Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 147:2028–2034 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ 2005 Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem 94:1639–1651 [DOI] [PubMed] [Google Scholar]
- Alonso M, Medina JH, Pozzo-Miller L 2004 ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem 11:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin M, Segal M 2003 Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci 17:2529–2539 [DOI] [PubMed] [Google Scholar]
- Sweatt JD 2004 Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14:311–317 [DOI] [PubMed] [Google Scholar]
- Thiels E, Klann E 2001 Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev Neurosci 12:327–345 [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA 2003 Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci 23:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE 2004 Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3–E1 osteoblasts. J Bone Miner Res 19:1181–1190 [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY 2005 Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25:11288–11299 [DOI] [PMC free article] [PubMed] [Google Scholar]