Abstract
To investigate how serotonin and leptin interact in the regulation of energy balance and glucose homeostasis, we generated a genetic mouse model, the OB2C mouse, which lacks functional serotonin 2C receptors and the adipocyte hormone leptin. The OB2C mice exhibited a dramatic diabetes phenotype, evidenced by a synergistic increase in serum glucose levels and water intake. The severity of the animals’ diabetes phenotype would not have been predicted from the phenotypic characterization of mice bearing mutations of either the leptin (OB mutant mice) or the serotonin 2C receptor gene (2C mutant mice). The synergistic impairment in glucose homeostasis developed at an age when OB2C mice did not differ in body weight from OB mice, suggesting that this impairment was not an indirect consequence of increased adiposity. We also demonstrated that the improvement in glucose tolerance in wild-type mice treated with the serotonin releaser and reuptake inhibitor fenfluramine was blunted in 2C mutant mice. These pharmacological and genetic findings provide evidence that the serotonin 2C receptor has direct effects on glucose homeostasis.
SUSCEPTIBILITY TO OBESITY and type 2 diabetes is known to be associated with a polygenic mode of inheritance (1). This is in accord with a complex set of interactions among the multiple pathways through which energy balance and glucose homeostasis are regulated. Insights into the pathophysiology of these disorders would therefore be facilitated by an understanding of the manner in which such pathways interact. For example, serotonin- and leptin-responsive pathways have both been implicated in the regulation of energy balance and glucose homeostasis, yet the extent to which they interact has been unclear.
Central serotonin [5-hydroxytryptamine (5-HT)] systems have long been implicated in the regulation of food intake and energy balance, as highlighted by the clinical use of the serotonin releaser and reuptake inhibitor fenfluramine as an appetite suppressant and weight loss medication (2). More recently a growing concern regarding the diabetogenic side effects of the atypical antipsychotics, which have nonspecific 5-HT receptor antagonist properties, has highlighted the need for an improved understanding of serotonin’s influence on glucose homeostasis (3). A role for serotonin in glucose homeostasis is also consistent with prior studies indicating that fenfluramine improves glucose tolerance and insulin action (4).
The serotonin 2C receptor (5HT2CR), one of at least 14 distinct subtypes of 5-HT receptors, has been shown to play an important role in serotonergic effects on energy balance. Expression of 5HT2CR is restricted to the central nervous system, in which it is found in both hypothalamic and extrahypothalamic regions implicated in energy balance and glucose regulation (5,6). Mice with targeted null mutations of the htr2c gene (2C mutant mice) are resistant to the anorectic effects of fenfluramine (7). In addition, 2C mutant mice are hyperphagic and develop mild obesity, hyperinsulinemia, and impaired glucose tolerance by the age of 6 months (8). These deficits are accelerated and exaggerated in 2C mutant mice fed a high-fat diet, consistent with the possibility that their hyperinsulinemia and glucose intolerance results indirectly as a consequence of their elevated adiposity (8).
Several lines of evidence support the possibility that serotonin and leptin may regulate energy balance and glucose homeostasis by influencing common neural pathways. Both leptin receptors and 5HT2CRs are found within multiple hypothalamic structures implicated in energy balance. These include the ventromedial, dorsomedial, paraventricular, lateral, and arcuate hypothalamic nuclei (5,6,9,10). Notably, leptin receptors expressed in the arcuate nucleus have been shown to play an important role in glucoregulation (11). 5HT2CRs are also expressed in this region, providing a potential site at which leptin and serotonin systems could interact to regulate glucose homeostasis.
To investigate potential interactions between 5HT2CR- and leptin-responsive pathways on the regulation of energy balance and glucose homeostasis, we crossed mice with null mutations of the htr2c gene with animals bearing null mutations of the leptin (lep) gene to generate mice deficient in both genes (OB2C double mutant mice). Phenotypic analysis of these animals and the effects of fenfluramine in wild-type and 2C mutant mice led us to report here that 5HT2CRs mediate central serotonergic influences on peripheral glucose homeostasis in a manner that is influenced by leptin signaling.
Materials and Methods
Animals
Male mice heterozygous for the obese spontaneous mutation (Lepob) were obtained from the Jackson Laboratory (B6.V-Lepob/J; Bar Harbor, ME) and bred with female mice heterozygous for a null mutation of the X-linked htr2c gene (12). Both mutations were congenic on a C57BL/6J background. From this cross, female mice heterozygous for both the Lepob and htr2c− mutations (htr2c−/htr2c+, Lepob/Lep+) and male mice heterozygous for the lep mutation (Lepob/Lep+) were then bred to produce the male experimental mice (WT: htr2c+/Y, Lep+/Lep+; 2C: htr2c−/Y, Lep+/Lep+; OB: htr2c+/Y, Lepob/Lepob; OB2C: htr2c−/Y, Lepob/Lepob) and heterozygous mice for additional breeding. For pharmacological studies, experimental mice were generated by breeding female mice heterozygous for the htr2c− mutation with male C57BL6/J mice obtained from the Jackson Laboratory. Genotyping for the htr2c− mutation was performed by PCR analysis using a primer within the neomycin resistance gene (NeoD: 5′-CACCTTGCTCCTGCCGAGAAA-3′) and flanking primers within the htr2c gene (2C forward: 5′-GCTCAGAATTCTGGAAATGTGT-3′; 2C reverse: 5′-CGGACTGCTAAATTGGGTC-3′) to produce a 114-bp band for WT mice and a 600-bp band for 2C mutant mice. Genotyping for the obese mutation was performed as previously described (13). All animals were housed at 20–24 C on a 12-h light, 12-h dark cycle (lights on at 0700 h) with free access to water and a standard chow diet (PicoLab Mouse Diet 20 5150; Purina Mills, Richmond, IN) except where indicated. Experiments were performed in accordance with the guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals and the University of California, San Francisco, Institutional Animal Care and Use Committee.
Food and water intake measurements
Mice were individually housed for 16 d in cages with feeders and water bottles mounted at one end. Animals were weighed before placement in the monitoring apparatuses and again at the end of data collection period. Intake of food and water were determined daily. Mean intake for the last 12 d of data collection was used for group comparisons to allow 4 d of acclimation to the housing conditions. The mice were run in 12 cohorts of three to 21 mice determined by the intermittent generation of mice from the breeding colony. The mice ranged in age from 2 to 8 months (2 months: WT, n = 10, 2C, n = 10, OB, n = 15, OB2C, n = 10; 3 months: WT, n = 4, 2C, n = 2, OB, n = 6, OB2C, n = 4; 4 months: WT, n = 7, 2C, n = 10, OB, n = 10, OB2C, n = 7; 5 months: WT, n = 3, 2C, n = 5, OB, n = 6, OB2C, n = 3; 6 months: WT, n = 3, 2C, n = 3, OB, n = 1, OB2C, n = 2; 7 months: WT, n = 2, 2C, n = 5, OB, n = 4; OB2C, n = 4; 8 months: WT, n = 4, 2C, n = 6, OB, n = 7; OB2C, n = 4), and where possible, individual mice were tested at multiple ages (one run: n = 47 mice; two runs: n = 29; three runs: n = 12; four runs: n = 4) to assess changes in intake with age.
Urine glucose
Mice were removed between 1300 and 1700 h from group housing in which food and water were available ad libitum and singly housed in a clean cage without bedding, food, or water. Urine produced was applied to a urinalysis reagent strip (Diastix; Bayer, Elkhart, IN) and scored on a color scale for glucose (0, 100, 250, 500, 1000, 2000 mg/dl). Mice were tested between 2 and 8 months of age (2 months: WT, n = 6, 2C, n = 14, OB, n = 13, OB2C, n = 11; 3 months: WT, n = 8, 2C, n = 9, OB, n = 6, OB2C, n = 7; 4 months: WT, n = 1, 2C, n = 6, OB, n = 6, OB2C, n = 7; 5 months: WT, n = 8, 2C, n = 8, OB, n = 12, OB2C, n = 10; 6 months: WT, n = 5, 2C, n = 8, OB, n = 8, OB2C, n = 6; 7 months: WT, n = 4, 2C, n = 1, OB, n = 5, OB2C, n = 4; 8 months: WT, n = 6, 2C, n = 4, OB, n = 2, OB2C, n = 8).
Fasting serum physiology
Mice were weighed and individually housed for 16 h before an 8-h fast beginning at 0700 h. Mice were then rapidly decapitated for collection of trunk blood. Samples were centrifuged to collect serum for measurement of glucose, insulin, glucagon, and corticosterone. Mice were tested from 2 to 10 months of age (2 months: WT, n = 7, 2C, n = 8, OB, n = 10, OB2C, n = 11; 4 months: WT, n = 6, 2C, n = 8, OB, n = 11, OB2C, n = 8; 6 months: WT, n = 6, 2C, n = 10, OB, n = 12, OB2C, n = 9; 10 months: WT, n = 11, 2C, n = 10, OB, n = 15, OB2C, n = 7). Glucose was measured using a GM7 Analox instrument (Analox Instruments Ltd., Lunenberg, MA) using the glucose oxidase method. Insulin was measured using the ALPCO ultrasensitive mouse insulin enzyme immunoassay no. 10-1150-01 (ALPCO Diagnostics, Salem, NH). Glucagon and corticosterone were measured by RIA using the Linco glucagon RIA kit GL-32K (Linco Research, St. Charles, MO), and a rat and mouse corticosterone RIA kit (no. 07-120103; MP Biomedicals, Solon, OH).
Weaning blood glucose
Mice were removed from their cages at 3 wk of age, and a drop of tail blood was applied to a One Touch Ultra glucometer (LifeScan Johnson & Johnson, Milpitas, CA) to measure blood glucose levels (WT: n = 8; 2C: n = 16; OB: n = 18; OB2C: n = 13).
Glucose tolerance tests
Beginning at 0700 h, mice were fasted for 8 h before blood collection from the tail vein for determination of baseline glucose levels (2 g/kg dose: WT, n = 15, 2C, n = 12, OB, n = 14, OB2C, n = 15; 0.2 g/kg dose: WT, n = 5, 2C, n = 5, OB, n = 7, OB2C, n = 10). Mice were then weighed and administered an ip injection of d-glucose (Sigma, St. Louis, MO) in distilled water. Subsequently blood was collected from the tail vein and centrifuged for serum collection at multiple time points for up to 2 h. For glucose tolerance tests with fenfluramine, 6 mg/kg fenfluramine or vehicle was administered ip immediately after baseline blood collection and before 2 g/kg glucose injection (WT, n = 8; 2C, n = 8). Fenfluramine, vehicle, and glucose were all injected at a volume of 10 μl/g of body weight. Fenfluramine was obtained from Sigma and was dissolved in 0.9% NaCl. Serum glucose was measured using a Trinder glucose oxidase kit (Mega Diagnostics, Los Angeles, CA).
Insulin tolerance tests
Insulin tolerance tests were carried out only on OB and OB2C mice because the dose of insulin required to lower serum glucose in these mice would be lethal to nondiabetic mice. Beginning at 0700 h, mice were fasted for 8 h before blood collection from the tail vein for determination of baseline glucose levels (OB, n = 9; OB2C, n = 5). Mice were then weighed and administered an ip injection of 12 U/kg human insulin (Sigma) in distilled water. Subsequently blood was collected from the tail vein and centrifuged for serum collection at multiple time points for up to 2 h.
Pancreatic histology
Mice (OB, n = 5; OB2C, n = 6) were anesthetized with avertin (2–2-2-tribromoethanol) and perfused with 0.9% NaCl followed by 4% paraformaldehyde. Histological analysis, quantification of tissue area, and counting of cells were performed as described previously (14). Immunohistochemical and immunofluorescence analyses were performed on paraffin sections as described previously (15). The following primary antibodies were used: guinea pig antiinsulin diluted 1:500 (Linco Research), rabbit antiglucagon diluted 1:500 (Linco Research). The following secondary antibodies were used for immunofluorescence: fluorescein isothiocyanate-conjugated anti-guinea pig (Invitrogen, Carlsbad, CA); Cy3-conjugated antirabbit diluted 1:500 (Invitrogen). Glucagon- and insulin-positive cells were quantitated using OpenLab software (Improvision, Lexington, MA).
Glucose-stimulated insulin release
Eleven-week-old mice (OB, n = 5; OB2C, n = 7) were euthanized by cervical dislocation, and pancreases were immediately inflated by injection of 3 ml of collagenase into the bile duct. The pancreases were removed and digested in collagenase at 37 C for 13–17 min followed by straining, centrifugation, and washing. Islets were then isolated by Ficoll gradient centrifugation, and six medium-sized islets were picked into 5-ml, round-bottom tubes containing RPMI 1640 media with 0.1% BSA. Between three and six tubes of islets were used for each animal at each concentration. Stock glucose was added to each tube to achieve concentrations of 75, 150, 300, and 600 mg/dl. Samples were then incubated with shaking for 1 h at 37 C followed by brief centrifugation and removal of supernatant for measurement of insulin by RIA using the Linco rat insulin RIA kit no. RI13K (Linco Research).
Statistics
Analyses of body weight, chow, and water intake were performed using a mixed-linear model (mixed procedure; SAS Institute Inc., Cary, NC) with mouse, htr2, and lep genotypes, and age in months defined as class variables and age as a repeated measure. The analysis of urine glucose levels was also carried out using a mixed-linear model with mouse, htr2c and lep genotypes, age in months, and urine glucose defined as class variables and age as a repeated measure. ANOVA was used for comparing fasting serum values (SPSS, Chicago IL). The analyses of the glucose tolerance tests, insulin tolerance tests, and glucose-stimulated insulin release used repeated-measures ANOVA (SPSS). In the case of the glucose tolerance tests with fenfluramine, each mouse received vehicle and drug treatment in a crossover design, with a 1-wk interval between tests, and the difference between the glucose levels for drug treatment and vehicle treatment at each time point was analyzed by repeated-measures ANOVA (SPSS).
Results
Regulation of food and water intake
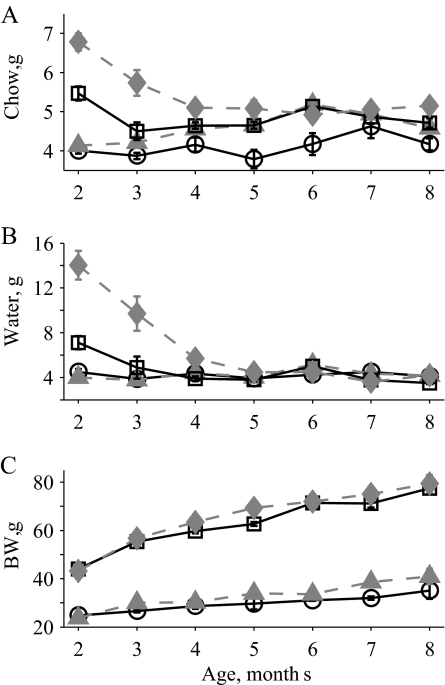
To determine how mutations in the htr2c and lep genes interact in the regulation of ingestion, daily food and water intake were measured in WT, 2C mutant, OB mutant, and OB2C double-mutant mice ranging in age from 2 to 8 months. These measurements revealed significant effects of age and the htr2c− and Lepob mutations on both food and water intake (Fig. 1, A and B). In addition, a significant synergistic interaction of the two mutations with age was observed for both food and water intake. OB2C double-mutant mice exhibited a large nonadditive increase in intake that declined slowly with age, compared with the WT and single-mutant mice (Fig. 1, A and B). This increase was greater than predicted based on the phenotypes of the single mutant lines and was particularly striking for water intake. For water intake, there was both a significant interaction of the two mutations with age and an interaction of the two genes across all ages. Thus, despite the decline in water intake to similar levels for all groups by 5 months of age, a significant interaction between the two genes across all ages is still detected [average water intake all mice from 2 to 8 months (mean ± se): WT, 4.3 ± 0.1; 2C, 4.2 ± 0.1; OB, 4.9 ± 0.3; OB2C, 8.0 ± 0.8]. A similar synergistic interaction was not observed for body weight. Although significant effects of age and htr2c and lep genotype on body weight were observed, OB2C mutant mice were not heavier than expected based on the phenotypes of the single mutants alone (Fig. 1C).
Figure 1.
Chow and water intake and body weight in WT (circles, solid line), 2C (triangles, dashed line), OB (squares, solid line), and OB2C (diamonds, dashed line) mice. A, Average daily chow intake. There was a significant effect of both htr2c and lep genotypes and a synergistic interaction of the two with age (htr2c, P < 0.0001; lep, P < 0.0001; age, P = 0.0001; htr2c × lep, P = 0.8; htr2c × age, P = 0.5; lep × age, P < 0.0001; htr2c × lep × age, P = 0.004). B, Average daily water intake. There was a significant effect of both htr2c and lep genotypes and a synergistic interaction of the two (htr2c, P < 0.0001; lep, P < 0.0001; age, P < 0.0001; htr2c × lep, P < 0.0001; htr2c × age, P = 0.0006; lep × age, P < 0.0001; htr2c × lep × age, P < 0.0001). C, Body weight (BW). There was a significant effect of both htr2c and lep genotypes without a significant interaction of the two (htr2c, P = 0.007; lep, P < 0.0001; age, P < 0.0001; htr2c × lep, P = 0.8; htr2c × age, P = 0.1; lep × age, P < 0.0001; htr2c × lep × age, P = 0.8).
Glucose homeostasis
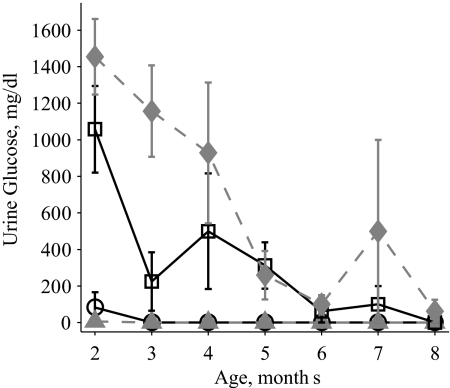
Glucose levels were determined in the urine of WT, 2C, OB, and OB2C mice from 2 to 8 months of age. A significant effect of age and the Lepob mutation was observed on urine glucose levels. In addition, there was a significant interaction between the htr2c− and Lepob mutations, with the OB2C double mutants exhibiting elevated urine glucose levels not predicted by a simple additive effect of the single gene mutations (Fig. 2). This interaction indicates that the combined effect of the two mutations produced a synergistic impairment in glucose homeostasis.
Figure 2.
Urine glucose in WT (circles, solid line), 2C (triangles, dashed line), OB (squares, solid line), and OB2C (diamonds, dashed line) mice. There was a significant effect of lep genotype and an interaction of lep and htr2c genotypes (htr2c, P = 0.051; lep, P < 0.0001; age, P < 0.0001; htr2c × lep, P = 0.04; htr2c × age, P = 0.99; lep × age, P < 0.0001; htr2c × lep × age, P = 0.99).
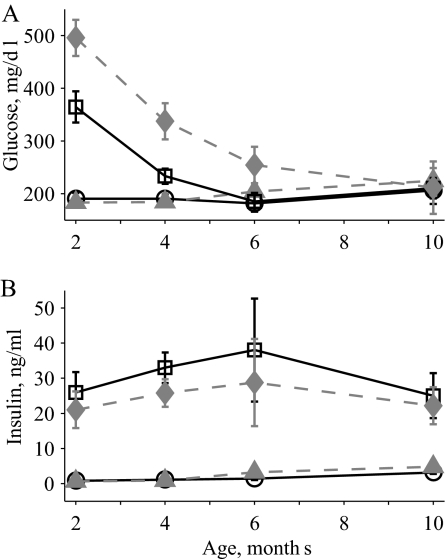
To confirm the presence of a synergistic impairment in the OB2C double mutants, fasting serum physiological parameters in WT, 2C, OB, and OB2C mice were investigated. There were significant effects of age and htr2c and lep genotype on fasting serum glucose levels. Again, a significant interaction between the two mutations on glucose levels was observed, with the OB2C mutants exhibiting a nonadditive increase in glucose levels relative to the WT and single-mutant mice (Fig. 3A).
Figure 3.
Fasting serum glucose and insulin over time in WT (circles, solid line), 2C (triangles, dashed line), OB (squares, solid line), and OB2C (diamonds, dashed line) mice. A, Serum glucose. There was a significant effect of both htr2c and lep genotypes and a synergistic interaction of the two (htr2c, P = 0.001; lep, P < 0.0001; age, P < 0.0001; htr2c × lep, P = 0.007; htr2c × age, P = 0.5; lep × age, P < 0.0001; htr2c × lep × age, P = 0.1). B, Serum insulin. There was a significant effect only of the lep genotype (htr2c, P = 0.5; lep, P < 0.0001; age, P = 0.7; htr2c × lep, P = 0.3; htr2c × age, P = 0.99; lep × age, P = 0.6; htr2c × lep × age, P = 0.99).
The elevated fasting glucose levels of the OB and OB2C mutant mice declined with age at a similar rate, and by 10 months no differences in glucose levels were detected among the groups (Fig. 3A). The improvement in fasting glucose with age in OB mice is consistent with previous work, indicating that the diabetes phenotype of OB mice on a C57BL/6 background normalizes with age (16). To investigate when the diabetes phenotype in these mice develops, blood glucose levels were determined at weaning when mice were 3 wk old. At this age, there were no significant effects of the htr2c or lep genotypes on blood glucose levels [blood glucose, milligrams per deciliter (mean ± se): WT, 151 ± 16; 2C, 169 ± 12; OB, 186 ± 11; OB2C, 182 ± 13; ANOVA: htr2c, P = 0.6, lep, P = 0.07, htr2c × lep P = 0.4]. This indicates that the diabetes phenotype develops after weaning, between the ages of 3 and 8 wk.
In contrast with the alterations in glucose levels, insulin levels were significantly altered only by the Lepob mutation (Fig. 3B). Glucagon and corticosterone were also measured at 2 months of age, when the difference in glucose levels between OB and OB2C mutants was largest. At this age, only the Lepob mutation resulted in significant elevations of insulin, glucagon, and corticosterone (Table 1). In contrast, there were significant effects of both htr2c− and Lepob mutations as well as a significant interaction between the mutations on glucose levels at this age.
Table 1.
Fasting serum physiological parameters at 2 months of age
| Measurement | WT | 2C | OB | OB2C | 2c, P value | lep Pvalue | 2c× lep, P value |
|---|---|---|---|---|---|---|---|
| Glucose, mg/dl | 191 ± 31 | 183 ± 29 | 364 ± 26 | 496 ± 25 | 0.03 | < 0.0001 | 0.02 |
| Insulin, ng/ml | 0.7 ± 5 | 0.6 ± 5 | 26 ± 4 | 20 ± 4 | 0.6 | < 0.0001 | 0.6 |
| Glucagon, pg/ml | 35 ± 23 | 38 ± 16 | 136 ± 16 | 91 ± 13 | 0.3 | < 0.0001 | 0.2 |
| Cort, ng/ml | 77 ± 25 | 68 ± 24 | 248 ± 21 | 301 ± 20 | 0.3 | < 0.0001 | 0.2 |
Measurements reported are the mean and sem. Significance values are from 1 × 2 ANOVA for each measurement.
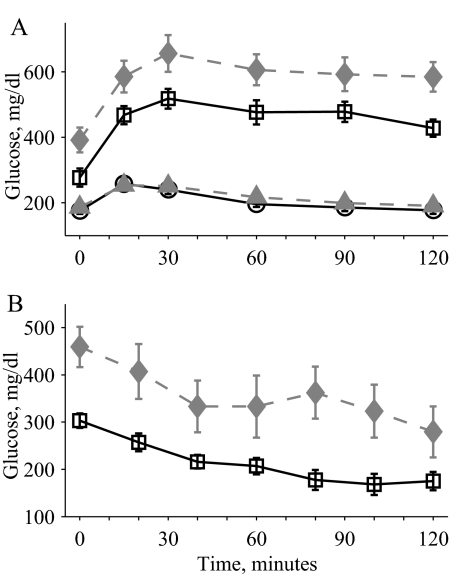
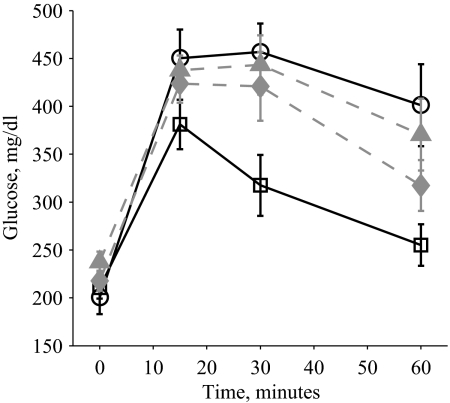
A glucose tolerance test performed on WT, 2C, OB, and OB2C mice with 2 g/kg glucose also revealed a significant nonadditive effect of the combined mutations on glucose levels (Fig. 4A). Glucose levels in both the OB and OB2C mutant mice returned to baseline values more slowly in comparison with the WT and 2C mutant mice, as revealed by the significant interaction of the Lepob mutation with time. In contrast, a three-way interaction between time and the two gene mutations was not significant, indicating that the significant interaction of the two mutations was attributable to the altered baseline glucose levels (Fig. 4A). Similar effects were seen with a lower glucose dose of 0.2 g/kg (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://endo.endojournals.org). An insulin tolerance test performed on OB and OB2C mice showed significant effects of time and htr2c− mutation and a significant interaction of the two (Fig. 4B), indicating that OB2C mice had altered sensitivity to an insulin challenge, compared with OB mice.
Figure 4.
A, Glucose tolerance test with 2 g/kg glucose in 2-month-old WT (circles, solid line), 2C (filled triangles, dashed line), OB (squares, solid line), and OB2C (filled diamonds, dashed line) mice. There was a significant effect of both htr2c and lep genotypes and a significant interaction between the two (htr2c, P = 0.008; lep, P < 0.0001; time, P < 0.0001; htr2c × lep, P = 0.03; htr2c × time, P = 0.7; lep × time, P < 0.0001; htr2c × lep × time, P = 0.9). B, Insulin tolerance test with 12 U/kg in 10-wk-old OB (squares, solid line) and OB2C (filled diamonds, dashed line) mice. There was a significant effect of htr2c (P = 0.008), time (P < 0.0001), and a significant interaction of htr2c × time (P = 0.03).
Pancreatic islet morphology and function
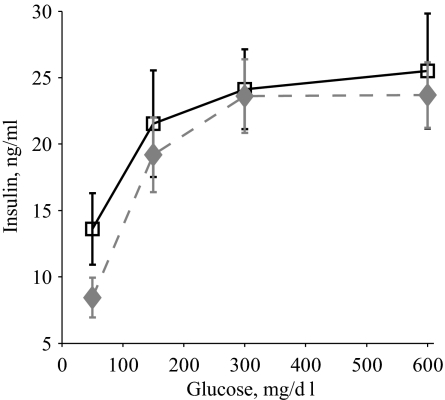
To determine whether the OB2C double-mutant phenotype was associated with alterations of pancreatic morphology, pancreatic tissue from OB and OB2C double mutants was examined at 11 wk of age, when phenotypic differences in glucose levels were largest. No significant difference in islet number or total islet area was observed (number, mean ± se: OB, 18.6 ± 3, OB2C, 15.6 ± 1.9, P = 0.5; normalized area, mean ± se: OB, 1 ± 0.3, OB2C, 0.6 ± 0.04, P = 0.2), nor were there differences in the ratios of insulin-positive to glucagon-positive cells (insulin/glucagon, mean ± se: OB, 2.3 ± 0.4, OB2C, 2.0 ± 0.4, P = 0.60). To determine whether the islets of OB and OB2C mutant mice differed in their ability to release insulin, a glucose-stimulated insulin release study was also performed. Consistent with the lack of difference in pancreatic morphology, there was no significant difference in glucose-stimulated islet insulin production between the two groups (Fig. 5).
Figure 5.
Glucose-stimulated insulin release from isolated pancreatic islets of 2-month-old OB (squares, solid line) and OB2C (diamonds, dashed line) mice. There was a significant effect of glucose but not htr2c genotype (htr2c, P = 0.3; glucose, P < 0.0001; htr2c × glucose, P = 0.9).
Glucose tolerance test with fenfluramine
The synergistic impairment of glucose homeostasis in OB2C mutants may be attributable to an indirect effect of increased adiposity or the result of a previously underappreciated role for 5HT2CRs in glucose homeostasis. However, no significant differences in the weights of adipose depots were observed between OB and OB2C mutants at 2 months of age (t tests, mean ± se, values in grams: epididymal OB, 3.8 ± 0.8, OB2C, 3.9 ± 1.2, P = 0.8; perirenal OB, 1.1 ± 0.5, OB2C, 1.4 ± 0.3, P = 0.1; omental OB, 1.1 ± 0.2, OB2C, 1.2 ± 0.2, P = 0.2; femoral OB, 1.5 ± 0.3, OB2C, 1.7 ± 0.3, P = 0.2) despite large differences in glucose levels. This suggests that 5HT2CRs may have a role in glucoregulation that is independent of phenotypic differences in adiposity. To further evaluate this hypothesis, we examined the effect of fenfluramine on glucose tolerance in young WT and 2C mutant mice at an age before the development of phenotypic differences in adiposity. 2C mutant mice and their WT littermates were injected with either vehicle or 6 mg/kg fenfluramine and then administered a glucose tolerance test. Fenfluramine treatment produced a more rapid decline toward baseline glucose levels in WT mice, compared with 2C mutant mice, as revealed by the significant interaction of the htr2c− mutation with time (Fig. 6). This differential response of the WT and 2C mutant mice to fenfluramine occurred at an age when the mice were not diverged in body weight (t test, mean ± se, values in grams: WT, 22.0 ± 0.7, 2C, 23.6 ± 0.6, P = 0.09), indicating that the 5HT2CR has a role in regulation of glucose homeostasis that is independent of its role in the regulation of food intake and body weight.
Figure 6.
Glucose tolerance test with 2 g/kg glucose with 6 mg/kg d-fenfluramine or vehicle in 3-month-old WT and 2C mice. Shown on graph are WT with vehicle (circles, solid line), WT with d-fenfluramine (squares, solid line), 2C with vehicle (triangles, dashed line), and 2C with d-fenfluramine (diamonds, dashed line). There was a significant interaction of htr2c genotype with time on the difference in glucose levels between saline and d-fenfluramine treatment (htr2c, P = 0.1; time, P < 0.0001; htr2c × time, P = 0.01).
Discussion
We report that combined mutations of the lep and htr2c genes results in a synergistic impairment of glucose homeostasis, accompanied by a synergistic increase in water intake in OB2C mice, indicating that leptin and the 5HT2CR interact in glucoregulation. The differences in glucose levels and food and water intake seen between OB and OB2C mice were not attributable to phenotypic differences in adiposity, suggesting a body weight-independent role of the 5HT2CR in regulation of glucose homeostasis. In this study, the elevation of body weight in 2C mutant mice, although significant, is somewhat less than reported earlier (8). Factors in the present study that may account for this include the exposure of animals to periods of individual housing and potential maternal effects of heterozygosity for the Lepob mutation in dams from which experimental animals were derived.
Several prior functional and neuroanatomical studies raised the possibility that interactions between leptin- and serotonin-responsive neuronal pathways could exist. OB mice were reported to have decreased expression of serotonin transporter mRNA in the dorsal raphé (17), and leptin treatment of OB mice increased the concentration of serotonin in hypothalamic and brain stem structures (18). In another study, a mouse line modeling leptin resistance (induced by constitutive leptin overexpression) was crossed with 2C mutant mice, resulting in enhanced sensitivity to high-fat diet-induced obesity and hyperinsulinemia (19). Notably, both the 5HT2CR and leptin receptors are expressed in hypothalamic regions implicated in energy balance and glucose homeostasis, including the arcuate nucleus of the hypothalamus, the dorsomedial hypothalamus, the paraventricular hypothalamus, and the lateral hypothalamus (5,6,9,10). Coexpression of 5HT2CRs and leptin receptors has also been shown to occur at the cellular level: leptin receptors are expressed on serotonergic neurons in the raphé nuclei (15,20,21), and these serotonergic neurons have been shown to accumulate leptin (22). 5HT2CRs are also expressed on leptin-sensitive proopiomelanocortin neurons in the arcuate nucleus (23), a brain region through which leptin regulates glucose homeostasis (11). The 5HT2CR does not appear to be expressed outside the central nervous system, and quantitative RT-PCR on mouse pancreas tissue did not reveal any expression of 5HT2CR mRNA, both in our hands (data not shown) and elsewhere (24).
At 2 months of age, OB2C double-mutant mice had dramatically increased food (124% of OB intake) and water (198% of OB intake) intake, compared with their OB littermates. In addition, OB2C double mutants exhibited elevated urine glucose levels as well as higher fasting glucose and impaired glucose tolerance, demonstrating a synergistic interaction of the lep and htr2c genes in the regulation of glucose homeostasis.
The impaired glucose homeostasis phenotype of both OB and OB2C mice ameliorates as the mice age. This finding had previously been observed in OB mice on the C57BL/6 background (16); however, the mechanisms underlying this age-dependent effect have not been fully delineated. Interestingly, serum insulin concentrations do not appear to change significantly with age (Fig. 3B), raising the possibility that euglycemia seen in older OB mice may result from improved insulin sensitivity, rather than increased insulin production. In parallel with the improvement of glucose levels with age, both OB and OB2C mice exhibit a normalization of food and water intake. Given the well-established effects of elevated blood glucose on osmotic regulation, it is likely that the normalization of water intake is secondary to normalization of serum glucose levels. Because hyperphagia is also linked to diabetes mellitus (25,26,27), the normalization of feeding in these mice may also occur secondary to their improvement in glucose homeostasis. Despite the marked hyperphagia of young adult OB2C mice, their body weights did not differ from those of OB mice. This is consistent with the notion that attempts to compensate for energetic losses from urinary glucose excretion contribute to hyperphagia in mice of both genotypes (25).
To better examine the synergistic interaction between the lep and htr2c genes, we focused attention on 2-month-old mice, the age at which their diabetes is maximal. We did not see any differences between 2-month-old OB and OB2C mice in pancreas morphology or the ability of islets to respond in vitro to glucose (Fig. 5), suggesting that the double mutant phenotype is unlikely attributable to a defect in pancreatic development or intrinsic islet cell function. However, this does not exclude the possibility that central regulation of pancreatic function may differ in OB and OB2C mice, i.e. through effects of central serotonin on the sympathetic and parasympathetic nervous systems (28).
It is also possible that phenotypic influences on insulin sensitivity contribute to the exacerbation of diabetes in OB2C mice. Consistent with this possibility, an insulin tolerance test indicated altered sensitivity to injected insulin in OB2C mice, compared with OB mice. However, interpretation of these insulin tolerance test results is confounded by the significantly elevated (relative to OB) fasting glucose levels of OB2C mice. It is noteworthy, however, that despite the higher fasting serum glucose levels of the double mutants, the fasting serum insulin levels of two groups do not differ. This is consistent with the possibility that reduced insulin sensitivity contributes to the enhanced disruption of glucose homeostasis in OB2C mice.
Whereas a null mutation of the htr2c gene does not result in impairment of glucose homeostasis in young, lean mice, Nonogaki et al. (8) showed that obese 2C mutant mice do develop impaired glucose homeostasis. However, it was unclear whether this reflected a direct impairment by the htr2c− mutation or an indirect consequence of enhanced adiposity, which is in and of itself a major risk factor for type 2 diabetes (29). Our observation that the effects of fenfluramine on glucose tolerance are blunted in 2C mutant mice indicates that manipulation of the 5HT2CR can play a role in glucose regulation in the absence of obesity. This suggests that young, lean 2C mutant mice, which have normal glucose tolerance, are somehow able to compensate for their chronic lack of 5HT2CR. This compensation appears to fail in diet-induced obesity [a condition accompanied by leptin resistance (8)], in a genetic model of leptin resistance (19), and in the absence of leptin (this study). These data suggest that the manner in which 2C mutant mice maintain glucose homeostasis is leptin dependent and that impairment of the leptin system augments the impact of 5HT2CR signaling on glucose homeostasis.
In summary, our findings indicate that serotonin and leptin interact synergistically to regulate glucose homeostasis. These findings may have significant clinical implications because diabetes and obesity associated with atypical antipsychotic drug treatment pose substantial obstacles in the treatment of schizophrenia and bipolar disorder (4). Atypical antipsychotic drugs are high-affinity antagonists of the 5HT2CR (30), and 5HT2CR occupancy has been found to correlate with the obesigenic and diabetogenic effects of these drugs (31). Furthermore, our finding that the 5HT2CR interacts with the leptin system to regulate glucose homeostasis further establishes the serotonin system as a possible target for the treatment of diabetes mellitus itself.
Acknowledgments
We thank the University of California, San Francisco Diabetes and Endocrine Research Islet Core for mouse pancreatic islet isolation; Janet Lau for assistance with pancreatic histology; and Elaine Storm, Elinor Sullivan, and Luna Abdallah for critical reading of the manuscript.
Footnotes
This work was supported by a National Alliance for Research on Schizophrenia and Depression Artworks Young Investigator Award (to E.H.G.) and an National Institutes of Health RO1 grant (to L.H.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 26, 2007
Abbreviations: 5-HT, 5-Hydroxytryptamine; 5HT2CR, serotonin 2C receptor; wild-type experimental mice.
References
- Kahn CR, Vincent D, Doria A 1996 Genetics non-insulin-dependent (type 2) diabetes mellitus. Annu Rev Med 47:509–531 [DOI] [PubMed] [Google Scholar]
- Davis R, Faulds D 1996 Dexfenfluramine. An updated review of its therapeutic use in the management of obesity. Drugs 52:696–724 [DOI] [PubMed] [Google Scholar]
- Ramaswamy K, Masand PS, Nasrallah HA 2006 Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry 18:183–194 [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Paolisso G, Salvatore T, Lefebvre PJ 1991 Improvement of insulin-induced glucose disposal in obese patients with NIDDM after 1-wk treatment with d-fenfluramine. Diabetes Care 14:325–332 [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E 1989 Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett 247:453–462 [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L 1995 Comparative localization of serotonin 1A, 1C and 2 receptor subtype mRNAs in rat brain. J Comp Neurol 351:357–373 [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH 1999 Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology 143:309–314 [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH 1998 Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med 4:1152–1156 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB 1998 Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG 1996 Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 1:63–72 [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D 1995 Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 374:542–546 [DOI] [PubMed] [Google Scholar]
- Erickson, JC, Hollopeter G, Palmiter RD 1996 Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274:1704–1707 [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, St. Jacques B, McMahon AP, Melton DA 2000 Regulation of pancreas development by Hedgehog signaling. Development 127:4905–4913 [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA 1997 Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol 62:377–383 [PubMed] [Google Scholar]
- Coleman DL, Hummel KP 1973 The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9:287–293 [DOI] [PubMed] [Google Scholar]
- Collin M, Hakansson-Ovesjo M-L, Misane I, Ogren SO, Meister B 2000 Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Mol Brain Res 81:51–61 [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Redmann SM Jr., Smagin GN, Smith SR, Rodgers E, Zachwieja JJ 1998 A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology 139:9–19 [DOI] [PubMed] [Google Scholar]
- Wang B, Chehab FF 2006 Deletion of the serotonin 2c receptor from transgenic mice overexpressing leptin does not affect their lipodystrophy but exacerbates their diet-induced obesity. Biochem Biophys Res Commun 351:418–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Rickard DG, Clifton DK, Steiner RA 2001 Serotonergic neurons are targets for leptin in the monkey. J Clin Endocrinol Metab 86:422–426 [DOI] [PubMed] [Google Scholar]
- Hay-Schmidt A, Helboe L, Larsen PJ 2001 Leptin receptor immunoreactivity is present in ascending serotonergic and catecholaminergic neurons of the rat. Neuroendocrinology 73:216–226 [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Diano S, Horvath TL, Garcia-Segura LM 2002 Leptin uptake by serotonergic neurones of the dorsal raphe. J Neuroendocrinol 14:429–434 [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD 2002 Activation of central melanocortin pathways by fenfluramine. Science 297:609–611 [DOI] [PubMed] [Google Scholar]
- Freeman TC, Dixon AK, Campbell EA, Tait TM, Richardson PJ, Rice KM, Maslen GL, Metcalfe AD, Streuli CH, Bentley DR 1998 Expression mapping of mouse genes. MGI direct data submission to Mouse Genome Database (MGD), MGI: 1199209. http://www.informatics.jax.org [Google Scholar]
- Friedman MI 1978 Hyperphagia in rats with experimental diabetes mellitus: a response to a decreased supply of utilizable fuels. J Comp Physiol Psychol 92:109–117 [DOI] [PubMed] [Google Scholar]
- Plum L, Belgardt BF, Bruning JC 2006 Central insulin action in energy and glucose homeostasis. J Clin Invest 116:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipols AJ, Baskin DG, Schwartz MW 1995 Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44:147–151 [DOI] [PubMed] [Google Scholar]
- Nonogaki K 2000 New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43:533–549 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM 2006 Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846 [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Cacchio M, Di Giulio C, Di Giovanni G, Esposito E 2002 Biochemical evidence that the atypical antipsychotic drugs clozapine and risperidone block 5-HT(2C) receptors in vivo. Pharmacol Biochem Behav 71:607–613 [DOI] [PubMed] [Google Scholar]
- Matsui-Sakata A, Ohtani H, Sawada Y 2005 Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet 20:368–378 [DOI] [PubMed] [Google Scholar]