Abstract
Dehydroepiandrosterone (DHEA) activates a plasma membrane receptor on vascular endothelial cells and phosphorylates ERK 1/2. We hypothesize that ERK1/2-dependent vascular endothelial proliferation underlies part of the beneficial vascular effect of DHEA. DHEA (0.1–10 nm) activated ERK1/2 in bovine aortic endothelial cells (BAECs) by 15 min, causing nuclear translocation of phosphorylated ERK1/2 and phosphorylation of nuclear p90 ribosomal S6 kinase. ERK1/2 phosphorylation was dependent on plasma membrane-initiated activation of Gi/o proteins and the upstream MAPK kinase because the effect was seen with albumin-conjugated DHEA and was blocked by pertussis toxin or PD098059. A 15-min incubation of BAECs with 1 nm DHEA (or albumin-conjugated DHEA) increased endothelial proliferation by 30% at 24 h. This effect was not altered by inhibition of estrogen or androgen receptors or nitric oxide production. There was a similar effect of DHEA to increase endothelial migration. DHEA also increased the formation of primitive capillary tubes of BAECs in vitro in solubilized basement membrane. These rapid DHEA-induced effects were reversed by the inhibition of either Gi/o-proteins or ERK1/2. Additionally, DHEA enhanced angiogenesis in vivo in a chick embryo chorioallantoic membrane assay. These findings indicate that exposure to DHEA, at concentrations found in human blood, causes vascular endothelial proliferation by a plasma membrane-initiated activity that is Gi/o and ERK1/2 dependent. These data, along with previous findings, define an important vascular endothelial cell signaling pathway that is activated by DHEA and suggest that this steroid may play a role in vascular function.
EXTENSIVE EVIDENCE FAVORS a vascular effect of the adrenal hormone dehydroepiandrosterone (DHEA). The evidence includes epidemiological studies showing that cardiovascular morbidity and mortality are inversely associated with plasma DHEA concentrations, particularly in males (1,2); animal studies showing antiatherogenic and vasculotonic effects of the steroid in diverse models of vascular dysfunction (2,3,4,5,6,7,8,9,10,11,12); and in vitro data suggesting beneficial effects of DHEA on vascular endothelium and smooth muscle (13,14,15) and key mediators of atherogenesis (16,17,18). Human studies support a beneficial effect of DHEA on angiographic evidence of atherosclerosis (19), markers of vascular risk (20,21,22,23,24), and vascular function (24,25).
The mechanisms underlying these findings remain unknown. A component of these effects may be due to conversion of the hormone to estrogens (26). However, DHEA has vascular effects that are independent of the estrogenic effects (25,26,27,28,29,30). We recently demonstrated that DHEA has a specific plasma membrane receptor on vascular endothelial cells (29). This G protein-coupled receptor is maximally activated by 1–10 nm DHEA to stimulate endothelial nitric oxide synthase (eNOS) and enhance nitric oxide production (30). This finding has been confirmed by others (28,31), and a similar G protein-coupled receptor has been identified in PC12 cells (32). We and others demonstrated that the release of nitric oxide depends on activation of ERK 1/2, a MAPK (28,30). Activation of ERK 1/2 is a crucial signaling event in a number of cellular functions, including proliferation, migration, cell growth, angiogenesis, survival, and apoptosis (33,34). In this study, we test the hypothesis that the effect of DHEA on the vasculature may relate in part to ERK 1/2-dependent activation of endothelial proliferation.
Materials and Methods
Reagents
The following reagents were used: DHEA, DHEA-17-carboxymethyl oxime-BSA conjugate (DHEA-BSA, 32:1 molar ratio of DHEA to BSA, according to manufacturer’s data sheet) from Steraloids (Newport, RI); ICI 182,780 from Tocris (St. Louis, MO); medium 199 (M199), fetal bovine serum (FBS), l-glutamine, and penicillin-streptomycin from Life Technologies, Inc.-BRL (Gaithersburg, MD); ERK 1/2, phospho-ERK 1/2 (Thr202/Tyr204), and phospho-p90 ribosomal S6 kinase (p90RSK; Ser380) antibodies from Cell Signaling Technology (Beverly, MA); Ki67 antibody from Chemicon (Temecula, CA); biotinylated antimouse antibody, avidin biotin complex kit, and diaminobenzidine substrate from Vector Laboratories (Burlingame, CA); matrigel and poly-l-lysine-coated coverslips from BD Biosciences (San Jose, CA); nitrocellulose membranes from Schleicher & Schuell (Keene, NH); CellTiter 96, an methylthiazolyldiphenyl-tetrazolium (MTT)-based cell proliferation assay kit from Promega (Madison, WI); SuperSignal chemiluminescence detection system from Pierce Chemical (Rockford, IL); pertussis toxin (PTX), PD098059, protease and phosphatase inhibitors, l-nitroarginine methyl ester (L-NAME), hydrogen peroxide, flutamide, and all other laboratory chemicals from Sigma Chemical (St. Louis, MO). All reagents used were of analytical grade. Stock solutions of steroids, at 10 mm in dimethyl sulfoxide, were stored at −20 C before use.
Cell culture
Bovine aortic endothelial cells (BAECs) were kindly provided by Dr. Robert Bar (University of Iowa, Iowa City, IA). Cells were grown in M199 supplemented with 20% FBS, 50 U/ml penicillin, and 0.05 mg/ml streptomycin and incubated at 37 C in a 5% CO2-95% air environment. The medium was changed every other day until the cells became confluent. BAECs were serially passaged after 0.05% trypsin treatment, and passages 4–8 were used in all experiments.
Western blotting
These assays were performed, as previously described (35), on BAECs that were grown in serum-free and phenol red-free M199 medium for 24 h before experimentation. Cells were incubated with various concentrations of DHEA or vehicle for 15 min (or other times as indicated). In some experiments, cells were preincubated with PTX (100 ng/ml) for 24 h or with PD098059 (20 μm) or vehicle (0.05% dimethyl sulfoxide) for 30 min before exposure to DHEA. Cells were sonicated, and 30 μg of detergent-extracted proteins were mixed with Laemmli buffer, heated for 5 min at 95 C, and resolved on 10% SDS-PAGE gels. Nitrocellulose membranes were probed with antibody against phospho-ERK 1/2 or phospho-p90RSK, according to the manufacturer’s instructions and the immunoreactive proteins detected by chemiluminescence. The membranes were stripped and reprobed with ERK 1/2 antibody to monitor for equal sample loading. The protein bands were quantitated with National Institutes of Health ImageJ software (Bethesda, MD). In one set of experiments, cytosolic and nuclear fractions, prepared by differential centrifugation (29), were used instead of total cell homogenates.
Cell proliferation assays
All media used during experiments were phenol red free, and the serum was charcoal stripped. Subconfluent BAECs were synchronized in serum-free medium for 24 h. For time-course experiments, BAECs were incubated with Hanks’ balanced salt solution (HBSS) for 30 min before addition of 1 nm DHEA for various times. After incubation with DHEA, cells were washed twice with HBSS and further incubated in M199 medium with 1% serum for up to 24 h. In parallel experiments, cells were incubated in 1% serum with vehicle or 5% serum as controls. For concentration-dependence experiments, cells were incubated with vehicle or various concentrations of DHEA in HBSS for 15 min, followed by M199 with 1% serum for up to 24 h. In some experiments, cells were preincubated with PTX (100 ng/ml) for 24 h or with PD098059 (20 μm), L-NAME (5 mm), ICI 182,780 (10 μm), or flutamide (10 μm) for 30 min and then coincubated for 15 min with DHEA or vehicle.
Cell proliferation was measured primarily by MTT-based cell proliferation assay or nuclear accumulation of Ki67 antigen. The MTT assays were carried out according to the manufacturer’s instructions, by assessing colorimetric change at absorbance of 490 nm in a 96-well plate format (FluoSTAR OPTIMA plate reader; BMG Labtech, Durham, NC). The cell numbers were calculated from a standard curve plotted with absorbance values for known cell numbers (counted manually with a hemocytometer) of BAECs and expressed as a percentage of the control. For immunocytochemical studies of Ki67 accumulation, BAECs were plated on poly-l-lysine-coated coverslips and placed in 24-well tissue culture plates. Cell proliferation conditions were as above. After 24 h of proliferation, the cells were fixed in 2% paraformaldehyde and endogenous peroxidase quenched with 3% hydrogen peroxide for 2 h at room temperature. The cells were solubilized in 0.25% Triton X-100 and endogenous biotin was blocked. The coverslips, preincubated with 1% BSA, were incubated with 1:300 primary antibody to Ki67 followed by a biotinylated secondary antibody. Control samples were incubated without the primary antibody. Avidin-biotin-peroxidase reagents and nickel-intensified diaminobenzidine were added to stain cells. Cells staining positively for Ki67 were counted manually using an LX71 microscope (Olympus, Center Valley, PA) under ×20 objective and expressed as a percentage of the total number of cells (stained and unstained cells). There were three or four coverslips per treatment for each experiment, and the experiment was repeated three times. Between 600 and 1000 cells were counted per treatment per experiment.
Cell migration assays
Cell migration was measured by two methods, a modified Boyden chamber and a monolayer wound assay. We used 24-well Transwell cell culture inserts with fibronectin-coated polycarbonate filters (pore size, 8 μm) (Costar, Corning, MA), as previously described (36), with modifications, for the modified Boyden chamber assay. Briefly, BAECs were serum starved for 6 h, harvested by trypsinization, washed with HBSS buffer, and placed in the upper Transwell compartment at 1 × 105 cells/well. Phenol red-free M199 containing DHEA or vehicle was added to the lower compartment of each well. In some experiments, cells were preincubated with or without PTX or PD098059, as above. After 2 h at 37 C in 5% CO2, nonmigrating cells on the upper surface of filter were removed. The cells that had traversed to the lower surface of the filters were fixed with ethanol, stained with trypan blue, and counted using a microscope with a ×20 objective. Each data point was the average number of cells in four randomly selected 1-mm2 fields. Each experiment was performed in triplicate, and migration was expressed as the average number of migrated cells counted per 1-mm2 field.
For the monolayer wound assay, near-confluent BAECs in 6-well plates were made quiescent in serum-free, phenol red-free media for 24 h and then further incubated in HBSS buffer for 30 min. The wound was created by drawing a 200-μl pipette tip in a line across the well, as described (37). The wounded monolayers were washed twice with HBSS buffer and incubated with DHEA or vehicle for 24 h. In some experiments, the cells were preincubated with PTX, PD098059, or vehicle, as above. The initial wounded areas were recorded with a digital camera. After 24 h incubation, the cells were fixed in 4% formaldehyde and randomly photographed for cell migration assessment. The images were analyzed with NIH ImageJ software. The wound healing effect was calculated as the cell-covered area after drug incubation divided by the cell-covered area after initial wounding, expressed as a percentage.
Angiogenesis
Angiogenesis was measured by two methods, growth of endothelial tubes in agarose and a chick chorioallantoic membrane (CAM) assay. We measured the formation of capillary-like structures of endothelial cells in growth factor-reduced Matrigel as described (38,39). BAECs were seeded on Matrigel (5 mg/ml)-coated, 12-well plates at 2 × 105 cells/well and incubated with DHEA or vehicle in phenol red-free M199 medium containing charcoal-stripped 1% FBS. In some experiments, cells were preincubated with PTX or PD098059 (as previously) before seeding on Matrigel. The formation of tube-like structures was optimal at 3 h. We photographed four random fields per condition using a phase-contrast microscope at ×10 magnification. The tubule length formed per field was measured with NIH ImageJ software, and the mean value was determined for each experiment.
The chick embryo CAM angiogenesis assay was performed as follows. Fertilized eggs were incubated at 37.8 C in 60–70% humidity. On d 5, a small square window was made in the region of the air sac and the shell was peeled off to expose the CAM of the chick embryo. The windows were sealed with plastic tape and incubation of the eggs was continued for another 24 h. On d 6, sterilized gelatin sponges of 5 mm diameter were soaked with 20 μl of sterile saline or DHEA diluted in saline and applied onto the top of the growing CAMs. Ten eggs were included in each group. The windows were resealed and the eggs incubated for anther 72 h. On d 9, 1 ml of fixative buffer, containing methanol-acetone [1:1, vol/vol)], was added via the window for 15 min, and then the CAMs were removed. Photos of each CAM, centered on the sponge, were taken with a digital camera (Coolpix 950; Nikon, Tokyo, Japan) through a stereomicroscope (Nikon SMZ800). The number of blood vessels that crossed the boundary of the gelatin sponge was counted and the mean value determined for each condition.
Transient transfection
BAECs were grown to 50% confluence in 12-well plates and then were transiently transfected with 1 μg of a dominant-negative ERK 2 plasmid, wild-type ERK 2 plasmid, or a control parent vector (p3XFLAG-CMV-7.1) (plasmids kindly provided by Dr. M. Cobb, University of Texas Southwestern Medical Center, Dallas, TX), using a liposome-mediated transfection procedure (Lipofectamine; Invitrogen, Carlsbad, CA). β-Galactosidase-encoding plasmids were cotransfected to normalize results for the transfection efficiency. After transfection, cells were incubated with complete medium containing 20% FBS for 24 h followed by synchronization for another 24 h in serum-deprived medium before stimulation with DHEA (1 nm) or vehicle for 15 min at 37 C. The activity of ERK 1/2 was determined in cell lysates by measuring the phosphorylation of p90RSK in Western blots (as above). DHEA-stimulated cell proliferation was determined in transfected cells as described above.
Statistical analysis
Data in each study were derived from at least three independent experiments and variation within treatments was expressed as the sem. All data were subjected to a one-way ANOVA using the general linear model procedure of SAS (Cary, NC) (or SPSS version 10 for CAM experiments; SPSS, Chicago, IL) and treatment differences were subjected to a Duncan’s multiple comparison test at the 5% probability.
Results
DHEA increases ERK 1/2 activity
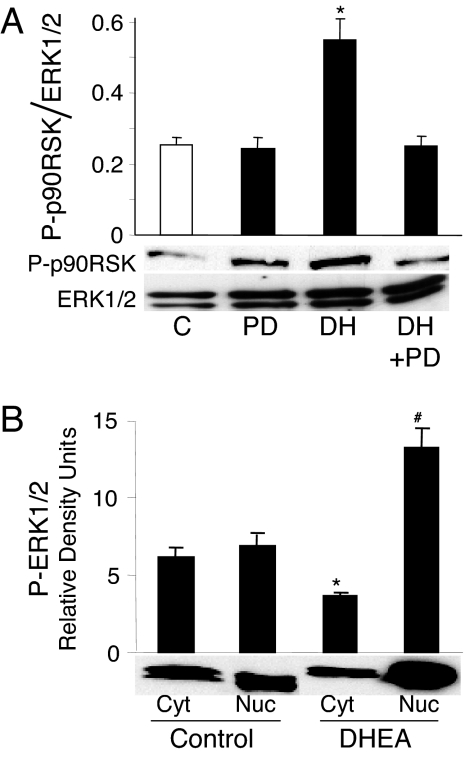
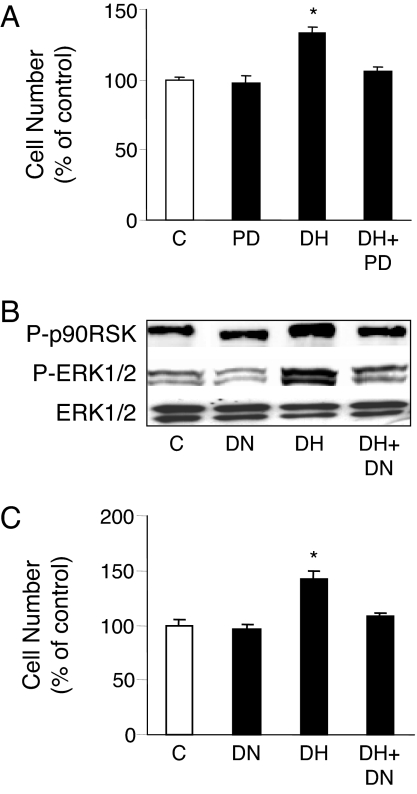
DHEA has recently been shown to rapidly phosphorylate the Raf-MAPK kinase (MEK)-ERK 1/2 signaling cascade in vascular endothelial cells (25,28,31). We confirmed these data and showed that the peak phosphorylation of ERK 1/2 in BAECs was at 15 min incubation and between 0.1 and 10 nm DHEA (data not shown). Upon activation, ERKs phosphorylate downstream kinases, including p90RSK and also translocate to the nucleus, in which they activate transcription factors (40). As shown in Fig. 1A, incubation of BAECs with DHEA (1 nm) for 15 min stimulated the phosphorylation of p90RSK. Inhibition of the ERK cascade with the MEK 1/2 inhibitor, PD098059, completely abolished the activation of p90RSK. Furthermore, DHEA stimulated the nuclear accumulation of phosphorylated-ERK 1/2 along with a decline in phosphorylated-ERK 1/2 in the cytosol (Fig. 1B). Coincident with these DHEA-induced changes in phosphorylated-ERK 1/2, the cytosolic total ERK 1/2 decreased by 40%, and the nuclear expression of total ERK 1/2 increased by 52.8% (data not shown). These data demonstrate that DHEA rapidly activates ERK 1/2 signaling and ERK 1/2 translocation to the nuclear compartment.
Figure 1.
DHEA stimulates phosphorylation of downstream kinases and nuclear translocation of activated ERK 1/2. A, Serum-deprived BAECs were preincubated with 20 μm PD098059 (PD) for 30 min before stimulation with 1 nm DHEA (DH) for 15 min. Western analysis was performed to detect phosphorylated p90RSK, which was normalized to total ERK 1/2 from the same sample. Means ± sem of normalized densitometry measurements from three separate experiments, along with representative blots, are shown. *, P < 0.05 vs. vehicle-treated controls (C) or PD098059 preincubated cells. B, Serum-deprived BAECs were incubated in the presence of 1 nm DHEA or vehicle at 37 C for 15 min. Cells were lysed and nuclear and cytoplasmic fractions were separated and collected by differential centrifugation. Western analysis was performed to detect phosphorylated ERK 1/2 in different fractions. Means ± sem of densitometry measurements from three separate experiments, along with representative blots, are shown. *, #, P < 0.05 vs. vehicle-treated cytosol (Cyt) and nuclei (Nuc), respectively.
Plasma membrane-initiated, Gi-protein-dependent effects of DHEA on the ERK 1/2 cascade
There is no known receptor for DHEA. We and others have pharmacologically characterized a high-affinity, plasma membrane DHEA receptor that couples to Gi-proteins and mediates rapid cellular actions of DHEA (29,30,32). To determine whether the DHEA effect on ERK 1/2 phosphorylation is initiated from the plasma membrane, we compared the acute effects of DHEA and the plasma membrane-impermeable DHEA-BSA on ERK 1/2 phosphorylation. As shown in Fig. 2A, DHEA-BSA (containing 1 nm DHEA) caused the same level of ERK 1/2 phosphorylation as DHEA, whereas BSA alone was without effect. We did not detect free DHEA in the DHEA-BSA used in this study (<3%; data not shown). These data suggest that the effect of DHEA on ERK 1/2 activation is dependent on a signal initiated from the cell surface.
Figure 2.
DHEA stimulates ERK 1/2 phosphorylation in BAECs by a plasma membrane-dependent and Gi protein-coupled action. Serum-starved BAECs were incubated with 1 nm DHEA, DHEA-BSA conjugate containing 1 nm DHEA, 1 nm BSA, or vehicle (C) for 15 min at 37 C (A) or preincubated with or without PTX (100 ng/ml) for 24 h and then incubated with 1 nm DHEA or vehicle for 15 min (B). Phosphorylated and total ERK 1/2 was determined by Western blotting. Means ± sem of normalized densitometry measurements from three separate experiments each, along with representative blots, are shown. *, P < 0.05 vs. vehicle-treated controls or PTX preincubated cells.
We also tested whether DHEA-induced ERK 1/2 phosphorylation was mediated through PTX-sensitive G-proteins, which can couple membrane receptors to activation of ERK 1/2 signaling (41). Preincubation of BAECs with PTX (100 ng/ml) for 24 h did not alter the basal phosphorylation of ERK 1/2 but fully inhibited the DHEA-induced phosphorylation (Fig. 2B).
DHEA effect on endothelial cell proliferation
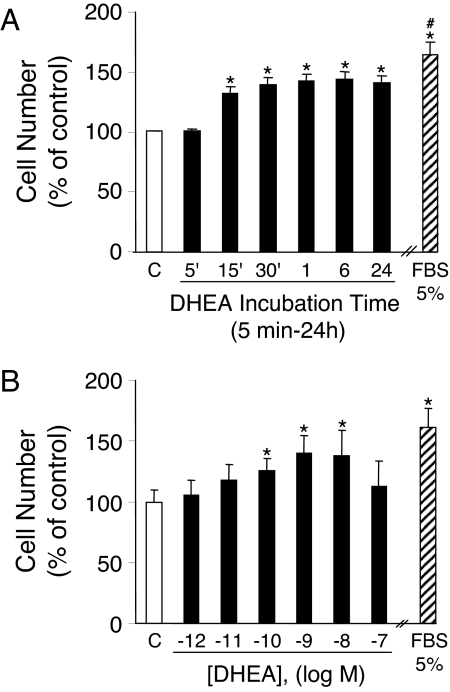
It is well recognized that the ERK 1/2 signaling pathway plays an important role in cell proliferation and growth (42,43). We therefore evaluated whether DHEA could stimulate the proliferation of BAECs. As shown in Fig. 3A, administration of DHEA (1 nm) time-dependently stimulated cell proliferation in an MTT-based cell proliferation assay. Exposure to DHEA for only 15 min increased the cell proliferation by 31% over the subsequent 24 h. Twenty-four hours of DHEA exposure was associated with a 41% increase in cell proliferation. Incubation of cells with 5% FBS for 24 h induced a 65% increase in proliferation over control. The effect of DHEA was concentration dependent, with 1 nm DHEA inducing maximal cell proliferation of 40% over control (Fig. 3B). The results on DHEA-induced endothelial cell proliferation were confirmed using both [3H]thymidine incorporation assays (43) and manual cell counting with a hemocytometer (data not shown).
Figure 3.
DHEA stimulates endothelial proliferation. BAECs were incubated with vehicle (C) or 5% FBS for 24 h or 1 nm DHEA for different times (A) or with various concentrations of DHEA for 15 min at 37 C (B). After stimulation, cell proliferation was determined as described in Materials and Methods using an MTT-based assay kit. Data were expressed as mean ± sem of four separate experiments. *, P < 0.05 vs. vehicle-treated control; #, P < 0.05 vs. DHEA-treated cells.
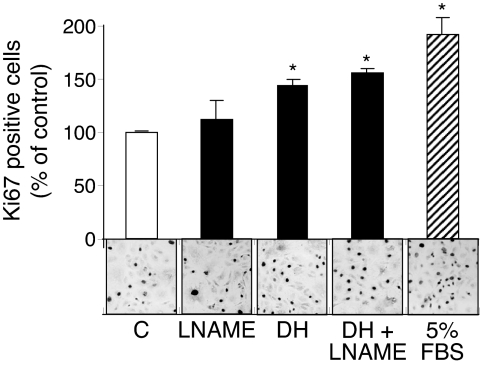
DHEA decreases apoptosis in BAECs under serum-free conditions (44). To confirm that the effect of DHEA in our experiments was proliferative, rather than a decrease in cell death, we immunohistochemically assessed the effect of DHEA on the nuclear expression of the Ki67 antigen. Consistent with the MTT assay, 1 nm DHEA induced a 44% increase in Ki67 stained nuclei (Fig. 4). Additionally, because endothelial proliferation may be nitric oxide dependent (45), we tested the effect of preincubation with L-NAME, an eNOS inhibitor. The DHEA-induced increase in endothelial proliferation was not affected by L-NAME, which we verified to block ligand-induced nitric oxide production in parallel studies (data not shown). We also confirmed (25) that the DHEA-induced increase in cell proliferation was not blocked by preincubation of the BAECs with estrogen or androgen receptor inhibitors (ICI181,780 or flutamide, data not shown). These data are consistent with a DHEA-specific pathway to cellular proliferation that is not dependent on the estrogen or androgen receptor or the known generation of nitric oxide by DHEA.
Figure 4.
DHEA stimulates endothelial proliferation independent of eNOS activity. BAECs were incubated with vehicle (C) or 5% FBS for 24 h or 1 nm DHEA for 15 min at 37 C for 24 h. L-NAME (5 mm) was added 30 min prior and also coincubated for 15 min with or without DHEA. After 24 h the cells were fixed and immunostained for Ki 67 proliferative antigen (as described in Materials and Methods). Data from three independent experiments with triplicate samples, along with representative fields at ×20 magnification, are shown. Data were expressed as means ± sem of Ki67-positive cells as a percentage of total cells (stained and unstained for Ki67) from at least 3000 total cells counted per condition. *, P < 0.05 vs. vehicle (C).
Plasma membrane-initiated and Gi-protein-dependent effects of DHEA on cell proliferation
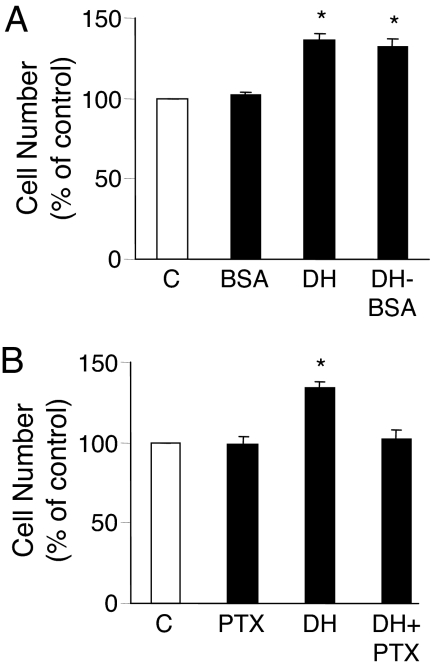
Because we have shown that the DHEA signaling to ERK 1/2 activation was initiated from the cell surface and involved PTX-sensitive G proteins and that ERK 1/2 signaling can mediate cellular proliferation (42,43), we determined whether the same signal transduction pathway mediated the DHEA-stimulated endothelial cell proliferation. The membrane impermeable ligand, DHEA-BSA, was equally potent with DHEA in stimulating BAEC proliferation, but BSA alone had no effect (Fig. 5A). Furthermore, preincubation of BAECs with PTX before stimulation with DHEA effectively inhibited DHEA-induced cell proliferation (Fig. 5B).
Figure 5.
DHEA increases BAEC proliferation by a plasma membrane-dependent and Gi protein-coupled action. Serum-starved BAECs were incubated with 1 nm DHEA, DHEA-BSA conjugate containing 1 nm DHEA, 1 nm BSA, or vehicle (C) for 15 min at 37 C (A) or preincubated with or without PTX (100 ng/ml) for 24 h and then incubated with or without 1 nm DHEA for 15 min (B). Cells were then incubated in phenol red-free medium plus 1% serum, and proliferation was measured 24 h later using an MTT-based assay kit. Data were expressed as mean ± sem of four separate experiments each. *, P < 0.05 vs. vehicle-treated controls or PTX preincubated cells.
ERK 1/2 cascade-dependent effects of DHEA on cell proliferation
Because DHEA both induced ERK 1/2 activity and stimulated cell proliferation, we next determined whether the activation of ERK 1/2 was involved in DHEA-induced cell proliferation. BAECs were preincubated with PD098059 for 30 min or transfected with plasmids expressing the dominant-negative ERK 2 cDNA. After 15 min stimulation with DHEA, cells were either lysed for Western blotting or maintained in phenol red-free M199 containing 1% charcoal-stripped serum for proliferation assays. Consistent with the PD098059 effects to inhibit DHEA-induced phosphorylation of ERK 1/2 and p90RSK (Fig. 1B and data not shown), the inhibition of MEK 1/2 blocked the DHEA-induced cell proliferation (Fig. 6A). Additionally, transfection with the dominant-negative ERK 2 plasmid largely prevented the DHEA-induced phosphorylation of ERK 1/2 and p90RSK (Fig. 6B) and decreased the DHEA-induced cell proliferation by 81%, whereas transfection with control plasmids was without effect (Fig. 6C). Transfection efficiency, based on β-galactosidase activity, was about 50%, similar to other reports with these reagents and cells (46).
Figure 6.
DHEA-stimulated endothelial proliferation is ERK 1/2 dependent. BAECs were preincubated with PD098059 (PD; 20 μm) for 30 min (A) or transfected with ERK 2 dominant-negative (DN) plasmid or vector alone (C) (B and C) before incubation with 1 nm DHEA (DH) or vehicle for 15 min at 37 C. Incubation was then either terminated to determine ERK 1/2 and p90RSK phosphorylation (B) or continued for cell proliferation assays (A and C). Data were expressed as mean ± sem of three separate experiments each. *, P < 0.05 vs. vehicle-treated control or PD098059 preincubated or ERK 2 dominant-negative plasmid-transfected cells.
DHEA effects on BAEC migration
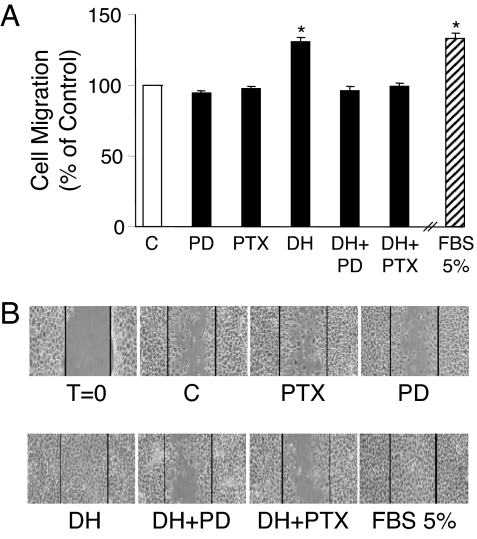
The chemotactic migration of endothelial cells is one of the critical steps in the process of angiogenesis and the repair of injured vessels. We therefore examined the effect of DHEA on endothelial cell migration by using a modified Boyden chamber. As shown in Fig. 7A, incubation of cells with 1 nm DHEA induced migration of BAEC by 33%, a magnitude comparable with its induction of cell proliferation. Preincubation of cells with PTX or PD098059 abolished the DHEA effect (Fig. 7A), suggesting that a Gi protein- and ERK 1/2-dependent signaling pathway also mediates the DHEA-induced cell migration.
Figure 7.
DHEA induces migration of BAECs that is dependent on Gi proteins and ERK 1/2 cascade. BAECs were preincubated with PD098059 (PD; 20 μm) or vehicle for 30 min or with PTX (100 ng/ml) or vehicle for 24 h. A, Cell migration was determined by modified Boyden chamber assay in the presence of 1 nm DHEA (DH) or vehicle (C) or 5% FBS, as described in Materials and Methods. Data were expressed as mean ± sem from three independent experiments, each assayed in duplicate. *, P < 0.05 vs. vehicle-treated control or inhibitor preincubated cells. B, Cell cultures were wounded and incubated in 1% FBS medium for 24 h, with or without 1 nm DHEA (DH) and in the continued presence or absence of PD098059 or PTX. Representative photographs are shown of cells immediately after wound induction (T = 0) or after 24-h incubations with the additives noted, from three independent experiments, each performed with duplicate samples.
We also used the monolayer wound healing assay to examine the effect of DHEA on endothelial proliferation and migration. A specific area of endothelial denudation was produced in near-confluent BAEC monolayers using a fine pipette tip. Incubation with DHEA (1 nm) significantly stimulated the migration of BAECs from the edge of the wound into the open area (Fig. 7B). After 24 h of incubation, the wounded area in cells treated with DHEA was completely covered by the migrated cells. DHEA was nearly as effective as 5% FCS, whereas only about 50% of wounded area was reendothelialized in control cultures. Preincubation of cultures with PTX or PD98059 blocked the effect of DHEA on wound healing of BAECs (Fig. 7B).
DHEA effects on in vitro angiogenesis
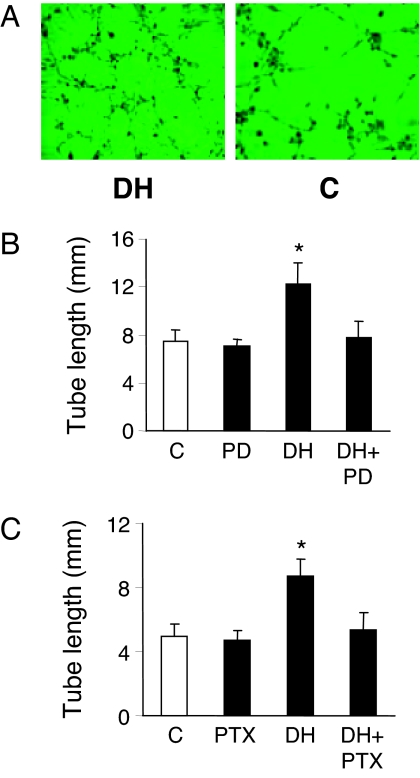
Angiogenesis is a complex process involving endothelial cell proliferation and migration, with the formation of vascular tube structures. We evaluated endothelial tube formation, an in vitro model of angiogenesis, by plating BAECs onto growth factor-reduced Matrigel and incubating with or without DHEA in phenol red-free medium containing 1% charcoal-stripped serum. Endothelial cells developed significantly more extensive tube/cord-like networks when incubated with DHEA rather than vehicle for 3 h (Fig. 8A). This effect was substantially ablated by MEK 1/2 inhibition using PD098059 (Fig. 8B). To determine whether Gi proteins were involved in this DHEA effect, BAECs were preincubated with PTX (200 ng/ml) for 6 h before being plated onto Matrigel and stimulated with DHEA. Preincubation of cells with PTX greatly reduced DHEA-induced tube-like structure formation (Fig. 8C). These results suggest an angiogenic effect of DHEA that is also mediated by a Gi protein-dependent ERK 1/2 signal transduction pathway.
Figure 8.
DHEA induces capillary tube-like formation that is dependent on Gi proteins and ERK 1/2 cascade. BAECs were plated on Matrigel and exposed to DHEA (DH) or vehicle (C) for 3 h. A representative low-magnification image from each condition is shown (A). BAECs were preincubated with PD098059 (PD; 20 μm) or vehicle for 30 min (B) or with PTX (200 ng/ml) or vehicle for 6 h (C) before incubation on Matrigel-covered culture dishes in the presence or absence of 1 nm DHEA for 3 h. The length of tubular structures from three randomly photographed fields (×10 objective) per well was measured using NIH ImageJ software. Data were expressed as mean ± sem from three independent experiments. *, P < 0.05 vs. vehicle-treated control.
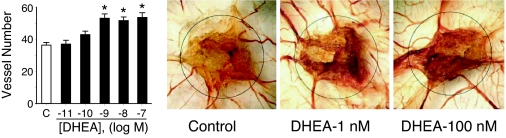
To confirm the angiogenic activity of DHEA using an in vivo technique, we assessed the effect of DHEA on blood vessel formation in the chick embryo CAM assay (Fig. 9). The CAMs were incubated with vehicle or DHEA (10 pm to 100 nm) for 3 d. Evaluation of the number of vessels crossing the rim of the DHEA- or vehicle-soaked sponge showed a DHEA concentration-dependent increase in vessel density in CAMs when compared with controls.
Figure 9.
DHEA induces angiogenesis in the chick embryo CAM assay. After 3 d incubation with DHEA (10 pm to 100 nm) or vehicle, the number of vessels crossing into the sponge area was counted. Representative photographs are shown along with cumulative data, expressed as mean ± sd from five to eight eggs in each group. *, P < 0.05 vs. vehicle-treated control.
Discussion
In this report we provide evidence linking DHEA effects at the endothelial plasma membrane with cellular proliferation and angiogenesis by a process mediated by PTX-sensitive G proteins and ERK 1/2. PTX completely blocked DHEA-induced ERK 1/2 activation, endothelial cell proliferation and migration, and vascular tube formation, suggesting that Gi proteins proximally link DHEA effects with these biological events in endothelial cells. Furthermore, both DHEA and the plasma membrane-impeded ligand, DHEA-BSA, activated ERK 1/2 and endothelial cell proliferation comparably at equimolar concentrations, suggesting that the biological effects of DHEA are initiated by events at the endothelial cell plasma membrane. Steroids coupled to BSA or other large proteins have been used extensively to characterize plasma membrane-initiated signaling (47,48,49,50,51). Whereas dissociation of steroid-BSA complexes with increased free steroid has been reported (52), there was no detectable free DHEA in the DHEA-BSA used in the present study (53). Collectively, our findings are consistent with a plasma membrane, Gi protein-coupled DHEA receptor. How the putative endothelial DHEA receptor, linked to PTX-sensitive G proteins, interacts with the ERK 1/2 pathway remains to be elucidated. However, Simoncini et al. (28) have shown that DHEA activates Raf-1, a kinase upstream of MEK and ERK 1/2. Raf-1, in turn, is downstream of nonreceptor tyrosine kinases, which we have shown to be involved in the activation of eNOS by DHEA (30) and which can be activated by Gi protein-linked receptors (54).
Vascular endothelial cell ERK 1/2 is activated in response to a large array of extracellular stimuli, including growth factors, estrogen, and shear stress (43,46,55). Activated ERK 1/2 plays a pivotal role in environmentally stimulated cellular responses, including cellular proliferation, growth, differentiation, and survival (33,34). The phosphorylation of ERK 1/2 by DHEA in endothelial cells has previously been described (25,28,31), and the rapid ERK 1/2-dependent activation of eNOS is independent of gene transcription and new protein synthesis (28). Our new data functionally link DHEA-induced activation of ERK 1/2 with cellular proliferation. Our time-course studies showed that peak activation of both ERK 1/2 and cellular proliferation was at 15 min exposure to DHEA. Simoncini et al. (28) previously noted a peak ERK 1/2 activation in human endothelial cells at 15 min. Similarly, the activation of ERK 1/2 by DHEA was maximal at between 0.1 and 10 nm DHEA, and the maximal stimulation of cell proliferation was at 1–10 nm DHEA. These concentrations are well within the plasma concentrations of DHEA in adult humans and are consistent with the binding kinetics of the putative plasma membrane DHEA receptor (29,30,32). Cellular proliferation was inhibited by either a specific inhibitor of the ERK 1/2 kinase, MEK 1/2, or expression of a dominant-negative mutant of ERK 2. These data indicate that short-term exposure to DHEA is sufficient to induce endothelial proliferation and that activation of ERK 1/2 is a necessary signaling component in this action.
The increased cellular proliferation, noted at 24 h after incubation with DHEA for 15 min or more, is consistent with data from studies of other cell proliferative agents (56,57,58). Jones et al. noted that a 30-min pulse of platelet-derived growth factor (PDGF) enhanced the proliferative response to subsequent incubation with PDGF. Similar to our data they show that there was little response to 10 h of PDGF alone without a preceding PDGF pulse and that there was no further increase in the proliferative effect if the pulse of PDGF was longer than 30 min. Note that our experiments involved incubation with DHEA in serum-free buffer for 15 min or more followed by incubation in 1% fetal bovine serum medium, which would have contained growth factors.
The mechanisms that link DHEA through ERK 1/2 to cellular proliferation remain unclear. However, in these studies we extended the signaling data on DHEA in vascular endothelial cells to show ERK 1/2-dependent phosphorylation of p90RSK, a directly targeted substrate of ERK 1/2, which phosphorylates nuclear transcription factors (59). We also show rapid nuclear translocation of activated ERK 1/2, a critical step in the transcriptional and cell proliferative effects of this kinase (60). Nuclear translocation of activated ERK 1/2 can phosphorylate transcriptional factors, such as c-myc and Elk-1, leading to cell proliferation and differentiation (61,62,63,64). Whether DHEA interacts directly with transcription factors to regulate cell proliferation, in addition to its plasma membrane-initiated kinase signaling effects, remains to be determined. Whereas the best-characterized effects of steroids are mediated by nuclear hormone receptors, which function as transcription factors on ligand interaction, there is increasing evidence that steroids activate plasma membrane receptors and cytosolic kinase cascades (47,48,50,51,65,66,67,68,69,70,71,72).
Previous studies examining the effect of DHEA on endothelial cell proliferation are in consensus that incubation of cells with concentrations of DHEA far above the normal range seen in human plasma (∼1–50 nm) are associated with decreased proliferation (Table 1). Furthermore, most studies report that endothelial cell incubation with concentrations of DHEA close to human plasma levels are associated with increased proliferation (Table 1). It should be noted that the actual intracellular concentration of DHEA will depend on the cell-specific expression and activity of key steroid metabolizing enzymes and thus may differ significantly from plasma concentrations in some tissues (73). Consistent with our data, three separate groups of researchers demonstrated phosphorylation of the proproliferative kinase ERK 1/2 at 0.1–100 nm DHEA (25,28,31). The biphasic effect of DHEA on cell proliferation and ERK 1/2 activation that we describe here is also consistent with the pattern of effects that we have shown for DHEA on eNOS and G protein activation (30). Similar biphasic effects of DHEA on proliferation are reported in other cell types (74,75,76), and these are seen also with other steroids, including estrogen (77). In contrast to our findings, the studies of Varet et al. (78) and Zapata et al. (79) showed no effect on proliferation at 1–100 nm DHEA concentrations. There are a number of variables that may account for the differences seen in these studies including cell-specific differences between immortalized microvascular cell lines and the primary cell cultures of large vessels (80,81,82), differential sensitivity of specific endothelial beds to specific ligands, based on differential receptor expression (83), species differences in the expression of key signaling molecules (84), and differences in ligand responses related to passage number (36). Another key difference with the studies of Varet et al. may be their use of 2.5 μm hydrocortisone in the culture medium because glucocorticoids and DHEA have been shown to be antagonistic in some systems (85).
Table 1.
Comparison of endothelial cell proliferation by specified concentrations and durations of incubation of DHEA
| Citation | Endothelial cell type | Proliferative effect at low DHEA concentration | Proliferative effect at high DHEA concentration |
|---|---|---|---|
| Mohan and Benghuzzi (14) | Rabbit aortic | ↑ at 17 nm × 24 h | ↓ at 174 nm × 48 h |
| Hinson and Khan (92) | Human microvascular | ↑ at 0.1–1 nma | ↓ at 10 μm × 72 h |
| Williams et al. (25) | BAEC | ↑ at 10–100 nm × 48 h | Not tested |
| Varet et al. (78) | Human microvascular | ↔ at 1–50 nm × 72 h | ↓ at 10–100 μm × 72 h |
| Zapata et al. (79) | Human umbilical vein | ↔ at 1–100 nm × 72 h | ↓ at 1–100 μm × 72 h |
| Liu (This study) | BAEC | ↑ at 0.1–10 nm × 24 h | ↔ at 100 nm × 24 h |
↑, Increased; ↓, decreased; ↔, no change in proliferation.
Personal communication from J. P. Hinson and Ref. 93.
DHEA is metabolized intracellularly to other biologically active steroids. Metabolites of DHEA include estradiol, which also induces vascular endothelial proliferation by activation of MAPKs (86). Testosterone, another metabolite of DHEA, does not acutely activate ERK 1/2 or increase vascular proliferation (87,88). Additionally, there are many other metabolites of DHEA (89), some of which have known biological activities, e.g. 7 oxo-DHEA (90) or androstenediol (91). The signaling mechanisms for these steroids in vascular endothelial cells or elsewhere are not known. Data that suggest that the acute vascular endothelial effects of DHEA demonstrated here are unrelated to its metabolic conversion include: 1) the effect of DHEA on endothelial ERK 1/2 activation, endothelial proliferation, and eNOS activation is not prevented by antagonism of the estrogen or androgen receptors (current study and Refs. 25 and 28,29,30,31); 2) cell-impermeable DHEA-BSA had a comparably potent and rapid effect on ERK 1/2 phosphorylation and cell proliferation as DHEA; 3) DHEA is not detectably metabolized to estradiol over a period of 48 h of coincubation of DHEA in human vascular endothelial cells (28); 4) DHEA metabolites including estradiol, testosterone, DHEA sulfate, and androstenedione do not bind the putative G protein-coupled DHEA receptor and, besides estradiol, do not stimulate endothelial nitric oxide production (29); and 5) because DHEA is effective at subnanomolar concentrations, any metabolite effect would need to be significantly more potent than DHEA, given the short time course for potential metabolic transformation. Our data are consistent with the concept of a plasma membrane G protein-coupled receptor with specificity for DHEA, which we previously proposed (29) and others have corroborated (32). It is evident that human plasma concentrations of DHEA are such that the putative receptor would usually be fully saturated, based on our in vitro pharmacokinetic studies (29). However, the concentration of DHEA in plasma that is available for receptor binding (free concentration) may be less than the total plasma concentration, similar to other steroids. Additionally, we anticipate that the specificity of DHEA action will be determined by tissue-specific DHEA receptor expression and close regulation of the expression and activity of the putative receptor by processes such as homologous or heterologous desensitization, expression of coreceptors or other interacting proteins, etc.
How our studies translate to human physiology remains to be determined. DHEA administration to humans does improve vascular endothelial function (24,25), but whether these effects are mediated by a specific DHEA receptor or intracellular metabolism to estrogen is unknown. DHEA does increase human vascular endothelial proliferation and tube formation on Matrigel, similar to the results with BAECs (data not shown).
In summary, DHEA acted in a novel, rapid, plasma membrane-initiated, Gi-dependent manner to stimulate the ERK 1/2 cascade in vascular endothelial cells. DHEA increased vascular endothelial proliferation, migration, and vascular tube formation by activating the ERK 1/2 pathway, which may be an important mechanism underlying the physiological effects of DHEA in the vasculature. These findings also provide the basis for further studies of the downstream signaling mechanisms from ERK 1/2 and evaluation of the genomic action of DHEA in angiogenesis, thereby defining the fundamental role of DHEA in the vascular system at the cellular and molecular levels. Finally, although endothelial proliferation is crucial in wound healing and development, it is also a component of pathological conditions such as diabetic retinopathy and tumor growth. Because DHEA is widely available in the community, it may be important to evaluate for possible side effects related vascular proliferation.
Acknowledgments
We acknowledge critical evaluations of the manuscript by Drs. R. Tomanek and K. Lamping and editorial assistance by Paul Casella.
Footnotes
This work was supported by grants from the Office of Research and Development, Department of Veterans Affairs (to J.S.D.), National Institutes of Health Grant AG55741 (to J.S.D.), American Heart Association (to J.S.D.), Virginia Polytechnic Institute and State University (to D.L.), and the Thomas F. Jeffress and Kate Jeffress Memorial Trust (to D.L.).
Current address for D.L.: Department of Human Nutrition, Foods, and Exercise, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24060.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 13, 2007
See editorial p. 886.
Abbreviations: BAEC, Bovine aortic endothelial cell; CAM, chorioallantoic membrane; DHEA, dehydroepiandrosterone; eNOS, endothelial nitric oxide synthase; FBS, fetal bovine serum; HBSS, Hanks’ balanced salt solution; L-NAME, l-nitroarginine methyl ester; M199, medium 199; MEK, MAPK kinase; MTT, methylthiazolyldiphenyl-tetrazolium; PDGF, platelet-derived growth factor; PTX, pertussis toxin.
References
- Barrett-Connor E, Goodman-Gruen D 1995 The epidemiology of DHEAS and cardiovascular disease. Ann NY Acad Sci 774:259–270 [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Haarbo J, Christiansen C 1996 The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis 125:1–13 [DOI] [PubMed] [Google Scholar]
- Arad Y, Badimon JJ, Badimon L, Hembree WC, Ginsberg HN 1989 Dehydroepiandrosterone feeding prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbit. Arteriosclerosis 9:159–166 [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C 1999 Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res 84:813–819 [DOI] [PubMed] [Google Scholar]
- Lohman R, Yowell R, Barton S, Araneo B, Siemionow M 1997 Dehydroepiandrosterone protects muscle flap microcirculatory hemodynamics from ischemia/reperfusion injury: an experimental in vivo study. J Trauma 42:74–80 [DOI] [PubMed] [Google Scholar]
- Eich DM, Nestler JE, Johnson DE, Dworkin GH, Ko D, Wechsler AS, Hess ML 1993 Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation 87:261–269 [DOI] [PubMed] [Google Scholar]
- Bednarek-Tupikowska G, Milewicz A, Kossowska B, Bohdanowicz-Pawlak A, Sciborski R 1995 The influence of DHEA on serum lipids, insulin and sex hormone levels in rabbits with induced hypercholesterolemia. Gynecol Endocrinol 9:23–28 [DOI] [PubMed] [Google Scholar]
- Kurzman ID, Panciera DL, Miller JB, MacEwen EG 1998 The effect of dehydroepiandrosterone combined with a low-fat diet in spontaneously obese dogs: a clinical trial. Obes Res 6:20–28 [DOI] [PubMed] [Google Scholar]
- Haffa AL, MacEwen EG, Kurzman ID, Kemnitz JW 1994 Hypocholesterolemic effect of exogenous dehydroepiandrosterone administration in the rhesus monkey. In Vivo 8:993–997 [PubMed] [Google Scholar]
- Christopher-Hennings J, Kurzman ID, Haffa AL, Kemnitz JW, MacEwen EG 1995 The effect of high fat diet and dehydroepiandrosterone (DHEA) administration in the rhesus monkey. In Vivo 9:415–420 [PubMed] [Google Scholar]
- Yorek M, Coppey L, Gellett J, Davidson E, Bing X, Lund D, Dillon J 2002 Effect of treatment of diabetic rats with dehydroepiandrosterone on vascular and neural function. Am J Physiol 283:E1067–E1075 [DOI] [PubMed] [Google Scholar]
- Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE 2003 Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100:9488–9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo M, Shan J, Pang PK, Resnick LM 1995 Effects of dehydroepiandrosterone sulfate on cellular calcium responsiveness and vascular contractility. Hypertension 26:1065–1069 [DOI] [PubMed] [Google Scholar]
- Mohan PF, Benghuzzi H 1997 Effect of dehydroepiandrosterone on endothelial cell proliferation. Biomed Sci Instrum 33:550–555 [PubMed] [Google Scholar]
- Yoneyama A, Kamiya Y, Kawaguchi M, Fujinami T 1997 Effects of dehydroepiandrosterone on proliferation of human aortic smooth muscle cells. Life Sci 60:833–838 [DOI] [PubMed] [Google Scholar]
- Khalil A, Lehoux JG, Wagner RJ, Lesur O, Cruz S, Dupont E, Jay-Gerin JP, Wallach J, Fulop T 1998 Dehydroepiandrosterone protects low density lipoproteins against peroxidation by free radicals produced by γ-radiolysis of ethanol-water mixtures. Atherosclerosis 136:99–107 [DOI] [PubMed] [Google Scholar]
- Mohan PF, Jacobson MS 1993 Inhibition of macrophage superoxide generation by dehydroepiandrosterone. Am J Med Sci 306:10–15 [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Yanase T, Kobayashi K, Takayanagi R, Nawata H 1996 Dehydroepiandrosterone markedly inhibits the accumulation of cholesteryl ester in mouse macrophage J774-1 cells. Atherosclerosis 126:143–154 [DOI] [PubMed] [Google Scholar]
- Herrington DM 1995 Dehydroepiandrosterone and coronary atherosclerosis. Ann NY Acad Sci 774:271–280 [DOI] [PubMed] [Google Scholar]
- Mortola JF, Yen SS 1990 The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 71:696–704 [DOI] [PubMed] [Google Scholar]
- Beer NA, Jakubowicz DJ, Matt DW, Beer RM, Nestler JE 1996 Dehydroepiandrosterone reduces plasma plasminogen activator inhibitor type 1 and tissue plasminogen activator antigen in men. Am J Med Sci 311:205–210 [DOI] [PubMed] [Google Scholar]
- Jesse RL, Loesser K, Eich DM, Qian YZ, Hess ML, Nestler JE 1995 Dehydroepiandrosterone inhibits human platelet aggregation in vitro and in vivo. Ann NY Acad Sci 774:281–290 [DOI] [PubMed] [Google Scholar]
- Lasco A, Frisina N, Morabito N, Gaudio A, Morini E, Trifiletti A, Basile G, Nicita-Mauro V, Cucinotta D 2001 Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol 145:457–461 [DOI] [PubMed] [Google Scholar]
- Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H 2003 Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab 88:3190–3195 [DOI] [PubMed] [Google Scholar]
- Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, Sudhir K, Komesaroff PA 2004 Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab 89:4708–4715 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Esaki T, Muto E, Kano H, Asai Y, Thakur NK, Sumi D, Jayachandran M, Iguchi A 2000 Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: the possible role of nitric oxide. Arterioscler Thromb Vasc Biol 20:782–792 [DOI] [PubMed] [Google Scholar]
- Williams MR, Ling S, Dawood T, Hashimura K, Dai A, Li H, Liu JP, Funder JW, Sudhir K, Komesaroff PA 2002 Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J Clin Endocrinol Metab 87:176–181 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR 2003 Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinol 144:3449–3455 [DOI] [PubMed] [Google Scholar]
- Liu D, Dillon JS 2002 Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gα(i2,3). J Biol Chem 277:21379–21388 [DOI] [PubMed] [Google Scholar]
- Liu D, Dillon JS 2004 Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids 69:279–289 [DOI] [PubMed] [Google Scholar]
- Formoso G, Chen H, Kim J, Montagnani M, Consoli A, Quon M 2006 Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol 20:1153–1163 [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Alexaki V, Lazaridis I, Dermitzaki E, Avlonitis N, Tsatsanis C, Calogeropoulou T, Margioris A, Castanas E, Gravanis A 2006 G protein-associated, specific membrane binding sites mediate the neuroprotective effect of dehydroepiandrosterone. FASEB J 20:577–579 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Turesson I, Book M, Gerwins P, Claesson-Welsh L 2002 p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J Cell Biol 156:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintucci G, Moscatelli D, Saponara F, Biernacki P, Baumann F, Bizekis C, Galloway A, Basilico C, Mignatti P 2002 Lack of ERK activation and cell migration in FGF-2-deficient endothelial cells. FASEB J 16:598–600 [DOI] [PubMed] [Google Scholar]
- Liu D, Homan L, Dillon J 2004 Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinol 145:5532–5539 [DOI] [PubMed] [Google Scholar]
- Wang F, Van Brocklyn J, Hobson J, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S 1999 Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 274:35343–35350 [DOI] [PubMed] [Google Scholar]
- Lee H, Goetzl E, An S 2000 Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol 278:C612–C618 [DOI] [PubMed] [Google Scholar]
- Lee M, Moon E, Lee S, Kim M, Kim K, Kim Y 2001 Angiogenic activity of pyruvic acid in in vivo and in vitro angiogenesis models. Cancer Res 61:3290–3293 [PubMed] [Google Scholar]
- Tang H, Hao Q, Fitzgerald T, Sasaki T, Landon E, Inagami T 2002 Pyk2/CAKbeta tyrosine kinase activity-mediated angiogenesis of pulmonary vascular endothelial cells. J Biol Chem 277:5441–5447 [DOI] [PubMed] [Google Scholar]
- Liu F, Austin D, Mellon P, Olefsky J, Webster N 2002 GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol 16:419–434 [DOI] [PubMed] [Google Scholar]
- Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F 2000 Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J 1:71–76 [PMC free article] [PubMed] [Google Scholar]
- Wu L, Mayo L, Dunbar J, Kessler K, Baerwald M, Jaffe E, Wang D, Warren R, Donner D 2000 Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem 275:5096–5103 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 1998 Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem 273:26722–26728 [DOI] [PubMed] [Google Scholar]
- Liu D, Si H, Reynolds K, Zhen W, Jia Z, Dillon J 2007 Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Gαi-protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of anti-apoptotic Bcl-2 expression. Endocrinology 48:3068–3076 [DOI] [PubMed] [Google Scholar]
- Shizukuda Y, Tang S, Yokota R, Ware J 1999 Vascular endothelial growth factor-induced endothelial cell migration and proliferation depend on a nitric oxide-mediated decrease in protein kinase Cδ activity. Circ Res 85:247–256 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER 2003 Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- Machelon V, Nome F, Tesarik J 1998 Nongenomic effects of androstenedione on human granulosa luteinizing cells. J Clin Endocrinol Metab 83:263–269 [DOI] [PubMed] [Google Scholar]
- Qiu J, Lou L, Huang X, Lou S, Pei G, Chen J 1998 Nongenomic mechanisms of glucocorticoid inhibition of nicotine-induced calcium influx in PC12 cells: involvement of protein kinase C. Endocrinology 139:5103–5108 [DOI] [PubMed] [Google Scholar]
- Peter W, Benten M, Lieberherr M, Sekeris C, Wunderlich F 1997 Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett 407:211–214 [DOI] [PubMed] [Google Scholar]
- Peter W, Benten M, Lieberherr M, Giese G, Sekeris C, Wunderlich F 1998 Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett 422:349–353 [DOI] [PubMed] [Google Scholar]
- Stefano G, Cadet P, Breton C, Goumon Y, Prevot V, Dessaint J 2000 Estradiol-stimulated nitric oxide release in human granulocytes is dependent on intracellular calcium transients: evidence of a cell surface estrogen receptor. Blood 95:3951–3958 [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE 1999 Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140:5455–5458 [DOI] [PubMed] [Google Scholar]
- Liu D, Ren M, Dillon J 2006 Dehydroepiandrosterone inhibits agonist-induced intracellular calcium release in INS-1 cells by a non-genomic mechanism. Steroids 71:691–699 [DOI] [PubMed] [Google Scholar]
- Mattingly R, Macara I 1996 Phosphorylation-dependent activation of the Ras-GRF/CDC25Mm exchange factor by muscarinic receptors and G-protein β/γ subunits. Nature 382:268–272 [DOI] [PubMed] [Google Scholar]
- Jo H, Sipos K, Go Y, Law R, Rong J, McDonald J 1997 Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gβ/γ-dependent signaling pathways. J Biol Chem 272:1395–1401 [DOI] [PubMed] [Google Scholar]
- Jones S, Kazlauskas A 2001 Growth-factor-dependent mitogenesis requires two distinct phases of signaling. Nat Cell Biol 3:165–172 [DOI] [PubMed] [Google Scholar]
- Otto A 1995 A one minute pulse of estradiol to MCF-7 breast cancer cells changes estrogen receptor binding properties and commits cells to induce estrogenic responses. J Steroid Biochem Mol Biol 54:39–46 [DOI] [PubMed] [Google Scholar]
- Wong J, Le H, Zsarnovszky A, Belcher S 2003 Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci 23:4984–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft S 1999 Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol 151:65–77 [DOI] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouyssagur J 1999 Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J 18:664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu G, Lalli M, Sussman M, Sadoshima J, Periasamy M 2000 Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J Mol Cell Cardiol 32:1447–1457 [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier J, Guibert B, Pages C, Besson M, Hipskind R, Caboche J 1999 Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol 19:136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J 1998 Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 273:31327–31336 [DOI] [PubMed] [Google Scholar]
- El-Dahr S, Dipp S, Baricos W 1998 Bradykinin stimulates the ERK→Elk-1→Fos/AP-1 pathway in mesangial cells. Am J Physiol 275:F343–F352 [DOI] [PubMed] [Google Scholar]
- Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW 1997 Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol 273:L119–L126 [DOI] [PubMed] [Google Scholar]
- Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR 1997 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res 81:885–892 [DOI] [PubMed] [Google Scholar]
- Chen F, Watson CS, Gametchu B 1999 Multiple glucocorticoid receptor transcripts in membrane glucocorticoid receptor-enriched S-49 mouse lymphoma cells. J Cell Biochem 74:418–429 [PubMed] [Google Scholar]
- Kim H, Lee J, Jeong J, Bae S, Lee H, Jo I 1999 Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem Biophys Res Commun 263:257–262 [DOI] [PubMed] [Google Scholar]
- Shaul PW 1999 Rapid activation of endothelial nitric oxide synthase by estrogen. Steroids 64:28–34 [DOI] [PubMed] [Google Scholar]
- Peter W, Benten M, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris C, Mossmann H, Wunderlich F 1999 Functional testosterone receptors in plasma membranes of T cells. FASEB J 13:123–133 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani A, Fornari L, Mannella P, Varone G, Caruso A, Liao J 2003 Non-genomic actions of sex steroid hormones. Eur J Endocrinol 148:281–292 [DOI] [PubMed] [Google Scholar]
- Norman A, Mizwicki M, Norman D 2004 Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41 [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B 1998 DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids 63:322–328 [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Tsatsanis C, Dermitzaki E, Alexaki VI, Castanas E, Margioris AN, Gravanis A 2004 Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc Natl Acad Sci USA 101:8209–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard F, Krug A, Ziegler C, Sperber S, Ehrhart-Bornstein M, Bornstein S 2006 Role of DHEA and growth factors in chromaffin cell proliferation. Ann NY Acad Sci 1073:312–316 [DOI] [PubMed] [Google Scholar]
- Sakakura Y, Nakagawa Y, Ohzeki T 2006 Differential effect of DHEA on mitogen-induced proliferation of T and B lymphocytes. J Steroid Biochem Mol Biol 99:115–120 [DOI] [PubMed] [Google Scholar]
- Banerjee S, Campbell D, Weston A, Banerjee D 1997 Biphasic estrogen response on bovine adrenal medulla capillary endothelial cell adhesion, proliferation and tube formation. Mol Cell Biochem 177:97–105 [DOI] [PubMed] [Google Scholar]
- Varet J, Vincent L, Akwa Y, Mirshahi P, Lahary A, Legrand E, Opolon P, Mishal Z, Baulieu EE, Soria J, Soria C, Li H 2004 Dose-dependent effect of dehydroepiandrosterone, but not of its sulphate ester, on angiogenesis. Eur J Pharmacol 502:21–30 [DOI] [PubMed] [Google Scholar]
- Zapata E, Ventura JL, De la Cruz K, Rodriguez E, Damian P, Masso F, Montano LF, Lopez-Marure R 2005 Dehydroepiandrosterone inhibits the proliferation of human umbilical vein endothelial cells by enhancing the expression of p53 and p21, restricting the phosphorylation of retinoblastoma protein, and is androgen- and estrogen-receptor independent. FEBS J 272:1343–1353 [DOI] [PubMed] [Google Scholar]
- Coulet F, Nadaud S, Agrapart M, Soubrier F 2003 Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem 278:46230–46240 [DOI] [PubMed] [Google Scholar]
- Frick M, Dulak J, Cisowski J, Jozkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher S, Pachinger O, Weidinger F 2003 Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis 170:229–236 [DOI] [PubMed] [Google Scholar]
- van der Schaft D, Seftor R, Seftor E, Hess A, Gruman L, Kirschmann D, Yokoyama Y, Griffioen A, MJ H 2004 Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst 96:1473–1477 [DOI] [PubMed] [Google Scholar]
- Obrig T, Louise C, Lingwood C, Boyd B, Barley-Maloney L, Daniel T 1993 Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem 268:15484–15488 [PubMed] [Google Scholar]
- Bouchet D, Tesson L, Menoret S, Charreau B, Mathieu P, Yagita H, Duisit G, Anegon I 2002 Differential sensitivity of endothelial cells of various species to apoptosis induced by gene transfer of Fas ligand: role of FLIP levels. Mol Med 8:612–623 [PMC free article] [PubMed] [Google Scholar]
- Lai GJ, McCobb DP 2002 Opposing actions of adrenal androgens and glucocorticoids on alternative splicing of Slo potassium channels in bovine chromaffin cells. Proc Natl Acad Sci USA 99:7722–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Bernatchez P, Tanguay J 2002 Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol 22:1585–1590 [DOI] [PubMed] [Google Scholar]
- Ling S, Dai A, Williams MR, Myles K, Dilley RJ, Komesaroff PA, Sudhir K 2002 Testosterone (T) enhances apoptosis-related damage in human vascular endothelial cells. Endocrinology 143:1119–1125 [DOI] [PubMed] [Google Scholar]
- Rubio-Gayosso I, Garcia-Ramirez O, Gutierrez-Serdan R, Guevara-Balcazar G, Munoz-Garcia O, Morato-Cartajena T, Zamora-Garza M, Ceballos-Reyes G 2002 Testosterone inhibits bradykinin-induced intracellular calcium kinetics in rat aortic endothelial cells in culture. Steroids 67:393–397 [DOI] [PubMed] [Google Scholar]
- Marwah A, Marwah P, Lardy H 2002 Ergosteroids-VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: a liquid chromatographic-mass spectrometric study. J Chromatogr B 767:285–299 [DOI] [PubMed] [Google Scholar]
- Shi J, Schulze S, Lardy HA 2000 The effect of 7-oxo-DHEA acetate on memory in young and old C57BL/6 mice. Steroids 65:124–129 [DOI] [PubMed] [Google Scholar]
- Padgett DA, Loria RM, Sheridan JF 1997 Endocrine regulation of the immune response to influenza virus infection with a metabolite of DHEA-androstenediol. J Neuroimmunol 78:203–211 [DOI] [PubMed] [Google Scholar]
- Hinson JP, Khan M 2004 Dehydroepiandrosterone sulphate (DHEAS) inhibits growth of human vascular endothelial cells. Endocr Res 30:667–671 [DOI] [PubMed] [Google Scholar]
- Hinson J, Renshaw D, Vakharia K, King P, Dehydroepiandrosterone sulphate (DHEAS) inhibits growth of human vascular endothelial cells. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004, p 186 (Abstract P1-132) [DOI] [PubMed] [Google Scholar]