Abstract
The relaxin receptor [leucine-rich repeat-containing G protein-coupled receptor 7 (LGR7)] belongs to the leucine-rich repeat containing G protein-coupled receptors subgroup C. Three new LGR7 splice variants have been cloned from the human fetal membranes and shown to be truncated versions of the full-length receptor, encoded by different lengths of the extracellular domain. The expression of their mRNAs has been confirmed by both qualitative and quantitative PCR and shown to be higher in the chorion and decidua before, compared with after, spontaneous labor. When HEK293 cells were transfected with each LGR7 splice variant, their proteins were retained within the endoplasmic reticulum. However, the protein for the shortest variant was also secreted into the medium. We have characterized the intracellular functions and effects of these LGR7 variants on the function of the wild-type (WT)-LGR7. In coexpression studies, each splice variant interacted directly with the WT-LGR7 and exerted a dominant-negative effect on cAMP accumulation by the WT-LGR7 after relaxin treatment. This interaction resulted in the sequestration of the WT-LGR7 inside the cells by down-regulation of its maturation and cell surface delivery. The constitutive homodimerization of WT-LGR7 has been shown here to take place in the endoplasmic reticulum, and the presence of any one of the splice variants decreased this by the formation of heterodimers with the WT-LGR7, supporting the view that homodimerization is a prerequisite for receptor trafficking to the cell surface. These data suggest that the dominant-negative effects of the LGR7 splice variants expressed in the chorion and decidua could be functionally significant in the peripartal period by inhibiting the function of WT-LGR7 and dampening the responsiveness of these tissues to endogenous relaxin.
RELAXIN IS NORMALLY considered to be the hormone responsible for the remodeling of the connective tissues of the reproductive tract during pregnancy (1). However, it has recently been shown to act as an autocrine/paracrine hormone in such diverse tissues as the heart (2), blood vessels (3), and kidney (4,5) as well as in thyroid and prostate cancers (6,7,8). Our work has focused on its production from the decidua and placenta (9,10) and its local action on the decidua and the juxtaposed fetal amnion and chorion (11). Studies with labeled human relaxin showed most binding to the decidua and chorionic cytotrophoblast of the fetal membranes, suggesting these as principal sites of relaxin action in the uterus (12).
The relaxin receptor [leucine-rich repeat (LRR)-containing G protein-coupled receptor (GPCR) 7 (LGR7)], also called RXFP1 (13), has recently been identified as a GPCR (14). It belongs to the LRR-containing GPCR subfamily of proteins, which have a characteristic large extracellular region with multiple LRRs and a rhodopsin receptor-like transmembrane (TM) domain. The LRR subfamily is divided into three classes: class A contains the glycoprotein hormone receptors FSH receptor (FSHR), LHR, and TSHR; class B consists of three orphan receptor proteins (LGR4, LGR5, and LGR6); and LGR7 and LGR8 form the C class of this subfamily. LGR7 and LGR8 are mosaic proteins in which their extracellular domains contain a unique low-density lipoprotein class A (LDL-A) module at their N termini followed by 10 LRRs and a seven-TM domain (15). LGR7 and LGR8 cause the production of cAMP upon the binding of their cognate ligands, relaxin and insulin-like peptide 3, respectively (14,16). In addition, LGR7 responds to relaxin by stimulation of protein kinase A (17). Functional characterization of the relaxin receptor has been carried out using chimeric receptor variants and by the introduction of point mutations in the LRR region. These studies showed that the LRR and TM domains are involved in both the binding and signaling of relaxin (18,19). Recent studies have shown that the LDL-A module is essential for receptor signaling (20,21) as well as for its maturation and cell surface delivery (21). It has also been shown that mutation of the extracellular domain of the LGR8 receptor affected the cell surface delivery of this receptor (22).
Alternative splicing is a common phenomenon within the LGR family of receptors. Splice variants have been reported for the glycoprotein hormone receptors FSHR (23,24), TSHR (25), and LHR (26,27). The alternative splicing of the LGR7 and LGR8 genes has also been reported (15,20,28). The coding sequence of LGR7 encompasses 18 exons (15,28). Prior work identified one splice variant lacking exon 3, which was cloned from the human ovary and testes (15) and recently designated as LGR7.10 (28). This variant neither binds relaxin nor stimulates cAMP production (15,28). Another two splice variants were recently cloned and designated as LGR7.1 and LGR7.2 (28). These authors grouped the LGR7 splice variants into two main classes: those that contain the TM region (LGR7.2 and LGR7.10) and those with only a truncated extracellular region (LGR7.1). They were shown to have different cellular localizations; LGR7.10 was expressed at a lower level at the cell surface compared with the wild-type LGR7 (WT-LGR7), whereas the other two isoforms, LGR7.1 and LGR7.2, were retained within the cells (28). In general, they exhibited a wide range of tissue expression but were unable to bind their ligands or to stimulate cAMP production. Their functional importance is largely unknown (28), although a truncated splice variant (designated as LGR7-truncate) in the mouse was recently shown to inhibit the function of the WT-LGR7 (20). This splice variant of LGR7 consisted only of the signal sequence and the LDL-A module and was shown to be expressed in a number of mouse reproductive tissues: cervix/vagina, uterus, myometrium, and endometrium (29). Functional analysis of this truncated mouse splice variant in transiently transfected HEK293 cells showed it to be secreted into the medium and capable of blocking the cAMP signaling of the WT-LGR7 after treatment with relaxin. This occurred extracellularly and therefore took place without any intracellular modulation of its cell surface expression or of the relaxin binding to the WT-LGR7. Two human truncated splice variants, structurally similar to the mouse LGR7-truncate, were cloned from the human uterus and brain (designated as LGR-truncate-2 and LGR-truncate-3) and may have similar functions to the mouse LGR7-truncate (20).
It has recently become clear that the splice variants and naturally occurring mutant forms of the GPCRs have a dominant-negative effect on the cell surface expression of their respective wild-type receptors. Dimerization of the GPCRs in the endoplasmic reticulum (ER) appears to be required for receptor maturation and their subsequent delivery to the cell surface (30). Thus, there are now several examples of an association between a splice variant with its wild-type receptor (26,27,31,32,33,34) or the naturally occurring mutant form and wild-type receptor proteins (35,36,37,38,39) to form a complex. This takes place early in their biosynthesis in the ER, and this complex can then inhibit the cell surface delivery and signaling of the wild-type receptor. It has also recently been shown that splice variants of the LGR subfamily of GPCRs can exert a dominant-negative effect on their own wild-type receptor (20,26,27). Thus, a splice variant of LHR lacking exon 9 is retained within the cell and interacts with and modulates the cell surface expression of the wild-type LHR (26). In addition, an LHR spice variant expressing only the extracellular domain is retained in the ER and modulates the cell surface expression of the wild-type receptor by misrouting it into a subcompartment of the ER (27).
We have recently shown the expression of LGR7 in the human chorion and decidua and its higher expression in the preterm period of human gestation than at term. However, expression was reduced after spontaneous labor and delivery in both tissues at preterm and term (40). In the current study, we have examined the alternative splicing of the LGR7 mRNA and identified three unique variants, all containing the extracellular region of the receptor; the LDL-A module and different lengths of the LRR region. Their levels of expression in separated fetal amnion and chorion and maternal decidua before and after the onset of labor has been quantitated by specific quantitative RT-PCRs (qRT-PCRs). Using their individual and combined transfection into HEK293 cells, the intracellular interactions between each of these variants and the WT-LGR7 has been studied and their dominant-negative effects on the function of the WT-LGR7 demonstrated. The mechanism of this interaction in the ER was further examined and allowed us to propose a model of LGR7 homodimerization, where interaction with a splice variant of LGR7 prevents homodimerization of LGR7 and its subsequent trafficking to the cell surface, causing a loss of receptor function.
Materials and Methods
Hormones, cell lines, and chemicals
Recombinant human relaxin H2 was a generous gift from BAS Medical Inc. (Palo Alto, CA). HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA; ATCC no. CRL-1573). Enzymes were purchased from New England Biolabs (Beverly, MA), and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Cloning of LGR7 splice variants from human fetal membrane
For the isolation of RNA for cloning of LGR7 splice variants and their qualitative and quantitative RT-PCR analysis, fetal membranes were collected from Kapiolani Medical Center for Women and Children (Honolulu, HI) with approval from the University Committee on Human Experimentation and the Hospital Institution Review Board. All fetal membranes were examined by a pathologist for histological evidence of infection and if positive were excluded. The region of the fetal membranes separating monozygotic twin pregnancies was used because this contained only pure fetal amnion (septum) devoid of fetal chorion and maternal decidua. Twin fetal membranes were collected before labor (at elective cesarean section). We separated the pure fetal septum (amnion) and placental basal plate (enriched in maternal decidua) cut from the uterine surface of the placenta after its expulsion. Total RNA was extracted with the RNeasy Midi kit (QIAGEN, Valencia, CA) and its purity determined on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). Template cDNA was prepared by RT in a total volume of 20 μl containing 1× PCR buffer II, 5 mm MgCl2, 500 μm each dNTPs, 2.5 μm random hexamers, 0.4 U/μl RNase inhibitor, 1.25 U/μl Multiscribe reverse transcriptase (all from Applied Biosystems, Foster City, CA) and 400 ng total RNA. The reaction mixture was incubated at 25 C for 10 min, 37 C for 120 min, and 95 C for 5 min. For cloning of LGR7 splice variants, a nested PCR approach with the same methodology was used as described previously (28). After selection of positive clones, sequence analysis (Biotechnology Core Facilities, University of Hawaii) was performed of both DNA strands to confirm the novel splice variants. To establish the exon organization and alternative splice sites of the variants, we compared the mRNA sequences with the genomic sequence of LGR7 (GeneID 59350) using Spidey web tool (www.ncbi.nlm.nih.gov/IEB/Research/Ostell/Spidey).
Qualitative mRNA detection of the LGR7 splice variants
mRNA expression of the splice variants was confirmed by nested PCR. Total RNA from the septum (amnion) and placental basal plate (decidua) of monozygotic twin pregnancies (n = 3) was isolated by the RNeasy midi kit (QIAGEN). Human uterus (total RNA) was purchased from Stratagene (La Jolla, CA). RT conditions were as described above for cloning the splice variants. Specific primer sets were designed to specifically amplify each variant using Oligo Explorer (Gene Link, Hawthorne, NY). Reverse primers were designed to span alternative spliced exon boundaries. Forward and reverse primers were used for the first PCR followed by a second PCR using nested forward and reverse primers (primer sequences shown in Table 1). Sequential PCRs were carried out in a total volume of 20 μl containing 2.5 U AmpliTaq Gold, 1× PCR buffer II, 5 mm MgCl2, 100 pmol primers, 250 μm of each dNTP (all from Applied Biosystems), and 1 μl cDNA template. The thermal cycle programs were 95 C for 10 min, followed by 35 cycles at 95 C for 15 sec, 58 C for 1 min 30 sec, and 72 C for 1 min and a final extension step at 72 C for 10 min. After the first round, 1 μl of the first PCR was used as a template in the nested PCR using identical conditions. For detection of the mRNA transcripts of WT-LGR7 and housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a single round of PCR with the same conditions was used. We used forward and reverse primers for LGR7 and GAPDH as described previously (14,41). Sequences of oligonucleotide primers are shown in Table 1. After amplification, products were separated on 2% agarose gels and visualized by ethidium bromide. The PCR products were isolated and confirmed by sequencing.
Table 1.
Sequence of oligonucleotide primers for qualitative and quantitative real-time PCR
| Sequences of oligonucleotide primers and probes (5′–3′) | |
|---|---|
| Detection by qualitative PCR | |
| LGR7-C | |
| Forward | GTCGAATTTCCCCACCAAC |
| Reverse | TACCCAAGAGGTTATGGTAC |
| Nested forward | GTCCTCACCCGTTTACCTGA |
| Nested reverse | ACAGCTCCTTCAGGTCCTTG |
| Nested PCR product size = 268 bp | |
| LGR7-D | |
| Forward | CTGTGGAGACAACAATGGAT |
| Reverse | GCTCCAATACTTTCTGTATCTTC |
| Nested forward | GGATGGTCTCTGCAATTTGAC |
| Nested reverse | GAAGCAATCAGGAGGAAGC |
| Nested PCR product size = 240 bp | |
| LGR7-F | |
| Forward | CTCTTGAGGCAGAAACAC |
| Reverse | GAGTCTCGTTGATTGAGATAC |
| Nested forward | GCAGAAACACCTGAATGT |
| Nested reverse | GGCTATTCAGTCCTCTGA |
| Nested PCR product size = 258 bp | |
| LGR7 | |
| Forward | CCCAATTCTCTATACTCTGACCACAAG |
| Reverse | TCATGAATAGGAATTGAGTCTCGTTGATT |
| PCR product size = 244 bp | |
| GAPDH | |
| Forward | GCATCCTGGGCTACACTGAG |
| Reverse | ACCACCTGTTGCTGTAGCC |
| PCR product size = 140 bp | |
| Detection by quantitative real-time PCR | |
| LGR7-C | |
| Forward | GGATGAATTGGATTTAGGAA |
| Reverse | CCAAGAGGTTATGGTACA |
| Probe | GATTGAAAATCTTCCACCGCTTATATTC |
| Wild-type reverse | TCTGGATTGGATTATAGGAA |
| LGR7-D | |
| Forward | TGACTGCAATGTCACTTC |
| Reverse | GCTCCAATACTTTCTGTATC |
| Probe | TGGAACTTAATAAGAAAGCTTCCTCCTG |
| Wild-type reverse | GAGATGGATGTAATCTTATTGTT |
| LGR7-F | |
| Forward | TCTCCATCTATGCTTTCAG |
| Reverse | GAATTGAGTCTCGTTGATTG |
| Probe | AGGACTGAATAGCCTTACTAAACTG |
| Wild-type reverse | CGGCTTCAGGAAGGTT |
The relative expression of each splice variant was expressed in comparison with the expression of full-length LGR7 in each RNA sample; therefore, the same forward primers and probes, but different reverse primers, were used for detection of variants and LGR7.
qRT-PCR analysis
To study the expression of the LGR7 splice variants in the fetal membranes at term, tissues were collected before labor at elective cesarean section (n = 5) and after normal-term spontaneous vaginal delivery (n = 5). The amnion was manually stripped from the choriodecidua, and the decidua was carefully scraped from chorion. The tissue samples were flash frozen in liquid nitrogen and stored at −80 C until use. Frozen tissues were pulverized with a BioPulverizer (BioSpec Products Inc., Bartlesville, OK) and the resulting powder used for total RNA extraction with RNeasy Fibrous MiniKit (QIAGEN) according to the manufacturer’s instructions. For analysis of BiP expression, RNA was isolated from stably transfected HEK293 cells with the RNeasy MiniKit (QIAGEN). The RNA quality was assessed on an Agilent Bioanalyzer 2100 (Agilent Technologies). Primers for BiP and the housekeeping gene (18S) were purchased from Applied Biosystems (TaqMan Assays on Demand). Primers for the splice variants and full-length LGR7 were designed using RealTime Design software (www.biosearchtech.com) and purchased from Biosearch Technologies (Novato, CA). The sequences of primers and probes are shown in Table 1. The relative expression of each splice variant was compared with expression of full-length LGR7 in each RNA sample; therefore, the same forward primers and probes but different reverse primers for detection of variants and LGR7 were used. Reverse primers to detect each splice variant were designed to span the alternative splice sites. The specificity of these primers was validated by using mRNAs from stably transfected HEK cells with cDNAs of the full-length LGR7, LGR7-C, LGR7-D, and LGR7-F. Each splice variant primer set amplified only cDNA prepared from the HEK cells stably transfected with the respective splice variant and did not amplify the cDNA prepared from the HEK cells transfected with the other splice variants or the full-length LGR7 (data not shown). The cDNAs were prepared using GeneAmp reagents (Applied Biosystems). The RT reaction contained 5 μg total RNA for splice variants and 100 ng total RNA for BiP and 18S detection, 1× PCR buffer II, 5 mm MgCl2, 250 μm of each dNTP, 2.5 μm random hexamers, 0.4 U/μl RNase inhibitor, and 1.25 U/μl Multiscribe reverse transcriptase. This was incubated at 25 C for 10 min, 37 C for 120 min, and 95 C for 5 min, and we used the ABI protocol for qRT-PCR with the following incubation: one cycle at 95 C for 10 min and 40 cycles at 60 C for 15 sec and 60 C for 1 min using an MJ Research Opticon Continuous Fluorescence Detector (MJ Research, Waltham, MA). Each reaction was performed in triplicate, and the results were normalized to the expression of 18S. The data were analyzed as described in the ABI User’s Bulletin no. 2 and represented as relative gene expression as mean values ± sem. Statistical analysis was performed by one-way ANOVA with Student’s t test using GraphPad Instat software (GraphPad Software Inc., San Diego, CA).
DNA constructs
The cDNAs of the splice variants were subcloned into pCR3.1 mammalian expression vector (Invitrogen, Carlsbad, CA). The PCR products generated with forward Kozprimer (5′-tcgctagcgatatcgccaccatggcatctggttctgtc-3′) and reverse primers for LGR7-C (5′-agactcgagtcagagatcttgggcagaata-3′), for LGR7-D (5′-agactcgagtcagagatctgatgataaatgcggcc-3′), and for LGR7-F (5′-agactcgagtcagagatcttcatgaatagg-3′), were digested with NheI and XhoI and cloned into pCR3.1 vector. To generate hemagglutinin (HA)-containing splice variants at the N terminus, the HA sequence was inserted by subcloning the HA-containing fragment from HA-WT-LGR7 (21) using NheI and HindIII restriction sites.
The splice variants bearing the green fluorescent protein (GFP) at the C terminus were generated by subcloning the cDNAs into pEGFP-N1 vector (BD Biosciences Clontech, Mountain View, CA). The splice variant cDNAs were amplified with the forward Kozprimer and reverse primers for LGR7-C (5′-attcctcgagatgggcagaata-3′), for LGR7-D (5′-agactcgagtcagagatctgatgataaatgcggcc-3′), and for LGR7-F (5′-attcctcgaggtcatgaatagg-3′). The PCR products were digested with NheI and XhoI and cloned into the pEGFP-N1 vector. The splice variant LGR7-D with a point mutation at Asp (36) (N36Q-D) was generated by excising the fragment bearing this mutation from N36Q-LGR7 (21) by restriction endonucleases NheI and HindIII and subcloning into the LGR7-D construct.
The LGR7-Rluc and LGR7-YFP receptors were created by subcloning the Renilla luciferase (Rluc) and yellow fluorescence protein (YFP) sequences into the LGR7 sequence. The Rluc was amplified from pRL-SV40 vector (Promega, Madison, WI) with forward primer (5′-atctcgagggaggcggtggaggcctagccaccatgacttcg-3′) and reverse primer (5′-gctcgaagcggccgctctag-3′), and YFP sequence was amplified from pEYFP-Tub vector (BD Biosciences Clontech) using forward primer (5′-atctcgagggaggcggtggaggcgcgctaccggtcgccacc-3′) and reverse primer (5′-tcgaagcggccgcctattagagtccggacttgtacagc-3′). The PCR products of Rluc and YFP were digested with XhoI and NotI and cloned into the vector containing the WT-LGR7-GFP sequence (21) exchanging the GFP sequence for Rluc or YFP. Sequencing was performed to confirm all DNA constructs.
Cell culture and transfection
HEK293 cells were grown routinely in DMEM supplemented with 10% fetal bovine serum and 100 μg/ml penicillin/streptomycin in a humidified atmosphere at 37 C and 95% air/5% CO2. Transient transfections of HEK293 cells were carried out using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s instructions. Transiently transfected cells were used 48 h after transfection. To correct for transfection efficiency, for intracellular cAMP assay and receptor expression ELISA, the receptor DNA constructs were cotransfected with 50 ng of the pEGFP-N1 plasmid (BD Biosciences Clontech) and the data normalized based on GFP fluorescence measured with a Victor2 plate reader (PerkinElmer Life Sciences, Wellesley, MA). Stable cell lines were established by transfecting with appropriate constructs containing HA-tag plasmid into HEK293 cells with Lipofectamine 2000 reagent under Geneticin (1000 μg/ml; Invitrogen) selection, according to the manufacturer’s instructions. After 10–14 d, individual clones were isolated using cloning cylinders (Genechoice, Frederick, MD) and expanded. The clones were tested for expression of the appropriate proteins by Western blotting.
Intracellular cAMP determination
Cells transfected with the splice variants or WT-LGR7 in the presence of each variant, LGR7-Rluc, and LGR7-YFP were stimulated for 30 min at 37 C with different concentrations of human relaxin as described previously (21). For characterization of the WT-LGR7 in the presence of each variant, we cotransfected HEK293 cells with WT-LGR7 construct (0.5 μg) and the same amount of the splice variant or empty vector (control). Each experiment was performed in duplicate and expressed as means ± sem for three observations, and data analysis was performed using GraphPad Prism software.
Immunoprecipitation of the splice variants from cell lysates and medium, membrane preparations, endoglycosidase digestion, and Western blot analysis
HEK293 cells were grown on petri dishes and transfected with each splice variant construct. Immunoprecipitations were performed 48 h after transfection with the ProFound Mammalian HA Tag IP/Co-IP Kit (Pierce, Rockford, IL). Briefly, for immunoprecipitation of the HA-tagged proteins from the cell lysates, cells were washed once with ice-cold Tris-buffered saline (TBS) buffer (25 mm Tris, 0.15 m NaCl, pH 7.2), lysed with 1 ml of the M-PER reagent, incubated for 5 min at room temperature, centrifuged for 30 min (16,000 × g) at 4 C to pellet the cell debris, and incubated with 6 μl anti-HA agarose slurry (10 μg anti-HA antibody) at 4 C overnight. For immunoprecipitation of the secreted HA-tagged proteins from medium, this was collected and concentrated to a total volume of 150 μl on Vivaspin15 columns (Sartorius Corp., Edgewood, NY), according to the manufacturer’s instructions. Concentrated samples were diluted with 450 μl M-PER reagent and centrifuged for 30 min (16,000 × g) at 4 C, and supernatants were incubated with 6 μl anti-HA agarose slurry (10 μg anti-HA antibody) at 4 C overnight. Cell lysates and media were washed three times with TBS-T buffer (TBS containing 0.05% Tween 20) and eluted in 30 μl with elution buffer provided with the kit, and samples were analyzed by Western blotting using mouse HA antibody (Covance HA.11 monoclonal antibody at 1:1000 dilution; Covance, Berkeley, CA).
Membrane preparations, endoglycosidase digestion, and Western blot analysis were performed as described previously (21). Detection of the splice variants was performed on SDS-PAGE with 10% separating gel and for the full-length receptor on 7.5% separating gel. Densitometric analyses were performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Cell surface expression ELISA
The expression of the HA-tagged splice variants and HA-WT-LGR7 in the presence of splice variants was performed by cell surface ELISA as described previously (21). In coexpression studies using different splice variant to HA-WT-LGR7 ratios, the total DNA concentrations were kept to 1 μg for each transient transfection.
Immunocytochemistry
HEK293 cells were grown in poly-d-lysine-coated eight-well chambers (Becton Dickinson Labware, Frankin Lakes, NJ) and transfected with 0.5 μg DNA construct per well using Lipofectamine 2000. For colocalization of the splice variants and full-length LGR7, cells were transfected with 0.25 μg of each GFP-tagged splice variant construct and 0.25 μg of the HA-WT-LGR7 construct. After 48 h, they were washed with PBS and fixed for 10 min with 4% paraformaldehyde (nonpermeabilized) and 4% paraformaldehyde containing 0.25% Triton X-100 (permeabilized). Cells were washed three times with PBS, blocked with PBS/BSA (PBS containing 1% BSA) for 1 h, and incubated with appropriate primary antibody. In colocalization studies, the ER was immunostained with antibody to calnexin (SPA-865 at 1:200 dilution; StressGen, Victoria, Canada) or calreticulin (Stressgen, SPA-600 at 1:200 dilution). An HA antibody (Covance HA.11 monoclonal antibody at 1:1000 dilution) was used to identify the HA-tagged proteins. Cells were washed three times, incubated with appropriate fluorescently labeled AlexaFluor antibody (at 1:1000 dilution; Molecular Probes, Eugene, OR) for 1 h, and washed three times with PBS. Slides were mounted, and laser scanning confocal microscopy was performed using a Zeiss LSM-5 system.
Coimmunoprecipitation
For coimmunoprecipitation studies, HEK293 cells were grown on 60-mm dishes and transfected with 4 μg HA-WT-LGR7 and 4 μg GFP-tagged splice variant or an empty GFP vector (pEGFP-N1). Forty-eight hours after transfection, the cells were washed with ice-cold TBS and lysed, and coimmunoprecipitation was performed with ProFound Mammalian HA Tag IP/Co-IP Kit as described above. After final washing, proteins were eluted with sample buffer [60 mm Tris/HCl (pH 6.8), 1% SDS, 10% glycerol, and 0.1 m dithiothreitol and lane marker tracing dye] and analyzed by Western blotting after separation on 7.5% gel. Detection of GFP-tagged proteins was performed with primary polyclonal antibody to GFP (ab6556 at 1:2000 dilution; Abcam, Cambridge, MA) together with a secondary horseradish peroxidase-conjugated rabbit antibody (at 1:2000 dilution; Amersham Biosciences, Arlington Heights, IL).
Bioluminescence resonance energy transfer (BRET) analysis
The dimerization of the LGR7 was assayed by BRET (42,43,44). The HEK293 cells were transfected with appropriate DNA constructs in six-well plates. For BRET titration curves, cells were cotransfected with a fixed amount of the LGR-Rluc and increasing amounts of the LGR7-YFP receptor. Empty vector (pCR3.1) was always added to transfect a constant amount of plasmid. Forty-eight hours after transfection, cells were washed with PBS, detached with PBS containing 5 mm EDTA, collected by centrifugation, and resuspended in PBS containing 0.1% glucose. Cells (200,000 per well) were distributed in a 96-well microplate (white isoplate; Wallac, Turku, Finland). Coelentarazine h (Molecular Probes) was added at a final concentration of 5 μm. Readings were taken using a Victor2 plate reader that allows the sequential integration of the signals detected in the 440- to 500-nm and 510- to 590-nm windows, using filters with the appropriate band pass. The BRET signal was determined by calculating the ratio of the light emitted by YFP (acceptor) over that emitted by the Rluc (donor). The measurements were always performed after addition of the substrate and 5, 10, and 15 min later. The net BRET values were obtained by subtracting the background signal detected when LGR7-Rluc construct was expressed alone. Total fluorescence and luminescence signals were also determined for all samples to asses the levels of LGR7-YFP and LGR7-Rluc expression. The YFP fluorescence was measured by using an excitation filter at 485 nm, an emission at 535 nm, and the following parameters: lamp energy 20,680 and reading time 1 sec. The total cell luminescence was measured after adding 5 μm coelenterazine h for 1 sec using Victor2. The net BRET signal was plotted as a function of the YFP to Rluc fusion proteins (YFP/Rluc). Each experiment was performed in duplicate and expressed as mean ± sem for three to four observations. The curves were fitted using a nonlinear regression equation with GraphPad Prism software.
Cellular fractionation by sucrose gradient centrifugation
Cellular fractionation was assessed by sucrose gradient centrifugation as described previously (45) with modification. Briefly, total membrane preparations from HEK293 cells transfected with constructs for either 4.8 μg LGR7-Rluc and 19.2 μg LGR7-YFP or 4.8 μg LGR7-Rluc and 19.2 μg LGR7 were used. These membrane preparations were supplemented with 2 m sucrose for a final concentration of 0.25 m sucrose. A discontinuous sucrose step gradient (0.5, 0.9, 1.2, 1.35, 1.5, and 2 m) was made using lysis buffer [50 mm Tris (pH 7.4), 50 mm mannitol, 2 mm EGTA, 0.5 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol and protease inhibitors (Roche Diagnostics, Indianapolis, IN)]. Total membrane protein preparations (200 μg) were applied to the top of the gradient and samples centrifuged for 16 h at 100,000 × g and 4 C. Fractions were collected from the top and subjected to BRET measurements as described previously. Samples containing plasma membrane (PM) and ER were identified by Western blotting. The fractionated samples were separated on SDS-PAGE (7.5% separating gels), Western blotting was performed with mouse monoclonal anti-calnexin (Abcam; ab2798 at 1:2000 dilution) to detect ER-enriched fractions and with mouse monoclonal anti-Na/K ATPase (at 1:2000 dilution; Upstate, Lake Placid, NY) to detect PM-enriched fractions. The secondary antibody was mouse horseradish peroxidase conjugated (dilution 1:3000; Bio-Rad, Hercules, CA).
Statistical analysis
Results were analyzed by one-way ANOVA with Student-Newman-Keuls multiple comparison methods, using GraphPad Instat software.
Results
Cloning LGR7 splice variants from the human choriodecidua and their qualitative detection
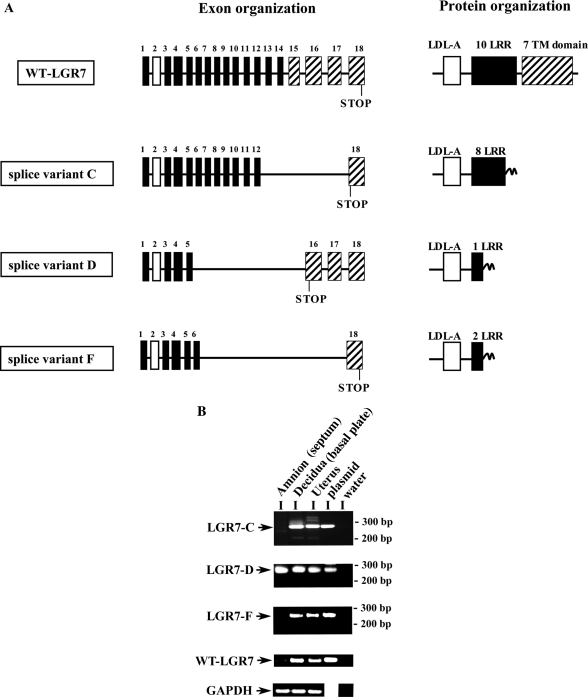
The LGR7 splice variants were cloned by nested PCR using cDNAs from the placental basal plate of twin pregnancies. We identified the WT-LGR7 and three different splice variants, termed LGR7-C, LGR7-D, and LGR7-F. Their nucleotide sequences did not correspond to any of the previously described LGR7 splice variant sequences (20,28). Schematic representation of their exons and predicted protein organization are shown in Fig. 1A. Comparisons with the WT-LGR7 showed that LGR7-C was alternatively spliced between exons 12 and 18, splice variant LGR7-D between exons 5 and 16 using cryptic splice sites of exonic sequences, and splice variant LGR7-F between exons 6 and 18 using cryptic splice sites of these exons. Their nucleotide and predicted protein sequences were aligned with the WT-LGR7 and are shown in supplemental Figs. 1 and 2 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). This alternative splicing of the LGR7 gene led to a shift in the open reading frame and to a premature stop codon and termination of protein synthesis in each case. Thus, the predicted protein sequence of LGR7-C contains the signal sequence followed by the LDL-A module, eight LRRs, and 13 new amino acids; splice variant LGR7-D contains the signal sequence, the LDL-A module, one LRR, and 25 new amino acids; and LGR7-F contains the signal sequence, the LDL-A module, two LRRs, and 10 new amino acids (Fig. 1A).
Figure 1.
A, Schematic representation of the exon and putative protein organization of the LGR7 splice variants cloned from the placental basal plate. The cDNA of the full-length WT-LGR7 spliced from 18 exons is shown for comparison (15). Exons are represented as boxes; exon 1 contains the signal sequence (black box), the LDL-A module encoded by exon 2 (white box), the 10 LRRs encoded from exons 5–14 (black boxes), and the TM domain encoded by the sequence from exons 15–18 (striped boxes). In all three splice variants, deletion of exons leads to shifts in the open reading frame and to premature termination of their putative polypeptides. Splice variants LGR7-D and LGR7-F use cryptic splice acceptor and splice donor sites. Shifts in the open reading frames resulted in novel amino acid sequences after the alternative splicing sites, not present in the full-length receptor. This alternative splicing resulted in proteins containing different lengths of the extracellular region of the LGR7. B, Expression of mRNAs for the splice variants and WT-LGR7 in amnion and decidua from monozygotic twin pregnancies (n = 3). Representative mRNAs from PCR analysis of one tissue are shown. The mRNA analysis of the splice variants was performed by nested PCR, WT-LGR7 and GAPDH transcripts by regular PCR. The oligonucleotide sequences of the primers are shown in Table 1. The amnion was isolated from the septum of the fetal membranes separating twin gestations that contained no chorion because these were monozygotic twins and decidua from the placental basal plates. Total mRNA from uterus was obtained commercially. Positive controls contained a reaction mix that included plasmids containing the cDNA sequences of the splice variants or WT-LGR7 as a template. No PCR products were detected in controls containing water instead of the cDNAs, and the housekeeping gene GAPDH was used. The sizes of PCR products were as follows: splice variant C, 268 bp; splice variant D, 240 bp; splice variant F, 258 bp; WT-LGR7, 244 bp; and GAPDH, 140 bp.
To confirm and characterize their mRNA expression, we used nested PCR. We detected mRNA transcripts for all three variants in the decidual basal plate; however, only LGR7-D was detected by this method in the amnion of the fetal membranes (Fig. 1B). The mRNA of WT-LGR7 was detected using primers previously described and designed to the C-terminal region of the receptor (14) and was detected in the decidua, although its expression was extremely low or undetectable in the amnion, confirming our previous work (40). LGR7 expression in the human uterus has been reported previously (14) and confirmed here, in addition to expression of all three novel LGR7 splice variants (Fig. 1B). The correct sizes of amplified fragments were confirmed by positive controls using templates that included plasmid containing cDNAs of each splice variant or WT-LGR7 (Fig. 1B). We did not detect any PCR products in negative control samples containing water instead of cDNAs. The PCR-amplified fragments of the three splice variants (LGR7-C, LGR7-D, and LGR7-F) and the WT-LGR7 were confirmed by sequencing.
qRT-PCR analyses of LGR7 splice variant mRNA expression in the fetal membranes at term and the effects of spontaneous labor and delivery
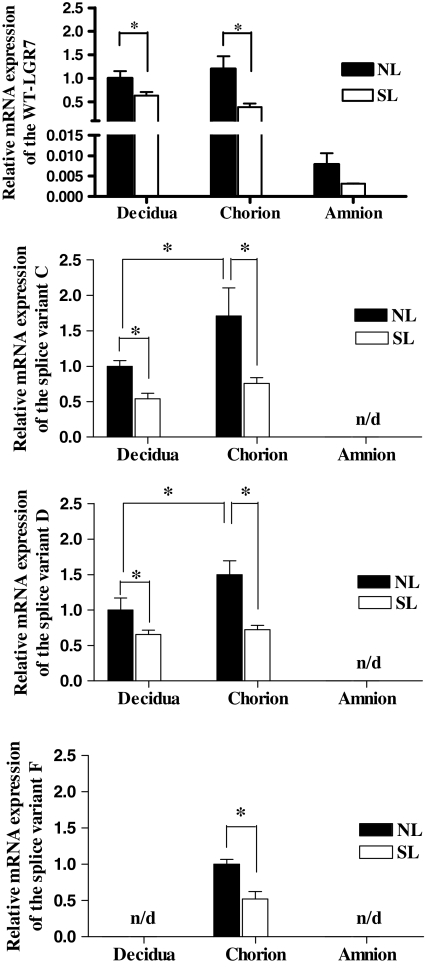
Expression of the mRNAs of WT-LGR7 and the splice variants were quantitated in separated decidua, chorion, and amnion at term and before and after spontaneous labor (Fig. 2). This confirmed our previous observations that labor reduced expression of LGR7 (40) and was extended here to show that labor also significantly reduced the expression of LGR7-C and LGR7-D (P < 0.05) in the chorion and decidua, shown in Fig. 2. Relatively lower levels of the variants were expressed in these tissues compared with the expression of WT-LGR7 (Table 2). However, LGR7-C was the most highly expressed variant in both the decidua and chorion, and although its expression was reduced by labor, the relative level of its mRNA remained about the same in both the chorion and decidua both before and after spontaneous labor, ranging between 26.7–27% and 19.8–21.7% of the WT-LGR7, respectively (Table 2). Interestingly, LGR7-F expression was detectable only in the chorion and was also significantly higher before labor than after (P < 0.05) (Fig. 2, lower panel). However, expression of the LGR7-F was detected in the decidua (Fig. 1) by qualitative PCR using a second nested PCR, which resulted in more sensitive detection compared with the qRT-PCR used for detection of the LGR7-F in Fig. 2. We were unable to detect any of the splice variant mRNAs in the amnion using qRT-PCR analysis. However, as shown in Fig. 1B, expression of LGR7-D was detected in the amnion by the more sensitive nested PCR.
Figure 2.
Comparative mRNA expression levels for the WT-LGR7 splice variants LGR7-C, LGR7-D, and LGR7-F in separated decidua, chorion, and amnion from singleton term pregnancies both before or no labor (NL) and after spontaneous labor (SL) (n = 5). Relative expression levels were determined by real-time quantitative RT-PCR using primers designed to amplify WT-LGR7 and each variant specifically (Table 1). Results are expressed as means ± sem of relative mRNA normalized to the levels of 18S RNA in each sample. The mean results of NL decidua for WT-LGR7, LGR7-C, and LGR7-D and the mean result of NL chorion for LGR7-F were arbitrarily assigned to value 1. No variant expression was detected (n/d) in the amnion, and LGR7-F was also undetectable in the decidua. *, P < 0.05.
Table 2.
Relative mRNA expression of splice variants by quantitative real-time PCR (percentage of WT-LGR7)
| LGR7-C
|
LGR7-D
|
LGR7-F
|
||||
|---|---|---|---|---|---|---|
| NL | SL | NL | SL | NL | SL | |
| Decidua | 19.8 | 21.7 | 0.5 | 0.6 | ND | ND |
| Chorion | 26.7 | 27 | 0.7 | 1.1 | 0.46 | 0.47 |
| Amnion | ND | ND | ND | ND | ND | ND |
ND, Not detectable; NL, no labor; SL, spontaneous labor.
Protein expression of the LGR7 splice variants in transiently transfected HEK293 cells
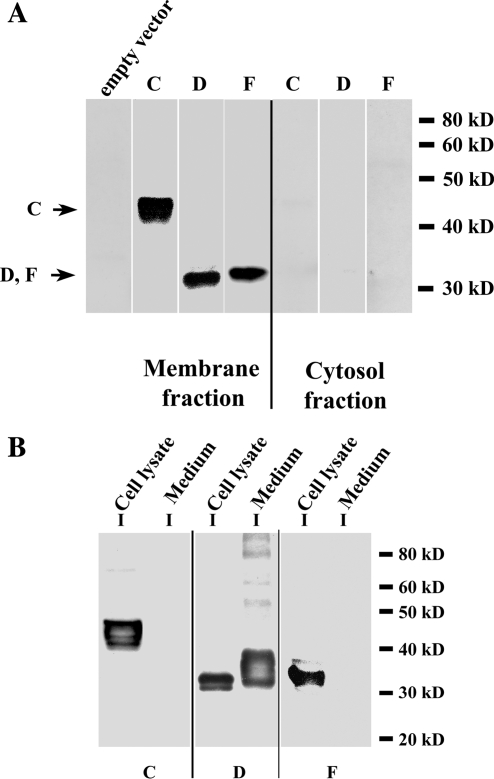
To characterize expression of the three splice variant proteins, we subcloned their cDNAs into a mammalian expression vector with the HA sequence inserted after the signal peptide for each cDNA. The membrane and cytosol fractions of transfected HEK293 cells were analyzed by Western blot showing their localization in the membrane fraction and not in the cytosol fraction of the transfected cells (Fig. 3A). None was detected in the membrane fractions of cells transfected with an empty vector (control), confirming that the detected bands represented the expressed splice variants.
Figure 3.
Detection of the splice variant proteins expressed in transfected HEK293 cells by Western blot. A, Total membrane and cytosol fractions from HEK293 cells transfected with each splice variant with the HA sequence inserted after the signal peptide for each cDNA. Samples (5 μg) of total membrane proteins and cytosol fractions were separated on 10% SDS-PAGE, immunoblotted, and incubated with an antibody to HA. All three splice variants were localized in the membrane fraction and none detected in the cytosol. No signal was detected in membrane fractions from cells transfected with an empty vector (control). B, Splice variant LGR7-D has a secreted protein form. Lysates and medium from HEK293 cells transfected with each splice variant cDNA with an HA tag were collected and subjected to immunoprecipitation with an anti-HA-agarose. Immunoprecipitates were separated in 10% gels and analyzed on Western blotting with an HA antibody. All three splice variants were detected in the cell lysates, and only splice variant LGR7-D was detected in medium and had a higher molecular weight compared with its intracellular form.
It was recently demonstrated that a mouse splice variant (LGR7-truncate) was secreted into media (20); we therefore investigated whether any of these human splice variants was secreted into the media of transfected cells. After 48 h of transfection, media were collected, and cell lysates were separated and used for immunoprecipitation with HA-agarose. The eluted samples were resolved on SDS-PAGE and the proteins detected by Western blotting. All variants were detected in the cell lysates (Fig. 3B); however, LGR7-D had two protein forms, one in the cell lysates and the other secreted into the medium. Secreted LGR7-D had a higher molecular weight compared with the intracellular LGR7-D protein, due to different amounts of glycosylation (see below). These results show that although all the variants were retained intracellularly, LGR7-D also had a secreted form.
Splice variants LGR7-C, LGR7-F, and intracellular LGR7-D localization in the ER
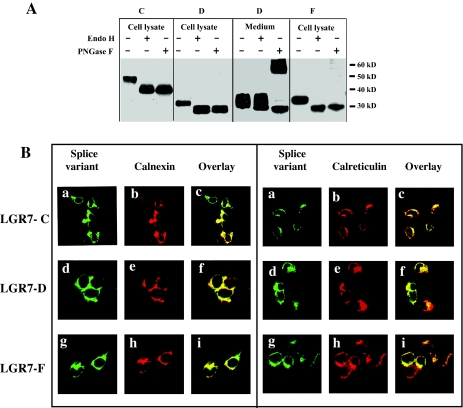
The splice variant forms expressed in HEK293 cells were further studied by subjecting immunoprecipitated samples to endoglycosidase treatment. As seen in Fig. 4A, all were sensitive to peptide N-glycosidase F (PNGase F) treatment, an enzyme that removes all types of N-linked oligosaccharides from glycoproteins (46). Removal of the glycan chains resulted in the appearance of completely deglycosylated proteins with lower molecular weights, indicating that all three were naturally subjected to N-linked glycosylation. Interestingly, PNGase F treatment of secreted LGR7-D resulted in the appearance of higher molecular weight species as well. Based on the molecular size, we predict that these forms of the secreted LGR7-D, after PNGase F treatment, represent dimers or aggregates. We hypothesize that PNGase F treatment of the secreted LGR7-D resulting in deglycosylation of this soluble splice variant can alter protein structure/stability, which leads to artifactual dimerization/aggregation which is SDS-PAGE resistant. The endoglycosidase enzyme endoglycosidase H (Endo H) removes unprocessed high-mannose-type N-linked oligosaccharides from glycoproteins but does not cleave complex fully processed glycans. The secreted form of LGR7-D was resistant to Endo H treatment (Fig. 4A), indicating that it contains fully processed complex type N-linked oligosaccharides and therefore would be transported to the medium through the Golgi complex. In contrast, treatment with Endo H showed sensitivity of the intracellular forms of all three splice variants (Fig. 4A), suggesting that they contain high-mannose-type N-linked glycans typical for glycoproteins residing in the ER.
Figure 4.
Localization of the splice variants to the ER. A, Lysates of cells transfected with LGR7-C, LGR7-D, or LGR7-F constructs containing the HA tag or medium from cells transfected with LGR7-D with the HA tag were immunoprecipitated with an antibody to HA-agarose. Immunoprecipitates were incubated with Endo H, PNGase F, or without enzymes, separated on 10% gels, blotted, and probed with the HA antibody. B, The splice variants colocalize with ER markers calnexin and calreticulin. HEK293 cells transfected with each splice variant with an HA-tag were fixed, permeabilized, and double labeled for confocal microscopy. The ER resident proteins were labeled with rabbit antisera to calnexin (left panel) or calreticulin (right panel). The splice variants LGR7-C (a–c), LGR7-D (d–f), and LGR7-F (g–i) were immunostained in parallel using a mouse antibody to HA. Secondary antibodies were AlexaFluor 488 (mouse) or AlexaFluor 546 (rabbit). Overlay pictures (c, f, and i) show strong colocalization of the three splice variants with calnexin and calreticulin, respectively, and their localization in the ER.
To further characterize the intracellularly retained splice variants, we colocalized them together with ER protein markers. Therefore, we tested the colocalization with two ER endogenous proteins, calnexin and calreticulin. HEK293 cells were transfected with each splice variant bearing the HA-epitope, and the cells were permeabilized and stained for the HA-epitope (Fig. 4B, a, d, and g, respectively) and ER markers calnexin (Fig. 4B, left panel: b, e, and h) and calreticulin (Fig. 4B, right panel: b, e, and h) and subjected to confocal microscopy. All three variants colocalized with calnexin (Fig. 4B, left panel: c, f, and i) and calreticulin (Fig. 4B, right panel: c, f, and i), supporting the view that LGR7-C, LGR7-F, and intracellular LGR7-D were retained in the ER of the transfected cells.
The secreted form of LGR7-D is delivered to the cell surface
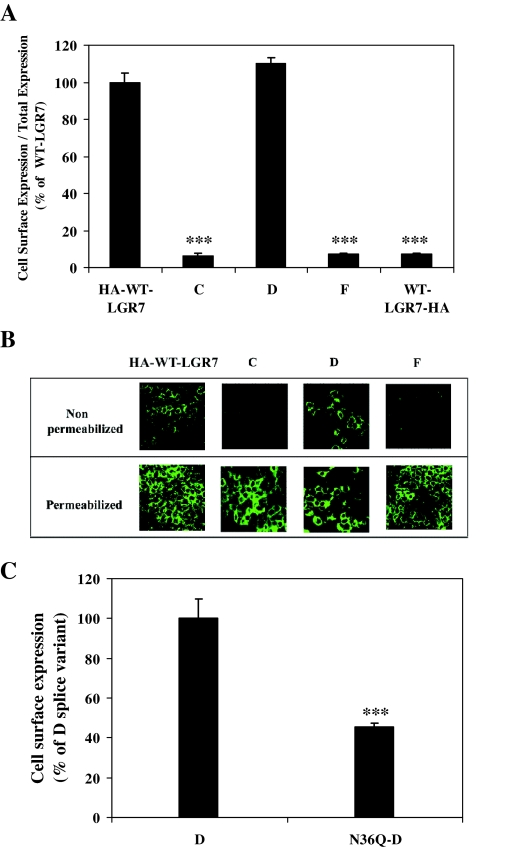
We also tested whether the secreted form of LGR7-D was delivered to the cell surface in transfected HEK293 cells by using a cell surface ELISA. HEK293 cells were transfected with WT-LGR7 constructs bearing the HA tag at the N terminus (HA-WT-LGR7) or with splice variants alone with the HA tag at their N termini and expression assessed with the cell surface ELISA. As seen in Fig. 5A, the relative level of cell surface expression compared with the total expression of LGR7-D was 110 ± 5% compared with HA-WT-LGR7, showing that secreted LGR7-D was indeed expressed at the cell surface. In contrast, the relative levels of cell surface expression of LGR7-C and LGR7-F were significantly lower (P < 0.001), 6.2 ± 1.7% and 7.5 ± 0.5%, respectively, compared with HA-WT-LGR7 (Fig. 5A). This confirmed the primary localization of LGR7-C and LGR7-F inside the cells. The sensitivity and specificity of the cell surface ELISA was confirmed with WT-LGR7 bearing the HA-epitope at the C-terminal end (WT-LGR7-HA). The HA tag would only be intracellular; therefore, in cells transfected with WT-LGR7-HA, antibody would be detected only as nonspecific labeling. Therefore, its relative cell surface expression compared with the total expression of WT-LGR7-HA was significantly lower (P < 0.001), 7.15 ± 0.85%, compared with the HA-WT-LGR7 (Fig. 5A) and very similar to LGR7-C and LGR7-F, supporting the concept that these two splice variants were indeed retained inside the cells. These results were confirmed by confocal microscopy (Fig. 5B). Nonpermeabilized (upper panel) and permeabilized (lower panel) HEK293 cells transfected with each variant were labeled with the HA antibody and visualized with AlexaFluor 488-conjugated secondary antibody. In nonpermeabilized conditions, only the HA-WT-LGR7 and LGR7-D were localized to the membrane. In contrast, cells transfected with LGR7-C or LGR7-F showed no cell surface localization (Fig. 5B, upper panel). However, in permeabilized HEK293 cells, all the variants were detected only intracellularly, demonstrating that each had portions retained within the cells (Fig. 5B, lower panel). These results confirm the localization of the secreted form of LGR7-D in the cell membrane.
Figure 5.
Secreted LGR7-D is delivered to the cell surface of transfected cells. A, HEK293 cells transfected with WT-LGR7, LGR7-C, LGR7-D, or LGR7-F bearing the HA epitope at their N termini as well as LGR7-HA bearing the HA epitope at its C terminus (control) were assayed by indirect ELISA using an antibody to the HA tag to show cell surface expression. Relative cell surface expression (cell surface expression to total expression) is shown as percentage of relative cell surface expression of the HA-WT-LGR7. ***, P < 0.001 compared with HA-WT-LGR7 by Student’s paired t test. B, Confocal microscopic images of nonpermeabilized (upper panels) and permeabilized (lower panels) HEK293 cells transfected with WT-LGR7, LGR7-C, LGR7-D, and LGR7-F bearing the HA tag at their N termini. The cells were stained with primary antibody to HA and with mouse AlexaFluor 488-conjugated secondary antibody, showing cell surface localization of the WT-LGR7 and LGR7-D in nonpermeabilized cells and the intracellular distribution of all constructs in the permeabilized cells. C, Expression analysis of an LGR7-D glycosylation mutant (N36Q-D) by cell surface ELISA. HEK293 cells transfected with WT-LGR7-D and N36Q-D bearing the HA tag at their N termini were assayed by cell surface ELISA. Cell surface expression was expressed as percentage of WT-LGR7-D and shows that cell surface expression of N36Q-D was reduced to 45.4 ± 1.8% compared with WT-LGR7-D. ***, P < 0.001 using Student’s paired t test.
We previously demonstrated the importance of glycosylation at the Asp36 for the cell surface delivery of the WT-LGR7 (N36Q-LGR7) (21). By analogy, we created a point mutant of LGR7-D by exchanging Asp36 to Gln (N36Q-D). The cell surface ELISA was then used to examine whether glycosylation at Asp36 was critical for the cell surface delivery of LGR7-D. As seen in Fig. 5C, this was significantly decreased (P < 0.001) to 45.4 ± 1.8% compared with LGR7-D cell surface expression, showing that N-linked glycosylation at Asp36 is important in the delivery of variant LGR7-D to the PM, and suggests that the secreted LGR7-D uses a similar biosynthetic pathway as WT-LGR7 for its cell surface delivery.
Functional characterization of LGR7 splice variants and their effects on the function of the WT-LGR7
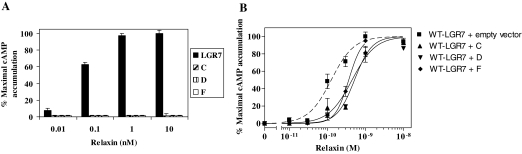
Previous studies have shown that relaxin treatment of cells expressing LGR7 induces cAMP production (21); therefore, we tested whether each splice variant expressed alone would respond to relaxin. Cells expressing WT-LGR7 showed dose-dependent increases in cAMP production (Fig. 6A), whereas the cells expressing LGR7-C, LGR7-D, or LGR7-F all failed to show any cAMP increases (Fig. 6A). The effects of each splice variant on the function of the WT-LGR7 were then investigated by cotransfecting the WT-LGR7 and one splice variant construct or WT-LGR7 and an empty vector (control) in HEK293 cells. The coexpression of WT-LGR7 with any one of the variants caused dose-dependent decreases in cAMP production compared with cells coexpressing WT-LGR7 and the empty vector (Fig. 6B). This resulted in significantly increased (P < 0.05) EC50 values for cells coexpressing WT-LGR7 with LGR7-C, LGR7-D, or LGR7-F (0.44 ± 0.08, 0.51 ± 0.06, and 0.36 ± 0.02 nm, respectively) compared with the control (0.1 ± 0.06 nm). These results show that each splice variant reduced the optimal efficacy of LGR7 and had a dominant-negative effect on the function of the WT-LGR7.
Figure 6.
Functional characterization of the LGR7 splice variants. A, Relaxin caused a dose-dependent stimulation of cAMP production from HEK293 cells transfected with WT-LGR7, but cells expressing LGR7-C, LGR7-D, or LGR7-F failed to show any effects. cAMP accumulation is expressed as percentage of the maximum relaxin response of WT-LGR7. B, Effects of the LGR7 splice variants on the function of the WT-LGR7. Relaxin stimulated the dose-dependent production of cAMP from cells cotransfected with WT-LGR7 and an empty vector (control, dashed line). Efficacy of relaxin-stimulated cAMP production of the WT-LGR7 was significantly decreased (P < 0.05) in the presence of each splice variant (solid lines) compared with WT-LGR7 with the empty vector (dashed line). Accumulation of cAMP was expressed as percentage of the maximum relaxin response in the presence of WT-LGR7 and empty vector.
LGR7 splice variants reduce the cell surface expression and maturation of the WT-LGR7
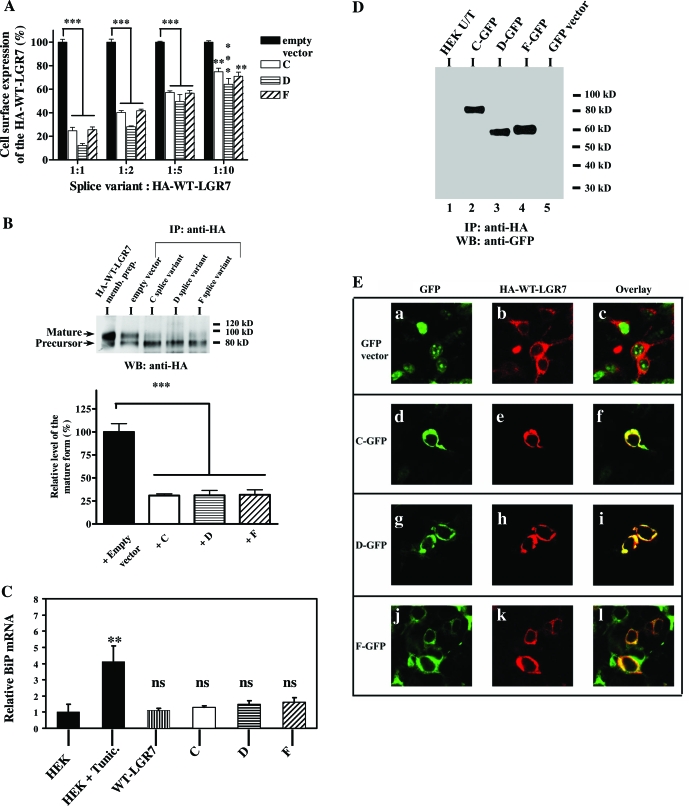
We also investigated whether the splice variants affected the cell surface delivery of the WT-LGR7. The cell surface ELISA was performed with cells transfected with varying ratios of splice variant to WT-LGR7. When each variant was coexpressed with WT-LGR7 at the ratio of 1:1, the cell surface expression of WT-LGR7 was significantly (P < 0.001) reduced to 24.7 ± 3, 12.3 ± 1.6, and 25.6 ± 2.3%, respectively, compared with expression of WT-LGR7 and an empty vector (control) (Fig. 7A). The coexpression of the splice variants had no significant effects on the total expression level of the WT-LGR7 compared with total expression level of WT-LGR7 with an empty vector; for coexpression with LGR7-C, total expression level of WT-LGR7 was 100.6 ± 7.7%; for coexpression with LGR7-D, total expression level of WT-LGR7 was 113 ± 5.1%; and for coexpression with LGR7-F, total expression level of WT-LGR7 was 95.6 ± 12.3% (data not shown). Furthermore, the cell surface delivery of the WT-LGR7 increased proportionately with decreasing ratios of splice variants to full-length receptor. However, such a dominant-negative effect of these splice variants was evident even when they were expressed at 10 times less than WT-LGR7 (Fig. 7A). Thus, coexpression of these splice variants together with LGR7 resulted in significantly decreased cell surface delivery of LGR7, although the total expression of LGR7 did not change, showing that the LGR7 was retained inside the cells in the presence of the splice variants confirming their dominant-negative effects on the LGR7.
Figure 7.
Effects of the LGR7 splice variants on the expression of the WT-LGR7. A, Effects of the splice variants on the cell surface delivery of WT-LGR7 by ELISA. HEK293 cells were cotransfected with HA-WT-LGR7 and either one splice variant construct or an empty vector (control) and assayed with the cell surface ELISA. Transfections were performed with different ratios of splice variants or empty vector to HA-WT-LGR7. Cell surface expression is presented as percentage of the HA-WT-LGR7 expression in the presence of the empty vector. ***, P < 0.001; ** P < 0.01 compared with HA-WT-LGR7 coexpressed with an empty vector by Student’s paired t test. B, The presence of a splice variant leads to decreased maturation of the full-length receptor. Lysates from cells coexpressing the HA-WT-LGR7 and one of the splice variants or an empty vector (control) were immunoprecipitated (IP) with HA-agarose and analyzed by 7.5% SDS-PAGE and Western blotting with an HA antibody (upper panel). The membrane preparations from cells transfected with HA-WT-LGR7 were also detected by Western blotting (WB). Two expressed molecular weight forms of HA-WT-LGR7 (95-kDa mature and 80-kDa precursor) were detected (upper panel). The relative intensities of the mature form were determined by densitometric analysis (mean ± sem of three independent experiments). Data are expressed as percentage intensity in cells coexpressing HA-WT-LGR7 and empty vector (lower panel). ***, P < 0.001 by Student’s paired t test analysis. C, The splice variants did not induce an UPR. Cells respond to ER stress by increasing transcription of genes encoding resident chaperones for facilitation of protein folding. mRNA from untreated HEK293 cells, HEK293 cells treated with tunicamycin (Tunic., an UPR-inducing reagent, 5 μg/ml) for 24 h (positive control for BiP induction), and HEK293 cells stably transfected with WT-LGR7, LGR7-C, LGR7-D, or LGR7-F were isolated and the BiP mRNA expression analyzed by quantitative real-time PCR. Data are shown as mean relative values of BiP/18S ± sem of three independent experiments. The mean result from untreated HEK293 cells was arbitrarily assigned a value of 1. No significant changes were observed in BiP expression in HEK293 cells compared with HEK293 cells with stable expression of each splice variant or WT-LGR7. In contrast, tunicamycin treatment led to a significant increase in BiP mRNA levels. **, P < 0.01 compared with untreated HEK293 cells; ns, not significant. D, The splice variants stably interact with WT-LGR7. Lysates from untransfected cells (HEK U/T, lane 1), cells cotransfected with HA-WT-LGR7 and one splice variant (lanes 2–4), and cells cotransfected with HA-WT-LGR7 and an empty GFP vector (lane 5) were immunoprecipitated with HA-agarose. The immunoprecipitates (IP) were analyzed by 7.5% SDS-PAGE and Western blotting (WB) using a rabbit antibody to GFP. Splice variants were detected in the immunoprecipitates from the cotransfected cells (lanes 2–4) but not from the untransfected controls or cells cotransfected with HA-WT-LGR7 and an empty vector. This shows the direct interactions between the splice variants and the WT-LGR7. E, The splice variants colocalize with WT-LGR7. Cells cotransfected with HA-WT-LGR7 and an empty GFP vector (a–c) or cotransfected with HA-WT-LGR7 and a splice variant tagged with GFP (splice variant C, d–f; splice variant D, g–i; and splice variant F, j–l) were fixed, permeabilized, and stained for the HA epitope with a primary mouse HA antibody and secondary mouse AlexaFluor 546 antibody. Colocalization of each variant and HA-WT-LGR7 is shown on the overlays (f, i, and l). No colocalization was detected in cells coexpressing HA-WT-LGR7 and the empty GFP vector (c).
We then investigated whether these splice variants were capable of decreasing the cell surface delivery of the full-length receptor and affect its biogenesis and maturation. Previously, we demonstrated that WT-LGR7 is expressed as two molecular weight forms, the immature or precursor form (80 kDa) was retained inside the cells, whereas the mature form (95 kDa) was delivered to the cell surface (21). After cotransfection of WT-LGR7 and each splice variant into HEK293 cells, total cell lysates were prepared and subjected to immunoprecipitation followed by Western blot analysis. As shown in Fig. 7B, coexpression of WT-LGR7 and each splice variant led to a significant (P < 0.001) decrease in the amount of the mature full-length receptor to 30.8 ± 1.8, 31 ± 5.4, and 31.8 ± 5.3% for each variant, respectively, compared with the mature receptor expressed by cells coexpressing WT-LGR7 and an empty vector (control). These data show that these splice variants decrease the cell surface delivery of the WT-LGR7 by inhibiting its maturation.
Expression of the splice variants in HEK293 cells does not induce an unfolded protein response (UPR)
The observed reduction in full-length receptor maturation and cell surface expression in the presence of LGR7 splice variants might be the consequence of altered protein synthesis or changes in the components of the ER quality control apparatus. Therefore, we tested whether their expression might cause UPR. The UPR is a typical cellular response involved in the up-regulation of ER chaperones and in proteins involved in ER-associated degradation (47,48). Cells respond to ER stress by increasing transcription of genes encoding ER-resident chaperones such as GRP78/BiP (glucose-regulated protein 78 kDa) to facilitate protein folding or by suppression of mRNA translation and synthesis of proteins (47,48). Therefore, we analyzed the BiP mRNA levels by quantitative RT-PCR. No significant changes were observed in the expression level of the BiP in HEK293 cells with stable expression of each splice variant or WT-LGR7, compared with the untransfected HEK293 cells (Fig. 7C). In contrast, the UPR-inducing reagent tunicamycin led to a significant (P < 0.01) increase in BiP mRNA levels compared with uninduced HEK cells (Fig. 7C). Thus, the inhibited expression of mature WT-LGR7 and decreased cell surface expression observed in cells expressing the splice variants is unlikely to result from impaired protein synthesis or changes in the ER quality control apparatus.
Splice variants dimerize and colocalize with the LGR7
To show whether the splice variants stably interact with the WT-LGR7, we used coimmunoprecipitation of lysates from cells cotransfected with WT-LGR7 bearing the HA-epitope and splice variants fused with the GFP at their C termini. Immunoprecipitation was carried out with anti-HA and a GFP antibody used for Western blotting and visualization. Splice variants were detected in the anti-HA immunoprecipitates from cotransfected cells (Fig. 7D) but not from untransfected control cells or cells cotransfected with WT-LGR7 and an empty GFP vector (Fig. 7D). Thus, these data show that the splice variants directly interact with the full-length receptor.
We also investigated the cellular colocalization of splice variants and full-length LGR7 by confocal microscopy. Cotransfected cells with WT-LGR7 and each splice variant fused with GFP were permeabilized, stained with anti-HA for detection of WT-LGR7, visualized with secondary AlexaFluor 546 antibody, and subjected to confocal microscopy. No colocalization was detected in cells cotransfected with an empty GFP vector and WT-LGR7 (Fig. 7E, a–c), but in cells cotransfected with WT-LGR7 and LGR7-C (Fig. 7E, d–f), LGR7-D (Fig. 7E, g–i), or LGR7-F (Fig. 7E, j–l), we detected strong colocalization of the splice variants and WT-LGR7. These data show that the dominant-negative effects of the splice variants on the function of the WT-LGR7 occur through direct interaction of the splice variant and WT-LGR7.
Homodimerization of the WT-LGR7
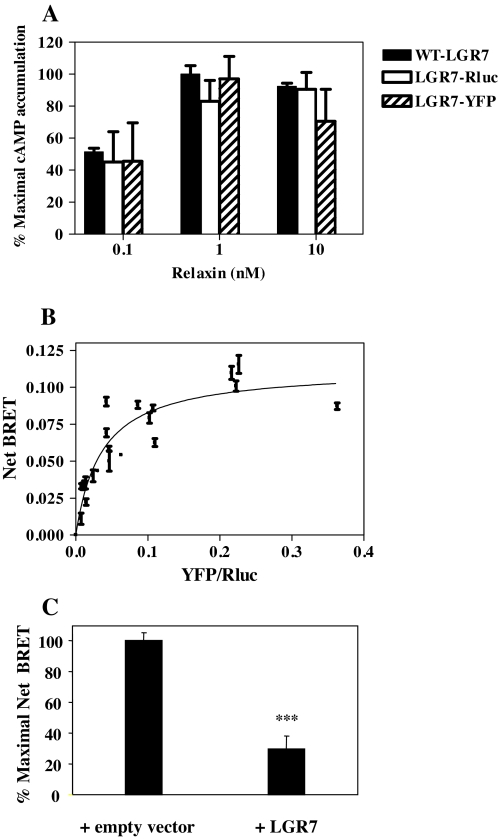
New evidence strongly suggests that effective GPCR dimerization is required at the level of the ER for receptor maturation and cell surface delivery to occur (30). Our demonstration of interaction between each splice variant and WT-LGR7, resulting in the drastic reduction of maturation and cell surface delivery of the WT-LGR7, suggested potential dimerization of LGR7. This was investigated, as well as whether dimerization of the full-length receptor was affected by the presence of any splice variants. Dimerization of WT-LGR7 was shown by BRET assay, and for this purpose, LGR7 was tagged at its C terminus with Renilla-luciferase (LGR7-Rluc) or YFP. Fusion of LGR7 to Rluc or YFP did not impair the signaling properties of LGR7 as assessed by the ability of relaxin to stimulate cAMP (Fig. 8A). Dimerization was studied in living cells by determining the transfer of energy between the Rluc and YFP fusion constructs expressed in HEK293 cells upon addition of the luciferase substrate, coelenterazine h. First, we assessed the dimerization state of the WT-LGR7 by BRET saturation assay; for this, a LGR7-Rluc construct was maintained at a constant level, whereas the concentration of the LGR7-YFP partner was gradually increased (Fig. 8B). A robust and very reproducible transfer was observed between LGR7 fusion proteins with BRETmax = 0.11 ± 0.008 and BRET50 = 0.04 ± 0.008. The BRET50 data reflected the concentration of LGR7-YFP construct reaching half-maximal BRET and provided a measure of the relative affinity of the partners for each other (44).
Figure 8.
Homodimerization of the LGR7. A, The WT-LGR7 was tagged at its C terminus with Renilla luciferase (LGR7-Rluc) or YFP (LGR7-YFP). Production of cAMP was assayed in cells transfected with WT-LGR7, LGR7-Rluc, and LGR7-YFP after treatment with different doses of relaxin (0.1, 1, and 10 nm). There were no significant differences in cAMP production in cells expressing LGR7-Rluc or LGR7-YFP compared with WT-LGR7. Thus, fusion of LGR7 to Rluc or YFP had no effect on the signaling of LGR7. B, Homodimerization of LGR7 assessed by BRET saturation assay. HEK293 cells were cotransfected with a constant amount of LGR7-Rluc and increasing DNA concentrations of LGR7-YFP. Data are shown as net BRET signals plotted over the relative expression levels (total fluorescence over luminescence, YFP/Rluc) and results expressed as the mean ± sem of three independent experiments. The curve was fitted using nonlinear regression equation assuming a single binding site (GraphPad Prism). C, BRET competition assays. HEK293 cells coexpressing a constant DNA level of LGR7-Rluc and LGR7-YFP constructs (concentrations determined to give a signal close to the BRET50) in the presence of an empty vector or untagged LGR7. The maximal net BRET measured in the presence of an empty vector was set at 100%. Data are expressed as a mean ± sem of three experiments. The BRET signal obtained in the presence of untagged LGR7 was significantly lower compared with the BRET signal measured in cells coexpressing LGR7-Rluc, LGR7-YFP, and an empty vector. ***, P < 0.001 compared with net BRET signal in the presence of an empty vector.
To further confirm the specificity of LGR7 dimerization, we carried out BRET competition assays. Cells were transfected with LGR7-Rluc and LGR7-YFP in the presence of untagged LGR7 or with an empty vector (control) (Fig. 8C). For each sample, total fluorescence and luminescence were also determined to ensure that the level of LGR7-Rluc and LGR7-YFP fusion constructs were the same between experiments (fluorescence to luminescence ratio was 0.04, indicating that the measurements were carried out in the linear phase of the saturation curve) (Fig. 8B). The BRET signal obtained in the presence of untagged LGR7 was significantly lower (P < 0.001), 30 ± 8.5%, compared with the BRET signal measured in cells coexpressing LGR7-Rluc, LGR7-YFP, and the empty vector (Fig. 8C), confirming the specificity of the dimerization of the LGR7.
Dimerization of the LGR7s occurs early in the biosynthetic pathway in the ER
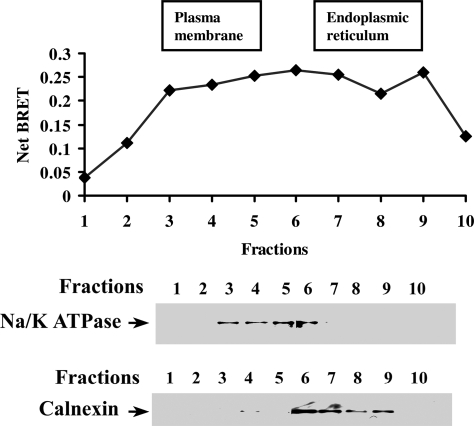
Biochemical and biophysical experimental approaches have recently demonstrated that dimerization of the GPCRs occurs in the ER during their biosynthesis (36,37,38,39,49,50). To investigate the site of LGR7 dimer formation, the subcellular distribution of the LGR7 dimers was studied by sucrose gradient fractionation of the crude membrane extracts prepared from cells coexpressing LGR7-Rluc and LGR7-YFP constructs by BRET assay. PM and ER fractions were separated by ultracentrifugation. Na/K-ATPase and calnexin were used as PM (fractions 2–5) and ER (fractions 7–9) markers, respectively (Fig. 9). We detected significant BRET between LGR7-Rluc and LGR7-YFP in both PM and ER fractions, indicating that dimers existed in both these compartments (Fig. 9, upper panel). These data show that LGR7 dimers exist early in the ER but were also found at the cell surface, showing that LGR7 dimerization may occur rapidly after the translocation of the newly synthesized receptor into the ER but is maintained throughout maturation and trafficking to the PM.
Figure 9.
Constitutive dimerization of the LGR7s occurs in the ER and PM. Total membrane preparations from HEK293 cells cotransfected with LGR7-Rluc and LGR7-YFP or LGR7-Rluc and WT-LGR7 were fractionated on a sucrose gradient and fractions subjected to BRET measurements (upper panel). The individual fractions were resolved on 7.5% SDS-PAGE and Western blotting performed using antibody markers for PM (Na/K ATPase) and ER (calnexin). The data shown are representative of two independent experiments.
Effects of the splice variants on dimerization of WT-LGR7
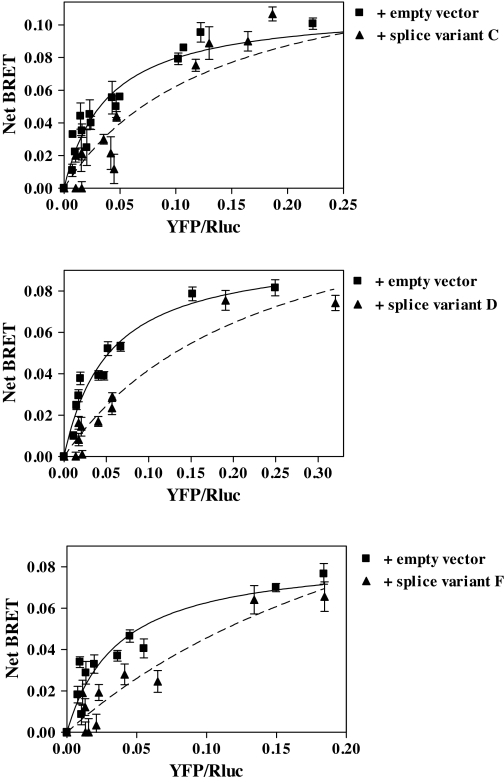
The effects of the splice variants on LGR7 dimerization was studied to establish a link between dimerization and the cell surface delivery of the WT-LGR7. LGR7 dimer formation in the presence of each splice variant construct or an empty vector (control) was investigated by BRET saturation assay on cells coexpressing a constant amount of LGR7-Rluc construct and increasing amounts of LGR7-YFP. In the BRET saturation assay, we assessed the BRET50 data, which reflects the relative affinity of LGR7s for each other and the ability to form a dimer (44). As seen in Fig. 10, the titration curves were shifted when each splice variant was coexpressed with WT-LGR7. The BRET50 data were significantly increased (P < 0.05) to 0.13 ± 0.03, 0.23 ± 0.09, and 0.3 ± 0.1 for each variant, respectively, compared with BRET50 data when LGR7 was coexpressed with an empty vector. Therefore, the splice variants inhibit formation of WT-LGR7 dimers. Taken together with the cell surface delivery data, these results show that splice variants exert a dominant-negative effect on the cell surface delivery of WT-LGR7 by decreasing formation of dimers and suggest that homodimerization of LGR7 in the ER is a prerequisite for the cell surface delivery of the WT-LGR7.
Figure 10.
Effects of LGR7 splice variants on dimerization of the WT-LGR7. BRET saturation assays for LGR7 dimerization were carried out in the presence of each splice variant. HEK293 cells were cotransfected with a constant amount of LGR7-Rluc and increasing DNA concentrations of LGR7-YFP in the presence of an empty vector (control) or the splice variants (LGR7-C, upper panel; LGR7-D, middle panel; and LGR7-F, lower panel). Data are shown as net BRET signals plotted over the relative expression levels (total fluorescence over luminescence; YFP/Rluc). The curves (solid line, in the presence of empty vector; dashed line, in the presence of splice variant) were fitted using nonlinear regression equation assuming a single binding site (GraphPad Prism). The titration curves were shifted to the right when splice variants were present, showing that each splice variant inhibited formation of WT-LGR7 dimers.
Discussion
We describe here three novel splice variants from the human fetal membranes that can decrease the homodimerization of the WT-LGR7 in the ER by the formation of heterodimers with the WT-LGR7. The consequence of this is the sequestration and inhibition of both maturation and cell surface delivery of WT-LGR7. Thus, these three novel LGR7 splice variants all exert a dominant-negative effect on the function of WT-LGR7. Although other LGR7 splice variants have been described previously, this is the first demonstration of an intracellular interaction between LGR7 splice variants and the WT-LGR7 to cause a loss of receptor function.
By analogy with two receptors in Drosophila melanogaster, the relaxin receptor (LGR7) is an archaic receptor of the rhodopsin GPCR family, predating the emergence of insects and vertebrates (17). This family of GPCRs is represented in both invertebrates and vertebrates, and the members are likely to have accumulated introns during their evolution, whereas newer GPCR genes appear to be predominantly without introns (51). Intron density analysis has shown that this LGR subfamily belongs to the intron-richest ancestral receptor group; thus, LGR7 contains 17 introns (51). Alternative splicing therefore is a common occurrence for members of this family, with reports of splice variants for FSHR (23,24), TSHR (25), and LHR (26,27) and most recently for LGR7 and LGR8 (20,28). Although we were unable to detect splice variant LGR7.10 (missing the third exon of LGR7 gene) in the human placenta or fetal membranes using regular PCR methodology (40), we now report the cloning, expression, and functional analysis of three new splice variants of the LGR7 in the human fetal membranes. All three variants contain different lengths of the extracellular region of the receptor and have no TM domain. Use of quantitative RT-PCR primers capable of distinguishing between them has allowed us to investigate their mRNA expression in separated amnion, chorion, and decidua, obtained both before and after spontaneous labor and delivery at term. This showed that labor significantly and coordinately decreased the expression of these splice variants and WT-LGR7. We previously showed that expression of the full-length receptor was highest in the decidua and chorion compared with the amnion, both before and after labor and delivery (40), and this result was confirmed here. We now show that expression of variants LGR7-C and LGR7-D were highest in the chorion before labor. Expression of LGR7-F was detected only in the chorion, and its mRNA levels were also significantly reduced by spontaneous labor. However, its level of expression was very low and could have been below detectability in the amnion and decidua. The results using RNAs from amnion and the decidua from the placental basal plate of monozygotic twin gestations for nested PCR gave positive results for LGR7-D in the amnion and LGR7-F in the decidua. We used these tissues to obtain pure fetal tissue, which is difficult to obtain by physical separation. This differed from the results obtained with the amnion and parietal decidua from singleton gestations collected before and after labor and qRT-PCR. The primers and PCR conditions, as well as the tissues, were quite different in these experiments and affected the ability to detect the relatively low expression of these splice variants by qRT-PCR. The existence of these splice variants and these differences in their mRNA expression over the peripartal period suggest their functional importance in these tissues. Therefore, their effects on cell surface expression and function of the full-length receptor has been studied.
Protein expression analysis showed all three splice variants localized with the cell membrane rather than with the cytosol. A recent study demonstrated a truncated LGR7 splice variant in the mouse that was secreted into medium in transfected HEK293 cells (20). We therefore investigated whether these human splice variants were similarly secreted. In contrast to expectations, splice variants LGR7-C and LGR7-F were shown to be retained inside the cells; however, splice variant LGR7-D had two protein forms, one retained inside the cells and the other secreted into the medium. Endoglycosidase analysis and colocalization experiments of the three intracellular splice variants showed them retained inside the cells and localized in the ER compartment. Splice variant LGR7-D consists of the LDL-A domain and one LRR, whereas LGR7-F and LGR7-C contain the LDL-A and two LRRs and eight LRRs, respectively, suggesting that the number of LRRs might affect their ability to be secreted. A splice variant of the related glycoprotein hormone receptor LHR, encoding the extracellular domain with 10 LRRs, was also retained in the ER compartment of transfected HEK293 cells (27). In contrast, a splice variant of the γ-aminobutyric acid GABAB receptor (GABAB1e) encoding only the extracellular domain of the receptor was also expressed in transfected HEK293 cells as two protein forms, one that was secreted and the other as a membrane-bound protein (52). It has recently been demonstrated that the binding sites for relaxin are localized within the four, five, six, and eight LRR repeats (19) and the LDL-A module is not involved in binding (20); hence, it is unlikely that this secreted LGR7-D form would bind and compete with WT-LGR7 for circulating relaxin. However, LGR7-D was also detected on the cell surface, suggesting that it becomes localized at this site, although its function is currently unknown. We demonstrated the presence of complex-type N-linked oligosaccharides in secreted LGR7-D, showing that it would be transported through the Golgi apparatus. However, site-directed mutagenesis of the Asp36 in its LDL-A module reduced its cell surface delivery, demonstrating the importance of the N-linked glycosylation at this site. We have previously shown that glycosylation at Asp36 in the WT-LGR7 is important for the cell surface delivery of the full-length receptor (21), suggesting that the secreted form of splice variant LGR7-D and the WT-LGR7 use a similar biosynthetic pathway.
There are now many studies documenting that alternative splice variants and naturally occurring mutant forms of the GPCRs inhibit the function of their respective wild-type receptors (26,27,31,32,33,34,35,36,37,38,39). It has recently been shown that the secreted mouse LGR7 splice variant, containing only the LDL-A module, inhibited function of the full-length receptor by its extracellular interaction with WT-LGR7 without modulating its cell surface expression (20); therefore, secreted LGR7-D might have a similar function, but further investigation is necessary to confirm this. We have concentrated here on the intracellular functions of the LGR7 splice variants and examined their intracellular interactions with the WT-LGR7, demonstrating that their coexpression significantly decreased the cAMP responses of the WT-LGR7 to relaxin treatment, showing their dominant-negative effects on the function of the WT-LGR7. This effect is consistent with the view that the expression of the splice variants is associated with a reduction in the number of WT-LGR7s present at the cell surface and supports the view that they inhibit its function by causing its intracellular retention. Indeed, this reduction of WT-LGR7 cell surface expression was shown to be dependent upon the ratio of the splice variant to the WT-LGR7, and the inhibitory effect was evident even when the splice variant was expressed at 10 times less than the WT-LGR7. It is not known whether the presence of these variants would have an additive effect on the WT-LGR7 cell surface expression. Our in vivo results show their concordant mRNA expression. However, the chorion and decidua both contain several different cell types, and it is not known whether they differentially express these LGR7 variants.
The ER is the main controller of protein folding and assembly. The LGR7 undergoes core (immature) N-linked glycosylation in the ER. Once properly folded, the core-glycosylated receptors transit to the Golgi apparatus where they undergo mature glycosylation and then expression on the cell surface. We previously demonstrated that LGR7 is expressed as two forms: an intracellularly retained precursor form bearing high-mannose-type oligosaccharides, characteristic for the ER proteins, and a mature form, delivered to the cell surface containing complex-type N-linked oligosaccharides (21). This N-linked glycosylation was shown to be essential for cell surface expression of the LGR7 and critical for signal transduction (21). Our data now show that the LGR7 splice variants can interfere with the intracellular sorting of the WT-LGR7, reducing its cell surface delivery and causing its retention inside the cells. Coexpression of any of the three novel splice variants with WT-LGR7 caused a significant reduction in the mature LGR7 bearing complex-type N-linked oligosaccharides, showing their interference with WT-LGR7 maturation. Our results show that the retention of WT-LGR7 due to a splice variant is an early event, occurring before posttranslational modification in the Golgi apparatus. Changes in the cell surface delivery and maturation of the WT-LGR7 in the presence of the splice variant were not a consequence of altered protein synthesis or changes in the components of the ER quality control apparatus, because expression of the variant was not found to cause UPR.
We also demonstrated by coimmunoprecipitation and colocalization that the splice variant and WT-LGR7 can form heterodimers. These data, and data showing the retention of the splice variants in the ER and their early inhibition of the maturation of the WT-LGR7, show that the interaction between the splice variants with the full-length receptor takes place in the ER compartment. Thus, each of the splice variants sequesters the WT-LGR7 by heterodimerization in the ER compartment.
Receptor dimerization is an emerging aspect of GPCR biosynthesis and trafficking. Dimerization was shown to be an early event in biosynthesis and occurs in the ER compartment for other GPCRs, indicating that it may be required for complete receptor maturation and cell surface delivery (30). To understand the role of LGR7 dimerization in its biosynthesis and show a linkage between its sequestration and reduced cell surface expression by the splice variants, we studied LGR7 homodimerization in living cells using BRET. The combination of BRET and subcellular fractionation has provided a powerful tool to determine the presence of LGR7 dimers in specific organelles. We measured BRET signals in ER and PM-rich fractions of cells expressing LGR7. These results showed that LGR7 forms dimers constitutively, that the earliest site of dimer formation is the ER compartment during biosynthesis, and that dimer stability is maintained during transit through the secretory pathway to the cell surface. Similar results have been shown with different biophysical and biochemical assays for other GPCR proteins (37,38,39,45,49,50).
Studies of dimerization of GPCRs to date have largely been performed in transfected immortalized cell lines rather than in primary cell culture. In part, this is because many immortalized cell lines are readily transfected with fluorescently tagged receptor protein-expressing plasmids. The suggestion that GPCR dimerization might be promoted at relatively high receptor expression levels and hence potentially be, at least partially, an artifact of overexpression has been raised by others. In studies on the extent of β-adrenergic receptor dimerization, it has been indicated that this is unaltered over a wide range of expression levels (44,53). Moreover, expression levels of GPCRs have been monitored in many similar dimerization studies, which showed that receptor dimerization indeed occurs at relatively low, physiologically relevant levels of expression (45,54,55). In our study, we performed the BRET titration assay in which the donor/acceptor ratio was varied, and the specificity of BRET signals allowed us to distinguish dimerization from random collision. This was verified by BRET competition assay, in which the occurrence of BRET between two partners was expressed at a given donor/acceptor ratio and could be inhibited by expression of the untagged partner. In our experiments, the transfection conditions were set to ensure that the receptor levels achieved were not excessive. Coexpression of any of the splice variants with WT-LGR7 resulted in inhibition of WT-LGR7 dimerization measured by BRET saturation assay. In the presence of any of these splice variants, we showed significant increases in BRET50 data; thus, the relative affinity of LGR7 for itself was reduced, and homodimerization therefore decreased. Taken together, these data show that interaction of these splice variants with the WT-LGR7 in the ER reduces the homodimerization of the full-length receptor, resulting in the reduction of its cell surface delivery and causing a dominant-negative effect on its function. Our results also support the current hypothesis that constitutive homodimerization of WT-LGR7 takes place early in the biosynthetic pathway in the ER compartment and is necessary for its trafficking to the cell surface. To accomplish heterodimerization of the WT-LGR7 and the splice variant, it would be necessary for their coexpression to occur in the same cell and possibly in the same region of the ER. In addition, temporal synthesis of such interacting proteins would also contribute to the selectivity process, because the two interacting proteins plausibly need to be synthesized at the same time and place to assemble into a heterodimer.
In conclusion, we have identified three novel LGR7 splice variants in the human fetal membranes and characterized their intracellular effects on the WT-LGR7, demonstrating their dominant-negative effects on its function. We have also demonstrated that the constitutive homodimerization of the WT-LGR7 takes place in the ER and that the presence of these splice variants decreases its ability to homodimerize, supporting the view that this is a prerequisite of receptor trafficking to the cell surface. The mRNA expression of each splice variant shows their concordant expression patterns in separated decidua and chorion during the peripartal period, suggesting their possible functional significance, especially in the chorion, where they are relatively well expressed over the period of spontaneous term labor. Their inhibition of the function of the full-length LGR7 would result in dampening of tissue responsiveness to decidual relaxin over this period of rapid local tissue changes required for successful parturition.
Acknowledgments
We thank the nurses and staff of the labor and delivery unit of Kapiolani Medical Center for Women and Children for their help with tissue collection. In particular, we thank J. Shimoda for assistance in the identification of potential patients and S. Yamamoto for the collection of tissues. We also acknowledge BAS Medical for the generous gift of human relaxin.
Footnotes
First Published Online December 13, 2007
Abbreviations: BRET, Bioluminescence resonance energy transfer; Endo H, Endoglycosidase H; ER, endoplasmic reticulum; FSHR, FSH receptor; GFP, green fluorescent protein; GPCR, G protein-coupled receptor; HA, hemagglutinin; LDL-A, low-density lipoprotein class A; LGR, leucine-rich repeat containing GPCR; LRR, leucine-rich repeat; PM, plasma membrane; PNGase F, peptide N-glycosidase F; Rluc, Renilla luciferase; TBS, Tris-buffered saline; TM, transmembrane; UPR, unfolded protein response; WT-LGR7, wild-type LGR7; YFP, yellow fluorescent protein.
This work was supported by a grant from the National Institutes of Health (HD24314).
Disclosure Statement: The authors have nothing to disclose.
References
- Sherwood OD 2004 Relaxin’s physiological roles and other diverse actions. Endocr Rev 25:205–234 [DOI] [PubMed] [Google Scholar]
- Samuel CS, Du XJ, Bathgate RA, Summers RJ 2006 ‘Relaxin’ the stiffened heart and arteries: the therapeutic potential for relaxin in the treatment of cardiovascular disease. Pharmacol Ther 112:529–552 [DOI] [PubMed] [Google Scholar]
- Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP 2006 Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 20:2352–2362 [DOI] [PubMed] [Google Scholar]
- Smith MC, Danielson LA, Conrad KP, Davison JM 2006 Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 17:3192–3197 [DOI] [PubMed] [Google Scholar]
- Samuel CS, Hewitson TD 2006 Relaxin in cardiovascular and renal disease. Kidney Int 69:1498–1502 [DOI] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Bialek J, Trojanowicz B, Weber E, Holzhausen HJ, Silvertown JD, Summerlee AJ, Dralle H, Hoang-Vu C, Klonisch T 2006 Relaxin enhances the oncogenic potential of human thyroid carcinoma cells. Am J Pathol 169:617–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM, Agoulnik AI 2007 Relaxin promotes prostate cancer progression. Clin Cancer Res 13:1695–1702 [DOI] [PubMed] [Google Scholar]
- Silvertown JD, Symes JC, Neschadim A, Nonaka T, Kao JC, Summerlee AJ, Medin JA 2007 Analog of H2 relaxin exhibits antagonistic properties and impairs prostate tumor growth. FASEB J 21:754–765 [DOI] [PubMed] [Google Scholar]
- Bogic LV, Yamamoto SY, Millar LK, Bryant-Greenwood GD 1997 Developmental regulation of the human relaxin genes in the decidua and placenta: overexpression in the preterm premature rupture of the fetal membranes. Biol Reprod 57:908–920 [DOI] [PubMed] [Google Scholar]
- Tashima LS, Yamamoto SY, Yasuda M, Millar LK, Bryant-Greenwood GD 2002 Decidual relaxins: gene and protein up-regulation in preterm premature rupture of the membranes by complementary DNA arrays and quantitative immunocytochemistry. Am J Obstet Gynecol 187:785–797 [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD, Millar LK 2000 Human fetal membranes: their preterm premature rupture. Biol Reprod 63:1575–1579 [DOI] [PubMed] [Google Scholar]
- Garibay-Tupas JL, A.Maaskant R, Greenwood FC, Bryant-Greenwood GD 1995 Characteristics of the binding of 32P-labelled human relaxins to the human fetal membranes. J Endocrinol 145:441–448 [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ 2006 International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev 58:7–31 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ 2002 Activation of orphan receptors by the hormone relaxin. Science 295:671–674 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ 2000 The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14:1257–1271 [DOI] [PubMed] [Google Scholar]
- Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ 2002 INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem 277:31283–31286 [DOI] [PubMed] [Google Scholar]
- Hsu SY 2003 New insights into the evolution of the relaxin–LGR signaling system. Trends Endocrinol Metab 14:303–309 [DOI] [PubMed] [Google Scholar]
- Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, Hsueh AJ 2003 H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem 278:7855–7862 [DOI] [PubMed] [Google Scholar]
- Büllesbach EE, Schwabe C 2005 The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J Biol Chem 280:14051–14056 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, Bathgate RA 2006 Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem 281:34942–34954 [DOI] [PubMed] [Google Scholar]
- Kern A, Agoulnik AI, Bryant-Greenwood GD 2007 The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology 148:1181–1194 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Ferlin A, Feng S, Truong A, Gianesello L, Foresta C, Agoulnik AI 2007 T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am J Physiol Endocrinol Metab 292:E138–E144 [DOI] [PubMed] [Google Scholar]
- Kraaij R, Verhoef-Post M, Grootegoed J, Themmen A 1998 Alternative splicing of follicle-stimulating hormone receptor pre-mRNA: cloning and characterization of two alternatively spliced mRNA transcripts. J Endocrinol 158:127–136 [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Manna PR, Huhtaniemi I 1999 Molecular cloning of the mouse follicle-stimulating hormone receptor complementary deoxyribonucleic acid: functional expression of alternatively spliced variants and receptor inactivation by a C566T transition in exon 7 of the coding sequence. Biol Reprod 60:1515–1527 [DOI] [PubMed] [Google Scholar]
- Graves PN, Tomer Y, Davies TF 1992 Cloning and sequencing of a 1.3 kb variant of human thyrotropin receptor mRNA lacking the transmembrane domain. Biochem Biophys Res Commun 187:1135–1143 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamashita S, Omori Y, Minegishi T 2004 A splice variant of the human luteinizing hormone (LH) receptor modulates the expression of wild-type human LH receptor. Mol Endocrinol 18:1461–1470 [DOI] [PubMed] [Google Scholar]
- Apaja PM, Tuusa JT, Pietilä EM, Rajaniemi HJ, Petäjä-Repo UE 2006 Luteinizing hormone receptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic reticulum. Mol Biol Cell 17:2243–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muda M, He C, Martini PG, Ferraro T, Layfield S, Taylor D, Chevrier C, Schweickhardt R, Kelton C, Ryan PL, Bathgate RA 2005 Splice variants of the relaxin and INSL3 receptors reveal unanticipated molecular complexity. Mol Hum Reprod 11:591–600 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Tregear GW, Bathgate RA 2005 LGR7-truncate is a splice variant of the relaxin receptor LGR7 and is a relaxin antagonist in vitro. Ann NY Acad Sci 1041:22–26 [DOI] [PubMed] [Google Scholar]
- Bulengera S, Marulloa S, Bouvier M 2005 Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 26:131–137 [DOI] [PubMed] [Google Scholar]
- Grosse R, Schöneberg T, Schultz G, Gudermann T 1997 Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol Endocrinol 11:1305–1318 [DOI] [PubMed] [Google Scholar]
- Karpa KD, Lin R, Kabbani N, Levenson R 2000 The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3–D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol 58:677–683 [DOI] [PubMed] [Google Scholar]
- Sarmiento JM, Añazco CC, Campos DM, Prado GN, Navarro J, González CB 2004 Novel down-regulatory mechanism of the surface expression of the vasopressin V2 receptor by an alternative splice receptor variant. J Biol Chem 279:47017–47023 [DOI] [PubMed] [Google Scholar]
- McElvaine AT, Mayo KE 2006 A dominant-negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology 147:1884–1894 [DOI] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM 2004 Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18:1787–1797 [DOI] [PubMed] [Google Scholar]
- Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L 2005 Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet 14:2991–3002 [DOI] [PubMed] [Google Scholar]
- Kaykas A, Yang-Snyder J, Héroux M, Shah KV, Bouvier M, Moon RT 2003 Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol 6:52–58 [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN 2006 Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15:2200–2209 [DOI] [PubMed] [Google Scholar]
- Sánchez-Laorden BL, Sánchez-Más J, Martínez-Alonso E, Martínez-Menárguez JA, García-Borrón JC, Jiménez-Cervantes C 2006 Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol 126:172–181 [DOI] [PubMed] [Google Scholar]
- Lowndes K, Amano A, Yamamoto S, Bryant-Greenwood GD 2006 The human relaxin receptor (LGR7): expression in the fetal membranes and placenta. Placenta 27:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R 2004 Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 89:324–334 [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Seeber RM, Eidne KA 2006 Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat Protoc 1:337–345 [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M 2000 Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci USA 97:3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M 2002 Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem 277:44925–44931 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M 2003 Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol 17:677–691 [DOI] [PubMed] [Google Scholar]
- Wheatley M, Hawtin S 1999 Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: its occurrence and role. Hum Reprod Update 5:356–364 [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ 2005 The mammalian unfolded protein response. Annu Rev Biochem 74:739–789 [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M 2004 Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat 28:67–78 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE 2006 Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem 281:27109–27116 [DOI] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagacé M, Marullo S, Bouvier M 2004 Homodimerization of the β2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem 279:33390–33397 [DOI] [PubMed] [Google Scholar]
- Fridmanis D, Fredriksson R, Kapaa I, Schiöthb HB, Klovins J 2007 Formation of new genes explains lower intron density in mammalian Rhodopsin G protein-coupled receptors. Mol Phylogenet Evol 43:864–880 [DOI] [PubMed] [Google Scholar]
- Schwarz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, Maki RA 2000 Characterization of γ-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J Biol Chem 275:32174–32181 [DOI] [PubMed] [Google Scholar]
- Breit A, Lagacé M, Bouvier M 2004 Hetero-oligomerization between β2- and β3-adrenergic receptors generates a β-adrenergic signaling unit with distinct functional properties. J Biol Chem 279:28756–28765 [DOI] [PubMed] [Google Scholar]
- Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, Labbé-Jullié C, Bouvier M, Marullo S 2002 Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem 277:34666–34673 [DOI] [PubMed] [Google Scholar]
- Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N 2005 Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem 280:9895–9903 [DOI] [PubMed] [Google Scholar]