Abstract
Activation of the glucagon-like peptide-1 receptor (GLP-1R) is associated with expansion of β-cell mass due to stimulation of cell proliferation and induction of antiapoptotic pathways coupled to β-cell survival. Although the GLP-1R agonist Exenatide (exendin-4) is currently being evaluated in subjects with type 1 diabetes, there is little information available about the efficacy of GLP-1R activation for prevention of experimental type 1 diabetes. We examined the consequences of exendin-4 (Ex-4) administration (100 ng once daily and 2 μg twice daily) on diabetes onset in nonobese diabetic mice beginning at either 4 or 9 wk of age prior to the onset of diabetes. Ex-4 treatment for 26 wk (2 μg twice daily) initiated at 4 wk of age delayed the onset of diabetes (P = 0.007). Ex-4-treated mice also exhibited a significant reduction in insulitis scores, enhanced β-cell mass, and improved glucose tolerance. Although GLP-1R mRNA transcripts were detected in spleen, thymus, and lymph nodes from nonobese diabetic mice, Ex-4 treatment was not associated with significant changes in the numbers of CD4+ or CD8+ T cells or B cells in the spleen. However, Ex-4 treatment resulted in an increase in the number of CD4+ and CD8+ T cells in the lymph nodes and a reduction in the numbers of CD4+CD25+Foxp3+ regulatory T cells in the thymus but not in lymph nodes. These findings demonstrate that sustained GLP-1R activation in the absence of concomitant immune intervention may be associated with modest but significant delay in diabetes onset in a murine model of type 1 diabetes.
BOTH TYPE 1 AND TYPE 2 diabetes mellitus (T2DM) are characterized by loss of β-cell function leading to inappropriately reduced insulin (type 2) or absent (type 1) insulin secretion. Current strategies for the treatment of type 2 diabetes include the use of agents that enhance insulin action or promote insulin secretion. Nevertheless, there is little evidence in human subjects with T2DM that antidiabetic agents directly target the β-cell to enhance β-cell regeneration and/or prevent further loss of functioning β-cells (1). Similarly, insulin replacement therapy remains the cornerstone of diabetes management for patients with type 1 diabetes, although islet transplantation may provide significant improvement in glucose control for several years in selected subjects (2).
More recently agents based on glucagon-like peptide (GLP)-1 action have been approved for the treatment of T2DM. GLP-1 is a gut peptide secreted in response to nutrient ingestion that functions as an incretin, enhancing glucose-dependent stimulation of insulin secretion after oral nutrient ingestion. GLP-1 also controls blood glucose via inhibition of glucagon secretion and gastric emptying, and long-term treatment with GLP-1R agonists reduces food intake and promotes weight loss in both preclinical experiments and clinical studies of subjects with T2DM (3).
GLP-1 exerts its actions through a specific GLP-1R expressed on islet β-cells. Considerable evidence using experimental models of T2DM demonstrates that therapy with GLP-1R agonists results in expansion of β-cell mass (4,5,6). The increase in β-cell mass observed after treatment with GLP-1R agonists is thought to arise from direct stimulation of β-cell proliferation and via enhancement of islet neogenesis (4). More recent evidence suggests that GLP-1 also preserves β-cell mass via inhibition of apoptosis (7). GLP-1R agonists reduce β-cell apoptosis in young db/db mice or in wild-type mice after administration of streptozotocin (8,9). Moreover, the antiapoptotic actions of GLP-1 are direct because treatment of rodent or human islets or isolated β-cells with GLP-1 or exendin-4 reduces β-cell death via engagement of antiapoptotic pathways (9,10,11).
The cytoprotective and regenerative properties of GLP-1R agonists have raised the possibility that prolonged treatment of patients with T2DM using GLP-1R agonists such as exendin-4 (Exenatide) might be associated with preservation or enhancement of β-cell mass, parameters that remain difficult to assess in human subjects. Furthermore, because type 1 diabetes is thought to result from progressive β-cell destruction and failure to regenerate β-cell mass, it remains possible that intervention with GLP-1R agonists may modify the natural course of β-cell destruction and/or regeneration, resulting in clinically detectable improvements in insulin secretion and glucose control. In this regard, there is evidence that experimentally induced changes in β-cell mass of nonobese diabetic (NOD) mice early in the course of the autoimmune process can inhibit the subsequent development of disease (12). To address whether the GLP-1R agonist exendin-4 modifies the development or progression of experimental type 1 diabetes, we carried out a series of studies using once- or twice-daily administration of exendin-4 to 4- and 9-wk-old NOD mice before the onset of clinically detectable diabetes.
Materials and Methods
Reagents
Synthetic Exendin-4 (Ex-4) was purchased from California Peptide Research Inc. (Napa, CA). Ex-4 was dissolved in PBS (pH 7.4) at a concentration of 1 mg/ml, aliquoted, and stored at −80 C until use. For flow cytometry studies, the following monoclonal antibodies were prepared as described (13): purified anti-CD16/CD32 (for Fc blocking), biotynylated anti-CD8α, fluorescein isothiocyanate anti-CD8α, phycoerythrin (PE) anti-CD25, fluorescein isothiocyanate anti-CD4, PE anti-CD4, allophycocyanin anti-H57 (T-cell receptor β-chain), and PE anti-187 (mouse κ-chain). Streptavidin-spectral red was purchased from Southern Biotech (Birmingham, AL). Allophycocyanin antimouse/rat Foxp3 staining set was purchased from eBioscience Inc. (San Diego, CA).
Mice
Three- and 7-wk-old female NOD/Ltj mice were purchased from the Jackson Laboratory (Bar Harbor, ME), housed in a specific pathogen-free facility, and maintained on a 12-h light, 12-h dark cycle, with free access to standard rodent chow and water, except when fasting. Mice were allowed to acclimatize to the animal facility for 1 wk before treatment. All experiments were carried out in accordance with protocols and guidelines approved by the Toronto General Hospital.
Treatment
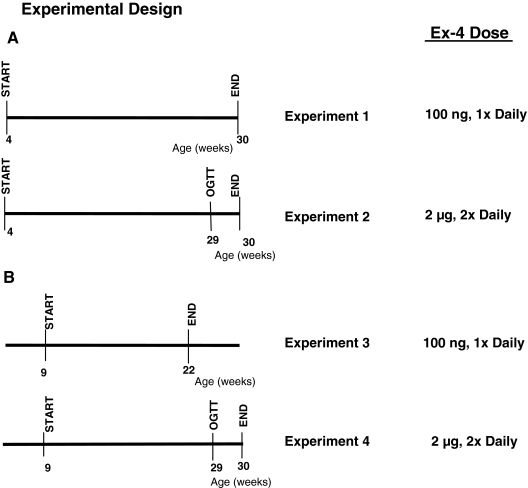
For analysis of the effects of Ex-4 on diabetes onset in young NOD mice, we treated groups of mice with either 100 ng once daily (experiment 1) or 2 μg twice daily (experiment 2) Ex-4 via the ip route, beginning at 4 wk of age when NOD islets are generally free of lymphocytic infiltration. Mice were monitored for diabetes onset, until they reached 30 wk of age. To determine whether Ex-4 modifies the development of diabetes in older mice with ongoing insulitis, separate experiments were initiated at 9 wk of age, a time when NOD mice already display histological evidence of insulitis and β-cell destruction (14,15,16,17), with either 100 ng Ex-4 once daily (experiment 3) or 2 μg Ex-4 twice daily (experiment 4). For experiment 3, the study was terminated by a predefined end point reached when 50% of mice in the saline-treated group were diagnosed as diabetic; all of the remaining nondiabetic animals were euthanized, and the pancreas and cardiac blood were collected. In experiment 4, mice were monitored for signs of diabetes until the age of 30 wk.
Ex-4 pharmacokinetic profile
To assess the pharmacokinetic profile of the two doses of Ex-4, a separate group of 10-wk-old female NOD mice were given a single ip injection of either 100 ng or 2 μg Ex-4 (or saline). Blood was drawn from a tail vein 1, 4, 8, and 12 h after the injection. Plasma levels of Ex-4 were quantified with an Ex-4 ELISA kit (Bachem, San Carlos, CA).
Diabetes monitoring
Morning ambient blood glucose levels were monitored three times per week, with an Ascencia Elite glucometer (Bayer Inc., Toronto, Ontario, Canada). Once blood glucose was greater than 10 mm, glucose levels were monitored daily. Onset of diabetes was defined as blood glucose levels equal to or greater than 17 mm that persisted for 3 consecutive days. Diabetic mice were euthanized and removed from the study.
Glucose tolerance tests
Oral (OGTT) or ip glucose tolerance tests were performed after an overnight fast (16–18 h), as described (18). Briefly, mice received a dose of 1.5 mg d-glucose per gram body weight via gavage (OGTT) or an ip injection (ip glucose tolerance test) at time 0. Blood was drawn from a tail vein at 0, 10, 20, 30, 60, 90, and 120 min, and glucose levels were measured as described above. At 10–20 min after glucose load, 100 μl of blood were collected for measurement of plasma insulin and glucagon. Insulin was measured using a mouse insulin ELISA kit (ALPCO Diagnostics, Salem, NH) with mouse insulin as standard. Plasma glucagon levels were determined using a mouse glucagon LINCOplex assay with mouse glucagon as standard (Linco, St. Charles, MO).
Pancreatic insulin content
A small piece of the distal tail of the pancreas was homogenized on ice twice in 5 ml of extraction medium [1 n HCl containing 5% (vol/vol) formic acid, 1% (vol/vol) trifluoroacetic acid, and 1% (wt/vol) NaCl], as described (18). The extracts were passed through a C18 silica cartridge (Waters Associates, Milford, MA), and peptides and small proteins were adsorbed. Adsorbed peptides were eluted with 4 ml of 80% (vol/vol) isopropanol containing 0.1% (vol/vol) trifluoroacetic acid. Pancreatic insulin levels were measured using a rat insulin RIA kit (Linco Research), according to the manufacturer’s protocol. Total protein levels in extracts were determined using the Bradford method (19) with dye reagent (Bio-Rad Laboratories, Inc., Hercules, CA).
Histology and insulitis scoring
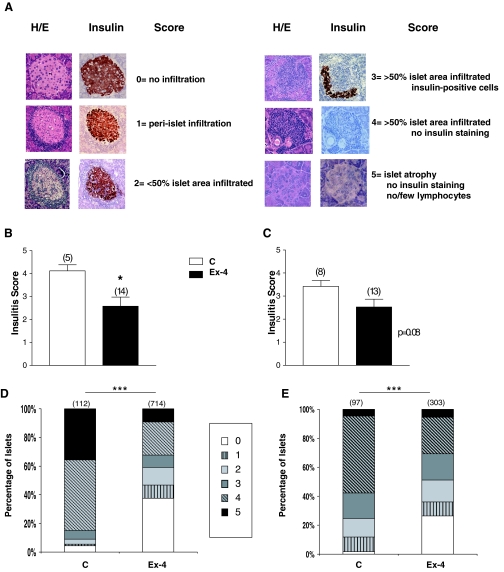
Pancreata were weighed, cut into eight to 10 pieces, and fixed overnight in 10% neutral buffered formalin and embedded in paraffin. Sections were obtained and stained with hematoxylin and eosin using standard protocols. Immunostaining for insulin was carried out as previously described (6). Insulin-positive area was determined using an image analysis system (Aperio Scanscope system, Vista, CA), as previously described (20). β-Cell area was calculated as the product of total insulin-positive area by the corresponding pancreatic weight. Islets were examined in a blinded manner using a Leica DMR microscope equipped with a Leica DC 300F camera (Leica Microsystems Canada Inc., Québec, Canada), to evaluate the degree of mononuclear cell infiltration. The severity of infiltration was classified into five scores, slightly modified from that published elsewhere (21) as follows: 0, no infiltration/insulitis; 1, periislet infiltration; 2, less than 50% of islet area showing infiltration; 3, more than 50% of islet area displaying invasive insulitis; 4, more than 50% of islet area displaying infiltration, no insulin positivity; 5, islet atrophy, no insulin staining, no/few lymphocytes (see Fig. 4A). The mean insulitis score was calculated by dividing the sum of the insulitis scores by the total number of islets examined for each mouse. For the calculation of both insulitis and β-cell mass, two sections were used from each pancreas, cut at least 250 μm apart.
Figure 4.
Treatment with Ex-4 results in reduction of islet infiltration. Pancreatic sections of normoglycemic mice that completed the treatment for experiment 2 (panels B and D) and experiment 3 (panels C and E) were assessed for the severity of lymphocytic infiltration. Panel A, Islets were given a score from 0 to 5, depending on the degree of lymphocytic infiltration and the proportion of islet surface area accounted for by insulin-positive cells. Serial sections of islets, stained with hematoxylin and eosin (H/E) and for insulin, are shown as a representation for each score. Data were plotted as the mean insulitis score (panels B and C) or the distribution of islets among insulitis scores (panels D and E). For panels B and C, insulitis scores were generated by dividing the sum of islet scores by the total number of islets of each mouse. The histograms for D and E represent the distribution of all islets analyzed among insulitis scores (n = 112–714 islets analyzed for panel D and 97–303 islets analyzed for panel E. The distribution of islets across insulitis scores for Ex-4-treated vs. control mice was analyzed using the χ2 test. Furthermore, the number of islets that were free of infiltration vs. infiltrated islets (i.e. islets that received a score of 0 vs. a score of > 0) was compared between Ex-4-treated and control mice, using the χ2 test. C, Saline-treated mice. Numbers above bar charts represent the number of animals (panels B and C) or total number of islets (panels D and E). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 Ex-4 vs. PBS-treated mice. For panels B and C, results displayed as mean ± sem.
Flow cytometry
Lymphocytes were prepared and stained according to slightly modified procedures described elsewhere (22). Briefly, single-cell suspensions were prepared from the spleen, lymph nodes, and thymus of NOD mice. Red blood cells were lysed and removed from the suspension by a 3-min exposure to ammonium chloride lysis buffer (0.15 m NH4Cl, 0.01 m KHCO3, 0.1 mm EDTA). Cells (106) were then incubated at room temperature with primary and secondary (in case of biotynylated primary) antibodies in PBS containing 2% calf serum. Cells were either fixed overnight in 1% paraformaldehyde or fixed/permeabilized and stained with the Foxp3 antibody according to the manufacturer’s protocol. Cells were acquired on a FACScalibur flow cytometer (BD Biosciences, San Diego, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR).
GLP-1R expression
RNA was extracted from the pancreas or single cell suspensions of lymphocytes, splenocytes, and thymocytes using TRIzol reagent (Life Technologies, Burlington, Ontario, Canada). Then 1.5 or 5 μg of total RNA were reverse transcribed at 42 C for 50 min using SuperScript II reverse transcriptase (Invitrogen, Burlington, Ontario, Canada) and random hexamer primers (Invitrogen Life Technologies). For each RNA sample, a positive (RT+) and negative (RT−, no enzyme added) reaction was set. Target cDNA was analyzed for the expression of mouse GLP-1R mRNA transcripts by the PCR method, using the following primer pairs and conditions: 5′-GTACCACGGTGTCCCTCTCA-3′ and 5′-CCTGTGTCCTTCACCTTCCCTA-3′, 94 C, 5 min; 94 C, 30 sec, 55 C, 30 sec, 72 C, 1 min 30 sec, 40 cycles; 72 C, 10 min. These primers result in the amplification of a 1.407-kb product spanning bases 79–1486 of the GLP-1R mRNA sequence. β-Actin cDNA was amplified using the primer pairs 5′-TGACATCCGTAAAGA-3′ and 5′-CAGCTCAGTAACAGTCC-3′, with the following parameters: 94 C, 5 min; 94 C, 30 sec, 45 C, 30 sec, 72 C, 30 sec, 40 cycles; 72 C, 4 min. The primers and conditions for CD3ε amplification are described in (23). The PCR products were electrophoresed in a 1% agarose gel, transferred to a Nytran Super Charge nylon membrane (Mandel Scientific, Guelph, Ontario, Canada), and hybridized overnight using the following internal primers labeled by T4 polynucleotide kinase reaction with γ-32P[ATP] (PerkinElmer, Wellesley, MA): GLP-1R 5′-GCTGTATCTGAGCATAGGCT-3′; β-actin 5′-GATCATTGCTCCTCCTGAGC-3′; CD3ε 5′-GAGCACCCTGCTACTCCTTG-3′. Blots were then washed, exposed overnight to a phosphoimaging cassette, and visualized using Storm 860 Phosphor Screen and Image Quant (version 5.0) software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
Results are expressed as mean ± sem. All statistical analyses were performed with Prism version 3.03 software (GraphPad Software Inc., San Diego, CA). Statistical significance was assessed by the Kaplan-Meier life table method and the log-rank test, one- or two-way ANOVA, and where appropriate, a two-tailed Student’s t test or a χ2 test. Differences were considered statistically significant at P ≤ 0.05.
Results
Ex-4 delays the onset of type 1 diabetes in NOD mice
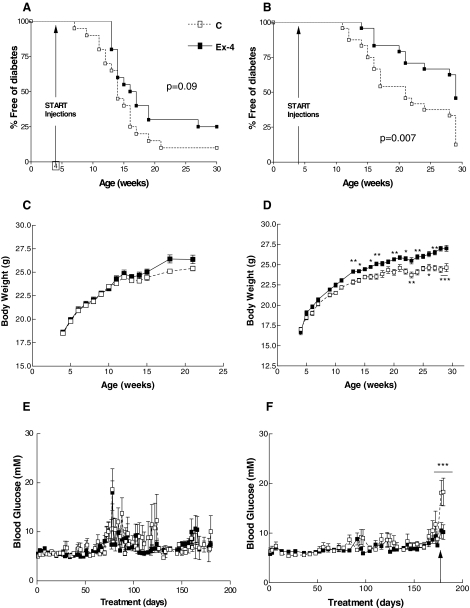
Four-week-old NOD mice treated with 100 ng Ex-4 once daily for up to 26 wk (experiment 1, Fig. 1) exhibited a delayed onset of diabetes (median onset of diabetes was 16.5 vs. 14 wk of age for Ex-4- vs. saline-treated mice); however, this difference was not statistically significant (P = 0.09, Fig. 2A). In contrast, a higher dose of Ex-4, 2 μg twice daily (experiment 2, Fig. 1), significantly delayed the onset of diabetes, as well as the cumulative incidence of diabetes, in NOD mice (29 vs. 21 wk, for Ex-4 vs. saline-treated mice, P = 0.007, Fig. 2B). Although mice treated with 100 ng of Ex-4 once daily did not exhibit significant differences in body weight, compared with saline-treated mice (Fig. 2C), body weight was significantly increased in mice treated with a higher dose of Ex-4, 2 μg twice daily (Fig. 2D). Ambient blood glucose levels were comparable in both groups of mice throughout the duration of both experiments (Fig. 2, E and F); however, glucose levels were significantly higher in saline- than Ex-4-treated mice after the glucose challenge at the end of the 25-wk treatment period in experiment 2 (18.1 ± 7.1 vs. 10.5 ± 5.9 mm, saline vs. Ex-4, P < 0.001, Fig. 2F).
Figure 1.
Summary of experimental design. The experiments were divided into two groups: in one set of experiments (A), Ex-4 administration was initiated when NOD mice were 4 wk of age; in a separate set of studies (B), Ex-4 administration initiated when NOD mice were 9 wk of age. Two different dose regimens of Ex-4, 100 ng once daily or 2 μg twice daily, were tested for each age group.
Figure 2.
Analysis of the effects of Ex-4 on diabetes onset in 4-wk-old NOD mice. The 4-wk-old female NOD mice with normal blood glucose levels were injected with an equal volume (100 μl) of either saline (denoted as C) or Ex-4, 100 ng once daily (experiment 1, A, C, and E) or a higher dose of Ex-4, 2 μg twice daily (experiment 2, B, D, and F) for a total of 26 wk. During treatment, nonfasting blood glucose levels were checked in the morning, and mice were diagnosed as diabetic if they had a blood glucose level 17 mm or greater for 3 consecutive days. Diabetic mice were euthanized and removed from the study. A and B, Cumulative incidence of diabetes was plotted against age (weeks). Body weight and ambient blood glucose over the course of the experiment are shown in C and D and E and F, respectively. For F, an OGTT was performed on surviving normoglycemic NOD mice on d 177 of treatment, as indicated by the arrow. After the glucose challenge, ambient blood glucose levels were monitored daily until euthanasia (n = 20 mice/treatment group at the onset of treatment, n = 4–6 at end point for experiment 1; n = 24 mice/treatment group at onset, n = 8–13 at end point for experiment 2). For C–F, results are displayed as mean ± sem. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, Ex-4 vs. control mice.
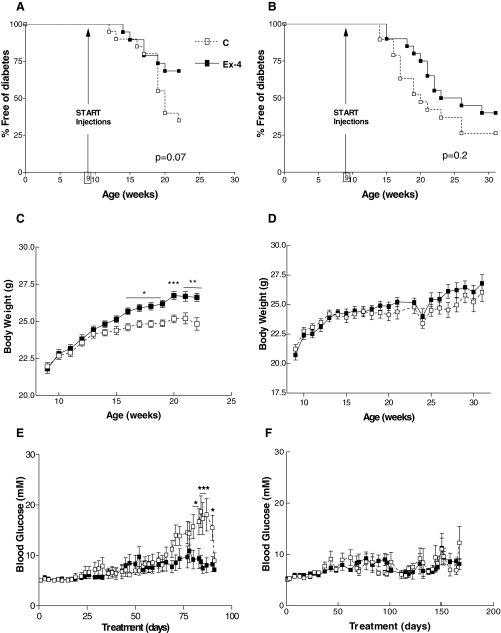
To determine whether Ex-4 administration was able to delay or prevent the onset of diabetes in older NOD mice that are more likely to exhibit histological evidence for active insulitis (14,15,16,17), 9-wk-old female NOD mice were randomized to treatment with daily injections of saline or Ex-4, either 100 ng once daily for 13 wk (Fig. 1, experiment 3) or 2 μg twice daily for 21 wk (Fig. 1, experiment 4). Treatment with Ex-4 reduced the cumulative incidence of diabetes in both experiments (Fig. 3, A and B); however, this difference was not statistically significant as assessed using Kaplan-Meier curves and the log-rank test (Fig. 3, A and B). Nevertheless, treatment of 9-wk-old NOD mice with 100 ng once daily Ex-4 was associated with greater weight gain (Fig. 3C) and a significant reduction in ambient levels of blood glucose (Fig. 3E), consistent with the trend toward reduced diabetes onset (Fig. 3A).
Figure 3.
Analysis of the effects of Ex-4 on diabetes onset in 9-wk-old NOD mice. Treatment with Ex-4, 100 ng once daily (experiment 3, A, C, and E) or 2 μg twice daily (experiment 4, B, D, and F), was initiated in normoglycemic female NOD mice at 9 wk of age. The entire experiment was terminated according to a predetermined end point when 50% of mice in the control group developed diabetes (experiment 3) or until NOD mice reached 30 wk of age (experiment 4). Mice were monitored for diabetes onset by checking ambient blood glucose levels three times a week. Once the mice were diagnosed as diabetic, they were euthanized and removed from the study. A and B, Onset of diabetes in mice treated with Ex-4 and saline (denoted as C). Body weight measurements and nonfasting blood glucose levels during the treatment period are shown in C and D and E and F, respectively (n = 19–20 mice/treatment group at onset for both experiments 3 and 4, n = 8–13 at end point for experiment 3; n = 4–8 at end point for experiment 4). For C–F, results are displayed as mean ± sem. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 Ex-4 vs. PBS-treated mice.
To ascertain whether treatment of NOD mice with Ex-4 was associated with expansion and/or protection of β-cell mass despite the development of autoimmune insulitis and β-cell destruction, we assessed islet histology (Fig. 4A) and insulitis scores in normoglycemic mice that had survived without diabetes until the end of experiments 2 and 3. Ex-4-treated mice displayed a significantly lower overall mean insulitis score, compared with control mice in both experiments (Fig. 4, B and C). Furthermore, Ex-4-treated mice exhibited a statistically significant increase in the number of islets without infiltration (Fig. 4, D and E) (P < 0.0001 for D and E).
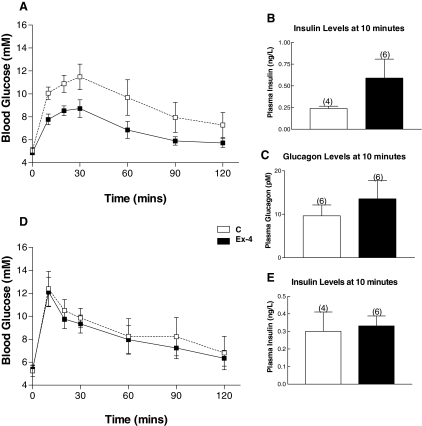
To determine whether prolonged therapy with Ex-4, 2 μg twice daily, was associated with improved glucose tolerance in NOD mice that had not yet developed diabetes, we carried out OGTTs at wk 29 in mice from experiments 2 and 4. After 25 wk of Ex-4 (experiment 2), the area under the curve glucose was significantly lower in Ex-4-treated mice (P < 0.05) (Fig. 5A). Glucose-stimulated insulin levels were 2-fold higher in Ex-4-treated mice (Fig. 5B), but this difference did not achieve statistical significance. No marked difference in plasma levels of glucagon was observed during the OGTT (Fig. 5C). In contrast, no difference was observed in the area under the curve or plasma insulin levels after oral glucose loading after 20 wk of either saline or Ex-4-treatment (experiment 4, Fig. 5, D and E).
Figure 5.
OGTTs in NOD mice treated with Ex-4, 2 μg twice daily. Panels A and D, An OGTT was performed at 29 wk of age on all mice [saline (C) and Ex-4-treated mice from experiments 2 and 4] that had a mean blood glucose less than 10 mm during week 28. Mice were fasted overnight, and an oral glucose load was administered by gavage (1.5 mg glucose per gram body weight). Tail blood was collected for insulin (panel B, experiment 2; panel E, experiment 4) and glucagon (panel C, experiment 2) determinations from 10–20 min after glucose administration. The numbers above the charts indicate the number of mice. For experiment 2 (panel A), the area under the curve for glucose measurements at time 0–60 min was 254.83 ± 33.12 vs. 399.58 ± 55.55 mm/min, P < 0.05 for Ex-4 vs. saline-treated mice. n = 4–9 mice per group for data shown in panels A–C and four to six mice per group for data shown in panels B and E. Results are displayed as mean ± sem.
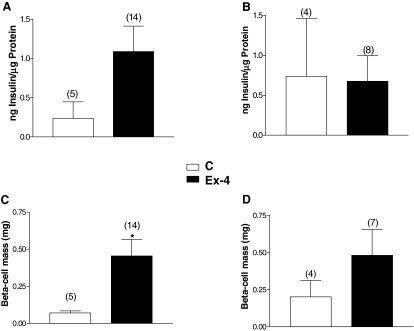
To determine whether prolonged Ex-4 treatment up-regulated pancreatic insulin biosynthesis in the setting of autoimmune insulitis, we measured pancreatic insulin content. In accordance with our results for improved glucose tolerance (Fig. 5A), the pancreatic insulin content of normoglycemic NOD mice treated for 26 wk with 2 μg Ex-4 (experiment 2) was higher, compared with saline-treated mice (Fig. 6A); however, due to the small number of nondiabetic mice, this difference did not reach statistical significance. Similarly, the Ex-4-treated mice from experiment 2 exhibited significantly increased β-cell mass (Fig. 6C). Conversely, there were no significant differences in pancreatic insulin levels and β-cell mass between normoglycemic Ex-4 vs. saline-treated mice that survived until the end of experiment 4 (Fig. 6, B and D).
Figure 6.
NOD mice treated with Ex-4 2 μg twice daily for 26 wk have increased pancreatic insulin content and β-cell mass. Pancreatic insulin content (panels A and B) and β-cell mass (panels C and D) were measured at the end of the treatment period in all normoglycemic NOD mice that survived until the end of experiment 2 (panels A and C) and experiment 4 (panels B and D). Numbers above bar charts represent the number of animals. *, P < 0.05 Ex-4 vs. control (C) mice. Results displayed as mean ± sem.
Ex-4-treated mice exhibit alterations in regulatory T cells
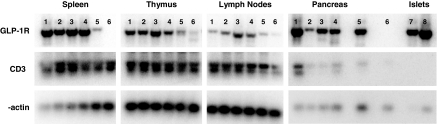
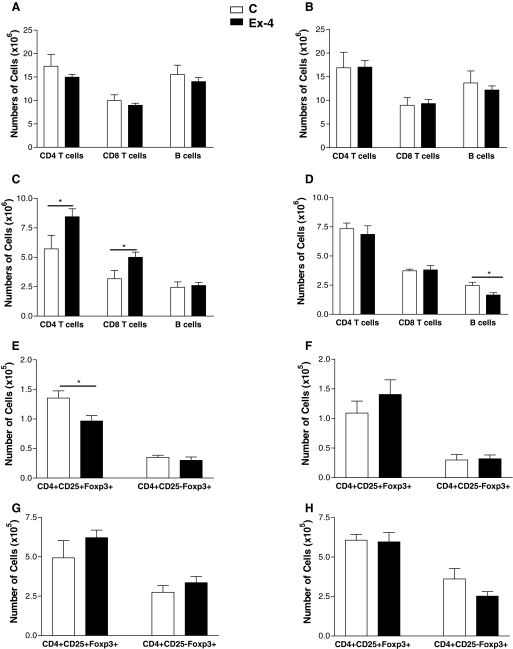
To ascertain whether the actions of Ex-4 in NOD mice may be mediated in part by modulation of the immune system, we first determined whether GLP-1R mRNA transcripts could be detected in immune tissues. Analysis of GLP-1R expression by RT-PCR demonstrated GLP-1R mRNA transcripts in RNA isolated from spleen, thymus, and lymph nodes from normoglycemic and diabetic NOD mice (Fig. 7). Because 26 wk of Ex-4 administration, 2 μg twice daily, was associated with a significant reduction in diabetes onset (experiment 2), we ascertained whether Ex-4 treatment was associated with changes in the absolute number of various lymphocyte populations isolated from the spleen, lymph nodes, and thymus. In addition, a similar comparison was performed in NOD mice from experiment 4, in which Ex-4 treatment (2 μg twice daily) was initiated after the development of lymphocyte-mediated islet attack. No significant differences were observed in splenocyte populations of CD4 and CD8 T cells or B cells from mice treated with Ex-4 or saline from either experiment (Fig. 8, A and B). Nevertheless, Ex-4 treatment, initiated at 4 wk of age, was associated with a significant increase in the number of CD4+ and CD8+ T cells (Fig. 8C) in peripheral lymph nodes. These changes in T cell populations were not observed when Ex-4 treatment was initiated at 9 wk of age (Fig. 8D). Furthermore, a significant reduction in the number of CD4+CD25+Foxp3+ regulatory T cells (Tregs) was detected in thymus but not lymph nodes of Ex-4-treated mice from experiment 2 (Fig. 8, E and G); however, no change in Tregs was observed in the thymus of Ex-4-treated mice from experiment 4 (Fig. 8, F and H). The number of CD4+CD25-Foxp3+ T cells was also examined because these cells have been shown to exhibit inhibitory actions in vitro and are thought to be the precursors for Tregs in vivo (24,25). However, no significant differences were observed in the numbers of these cells in either thymic or lymph node populations of Ex-4 vs. control mice from both experiments (Fig. 8, E–H). Similar trends were observed for all lymphocyte subpopulations when the results were plotted as percentages of cells, or relative numbers, rather than absolute numbers.
Figure 7.
GLP-1R mRNA transcripts are expressed in lymphoid tissue. Pancreas, islets, and lymphoid tissue were isolated for preparation of total cellular RNA, and the expression of GLP-1R transcripts was assessed in control and Ex-4-treated diabetic female NOD mice (lanes 1 and 2, respectively); control and Ex-4-treated normoglycemic female NOD mice (lanes 3 and 4); and age- and sex-matched C57BL/6 GLP-1R+/+ and GLP-1R−/− mice (lanes 5 and 6) as well as in islets of 12-wk-old NOD and C57BL/6 mice (lanes 7 and 8).
Figure 8.
Lymphocyte subpopulations in NOD mice treated with saline (C) or 2 μg Ex-4. Single-cell populations from spleen, thymus, and lymph nodes of NOD mice that survived until the end of experiment 2 (panels A, C, E, and G) and experiment 4 (panels B, D, F, and H) were used for flow cytometry. The number of B and T cells were examined in the spleen (panels A and B) and lymph nodes (panels C and D). The absolute number of CD4+CD25+Foxp3+ cells (Tregs) and CD4+CD25-Foxp3+ cells was determined for the thymic (panels E and F) and lymph node (panels G and H) populations (n = 5–14 mice per group for data shown in panels A, C, E, and G and n = 4–8 mice per group for data shown in panels B, D, F, and H). Values are expressed as mean ± sem. Statistical differences between treatment and control animals in lymphocyte subpopulations were calculated using Student’s t test. *, P < 0.05.
Discussion
Initial observations that GLP-1R agonists robustly and rapidly increase β-cell mass in preclinical models of type 2 diabetes were followed by studies demonstrating that GLP-1R activation leads to enhancement of β-cell cytoprotection in multiple rodent models characterized by apoptotic β-cell death. Moreover, GLP-1R activation directly reduces apoptotic cell death after exposure to diverse toxins and cytokines (9,26), agents that likely contribute to β-cell destruction in the setting of type 1 diabetes. These observations have raised questions as to whether GLP-1R agonists may be able to prevent β-cell death and/or restore β-cell function in subjects with type 1 diabetes. The demonstration that NOD islets are capable of responding to GLP-1R activation with improved insulin secretion (27), taken together with our data showing that exendin-4 improves glucose tolerance in prediabetic NOD mice (data not shown), prompted us to determine whether prolonged GLP-1R activation might reduce the onset of diabetes in the setting of autoimmune insulitis.
Transient administration of Ex-4 has been used to treat NOD mice alone or in combination with antilymphocyte serum (ALS) after the onset of diabetes (28). Ex-4 (12 nmol/kg/day) was administered for two consecutive 4-d periods to hyperglycemic NOD mice either alone or in combination with ALS, with all mice also receiving concomitant insulin administration to reduce blood glucose levels less than 11 mm. Ex-4 alone did not produce disease remission or significant improvement in diabetes in any of the mice examined (28). Although treatment with ALS alone achieved remission in about 40% of NOD mice, the combination of Ex-4 plus ALS-induced remission of diabetes in 23 of 26 mice. Adoptive transfer experiments demonstrated that the combined therapy with exendin-4/ALS inhibited the induction of diabetes by diabetogenic T cells; however, the contribution of Ex-4 to the reduction in diabetes onset and/or development of immune tolerance was not delineated in these experiments (28).
Ex-4 (18 nm/d) has also been coadministered with an antiinflammatory compound (lisofylline) to NOD mice. Ex-4 plus lisofylline significantly reduced blood glucose less than 250 mg/dl, the prespecified cutoff for normoglycemia, when continuously administered to diabetic NOD mice for 28 d via sc infusion (29). Neither Ex-4 nor lisofylline alone was able to significantly reverse hyperglycemia in diabetic NOD mice. Remarkably, blood glucose remained normal in NOD mice for up to 6 wk after discontinuation of the Ex-4/ lisofylline infusion, and clusters of insulin-immunopositive islet cells devoid of mononuclear cell infiltration were detected in the pancreases of these mice (29). Whether the beneficial effects of Ex-4/lisofylline were due to actions on islet cells and/or the immune system was not determined.
Ex-4 has also been combined with anti-CD3 immunotherapy for 10 d, resulting in an improved rate of diabetes remission in NOD mice with established diabetes (30). Ex-4 plus anti-CD3 treatment had no positive effects on β-cell mass, β-cell replication, or apoptosis but did enhance insulin content and glucose-stimulated insulin release. No significant effects of Ex-4 alone were detected in this 10-d study, and Ex-4 had no effect on the frequency of diabetogenic or regulatory T cells (30). The authors concluded that the beneficial effects of Ex-4 were attributable to repletion of insulin stores of residual β-cells. A recent study has also reported that continuous administration of native GLP-1 to prediabetic NOD mice via an implanted miniosmotic pump for 4 or 8 wk lowers blood glucose, in association with enhanced β-cell proliferation and reduced β-cell apoptosis (31). Whether GLP-1 treatment enhanced pancreatic insulin content or modified immune cells subsets was not examined in this study. Whether the differences in outcomes in this study using GLP-1, compared with our observations with exendin-4 reported here, reflect differences in continuous vs. intermittent receptor activation, different peak levels of peptide, or other biological differences in GLP-1 vs. exendin-4 therapy, requires further study.
The experiments reported here differ significantly from previous studies in a number of important aspects. First, we initiated treatment with Ex-4 administered in an intermittent manner, consistent with the protocol used for a recent study of Ex-4 action in human subjects with type 1 diabetes (http://clinicaltrials.gov/ct/show/NCT00064714?order=25). We examined the pharmacokinetic profile of Ex-4 in NOD mice; plasma immunoreactive Ex-4 levels were significantly higher after the 2 μg vs. the 100 ng vs. saline injections (23.4 vs. 1.4 vs. 0.7 ng/ml, respectively). However, plasma immunoreactive Ex-4 was no longer detectable at 4 or 8 h after Ex-4 injection. Hence, different delivery strategies may be required to optimize GLP-1R activation for stimulation of β-cell regeneration or prevention of β-cell apoptosis.
Second, we examined the efficacy of a number of different dosing regimens, and used a prolonged treatment period of several months, whereas previous intervention studies involved much shorter administration regimens ranging from 8 d to 8 wk (28,29,31). Our dosing regimens were chosen based on published work demonstrating the regenerative actions of Ex-4 on β-cells using a wide range of Ex-4 concentrations. Third, we also examined whether Ex-4 alone modulates numbers of T cells and B cells in different immune compartments. Our data clearly demonstrate that Ex-4 exerts a modest but detectable effect on diabetes onset in NOD mice in the absence of concomitant immunotherapy. The effect of Ex-4 on delayed diabetes onset is associated with enhanced β-cell mass and pancreatic insulin content, improved glucose tolerance on oral glucose challenge, and a significant reduction in the severity of insulitis.
The mechanism whereby Ex-4 in our study, or GLP-1 in a recent study (31), reduces the extent of insulitis remains unknown. Our finding that Ex-4 alone enhanced insulin content in the NOD pancreas is consistent with data from studies using Ex-4 in combination with immunomodulatory agents and suggests that GLP-1 receptor activation preserves β-cell mass by reducing β-cell apoptosis (31) and also up-regulates insulin biosynthesis in existing surviving β-cells (30). Taken together, the coupling of GLP-1R activation to enhanced β-cell survival and/or increased insulin biosynthesis are both desirable actions for the treatment of type 1 diabetes.
A preliminary report has demonstrated the expression of the GLP-1 receptor on human T lymphocytes and exogenous GLP-1 modulated CD8+ T lymphocyte migration in vitro (32). Our data extend these findings by demonstrating that GLP-1R mRNA transcripts are detectable in RNA isolated from murine spleen, thymus, and lymph nodes. Although we did not detect consistent changes in T cell subsets that might account for the reduction in diabetes onset seen in some of our experiments, the potential for GLP-1R activation to modify one or more lymphocyte subsets, thereby contributing to the development of immune tolerance, requires further investigation.
Our data may have implications for future clinical studies using GLP-1R agonists for the treatment of type 1 diabetes. GLP-1 reduces blood glucose in short-term studies of patients with type 1 diabetes (33), and Exenatide modestly improves insulin secretion in subjects with type 1 diabetes after islet transplantation (34). Furthermore, Exenatide therapy has been studied in human subjects with islet transplants in an attempt to preserve graft survival and β-cell function after transplantation (35), and Exenatide therapy, alone or in combination with sirolimus and tacrolimus, has been studied in C-peptide positive human subjects with type 1 diabetes. Hence, it seems reasonable to suggest that GLP-1R agonists may be considered for their potential to delay the onset of diabetes in genetically predisposed individuals. Our data suggest that GLP-1 receptor activation alone is unlikely to produce substantial antiapoptotic or immunomodulatory actions necessary for the prevention of autoimmune β-cell destruction, implying that future studies using GLP-1R agonists in combination with additional agents seems prudent.
Footnotes
This work was supported by an operating grant from the Juvenile Diabetes Research Foundation (to D.J.D.). D.J.D. is supported in part by the Canada Research Chairs Program.
Disclosure Statement: D.J.D. has served as an advisor or consultant within the past 12 months to Amgen Inc., Amylin Pharmaceuticals, Arisaph Pharmaceuticals Inc., Chugai Inc., Conjuchem Inc., Eli Lilly Inc., Emisphere Technologies Inc., Glaxo Smith Kline, Glenmark Pharmaceuticals, Isis Pharmaceuticals Inc., Johnson & Johnson, Merck Research Laboratories, Merck Frosst, Novartis Pharmaceuticals, Novo Nordisk Inc., NPS Pharmaceuticals Inc., Phenomix Inc., Takeda, and Transition Pharmaceuticals Inc. D.J.D. has also received operating grant support for other projects from Merck, Novartis, Novo Nordisk, and Amylin/Eli Lilly. I.H. and P.P. have nothing to disclose; L.L.B. has served as a consultant and speaker for Merck Frosst Inc.
First Published Online December 6, 2007
Abbreviations: ALS, Antilymphocyte serum; Ex-4, exendin-4; GLP, glucagon-like peptide; GLP-1R, glucagon-like peptide-1 receptor; NOD, nonobese diabetic; OGTT. oral glucose tolerance test; PE, phycoerythrin; T2DM, type 2 diabetes mellitus; Treg, regulatory T cell.
References
- Baggio LL, Drucker DJ 2006 Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med 57:265–281 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR 2006 International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA 2006 The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705 [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S 1999 Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Egan JM, Bonner-Weir S, Habener JF 2000 Insulinotropic glucagon-like peptide-1 agonists stimulate expression of homeodomain protein IDX-1 and increase β-cell mass in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jette L, Benquet C, Drucker DJ 2003 Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes 52:751–759 [DOI] [PubMed] [Google Scholar]
- Drucker DJ 2003 Glucagon-like peptide-1 and the islet β-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology 144:5145–5148 [DOI] [PubMed] [Google Scholar]
- Wang Q, Brubaker PL 2002 Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45:1263–1273 [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ 2003 Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J Biol Chem 278:471–478 [DOI] [PubMed] [Google Scholar]
- Wang Q, Li L, Xu E, Wong V, Rhodes CJ, Brubaker PL 2004 Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic (INS-1) β-cells. Diabetologia 47:478–487 [DOI] [PubMed] [Google Scholar]
- Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M 2004 Glucagon-like peptide-1 prevents β cell glucolipotoxicity. Diabetologia 47:806–815 [DOI] [PubMed] [Google Scholar]
- Itoh A, Maki T 1996 Protection of nonobese diabetic mice from autoimmune diabetes by reduction of islet mass before insulitis. Proc Natl Acad Sci USA 93:11053–11056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P, Ning T, Murphy T, Dabrowski D, Ramanathan S 2005 Impaired post-thymic development of regulatory CD4+25+ T cells contributes to diabetes pathogenesis in BB rats. J Immunol 174:4081–4089 [DOI] [PubMed] [Google Scholar]
- Reddy S, Bibby NJ, Elliott RB 1990 Early nicotinamide treatment in the NOD mouse: effects on diabetes and insulitis suppression and autoantibody levels. Diabetes Res 15:95–102 [PubMed] [Google Scholar]
- Reddy S, Liu W, Thompson JM, Bibby NJ, Elliott RB 1992 First phase insulin release in the non-obese diabetic mouse: correlation with insulitis, β cell number and autoantibodies. Diabetes Res Clin Pract 17:17–25 [DOI] [PubMed] [Google Scholar]
- Debussche X, Lormeau B, Boitard C, Toublanc M, Assan R 1994 Course of pancreatic β cell destruction in prediabetic NOD mice: a histomorphometric evaluation. Diabet Metab 20:282–290 [PubMed] [Google Scholar]
- Signore A, Procaccini E, Toscano AM, Ferretti E, Williams AJ, Beales PE, Cugini P, Pozzilli P 1994 Histological study of pancreatic β-cell loss in relation to the insulitis process in the non-obese diabetic mouse. Histochemistry 101:263–269 [DOI] [PubMed] [Google Scholar]
- Baggio L, Kieffer TJ, Drucker DJ 2000 Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology 141:3703–3709 [DOI] [PubMed] [Google Scholar]
- Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Chem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Kiraly MA, Bates HE, Yue JT, Goche-Montes D, Fediuc S, Park E, Matthews SG, Vranic M, Riddell MC 2007 Attenuation of type 2 diabetes mellitus in the male Zucker diabetic fatty rat: the effects of stress and non-volitional exercise. Metabolism 56:732–744 [DOI] [PubMed] [Google Scholar]
- Fox CJ, Paterson AD, Mortin-Toth SM, Danska JS 2000 Two genetic loci regulate T cell-dependent islet inflammation and drive autoimmune diabetes pathogenesis. Am J Hum Genet 67:67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle E, Fuks A, Poussier P, Edouard P, Guttmann RD 1992 Polygenic nature of spontaneous diabetes in the rat. Permissive MHC haplotype and presence of the lymphopenic trait of the BB rat are not sufficient to produce susceptibility. Diabetes 41:1617–1623 [DOI] [PubMed] [Google Scholar]
- Fox CJ, Danska JS 1997 IL-4 expression at the onset of islet inflammation predicts nondestructive insulitis in nonobese diabetic mice. J Immunol 158:2414–2424 [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY 2005 Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22:329–341 [DOI] [PubMed] [Google Scholar]
- Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J 2005 Foxp3+ CD25-CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA 102:4091–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, El-Kholy W, Rhodes CJ, Brubaker PL 2005 Glucagon-like peptide-1 protects β cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia 48:1339–1349 [DOI] [PubMed] [Google Scholar]
- Linn T, Schneider K, Goke B, Federlin K 1996 Glucagon-like-peptide-1 (7–36) amide improves glucose sensitivity in β-cells of NOD mice. Acta Diabetol 33:19–24 [DOI] [PubMed] [Google Scholar]
- Ogawa N, List JF, Habener JF, Maki T 2004 Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 53:1700–1705 [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Carter JD, Nunemaker CS, Garmey JC, Kimble SD, Nadler JL 2006 Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun 344:1017–1022 [DOI] [PubMed] [Google Scholar]
- Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC 2007 Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 mAb by enhancing recovery of β cells. Endocrinology 148:5136–5144 [DOI] [PubMed] [Google Scholar]
- Zhang J, Tokui Y, Yamagata K, Kozawa J, Sayama K, Iwahashi H, Okita K, Miuchi M, Konya H, Hamaguchi T, Namba M, Shimomura I, Miyagawa JI 2007 Continuous stimulation of human glucagon-like peptide-1 (7–36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia 50:1900–1909 [DOI] [PubMed] [Google Scholar]
- Masur K, Beinborn M, Zaenker KS 2004 Glucagon-like peptide-1 (GLP-1) is not only an important incretin hormone but also a modulator of the cellular immune system. Diabetes 53:A49-LB [Google Scholar]
- Dupre J, Behme MT, Hramiak IM, McFarlane P, Williamson MP, Zabel P, McDonald TJ 1995 Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 44:626–630 [DOI] [PubMed] [Google Scholar]
- Fung M, Thompson D, Shapiro RJ, Warnock GL, Andersen DK, Elahi D, Meneilly GS 2006 Effect of glucagon-like peptide-1 (7–37) on β-cell function after islet transplantation in type 1 diabetes. Diabetes Res Clin Pract 74:189–193 [DOI] [PubMed] [Google Scholar]
- Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM 2007 Effect of exenatide on β cell function after islet transplantation in type 1 diabetes. Transplantation 83:24–28 [DOI] [PubMed] [Google Scholar]