Abstract
Therapeutic vaccination is a potentially powerful strategy to establish immune control and eradicate persistent viral infections. Large and multifunctional antiviral T cell responses are associated with control of viral persistence; however, for reasons that were mostly unclear, current therapeutic vaccination approaches to restore T cell immunity and control viral infection have been ineffective. Herein, we confirmed that neutralization of the immunosuppressive factor interleukin (IL)-10 stimulated T cell responses and improved control of established persistent lymphocytic choriomeningitis virus (LCMV) infection. Importantly, blockade of IL-10 also allowed an otherwise ineffective therapeutic DNA vaccine to further stimulate antiviral immunity, thereby increasing T cell responses and enhancing clearance of persistent LCMV replication. We therefore propose that a reason that current therapeutic vaccination strategies fail to resurrect/sustain T cell responses is because they do not alleviate the immunosuppressive environment. Consequently, blocking key suppressive factors could render ineffective vaccines more efficient at improving T cell immunity, and thereby allow immune-mediated control of persistent viral infection.
Viral infections trigger robust T cell responses, which are crucial to clear infection. However, in response to some viral infections, antiviral CD4 and CD8 T cells become unresponsive to viral antigens and are either physically deleted or persist in a nonfunctional (exhausted) state. This is characterized by the inability to produce antiviral and immune-stimulatory cytokines, lyse virally infected cells, or proliferate (1–3). Multiparameter loss of T cell function directly facilitates persistence, as indicated by the fact that prolonged T cell responses strongly correlate with clearance and control of hepatitis C virus (HCV) and HIV infections in humans and lymphocytic choriomeningitis virus (LCMV) infection in mice (4–8). Thus, restoration of T cell activity represents a potentially powerful approach to control established persistent viral infections. Unfortunately, however, vaccines to restore antiviral T cell activity and control persistent viral infections have only been marginally successful (9). The reasons underlying the failures are unclear because these same vaccines are often immunogenic when administered before infection. One explanation is that although the vaccines are designed to stimulate T cells, they do not alleviate the immunosuppressive environment and, as a result, vaccine-activated T cells rapidly succumb to the same constraints that previously limited their responsiveness. By neutralizing the suppressive factors and alleviating the immunosuppressive environment, antiviral T cells may become receptive to vaccine stimulation and enabled to fight viral replication.
Recently, based on genetic deletion and antibody blockade studies, IL-10 was identified as a single, dominant factor that determines whether a virus infection is cleared acutely or persists (5, 6). Antibody blockade of IL-10 during an established persistent infection and after T cell exhaustion restored T cell function, leading to enhanced clearance of virus. During persistent viral infection, programmed death-1–programmed death-ligand 1 (PD-1–PD-L1) interactions further limit T cell function, and antibody blockade of PD-L1 can stimulate T cell activity (4). Thus, multiple immunosuppressive pathways are invoked during a persistent viral infection that inhibit T cell immunity and prevent virus clearance. The ability to restore T cell immunity through blockade of IL-10 or PD-L1 pathways appears distinct based on findings that PD-1 levels remained high in IL-10–deficient mice early after infection and that simultaneous blockade of IL-10 and PD-1 synergized to stimulate T cell activity during an established persistent viral infection (unpublished data). This is particularly important considering increased IL-10 expression is observed during many persistent viral infections in humans (e.g., HIV, HCV, and HBV) and is directly correlated with decreased T cell responsiveness and the failure to control viral replication (for review see [10]). Thus, IL-10 might serve as a therapeutic target during many persistent viral infections to restore T cell responsiveness to vaccination and ultimately establish control of viral replication.
RESULTS AND DISCUSSION
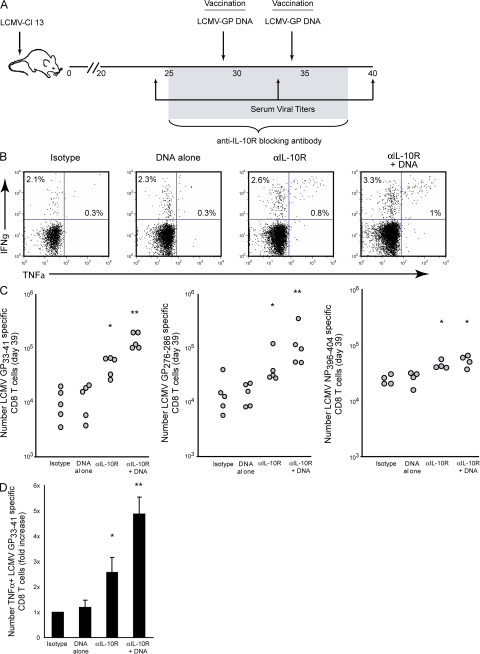
To determine how the immunosuppressive environment affects T cell responsiveness to vaccination during persistent viral infection, C57BL/6 mice were infected with LCMV Clone 13 (Cl 13). Infection with LCMV-Cl 13 rapidly induces high-level expression of IL-10 that suppresses antiviral immunity and leads to viral persistence (5, 6, 11). The profound effect IL-10 had on suppressing antiviral T cell activity and potentiating viral persistence, coupled with the continued high-levels of IL-10 expression throughout persistent infection (unpublished data) suggested that blocking IL-10–mediated immunosuppression might now make T cells receptive to exogenous stimulation (i.e., therapeutic vaccination). To determine whether IL-10 inhibits responsiveness to therapeutic vaccination, LCMV-Cl 13 persistently infected mice were treated with isotype control antibody alone; treated with isotype control antibody and vaccinated with a DNA plasmid encoding the entire glycoprotein (GP) sequence of LCMV; treated with anti–IL-10R blocking antibody; or treated with a combination of anti–IL-10R blocking antibody plus DNA vaccination. Antibody treatment was initiated on day 25 after Cl 13 infection and administered every 3 d for 5–6 treatments. DNA vaccination was administered on day 29 and 34 after virus infection (i.e., 4 and 9 d after the initiation of anti–IL-10R therapy). The treatment regimen is illustrated in Fig. 1 A.
Figure 1.
IL-10R blockade enables effective stimulation of antiviral T cell responses by therapeutic vaccination. (A) Schematic representation of anti–IL-10R antibody treatment and DNA vaccination. LCMV-Cl 13–infected mice were treated with an isotype control antibody, treated with isotype control antibody and DNA vaccine (encoding the entire LCMV-GP), treated with an anti–IL-10R blocking antibody, or cotreated with DNA vaccine plus anti–IL-10R antibody. Anti–IL-10R treatment was initiated on day 25 after infection and continued every 3 d for 2 wk. DNA vaccination was administered on day 29 and 34 after infection. T cell responses were then analyzed on day 39 after infection. (B) Cytokine production was quantified by ex vivo peptide stimulation and intracellular staining. Flow plots are gated on CD8 T cells and illustrate the frequency of IFNγ- and TNFα-producing, LCMV-GP33-41–specific CD8 T cells. Data are representative of four to five mice per group. (C) The graphs indicate the number of IFNγ-producing LCMV-GP33-41-, GP276-286-, and NP396-404-specific CD8 T cells after each treatment regimen. Circles represent individual mice. *, P < 0.05 compared with untreated and DNA vaccination alone. **, P < 0.05 compared with all other treatment groups. (D) The bar graph represents the mean fold increase in the number of TNFα-producing, LCMV-GP33-41–specific CD8 T cells in each treatment group compared with isotype treatment (which is set to 1). Values are the mean ± the SD of three experiments using four to six mice. *, P < 0.05 compared with untreated and DNA vaccination alone; **, P < 0.05 compared with all other treatment groups.
Consistent with the inability of therapeutic vaccination to stimulate T cell responses during persistent infection (12), DNA vaccination alone had no effect on the frequency or number of virus-specific CD8 T cells compared with untreated animals (Fig. 1, B and C). On the other hand, IL-10R blockade alone increased the frequency and number of IFNγ-producing CD8 T cells against multiple LCMV epitopes (Fig. 1, B and C), indicating that IL-10 actively suppresses T cell responses throughout persistent infection and that blocking IL-10R alone enhances T cell immunity. Importantly, IL-10R blockade combined with DNA vaccination dramatically enhanced T cell responses compared with either DNA vaccine or anti–IL-10R therapy alone (Fig. 1 C). Anti–IL-10R plus DNA vaccine dual therapy induced a twofold increase in the frequency and a greater than fivefold increase in the number of IFNγ-producing, virus-specific CD8 T cells. A similar enhancement of CD8 T cell immunity was observed by MHC class I tetramer staining (unpublished data). Although IL-10R blockade alone increased the number of LCMV nucleoprotein (NP) 394-404-specific CD8 T cells compared with isotype treatment, DNA vaccine (which contained only LCMV-GP epitopes and not LCMV-NP epitopes) plus anti–IL-10R therapy did not enhance LCMV-NP396-404–specific CD8 T cell responses (Fig. 1 C). Moreover, vaccination with the parental control DNA plasmid (i.e., that did not encode LCMV sequences) failed to further enhance T cell responses when combined with IL-10R blockade (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071948/DC1). Thus, T cell stimulation resulted from the LCMV sequences encoded by the DNA vaccine and not a secondary response to heightened immune activation or nonspecific activation by immunostimulatory sequences present in the DNA vaccine.
IL-10R blockade followed by vaccination also significantly increased the number of functional virus-specific CD8 T cells (Fig. 1 D). Whereas vaccination alone did not enhance virus-specific CD8 T cell function, and IL-10R blockade alone only increased the number of TNFα-producing, virus-specific T cells by approximately twofold, IL-10R blockade combined with vaccination stimulated a fourfold increase in the number of functional CD8 T cells that produced TNFα (Fig. 1 D). Interestingly, the treatments did not substantially increase the frequency of TNFα-producing CD8 T cells as a proportion of virus-specific cells (Fig. 1 B). Rather, a dramatic increase in the absolute number of functional, cytokine-producing CD8 T cells was observed (Fig. 1 D).
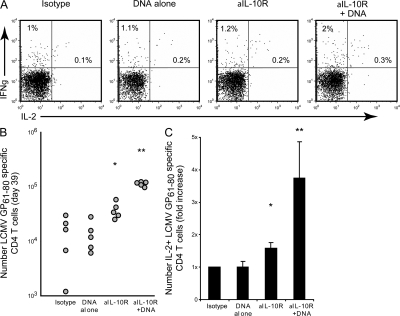
IL-10R blockade alone had a less dramatic effect on CD4 T cells than that observed for CD8 T cells, inducing a small but significant (P < 0.05) 1.5–2-fold increase in the number of IFNγ-producing CD4 T cells (Fig. 2, B and C). On the other hand, IL-10R blockade plus DNA vaccine therapy substantially increased the frequency and particularly the number (a sixfold increase compared with isotype treatment) of IFNγ-producing CD4 T cells (Fig. 2, B and C). Although, similar to virus-specific CD8 T cell responses, IL-10R blockade alone or in combination with DNA vaccination only minimally enhanced the frequency of IL-2–producing CD4 T cells and DNA vaccine or anti–IL-10R therapy alone only modestly affected the number of IL-2 producing CD4 T cells; in contrast, cotreatment stimulated a robust fourfold increase in the number of functional virus-specific CD4 T cells (Fig. 2, A and C). These data demonstrate that an otherwise ineffective vaccination strategy can stimulate robust T cell responses during persistent viral infection if IL-10–mediated immunosuppression is neutralized.
Figure 2.
CD4 T cell responses are enhanced by IL-10R blockade and vaccination. (A) LCMV-Cl 13–infected mice were treated and analyzed as described in Fig. 1. The frequency of cytokine-producing, LCMV-GP61-80–specific CD4 T cells was quantified by ex vivo peptide stimulation and intracellular staining. Flow plots are gated on CD4 T cells and illustrate the frequency of IFNγ- and IL-2–producing, LCMV-GP61-80–specific CD4 T cells after each treatment (39 d after infection). Data are representative of 4–5 mice per group. (B) The graph illustrates the number of IFNγ-producing, LCMV-GP61-80–specific CD4 T cells (quantified as described in Fig. 1 C). *, P < 0.05 compared with untreated and DNA vaccination alone. **, P < 0.05 compared with all other treatment groups. (C) The bar graph illustrates the mean fold increase in the number of IL-2–producing, LCMV-GP61-80–specific CD4 T cells after each treatment regimen compared with isotype treatment. Individual bars represent the mean value ± the SD of 3 experiments containing for 4–6 mice per group. *, P < 0.05 compared with untreated and DNA vaccination alone. **, P < 0.05 compared with all other treatment groups.
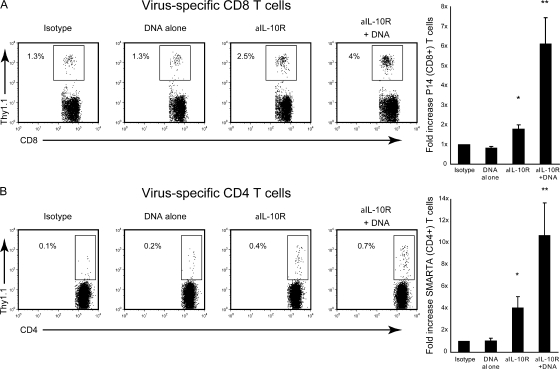
We next addressed whether the increased T cell function was caused by reactivation of previously exhausted T cells or from de novo priming of naive T cell precursors. To address this issue, Thy1.1+ TCR transgenic (tg) CD4 T cells (SMARTA cells; specific to LCMV-GP61-80 peptide) and Thy1.1+ TCR tg CD8 T cells (P14 cells; specific to LCMV GP33-41 peptide) were cotransferred into Thy1.2+ C57BL/6 mice and the mice were subsequently infected with LCMV-Cl 13. The cotransfer enabled analysis of LCMV-specific CD4 and CD8 T cells present from the beginning of infection and which could be distinguished from endogenous (i.e., host-derived) antiviral T cells based on Thy1.1 versus Thy1.2 expression. Physiological numbers of these tg T cells were transferred to ensure that they respond similarly to their endogenous CD4 (Fig. 2) and CD8 (Fig. 1) T cell counterparts (13). Similar to endogenous T cell responses (Fig. 1 and 2), DNA vaccine alone did not increase the number of virus-specific tg CD8 or CD4 T cells, whereas IL-10R blockade stimulated a two- and fourfold increase in the number of tg CD8 and CD4 T cells, respectively (Fig. 3). IL-10R blockade in combination with DNA vaccination induced a dramatic sixfold increase in the number of tg virus-specific CD8 T cells compared with isotype control or DNA vaccine alone–treated mice and an approximately threefold increase when compared with IL-10R blockade alone (Fig. 3 A). Similarly, IL-10R blockade in combination with vaccination stimulated an 11-fold increase in the number of virus-specific tg CD4 T cells compared with isotype control or vaccine alone and a fourfold increase versus IL-10R blockade therapy alone (Fig. 3 B). Like their endogenous counterparts, the combination therapy also elevated the number of functional virus-specific T cells (i.e., TNFα-producing tg CD8 T cells and IL-2–producing tg CD4 T cells; not depicted). Thus, IL-10R blockade permits the previously exhausted T cells to respond to vaccination.
Figure 3.
IL-10R blockade combined with vaccination restores T cell function. Before infection, mice were seeded with LCMV-specific, Thy1.1+, TCR tg CD8+ (P14; A), and CD4+ (SMARTA; B) T cells and infected with LCMV-Cl 13. Mice were treated with isotype control antibody, anti–IL-10R blocking antibody, and/or DNA vaccine, as described in Fig. 1 A. Bar graphs indicate the fold increase in the number of P14 and SMARTA cells on day 39 after infection. Individual bars represent the mean ± the SD of five mice per group. *, significant (P < 0.05) increase in the mean number versus isotype and DNA vaccine alone. **, significant (P < 0.05) increase versus all other conditions.
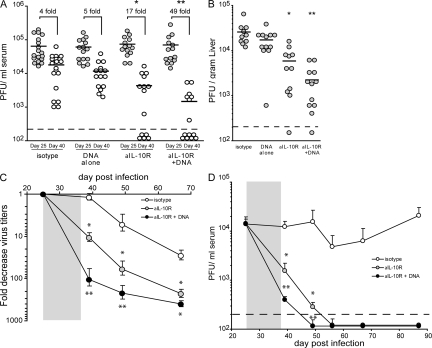
To determine if IL-10R blockade and vaccination enhanced control of infection, virus titers were quantified after treatment and compared with pretreatment levels. After treatment, viral titers were similar in isotype and DNA vaccinated mice through 40 d after infection, whereas viral replication was significantly (P < 0.05) decreased in anti–IL-10R–treated mice, falling 17-fold after the resolution of therapy (Fig. 4 A), indicating that the T cell responses elicited by IL-10R blockade rapidly (i.e., within the 2-wk treatment regimen) impacted and decreased persistent viral replication. Importantly, anti–IL-10R plus DNA vaccine treatment resulted in an even greater enhanced ability to control and eliminate persistent viral replication, ultimately resulting in a 49-fold decrease in virus replication within the 2-wk timeframe between the initiation and completion of therapy (Fig. 4 A). Enhanced control of virus replication was also rapidly observed in the liver immediately after IL-10R blockade alone, and virus titers were decreased even further in the liver after IL-10R blockade in combination with the DNA vaccine (Fig. 4 B), demonstrating control of virus replication in nonlymphoid/effector sites of infection. There was a discrepancy in the ability of some animals to eliminate virus infection after IL-10R blockade alone or in combination with DNA vaccination (Fig. 4 A). Whereas some animals completely cleared virus infection after therapy, other similarly treated animals still retained virus replication. It should be noted that Fig. 4 (A and B) illustrates the combined data from multiple experiments and that pretherapy virus titers varied between experiments. However, in general, animals with higher pretreatment virus titers corresponded to higher levels of virus replication after treatment, and results were significant within experiments. The difference in virus clearance kinetics after treatment was not caused by the gender or the age of the mice (unpublished data). Importantly, when the fold decrease in virus titers were quantified, the enhanced antiviral effect of IL-10R blockade plus DNA vaccination became even more evident (Fig. 4 C), illustrating that the control of persistent virus replication was maintained well after the cessation of therapy and until virus infection was cleared. IL-10R treatment alone was highly effective to control persistent viral infection; however, IL-10R therapy plus DNA vaccination enhanced control of virus replication even further (Fig. 4 D). By day 65 after infection (28 d after the completion of therapy), the fold decrease in virus titers was similar in mice that received IL-10R treatment alone and those that received IL-10R plus DNA vaccine (Fig. 4, C and D) because by this time virus had been cleared in both groups. However, it should be noted that the fold decrease in virus titers in the cotreated animals remained significantly lower than IL-10R–treated animals alone well after the completion of therapy and only became equal after viral clearance (i.e., once there was no further decrease that could occur). Thus, although both treatments were highly effective at controlling persistent viral replication, the ability to further increase T cell responses by vaccination enhanced control of persistent viral infection. Viral replication remained absent after IL-10R blockade (>150 d after infection) and treated mice were protected from subsequent reinfection (not depicted). Interestingly, anti–IL-10R antibody treatment every 3 d boosted T cell responses, leading to control of persistent viral infection, whereas weekly treatments, including treatment with twice the required dose of antibody, were ineffective (unpublished data), indicating that the timing of antibody therapy is an important determinant for therapeutic efficacy. IL-10R blockade in combination with the control DNA vaccine (i.e., not encoding LCMV-GP) did not affect virus titers compared with anti–IL-10R treatment alone, again demonstrating that the enhanced effect of DNA vaccine was caused by stimulation of LCMV-specific cells (not depicted). Thus, neutralizing IL-10–mediated immunosuppression facilitates the induction of robust virus-specific T cell responses with an enhanced ability to control persistent viral infection.
Figure 4.
Accelerated control of persistent viral infection by alleviating the immunosuppressive environment and vaccinating to stimulate T cell responses. (A) LCMV-Cl 13–infected mice were infected and treated as described in Fig. 1 A. Serum viral titers were quantified on day 25 (gray circles, before treatment) and day 40 (white circles, after the completion of all therapeutic treatments) after infection. Each circle represents the virus titers in a single mouse on day 25 and 40 after infection. The dashed line indicates the level of detection of the plaque assay (200 PFU/ml). *, indicates a significant (P < 0.05) decrease in virus titers on day 40 after infection compared with untreated or to DNA vaccination alone. **, indicates a significant (P < 0.05) decrease in virus titers on day 40 after infection compared with isotype antibody treatment, DNA vaccine alone, or IL-10R antibody treatment alone. The numbers above each group indicate the mean fold decrease in virus titers between day 25 and 40 after infection. The graph contains data from three separate experiments. (B) The graph illustrates the virus titer in the liver of individual mice 3 d after the withdrawal of therapy. Each circle represents the liver virus titers in an individual mouse on day 40 after infection. The dashed line indicates the level of detection of the plaque assay (200 PFU/ml). *, indicates a significant (P < 0.05) decrease in virus titers on day 40 after infection compared with untreated mice or with DNA vaccination alone. **, indicates a significant (P < 0.05) decrease in virus titers on day 40 after infection compared with isotype antibody treatment, DNA vaccine alone, or IL-10R antibody treatment alone. (C) The graph illustrates a longitudinal analysis of the mean fold decrease in serum virus titers after isotype antibody treatment (white circles), IL-10R antibody blockade (gray circles), or IL-10R antibody blockade plus DNA vaccination (black circles). The mean fold decrease in each group was determined by dividing the pretreatment virus titers in each mouse (on day 25 after infection) by the virus titer in the same mouse on the indicated day after infection. Each circle represents the mean fold decrease ± the SD in viral titers of four to five mice per group. The gray box indicates the duration of antibody treatment. (D) Longitudinal analysis of serum viral titers were performed after LCMV-Cl 13 infection after isotype antibody treatment (white circles), IL-10R antibody blockade (gray circles), or IL-10R antibody blockade plus DNA vaccination (black circles). To better standardize virus titers, all groups were initially normalized to the virus titer of the isotype-treated group on day 25 after infection (i.e., before treatment). Each circle represents the mean virus titer of 4–5 mice on the indicated day after infection. The gray box indicates the duration of antibody treatment. The dashed line indicates the level of detection of the plaque assay (200 PFU/ml).
In this study, we establish the concept that the immunosuppressive environment inhibits the ability of vaccines to restore T cell immunity during an established persistent viral infection. Our results shed light on the enigma of why therapeutic administration of a vaccine agent is unsuccessful, whereas administration of the same vaccine to pathogen-naive individuals (in which no immunosuppressive environment exists) is highly effective. Once the immunosuppressive signals that limit T cell function are neutralized, T cells become receptive to stimulation by vaccines. Specifically, we demonstrate that antibody neutralization of IL-10 activity during persistent viral infection allows an otherwise ineffective DNA vaccine to potently stimulate antiviral CD4 and CD8 T cell responses and control viral replication. Using tg cells, we showed that IL-10R blockade in combination with vaccination improved and expanded effector activity in previously exhausted T cells even after prolonged exposure to an immunosuppressive milieu. An independent finding (see Ha et al. [14] on p. 543 of this issue) demonstrated that blockade of a different host immunosuppressive molecule (PD-L1) was similarly able to restore efficacy of therapeutic vaccination and stimulate antiviral T cell responses during persistent viral infection. Together, these findings establish the concept that the immunosuppressive environment inhibits therapeutic vaccination, and indicates a potentially powerful approach to enhance T cell immunity and combat persistent viral infections.
Multiple lines of evidence indicate that IL-10 mediates suppression during chronic infections in humans. Elevated IL-10 levels are observed during HIV, HCV, and HBV infections and, similar to LCMV infection, the extent of IL-10 expression correlates with diminished T cell activity and increased virus replication (10). Further, in vitro blockade of IL-10 in peripheral blood mononuclear cells from HIV and HCV chronically infected human's restored T cell activity (15–18). Recent studies also indicate that genetic polymorphisms in the IL-10 promoter that reduce IL-10 production are associated with clearance of HCV infection and enhanced virus control during chronic HCV, HBV, HIV, and EBV infections (19–22). Thus, IL-10 neutralization in persistently infected humans might enable vaccines to restore antiviral T cell immunity and control persistent infections.
It is interesting that IL-10R blockade alone or in combination with vaccination increased the number of functional, cytokine-producing T cells, but at the population level did not substantially increase the overall frequency of cytokine-producing, virus-specific T cells. Nevertheless, the increased number of functional cells was able to control infection. These findings have important implications for the treatment of persistent infections because they suggest that complete functional restoration at the cellular level may not be required to control viral replication. Rather, it might be sufficient to therapeutically elevate the absolute number of functional virus-specific T cells to control viral persistence. Thus, in human clinical trials testing candidate vaccines, increases in the absolute number of antiviral T cells should be carefully considered as a therapeutic endpoint.
Given that high levels of viral antigen exist during persistence, it is surprising that the addition of more antigen through vaccination can actually further elevate antiviral T cell numbers. Our results with IL-10R blockade suggest that the failure of T cell activity is not necessarily caused by the high levels of viral antigen per se, but rather to the milieu in which viral antigens are being processed and/or presented to T cells. The increased T cell activity observed after vaccination may be a function of multiple factors. First, viral antigen during persistent infection may not be presented in a manner that robustly stimulates T cell responses. Because DCs are a primary target of LCMV-Cl 13 (23), the majority of DCs presenting viral antigens may still exhibit some level of dysfunction even after IL-10R blockade. DCs can present DNA-derived antigens after vaccination (24, 25). Consequently, newly recruited DCs (which have not been rendered dysfunctional by LCMV) may be better equipped to stimulate T cell activity. Second, because DNA vaccines bypass the requirement for antigen-specific recognition, non-LCMV–specific B cells may also become a source of antigen presentation and further amplify T cell activity. Third, additional APC populations (i.e., macrophages, B cells, and different DC subsets) that would otherwise not present to T cells during infection may acquire antigen derived from DNA vaccines and stimulate T cells. We are currently exploring these scenarios. Ultimately, the determination of the cell types that present DNA-derived antigens and how IL-10 dysregulates antigen presentation should yield insights into the cellular interactions that potentiate viral persistence and aid in the development of immunotherapies to correct antigen presentation and enhance immunity.
In our system, IL-10R blockade in combination with DNA vaccine stimulated a more rapid viral clearance than IL-10R blockade alone, although IL-10R blockade alone does effectively control persistent viral replication. These data are important because it illustrates that solely blocking IL-10–mediated immunosuppression elicits T cell responses capable of controlling persistent LCMV infection. However, clearance of LCMV is particularly dependent on and susceptible to the levels of T cell activity. In other infections, such as HIV and HCV, in which T cells are important for virus control but not necessarily as effective as against LCMV, the ability to further elevate T cell responses through vaccination is particularly desirable. Moreover, LCMV-Cl 13 infection is naturally cleared within 60–150 d (26). Thus, although, IL-10R blockade alone increases T cell responses to a sufficient level to accelerate LCMV clearance, stronger T cell responses will likely be required to control other persistent viral infections that are not naturally resolved. Although the increase in virus-specific T cells after IL-10R blockade alone eliminates persistent LCMV infection, when larger T cell responses are required to control other virus infections, then our data establish that blocking IL-10R and stimulating with a vaccine will be highly effective. For example, IL-10R blockade in combination with DNA vaccine stimulated a 5–10 fold increase in the total number of virus-specific T cells (which is greater than IL-10R blockade alone; Figs. 2 and 3) and, importantly, increased the population of T cells able to simultaneously produce multiple cytokines in response to viral antigen. It is these polyfunctional T cells that are associated with enhanced control of HIV infection (27). In fact, the further enhancement of T cell responses may be the thing that ultimately tips the balance to immune control and clearance of persistent viral replication in humans. In essence, our study illustrates that in situations in which maximal T cell responses are required to control virus replication, neutralization of the immunosuppressive environment is essential to allow T cell–stimulating agents to elicit their greatest response.
The failure of vaccines to trigger T cell immunity when administered during an established persistent viral infection has largely been enigmatic because these same vaccines are often immunogenic and can stimulate T cell responses when given prophylactically. Our data now clarifies this discrepancy. Although a vaccine may inherently stimulate T cell activity, when it is administered in the context of a persistent viral infection, the immunosuppressive constraints continue to inhibit T cell responsiveness. Consistent with our findings, when a vaccine is administered to HIV-infected patients in which virus replication is suppressed by antiretroviral therapy, T cells are receptive to stimulation (for review see [28]). However, T cell activity wanes as both virus and immunosuppressive factors rebound after the cessation of therapy. Such a loss of T cell function may be avoided through the use of IL-10 blockade and periodic vaccination to further boost T cell immunity. Lastly, an initial immunogenic challenge likely also elicits a counter immunosuppressive response to limit the immune response and prevent excessive immunopathology. Thus, blockade of immunosuppressive molecules like IL-10 in conjunction with prophylactic vaccination of a naive recipient enhances the host's immune response above vaccination alone (unpublished data). Ultimately, a similar dual immunotherapeutic adjuvant/vaccination strategy that alleviates immunosuppressive signals while boosting immunity may stimulate effective, long-term, immune-mediated control of persistent viral infections in humans, and may also be an adjuvant for primary vaccination.
MATERIALS AND METHODS
Mice and virus.
C57BL/6 mice were obtained from the Rodent Breeding Colony at The Scripps Research Institute. The LCMV-GP61-80–specific CD4+ TCR tg (SMARTA) mice and LCMV-GP33-41–specific CD8+ TCR tg (P14) mice have been previously described (29). All mice were housed under specific pathogen–free conditions. Mouse handling conformed to the requirements of the National Institutes of Health and The Scripps Research Institute Animal Research Committee. The animal experiments were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute. Mice were infected intravenously with 2 × 106 PFU of LCMV-Cl 13. To test immunological memory and protection, mice were rechallenged with 2.5 × 106 PFU LCMV-Cl 13 i.v. Virus stocks were prepared and viral titers were quantified as previously described (26). For analysis of liver viral titers, the mice were perfused with 25–30 ml of 0.9% saline by direct cardiac injection to remove blood from the tissue.
T cell isolation and transfer.
CD4 and CD8 T cells were purified from the spleens of naive SMARTA and P14 mice, respectively, by negative selection (StemCell Technologies), and 1,000 purified cells from each population were cotransferred i.v. into C57BL/6 mice. We avoided the problems associated with using large, nonphysiological numbers of transferred tg T cells (30) by cotransferring 1,000 CD4 and CD8 T cells. Each of these tg T cell populations behave similarly to their endogenous (i.e., host-derived) T cell counterparts, based on tetramer analysis and intracellular cytokine staining [3, 26] (unpublished data). The number of SMARTA and P14 cells in the spleen was determined by multiplying the frequency of Thy1.1+ cells (determined flow cytometrically) by the total number of splenocytes.
Quantitative RT-PCR.
RNA from total splenic mononuclear cells was obtained and amplified as previously described (5). In brief, RNA expression was normalized by input concentration and amplified using the One-Step RT-PCR kit (QIAGEN). The Assays-on-Demand Real-Time IL-10 expression kit (Applied Biosystems) was used to amplify IL-10 RNA. The RT-PCR reaction did not amplify DNA (unpublished data). To quantify IL-10 RNA, a standard curve was generated by 10-fold serial dilutions of total splenic RNA (1 μg to 1 pg total RNA; standard curve, r 2 > 0.99) from Cl 13–infected splenocytes and a relative number of IL-10 RNA determined. Amplifications were performed on the ABI7700 (Applied Biosystems).
Intracellular cytokine analysis and flow cytometry.
Splenocytes were stimulated for 5 h with 5 μg/ml of the MHC class II–restricted LCMV-GP61-80 or 2 μg/ml of the MHC class I restricted LCMV-NP396–404, GP33–41, or GP276–286 peptide (all >99% pure; Synpep,) in the presence of 50 U/ml recombinant murine IL-2 (R&D Systems) and 1 mg/ml brefeldin A (Sigma-Aldrich). Addition of IL-2 to the ex vivo stimulation did not alter cytokine production (unpublished data). Cells were stained for surface expression of CD4 (clone RM4-5; BD Biosciences) and CD8 (clone 53–6.7; Caltag). Cells were fixed, permeabilized, and stained with antibodies to TNF-α (clone MP6-XT22), IFN-γ (clone XMG1.2), and IL-2 (clone JES6-5H4). Flow cytometric analysis was performed using the Digital LSR II (Becton Dickinson). The absolute number of virus-specific T cells was determined by multiplying the frequency of cytokine+ cells by the total number of cells in the spleen.
In vivo IL-10R–specific antibody treatment.
C57BL/6 mice received 250 μg/mouse/injection i.p. of IL-10R–specific antibody (clone 1B1.3a; provided by Schering-Plough) or rat IgG1 isotype control antibody (clone KM1.GL113 [anti–Escherichia coli β-galactosidase]; provided by Schering-Plough) beginning 25 d after infection and continuing every 3 d for 5–6 treatments.
DNA vaccinations.
Plasmid pCMV-GP encodes the entire LCMV glycoprotein. The parental control vector pCMV is the same, except that it contains no LCMV sequences. The plasmids were provided by Lindsay Whitton (The Scripps Research Institute, La Jolla, CA), and the construction of these plasmids has been previously described (31). pCMV-GP encodes both CD4 and CD8 T cell LCMV GP epitopes, but not CD4 and CD8 T cell LCMV NP epitopes. Plasmids were propagated in parallel in E. coli and purified using an endotoxin-free plasmid purification kit (QIAGEN). C57BL/6 mice received DNA injections (bilateral 50 μl injections of plasmid DNA in saline (100 μg plasmid/mouse) into the anterior tibial muscles) on days 29 and 34 after LCMV-Cl 13 infection.
Statistical analysis.
Student's t tests and Mann-Whitney Rank Sum tests were performed using SigmaStat 2.0 software (Systat Software, Inc.).
Online supplemental material.
Fig. S1 shows that the LCMV sequences in the DNA vaccine are responsible for stimulating antiviral T cell responses. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071948/DC1.
Supplemental Material
Acknowledgments
We thank Stephanie Harkins and Dae Young for technical assistance and Lindsay Whitton for the DNA plasmids.
This work was supported by National Institutes of Health (NIH) grants AI09484, AI45927 (M.B.A. Oldstone), and AI077012-01 (D.G. Brooks); NIH training grants AI07244-22 (D.G. Brooks), AI062718-01 (D.B. McGavern), and NS048866-01 (D.B. McGavern); and a Dana Foundation grant (D.B. McGavern).
The authors have no conflicting financial interests.
References
- 1.Zajac, A.J., J.N. Blattman, K. Murali-Krishna, D.J. Sourdive, M. Suresh, J.D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore, A., A. Glithero, A. Godkin, A.C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, D.G., M.J. Trifilo, K.H. Edelmann, L. Teyton, D.B. McGavern, and M.B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejrnaes, M., C.M. Filippi, M.M. Martinic, E.M. Ling, L.M. Togher, S. Crotty, and M.G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi, R.T., and B.D. Walker. 2002. Immunologic control of HIV-1. Annu. Rev. Med. 53:149–172. [DOI] [PubMed] [Google Scholar]
- 8.Shoukry, N.H., A.G. Cawthon, and C.M. Walker. 2004. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58:391–424. [DOI] [PubMed] [Google Scholar]
- 9.Autran, B., G. Carcelain, B. Combadiere, and P. Debre. 2004. Therapeutic vaccines for chronic infections. Science. 305:205–208. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn, S.D., and E.J. Wherry. 2007. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 15:143–146. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed, R., A. Salmi, L.D. Butler, J.M. Chiller, and M.B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry, E.J., J.N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks, D.G., D.B. McGavern, and M.B. Oldstone. 2006. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha, S.-J., S.N. Mueller, E.J. Wherry, D.L. Barber, R.D. Aubert, A.H. Sharpe, G.J. Freeman, and R.F. Ahmed. 2008. Enhancing vaccination by blocking PD-1–mediated inhibitory signals during chronic infection. J. Exp. Med. 205:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cacciarelli, T.V., O.M. Martinez, R.G. Gish, J.C. Villanueva, and S.M. Krams. 1996. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 24:6–9. [DOI] [PubMed] [Google Scholar]
- 16.Clerici, M., T.A. Wynn, J.A. Berzofsky, S.P. Blatt, C.W. Hendrix, A. Sher, R.L. Coffman, and G.M. Shearer. 1994. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Invest. 93:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landay, A.L., M. Clerici, F. Hashemi, H. Kessler, J.A. Berzofsky, and G.M. Shearer. 1996. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J. Infect. Dis. 173:1085–1091. [DOI] [PubMed] [Google Scholar]
- 18.Rigopoulou, E.I., W.G. Abbott, P. Haigh, and N.V. Naoumov. 2005. Blocking of interleukin-10 receptor–a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin. Immunol. 117:57–64. [DOI] [PubMed] [Google Scholar]
- 19.Cheong, J.Y., S.W. Cho, I.L. Hwang, S.K. Yoon, J.H. Lee, C.S. Park, J.E. Lee, K.B. Hahm, and J.H. Kim. 2006. Association between chronic hepatitis B virus infection and interleukin-10, tumor necrosis factor-alpha gene promoter polymorphisms. J. Gastroenterol. Hepatol. 21:1163–1169. [DOI] [PubMed] [Google Scholar]
- 20.Helminen, M., N. Lahdenpohja, and M. Hurme. 1999. Polymorphism of the interleukin-10 gene is associated with susceptibility to Epstein-Barr virus infection. J. Infect. Dis. 180:496–499. [DOI] [PubMed] [Google Scholar]
- 21.Paladino, N., H. Fainboim, G. Theiler, T. Schroder, A.E. Munoz, A.C. Flores, O. Galdame, and L. Fainboim. 2006. Gender susceptibility to chronic hepatitis C virus infection associated with interleukin 10 promoter polymorphism. J. Virol. 80:9144–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin, H.D., C. Winkler, J.C. Stephens, J. Bream, H. Young, J.J. Goedert, T.R. O'Brien, D. Vlahov, S. Buchbinder, J. Giorgi, et al. 2000. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc. Natl. Acad. Sci. USA. 97:14467–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevilla, N., D.B. McGavern, C. Teng, S. Kunz, and M.B. Oldstone. 2004. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 113:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casares, S., K. Inaba, T.D. Brumeanu, R.M. Steinman, and C.A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Condon, C., S.C. Watkins, C.M. Celluzzi, K. Thompson, and L.D. Falo Jr. 1996. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2:1122–1128. [DOI] [PubMed] [Google Scholar]
- 26.Brooks, D.G., L. Teyton, M.B. Oldstone, and D.B. McGavern. 2005. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79:10514–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichterfeld, M., X.G. Yu, M.T. Waring, S.K. Mui, M.N. Johnston, D. Cohen, M.M. Addo, J. Zaunders, G. Alter, E. Pae, et al. 2004. HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood. 104:487–494. [DOI] [PubMed] [Google Scholar]
- 28.McMichael, A.J. 2006. HIV Vaccines. Annu. Rev. Immunol. 24:227–255. [DOI] [PubMed] [Google Scholar]
- 29.Oxenius, A., M.F. Bachmann, R.M. Zinkernagel, and H. Hengartner. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400. [DOI] [PubMed] [Google Scholar]
- 30.Marzo, A.L., K.D. Klonowski, A. Le Bon, P. Borrow, D.F. Tough, and L. Lefrancois. 2005. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 6:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama, M., J. Zhang, and J.L. Whitton. 1995. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J. Virol. 69:2684–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.