Abstract
Therapeutic vaccination is a potentially promising strategy to enhance T cell immunity and viral control in chronically infected individuals. However, therapeutic vaccination approaches have fallen short of expectations, and effective boosting of antiviral T cell responses has not always been observed. One of the principal reasons for the limited success of therapeutic vaccination is that virus-specific T cells become functionally exhausted during chronic infections. We now provide a novel strategy for enhancing the efficacy of therapeutic vaccines. In this study, we show that blocking programmed death (PD)-1/PD-L1 inhibitory signals on exhausted CD8+ T cells, in combination with therapeutic vaccination, synergistically enhances functional CD8+ T cell responses and improves viral control in mice chronically infected with lymphocytic choriomeningitis virus. This combinatorial therapeutic vaccination was effective even in the absence of CD4+ T cell help. Thus, our study defines a potent new approach to augment the efficacy of therapeutic vaccination by blocking negative signals. Such an approach may have broad applications in developing treatment strategies for chronic infections in general, and perhaps also for tumors.
Persistent viral infections such as HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) in humans, simian immunodeficiency virus (SIV) in monkeys, and lymphocytic choriomeningitis virus (LCMV) in mice are associated with functional exhaustion of virus-specific CD8+ T cells (1–3). This defect in responding T cells is one of the primary reasons for the inability of the host to eliminate the persisting pathogen. Because many chronic viral infections cannot be cleared by antiviral therapy alone (4, 5), therapeutic vaccination, which aims to enhance the patient's own antiviral immune response, has been considered as an alternative therapy. However, the efficacy of such strategies has so far been disappointing (6–9). Recent work suggests that the decreased proliferative potential of virus-specific CD8+ T cells generated during chronic infection, and high viral load at the time of vaccination might explain the inefficient responses to therapeutic vaccination (6, 8, 10–12). Thus, it is important to develop a therapeutic vaccine strategy to more effectively boost endogenous T cell responses to control persistent viral infections.
We have recently demonstrated that exhausted virus-specific CD8+ T cells up-regulate the inhibitory programmed death-1 (PD-1) during chronic infection with LCMV clone-13 (CL-13) (13). Furthermore, in vivo blockade of PD-1 restores the function of virus-specific CD8+ T cells, resulting in enhanced viral clearance. Similarly, virus-specific CD8+ T cells significantly up-regulate PD-1 expression during chronic infections such as HIV (14–17), HCV (18–20), and HBV in humans (21) and SIV in monkeys (22). This expression correlates with viral load in the blood plasma in HIV-infected patients (14, 16, 17) and SIV-infected monkeys (22, 23). Strikingly, blocking the interaction between PD-1 and its ligands in vitro partially restored effector function and improved the proliferative capacity of exhausted CD8+ T cells in these chronic infections (14–23). Collectively, these data suggest that PD-1 signaling on T cells is a major inhibitory pathway operating during chronic infection and blockade in vivo may be useful for the treatment of chronic viral infections.
In this study, we examined whether blockade of the PD-1 pathway in combination with therapeutic vaccination could enhance CD8+ T cell immunity and resolution of a chronic infection in mice. Mice that were persistently infected with LCMV CL-13 were vaccinated with a recombinant vaccinia virus expressing the LCMV gp33-41 epitope and treated with anti–PD-L1 blocking antibody. This combinatorial therapeutic vaccination synergistically enhanced epitope-specific CD8+ T cell responses and resulted in accelerated viral clearance. Responding CTL demonstrated increased cytokine production, increased expression of the interleukin-7 receptor-α (CD127) and decreased expression of PD-1, correlating with enhanced viral control. Moreover, therapeutic vaccination combined with PD-L1 blockade also enhanced CD8+ T cell responses and viral control in the absence of CD4+ T cells. Together, this provides a promising strategy for the treatment of chronic viral infections, including those that induce pronounced CD4+ T cell deficiency, such as HIV.
RESULTS
PD-L1 blockade synergizes with therapeutic vaccination to enhance T cell immunity and clearance of persistent viral infection
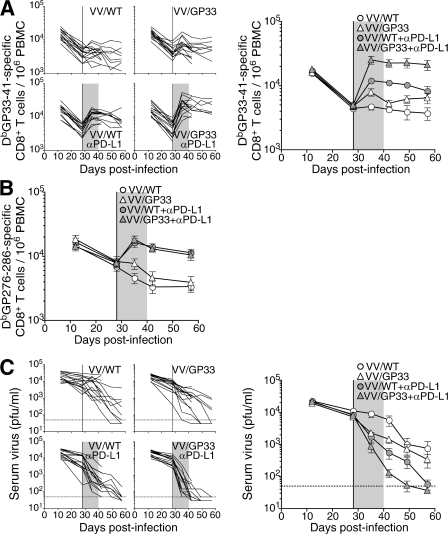
To determine the effect of therapeutic vaccination in combination with PD-L1 blockade during chronic infection, mice were infected with LCMV CL-13. 4 wk after infection, when viral loads were between ∼103 and 105 PFU/ml of serum, mice were vaccinated with a recombinant vaccinia virus expressing the LCMV gp33-41 CD8+ T cell epitope (VV/GP33). For the combinatorial therapeutic vaccination, mice were treated with anti–PD-L1 blocking antibody (αPD-L1) after vaccination. Control mice were vaccinated with WT vaccinia virus (VV/WT) with or without PD-L1 blockade. This experimental setting allowed us to follow antigen-specific CD8+ T cell responses to the therapeutic vaccine (gp33-specific) alongside responses to other LCMV epitopes not found within the vaccine vector (gp276-specific).
We longitudinally monitored individual mice for DbGP33- and DbGP276-specific T cell responses, as well as serum viral load in the blood after treatment. Consistent with our previous study (11), therapeutic vaccination (VV/GP33) induced little expansion of the DbGP33-specific CD8+ T cell population (Fig. 1 A; P > 0.05, 2 wk after vaccination) and no change in gp276 responses (Fig. 1 B), compared with control VV/WT vaccinated mice. PD-L1 blockade of control mice (VV/WT + αPD-L1) resulted in a significant increase of both DbGP33- and DbGP276-specific CD8+ T cell response (P < 0.01 in both, 2 wk after therapy), as previously reported (13). Importantly, combinatorial therapeutic vaccination (VV/GP33 + αPD-L1) induced a significant increase in the DbGP33-specific CD8+ T cell population (P < 0.01, 2 wk after therapy) over that in VV/WT + αPD-L1 mice (Fig. 1 A). This was sustained even after cessation of the αPD-L1 treatment. In contrast, DbGP276-specific CD8+ T cell responses were not enhanced after combinatorial therapeutic vaccination compared with PD-L1 blockade of VV/WT-treated mice. Thus, vaccination with VV/GP33 enhanced CD8+ T cell response specific to gp33, but not gp276, indicating epitope-specific responses to therapeutic vaccination (Fig. 1 B). Mice receiving combinatorial therapeutic vaccination showed accelerated clearance of virus from the blood compared with αPD-L1–treated mice vaccinated with VV/WT (P < 0.01, 2 wk and 3 wk after therapy; P < 0.05, 4 wk after vaccination; Fig. 1 C). Conversely, 75% of VV/WT- and 50% of VV/GP33-vaccinated mice remained viremic by 4 wk after vaccination. These results demonstrate that a combination of therapeutic vaccine and PD-L1 blockade synergistically enhanced expansion of CD8+ T cells and promoted clearance of persisting virus during chronic infection.
Figure 1.
Synergistic effect of PD-L1 blockade and therapeutic vaccination on T cell responses and viral control. LCMV CL-13–infected mice were vaccinated with wild-type vaccinia virus (VV/WT) or LCMV GP33-41 epitope-expressing vaccinia virus (VV/GP33) at 4 wk after infection (vertical line). Cohorts of mice were also treated for 12 d with αPD-L1, starting at 4 wk after infection (shaded region). The number of DbGP33-41 (A) and DbGP276-286 tetramer-positive cells (B) or viral titer (C) was determined in the blood at the indicated time points. The numbers of DbGP33-41 tetramer-specific CD8+ T cells and viral titers from individual mice are depicted on the left in A and C. Dashed lines represent virus detection limit. Results are pooled from three independent experiments.
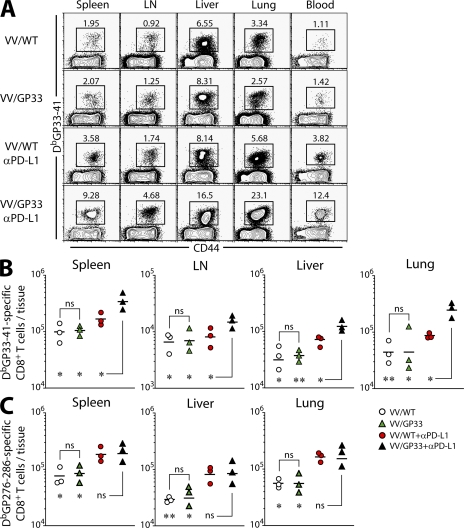
To examine whether combinatorial therapeutic vaccination resulted in increased responses in the tissues, we analyzed DbGP33-specific CD8+ T cell responses in different tissues 2 wk after vaccination. In nonlymphoid tissues, such as liver and lung, as well as in lymphoid tissues, including spleen and LN, the combination of PD-L1 blockade with therapeutic vaccination resulted in a significant increase in DbGP33-specific CD8+ T cells compared with control VV/WT vaccination, as well as VV/WT + αPD-L1 (P < 0.05 in all tissues examined; Fig. 2, A and B). VV/GP33 therapeutic vaccination alone did not increase numbers of DbGP33-specific CD8+ T cells in the tissues (P > 0.05), which is consistent with our previous study (11). Importantly, there was no comparable increase in DbGP276-specific CD8+ T cells between PD-L1 blockade and combinatorial therapeutic vaccine groups, confirming that the synergistic effect on expansion of CD8+ T cells was epitope specific (Fig. 2 C).
Figure 2.
Expansion of epitope-specific CD8+ T cells in multiple tissues. (A) The frequency and total number of DbGP33-41 (B) and DbGP276-286 (C) tetramer-positive cells in the indicated tissues at 2 wk after therapy. Data are representative of two independent experiments. n = 3 mice per group in each experiment. Results are pooled from two experiments. *, P < 0.05; **, P < 0.01.
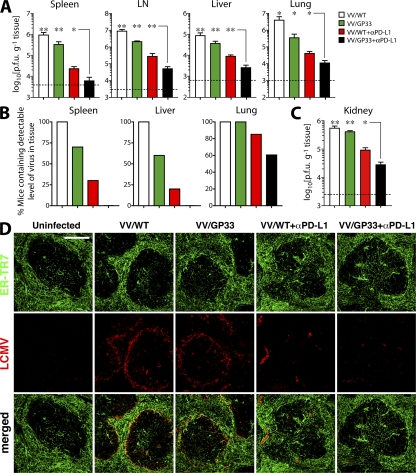
The aforementioned results suggested that PD-L1 blockade enhanced epitope-specific responses to therapeutic vaccination by rejuvenating circulating exhausted CD8+ T cells. Next, we assessed whether expansion of the DbGP33-specific CD8+ T cells after combinatorial therapeutic vaccination enhanced viral control in these tissues. Mice vaccinated with VV/GP33 alone demonstrated a reduction in viral titers in the tissues (Fig. 3 A; P < 0.05 in all tissues), despite no detectable increase in DbGP33-specific T cells in these mice (Fig. 2, A and B). This suggests that therapeutic vaccination may lead to qualitative changes to the antigen-specific CD8+ T cells. As expected, PD-L1 blockade of VV/WT-vaccinated mice improved viral control (Fig. 3 A; P < 0.05 in all tissues). Importantly, combinatorial therapeutic vaccination induced a greater reduction in viral load in tissues including spleen, liver, lung, and LN as early as 2 wk after vaccination compared with the other treatment groups (Fig. 3 A; P < 0.05 in all tissues). Moreover, the mice completely controlled viremia in spleen and liver within 4 wk after combinatorial therapeutic vaccination, whereas 70 and 60% of VV/GP33-vaccinated mice remained viremic in spleen and liver, respectively, at this time point (Fig. 3 B). We also measured viral titers at a later time point (14 wk after therapy) to confirm the efficacy of combined therapy. The kidney is a heavy reservoir of CL-13 infection and is a good target organ to monitor long-term persistence (3). There were high levels of virus in the kidney of CL-13–infected mice treated with control vaccine (Fig. 3 C). In contrast, mice given the combinatorial therapeutic vaccine showed a substantial decrease of virus (P < 0.01 and P < 0.05 compared with VV/WT and VV/WT + αPD-L1 groups, respectively), indicating that the efficacy of combination therapy is durable to a later time point and better than PD-L1 blockade alone.
Figure 3.
Enhanced viral control in tissues after combinatorial therapeutic vaccination. (A) Viral titers in the indicated tissues at 2 wk after therapy. Dashed lines represent virus detection limit. (B) Percentage of mice containing virus above detectable limit in different tissues at 4 wk after therapy. (C) Viral titers in the kidney at a later time point (14 wk after therapy). n = 6 mice per group. Results are pooled from two experiments. ns, P > 0.05; *, P < 0.05; **, P < 0.01. (D) Immunostaining of spleen with ERTR7 (green) and αLCMV antigens (red) at 2 wk after therapy. Bar, 100 μm.
To further examine the resolution of viremia after therapeutic vaccination, we performed immunostaining on tissue sections from the spleen at 2 wk after therapy (Fig. 3 D). LCMV CL-13 antigen was found in the marginal zones of the white pulp and associated with vasculature in the spleen after control VV/WT vaccination. We observed similar viral antigen staining after vaccination with VV/GP33, whereas PD-L1 blockade appeared to induce clearance from the splenic parenchyma, although antigen remained associated with vasculature. Moreover, the combination of PD-L1 blockade and therapeutic vaccination induced a noticeably greater reduction in viral antigen in the spleen. A similar pattern of viral antigen staining was also observed in the liver in the different treatment groups (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071949/DC1).
Functional and phenotypic changes in exhausted CD8+ T cells after combinatorial therapeutic vaccination
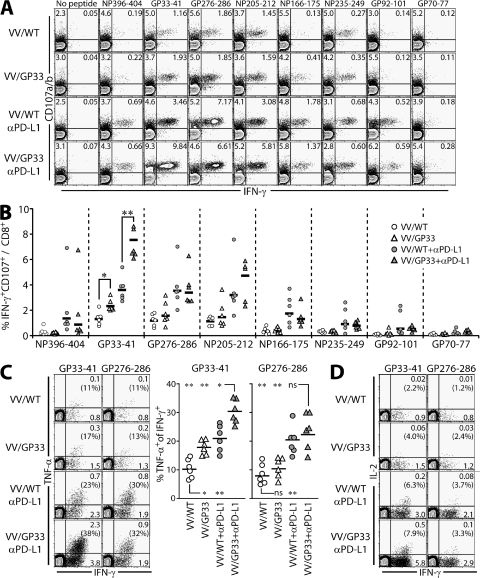
We next determined whether the combination of PD-L1 blockade with therapeutic vaccine improved the function of epitope-specific CD8+ T cells during persistent infection. We measured the ability of virus-specific CTLs to produce the cytokine IFN-γ and to degranulate by monitoring CD107a/b expression at 2 wk after therapy (Fig. 4, A and B). Although therapeutic vaccination alone enhanced few DbGP33-specific CD8+ T cell numbers (Fig. 2), IFN-γ production and CD107a/b expression by CD8+ T cells specific for GP33 was increased compared with control VV/WT-vaccinated mice (Fig. 4, A and B; P < 0.05). This indicates that therapeutic vaccination can improve CD8+ T cell function in an epitope-specific manner because IFN-γ production and CD107a/b expression by CD8+ T cells responding to other epitopes were not increased. PD-L1 blockade induced an increase in functional CD8+ T cells responding to multiple epitopes, as previously reported (13). Importantly, combinatorial therapeutic vaccination significantly increased the proportion of IFN-γ+ CD107a/b+ GP33-specific CD8+ T cells over that in all other groups (Fig. 4, A and B; P < 0.01). An increase in IFN-γ production responding to GP33 peptide appeared to be correlated to viral titer observed in tissues (Fig. 3), which is consistent with the study confirming the critical role of IFN-γ in reducing viral loads (24). No such increase was seen in CD8+ T cells responding to other epitopes (P > 0.05).
Figure 4.
Function of exhausted CD8+ T cells after therapeutic vaccination and PD-L1 blockade. (A) IFN-γ production and degranulation by CD8+ T cells in vaccinated mice at 2 wk after therapy. Splenocytes were stimulated with the indicated peptides in the presence of αCD107a/b antibodies, and then costained for IFN-γ. The cells shown in plots are gated on CD8+ T cells. (B) The percentages of IFN-γ+CD107+ CD8+ T cells specific for each of the LCMV peptides from A are summarized for multiple mice (n = 6 per group). TNF-α (C) and IL-2 (D) production by IFN-γ+ CD8+ T cells in vaccinated mice at 2 wk after therapy. Splenocytes were stimulated with GP33-41 or GP276-286 peptides. Percentages of cytokine-producing cells among CD8+ T cells are shown in the plots and numbers in parentheses on plots indicate the percentages of cytokine coproducers out of the total IFN-γ+ population. (C, right) Summarizes the data for multiple mice (n = 6 for each response). *, P < 0.05; **, P < 0.01.
To further determine whether combinatorial therapeutic vaccination improved CD8+ T cell function, we assessed the ability of IFN-γ–producing CD8+ T cells to produce TNF-α on a per cell basis (Fig. 4 C). After stimulation with GP33 peptide, but not with GP276 peptide, the population of TNF-α+ IFN-γ+ CD8+ T cells increased in VV/GP33 vaccinated mice compared with VV/WT mice (P < 0.05). PD-L1 blockade enhanced TNF-α production by GP33- and GP276-specific CD8+ T cells (P < 0.01). Combinatorial therapeutic vaccination further improved the ability of IFN-γ–producing CD8+ T cells to produce TNF-α in response to GP33 peptide (P < 0.05), but not to GP276 peptide (P > 0.05). In addition, we observed that IL-2 production specific to GP33 peptide was improved in the mice vaccinated with VV/GP33 + αPD-L1 (20- and 2.5-fold compared with VV/WT and VV/WT + αPD-L1, respectively), even though the percentage of IL-2/IFN-γ coproducers among IFN-γ–producing CD8+ T cells did not dramatically increase compared with that of PD-L1 blockade alone (Fig. 4 D). Together, these results demonstrate that the combination of therapeutic vaccination and PD-L1 blockade greatly improved CD8+ T cell function during chronic viral infection.
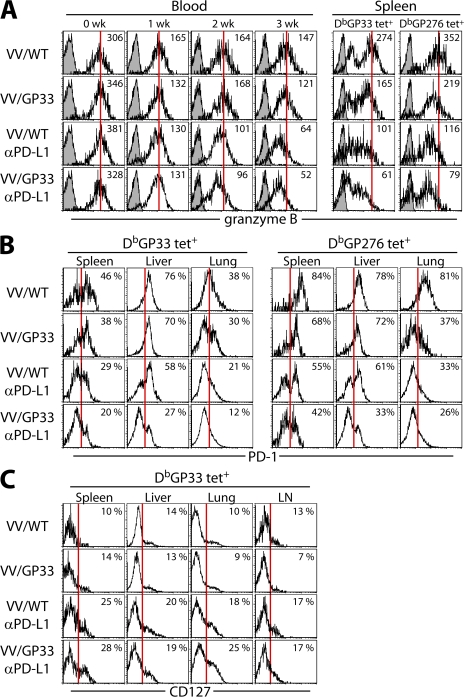
Granzyme B expression on DbGP33+ CD8+ T cells in the blood was gradually down-regulated in control VV/WT-vaccinated mice as serum viremia decreased (Fig. 5 A). The MFI of granzyme B was lower in both blood and spleen in mice treated with αPD-L1, with or without therapeutic vaccination (P < 0.01). This decreased granzyme B expression was observed on DbGP276+, as well as DbGP33+, CD8+ T cells after PD-L1 blockade alone and combinatorial therapeutic vaccination (Fig. 5 A). Importantly, PD-1 expression on GP33-specific CD8+ T cells was significantly down-regulated in the spleen, liver, and lung in mice treated with the combinatorial vaccine, in comparison with the other groups (Fig. 5 B). Decreased level of PD-1 after combinatorial therapeutic vaccination was also observed on DbGP276+ CD8+ T cells (Fig. 5 B). Collectively, reduced expression of granzyme B and PD-1 tightly correlated with reduced viral titer after combinatorial therapeutic vaccination (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071949/DC1).
Figure 5.
Phenotypic changes in antigen-specific CD8+ T cells after combinatorial therapeutic vaccination. (A) Expression of granzyme B on DbGP33-41 tetramer-positive cells in PBMC at the indicated times after therapy and on DbGP33-41 or DbGP276-286 tetramer-positive cells in spleen at 4 wk after therapy. Open histograms, granzyme B; filled histograms, isotype control. Numbers represent mean fluorescence intensity of granzyme B expression. PD-1 expression on DbGP33-41 or DbGP276-286 tetramer-positive cells (B) and CD127 expression on DbGP33-41 tetramer-positive cells (C) in different tissues at 4 wk after therapy. Numbers represent the frequency of tetramer-positive cells expressing CD127 or PD-1. The data are representative of two independent experiments.
Exhausted CD8+ T cells generated during persistent infection display a very different phenotype from long-lived memory cells. Chronically stimulated CD8+ T cells down-regulate IL-7Rα (CD127), which is indicative of reduced responsiveness to prosurvival/homeostatic signals delivered by IL-7 (16, 19, 20, 25–27). We observed increased expression of CD127 on a population of DbGP33+ CD8+ T cells in the spleen after αPD-L1 treatment (Fig. 5 C; P < 0.05). The combination of therapeutic vaccine with PD-L1 blockade did not further increase this CD127hi population. We also found that CD127 expression in other tissues including liver, lung, and LN increased after PD-L1 blockade regardless of therapeutic vaccination. However, combinatorial therapeutic vaccination did not induce CD62L expression on these cells (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20071949/DC1).
Efficacy of combinatorial therapeutic vaccination in the absence of CD4+ T cells
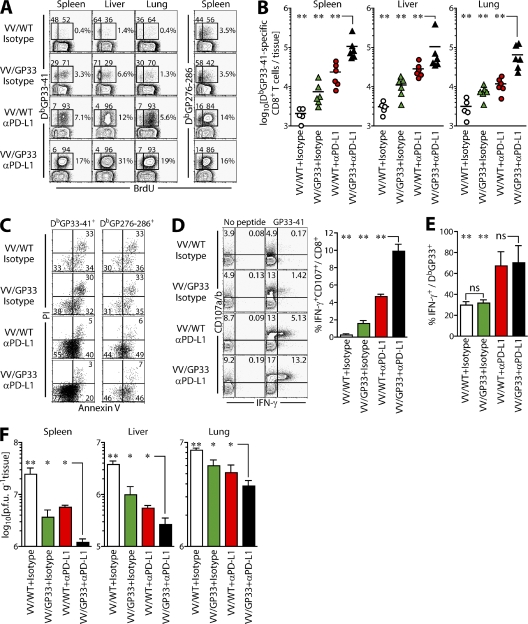
Under such “helpless” conditions, chronic CL-13 infection leads to more pronounced exhaustion of antigen-specific CD8+ T cells and is relevant to persistent infections, such as HIV in humans. We examined whether the combination of therapeutic vaccination and PD-L1 blockade improved CD8+ T cell responses under conditions of CD4+ T cell deficiency. In our CD4 helpless model, CD4+ T cells are only transiently depleted (>99%) using αCD4 antibody (GK1.5) at the time of infection, and CD4+ T cell numbers return to nearby normal levels within a month after infection. However, although total numbers of CD4+ T cells are restored at a later time point after infection with CL-13, the virus-specific CD4+ T cell response is severely impaired compared with memory CD4+ T cell responses after LCMV acute infection, and is hardly recovered even after PD-L1 blockade (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20071949/DC1), showing that this model provides a situation where CD4+ T cell help is very restricted for a long period of time. When CD4+ T cell help was absent, therapeutic vaccination with VV/GP33 alone induced expansion of epitope-specific CD8+ T cells (Fig. 6, A and B; P < 0.01). Importantly, combinatorial therapeutic vaccination further increased the frequency of DbGP33-specific CD8+ T cells in helpless mice at least twofold compared with therapeutic vaccination or PD-L1 blockade alone, in both lymphoid and nonlymphoid tissues (Fig. 6, A and B). The number of DbGP33+ CD8+ T cells was approximately sixfold higher in lung and threefold higher in spleen and liver, respectively, after combinatorial therapeutic vaccination (Fig. 6 B; P < 0.001). However, there was no difference in the frequency of DbGP276-specific CD8+ T cells between combinatorial therapeutic vaccination and αPD-L1 treatment, indicating epitope-specific CD8+ T cell expansion by combinatorial therapeutic vaccination (Fig. 6 A; P > 0.05).
Figure 6.
Combinatorial therapeutic vaccination enhances CD8+ T cell responses in the absence of CD4+ T cells. Mice were depleted of CD4+ T cells, infected with LCMV CL-13, and vaccinated with VV/WT or VV/GP33 at 7–10-wk after infection, with or without αPD-L1 treatment. 2 wk after treatment, mice were killed for analysis. (A) BrdU incorporation in different tissues. The ratio of BrdU-negative and -positive cells on DbGP33-41 tetramer-positive cells and the frequency of tetramer-positive cells are shown on plots. (B) Total number of DbGP33-41 tetramer-positive cells in different tissues. (C) Live/dead analysis of DbGP33-41 tetramer-positive cells in spleen by Annexin V and PI staining. (D) IFN-γ and CD107 expression by GP33-specific CD8+ T cells in the spleen after treatment. (E) Proportion GP33-specific CD8+ T cells producing IFN-γ in the spleen. (F) Viral titers in the indicated tissues. All plots are representative of two experiments and summarized results are pooled from two experiments (n = 6 mice per group). *, P < 0.05; **, P < 0.01.
To examine in detail how combinatorial therapeutic vaccination increased the epitope-specific CD8+ T cell population, we measured the proliferation of antigen-specific CD8+ T cells after therapeutic vaccination by BrdU incorporation (Fig. 6 A). After PD-L1 blockade, >90% of DbGP33+ and 80% of DbGP276+ cells incorporated BrdU, as previously reported (13). Combinatorial vaccination also induced a similar level of BrdU incorporation. These data suggested that the exhausted CD8+ T cells proliferated after PD-L1 blockade, and that this proliferation might be accelerated by simultaneous therapeutic vaccination resulting in more DbGP33-specific CD8+ T cells (Fig. 6, A and B).
Increased expression of PD-1 on CD8+ T cells during chronic infection is also known to be associated with susceptibility to apoptosis upon antigen-specific TCR stimulation (22, 28). We found that PD-L1 blockade reduced the frequency of Annexin V+ propidium iodide (PI)+ apoptotic cells (P < 0.01), as well as cells in the early stages of apoptosis (Annexin V+ PI−, P < 0.02) and increased numbers of viable Annexin V− PI− cells (Fig. 6 C). This indicated that this treatment enhanced the survival of the antigen-specific CD8+ T cells. Interestingly, this increase in viable (annexin V− PI−) cells was further increased among DbGP33+ CD8+ T cells, but not DbGP276+ CD8+ T cells, in mice receiving combinatorial therapy, compared with mice treated with αPD-L1 alone (Fig. 6 C; P < 0.02). These data suggest that the increased population of epitope-specific CD8+ T cells after combinatorial therapeutic vaccination may result from both increased proliferation and decreased susceptibility to cell death.
We next measured the effect of combinatorial therapeutic vaccination on the ability of epitope-specific CD8+ T cells to produce IFN-γ and to induce degranulation in the absence of CD4+ T cell help. A significant increase of IFN-γ production and CD107a/b expression was observed after combinatorial therapeutic vaccination (Fig. 6 D). Interestingly, increased degranulation was also observed in mice treated with VV/GP33 or PD-L1 blockade alone, although to a lesser extent. However, combinatorial therapy did not further improve IFN-γ production by DbGP33+ CD8+ T cells when compared with PD-L1 blockade alone (Fig. 6 E), unlike that in the presence of CD4+ T cell help (Fig. 4 C). Similarly, therapeutic vaccination did not increase IFN-γ production by CD8+ T cells compared with control vaccination. Nevertheless, the clearance of virus in these mice correlated with their degranulation capacity. Thus, we observed a significant reduction in virus titer in the spleen, liver, and lung after VV/GP33 + αPD-L1 treatment compared with therapeutic vaccination or PD-L1 blockade alone (Fig. 6 F; P < 0.05 in multiple tissues). These results demonstrate that the combination of therapeutic vaccination and PD-L1 blockade more efficiently enhanced CD8 + T cell responses and viral control than PD-L1 blockade alone, both in CD4+ T cell help–sufficient and –deficient hosts.
Effect of therapeutic vaccination on antigen presentation by dendritic cells
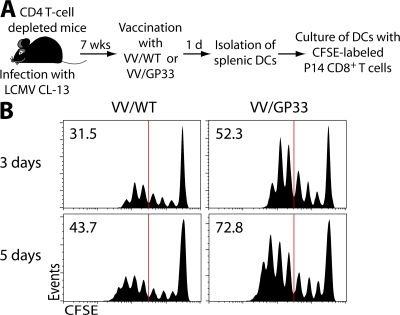
Mice chronically infected with LCMV CL-13 contain high levels of virus in multiple tissues, and there is clearly sufficient antigen in these mice to drive virus-specific T cell responses (29), especially after blockade of the PD-1–PD-L1 pathway (13). It is unlikely that the amount of gp33-41 epitope expressed in vivo by the recombinant VV/GP33 would exceed the total amount of gp33-41 epitope present in the chronically infected mice. This raised the interesting question as to how therapeutic vaccination can provide synergism with PD-L1 blockade even if therapeutic vaccination itself seems to be ineffective. Because CD8+ T cells specific for LCMV epitopes that were not expressed by the vaccine (i.e., DbGP276-286 tetramer-positive cells) were not boosted after therapeutic vaccination, as shown in Fig. 6 A, it is likely that vaccine-induced inflammation alone is not sufficient to enhance the CD8+ T cell response. Thus, we hypothesized that APCs after therapeutic vaccination can present antigen more efficiently compared with the APCs present in the chronically infected mice. To test this, we determined whether antigen presentation by APCs is improved after therapeutic vaccination during chronic infection. DCs were isolated from spleens of LCMV CL-13–infected mice vaccinated with VV/WT or VV/GP33 and co-cultured with CFSE-labeled naive P14 CD8+ T cells (DbGP33-41–specific; Fig. 7 A). DCs from mice given the therapeutic vaccine (VV/GP33) were able to induce more proliferation of P14 cells compared with DCs from the chronically infected mice given VV/WT (Fig. 7 B). These data demonstrate that DCs generated after therapeutic vaccination could present antigen more efficiently than the DCs present in the CL-13–infected mice. These results suggest that the mechanism of synergism between therapeutic vaccination and PD-L1 blockade could be that DCs can efficiently present epitopes encoded by therapeutic vaccination and subsequently reinforce the proliferation of antigen-specific CD8+ T cells restored by αPD-L1 treatment.
Figure 7.
Improved antigen presentation after therapeutic vaccination of chronically infected mice. (A) Mice chronically infected with LCMV CL-13 (7 wk after infection) were vaccinated with VV/WT or VV/GP33. 1 d later, DCs were isolated from spleens of both groups of mice and co-cultured with CFSE-labeled P14 Thy1.1+CD8+ T cells. (B) Proliferation of P14 cells was analyzed at day 3 (top) and 5 (bottom) after co-culture. Data are representative from three different samples. The percentage of cells showing more than four divisions is indicated in the top left corner of each histogram.
DISCUSSION
The goal of therapeutic vaccination is to enhance adaptive immune responses during persistent infection and to reduce the viral burden. However, such strategies are often hampered by the reduced responsiveness and low proliferative potential of antigen-specific T cells (11), suggesting the need for alternative strategies to enhance immune function during chronic infection. In this study, we evaluated the efficacy of therapeutic vaccination in combination with blockade of the inhibitory PD-1 pathway, which plays a role in T cell exhaustion during chronic viral infection. Administration of a therapeutic vaccine in combination with PD-L1 blockade enhanced expansion and improved the function of epitope-specific CD8+ T cells in mice persistently infected with LCMV CL-13. This combinatorial therapeutic vaccination accelerated viral control compared with therapeutic vaccine only or PD-L1 blockade alone. These results define a novel strategy for effective therapeutic vaccination of chronic viral infections.
When viral load is high and T cell dysfunction is severe, therapeutic vaccination alone is unlikely to be highly beneficial (11). Indeed, poor responses have been reported for the therapeutic vaccination of human HBV carriers (8). Conversely, at lower viral loads, when T cell exhaustion is less severe, therapeutic vaccination may be more effective (11). Studies with nonhuman primates support this concept because therapeutic vaccination is more effective after antiviral therapy of SIV replication (30, 31). Although suppression of chronic viral replication using antiviral drugs causes a corresponding increase of T cell activity, it can involve drug toxicity, emergence of drug resistance mutations, and rebounding viremia after cessation of antiviral therapy (32–34). In these circumstances, our approach using αPD-L1 might minimize a deleterious effect on T cells, maximizing a therapeutic effect. As shown in our results, therapeutic vaccination most efficiently boosted epitope-specific CD8+ T cells when combined with PD-L1 blockade. Expansion of epitope-specific CD8+ T cells and accelerated control of virus were also observed in multiple tissues in epitope-specific manner, indicating that the effectiveness of combinatorial therapeutic vaccination is valid systemically. Such an approach could be more effective in reducing viral load when multiple epitopes are introduced to therapeutic vaccine vector.
Recent studies of HIV infection showed that PD-1 is significantly up-regulated on HIV-specific CD8+ T cells in blood (14–17). Furthermore, the proportion of CD8+ T cells expressing PD-1 and the levels of PD-1 on HIV-specific T cells strongly correlated with viral load in the blood plasma (14, 16). Antiretroviral treatment resulted in the dramatic decline of plasma viral load, coincident with a decrease in the level of PD-1 expression on HIV-specific CD8+ T cells (14, 16). Similarly, Urbani et al. showed that although PD-1 declined on HCV-specific CD8+ T cells during recovery, its expression remained high when HCV persisted (20). Thus, PD-1 expression level appears to be a good indicator of viral load and disease progression during persistent infection. In this study, we show decreased PD-1 expression on antigen-specific CD8+ T cells after combinatorial therapeutic vaccination, which correlated with viral control. PD-1 was also down-regulated on DbGP276+ as well as DbGP33+ CD8+ T cells, suggesting that down-regulation of PD-1 occurred as a consequence of viral control. Nevertheless, PD-1 expression on gp33-specific CD8+ T cells in different tissues was not always proportional to virus titer, suggesting that other factors in the tissue environment may also affect PD-1 expression on T cells.
PD-1 expression is also associated with altered survival and expansion of HIV-specific cells (14–16). We show that combinatorial therapeutic vaccination induced lower numbers of Annexin V+ gp33-specific T cells, suggesting reduced susceptibility to apoptosis and enhanced survival. During chronic LCMV infection, antigen-specific CD8+ T cells demonstrate reduced responses to the homeostatic cytokine IL-7, and an increased dependence upon antigenic signals for survival (29). Recent studies also demonstrate that down-regulation of CD127 occurs on LCMV-, HIV-, and HCV-specific CD8+ T cells (19, 27, 35). Interestingly, CD127 was found to be up-regulated on a population of antigen-specific CD8+ T cells after PD-L1 blockade and combinatorial therapeutic vaccination, suggesting that CD8+ T cell differentiation was altered after treatment. Functional programming of T cells during persistent infection appears to be an ongoing event, rather than restricted to signals acquired during priming (36). Collectively, our data suggest that combinatorial therapeutic vaccination generated CD8+ T cells with improved phenotype and function.
We also demonstrate that DCs generated after therapeutic vaccination with VV/GP33 provide critical positive signals that can now synergize with blocking PD-1-mediated negative signals. It is worth noting that these positive signals from DCs require the generation of new peptides from the therapeutic vaccine and are not simply caused by some inflammation induced by the VV infection because (a) the response specific to DbGP276-286 epitope was not changed after VV/GP33 therapeutic vaccination and (b) LCMV-specific CD8+ T cells were not affected by vaccination with wild-type vaccinia virus. Indeed, there was no difference in the levels of inflammatory cytokines (i.e., TNF-α, IFN-γ, MCP-1, and IL-10) after vaccination with VV/WT or VV/GP33 (unpublished data).
Though therapeutic vaccination alone is not sufficient to boost the ongoing CD8+ T cell responses caused by the abundance of immunosuppressive signals, blockade of such signals may be enough to tip the balance toward positive amplification of the response. Based on this original concept, it would be interesting to block other immunosuppressive factors in combination with therapeutic vaccination. IL-10 blockade is one potential candidate because IL-10 is elevated during LCMV, HIV, HCV, and HBV infections (37–41) and its blockade can improve viral clearance (42, 43). We also observed that IL-10 mRNA levels are elevated around twofold in the mice chronically infected and vaccinated, compared with the naive mice (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20071949/DC1), suggesting the possibility to increase the efficacy of therapeutic vaccine when combined with blocking IL-10 signaling. Indeed, Brooks et al. (on p. 533 of this issue) demonstrates that neutralization of immunosuppressive IL-10 signals can also significantly enhance the efficacy of therapeutic vaccination (44). Thus, their study, along with ours, establishes that blocking immune inhibitory signals might overcome the failure of current therapeutic vaccination strategies to treat persistent viral infections. Interestingly, in vivo PD-L1 blockade does not seem to affect IL-10 level during chronic infection (Fig. S5), even though PD-1/PD-L1 blockade has been reported to down-regulate IL-10 but up-regulate IL-2 and IFN-γ during in vitro stimulation (45, 46). These data suggest that inherent suppression on exhausted T cells by PD-1 and environmental suppression by IL-10 might be regulated independently. Thus, it would be interesting to test the effect of synergism between PD-1 blockade and IL-10 blockade on the induction of CD8+ T cell response during chronic infection.
In summary, our study shows that therapeutic vaccination during chronic viral infection, in combination with PD-L1 blockade, synergistically boosts epitope-specific CD8+ T cell responses and promotes viral control. These experiments demonstrate that blocking this major inhibitory pathway during chronic infection improves the efficacy of therapeutic vaccination. This strategy may prove useful for the treatment of persistent infections, in general, as well as to improve immune responses to tumors.
MATERIALS AND METHODS
Mice and infections.
4–6-wk-old female C57BL/6 mice were purchased from The Jackson Laboratory. For infection, mice received 2 × 106 PFU of LCMV CL-13 i.v. LCMV titers were determined by plaque assays, as described previously (11). All mice were used in accordance with National Institutes of Health and the Emory University Institutional Animal Care and Use Committee guidelines.
In vivo antibody blockade and therapeutic vaccination.
For therapeutic vaccination, 2 × 106 PFU of VV/GP33 as therapeutic vaccine or VV/WT as control vaccine was given i.p., as described previously (11). For PD-L1 blockade, 200 μg of rat anti–mouse PD-L1 antibody (10F:9G2) or rat IgG2b isotype control were administered i.p. 5 times every 3 d, beginning on the day of vaccination. For depletion of CD4+ T cells, mice were given 500 μg of anti–mouse CD4 antibody (GK1.5; BioExpress) i.p. on days −1 and 0 after infection with CL-13.
Cell isolation and in vitro proliferation assay.
Lymphocytes were isolated from tissues including spleen, liver, lung, inguinal LN, and blood, as previously described (13). Liver and lung were perfused with ice-cold PBS before removal for lymphocyte isolation. For DC isolation, spleen was digested in complete media containing 1 mg/ml collagenase type II (Worthington Biochemicals) and 1 mg/ml DNase (Sigma-Aldrich) as previously described (47). DCs were enriched using CD11c beads (Miltenyi Biotec). To measure the ability of antigen presentation by DCs, 5 × 105 P14 Thy1.1+ CD8+ T cells labeled with CFSE were cultured with 2.5 × 104 DCs. Proliferation was determined by flow cytometry after 3 or 5 d in culture.
Antibodies and flow cytometry.
MHC class I tetramers were generated and used as previously described (3). All antibodies were obtained from BD Biosciences except for granzyme B (Caltag Laboratories) and CD127 (eBioscience). All surface and intracellular cytokine staining was performed as previously described (3). To detect degranulation, splenocytes were stimulated for 5 h in the presence of brefeldin, monensin, anti–CD107a-FITC, and anti–CD107b-FITC. For in vivo BrdU incorporation, CL-13–infected mice were fed 1 mg/ml BrdU in their drinking water every day for 2 wk from the time point of therapeutic vaccination. Intracellular staining with a BrdU-specific antibody was performed as previously described (48). For analysis of direct ex vivo apoptosis, splenocytes were isolated and briefly incubated with Annexin V and PI as previously described (48).
Confocal microscopy.
Spleens were removed from mice and frozen in OCT (TissueTek). 20-μm cryostat sections were fixed in ice-cold acetone for 10 min. Sections were stained with ER-TR7 antibody (Biogenesis) and goat anti–rat Alexa Fluor 488 (Invitrogen) to detect reticular cells, and with polyclonal anti-LCMV guinea pig sera plus goat anti–guinea pig Alexa Fluor 568 (Invitrogen). Sections were analyzed by confocal microscopy (LSM510 Meta; Carl Zeiss, Inc.). Images were prepared using ImageJ (National Institutes of Health) and Photoshop (Adobe).
Quantitative real-time RT-PCR.
Sequences of PCR primer pairs for IL-10 and HPRT are as follows: IL-10 (forward, 5′-GGTTGCCAAGCCTTATCGGA-3′; reverse, 5′-ACCTGCTCCACTGCCTTGCT-3′) and HPRT (forward: 5′-AGGTTGCAAGCTTGCTGGT-3′; reverse, 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′). Total RNA were extracted from whole spleens using RNeasy mini kit (QIAGEN), and then reverse transcribed by Superscript II (Invitrogen). Real-time quantitative RT-PCR for IL-10 mRNA using SYBR Green (Applied Biosystems) and detected using an iCycler (Bio-Rad Laboratories) as previously described (47). IL-10 expression was quantified, using HPRT as a reference, by the comparative CT method, as per the manufacturer's instructions (Applied Biosystems).
Statistical analysis.
Statistical analysis was performed with two-tailed unpaired Student's t tests using Prism (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 provides immunostaining data of liver with ERTR7 (green) and αLCMV antigens (red) at 2 wk after therapy. Fig. S2 displays a correlation between virus titer and granzyme B/PD-1 expression. Fig. S3 shows CD62L expression on antigen-specific CD8+ T cells after combinatorial therapeutic vaccination. Fig. S4 shows that there is no induction of antigen-specific CD4+ T cell immune response during chronic infection when CD4+ T cells are depleted at the same time of infection. Fig. S5 demonstrates no change of IL-10 mRNA level after therapeutic vaccination and/or αPD-L1 treatment. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071949/DC1.
Supplemental Material
Acknowledgments
We thank for B. T. Konieczny and Y. Blinder for technical assistance and members of the Ahmed laboratory for helpful discussions.
This work was supported by funding from the Foundation for the National Institutes of Health (NIH) through the Grand Challenges in Global Health Initiative and NIH grants AI30048 and AI56299 (to G.J. Freeman, A.H. Sharpe, and R. Ahmed) and a postdoctoral fellowship from Korean Engineering and Science Foundation (to S.-J. Ha) and a CJ Martin fellowship from the National Health and Medical Research Council of Australia (to S.N. Mueller).
The authors have no conflicting financial interests.
Abbreviations used: HBV, hepatitis B virus; HCV, hepatitis C virus; LCMV, lymphocytic choriomeningitis virus; PD, programmed death; PI, propidium iodide; SIV, simian immunodeficiency virus.
E.J. Wherry's present address is Immunology Program, The Wistar Institute, Philadelphia, PA 19104.
D.L. Barber's present address is Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious diseases, National Institutes of Health, Bethesda, MD 20892, USA.
References
- 1.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879. [DOI] [PubMed] [Google Scholar]
- 2.Wherry, E.J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi, R.T., and B.D. Walker. 2002. Promises and pitfalls in the reconstitution of immunity in patients who have HIV-1 infection. Curr. Opin. Immunol. 14:487–494. [DOI] [PubMed] [Google Scholar]
- 5.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229. [DOI] [PubMed] [Google Scholar]
- 6.von Herrath, M.G., D.P. Berger, D. Homann, T. Tishon, A. Sette, and M.B. Oldstone. 2000. Vaccination to treat persistent viral infection. Virology. 268:411–419. [DOI] [PubMed] [Google Scholar]
- 7.Lindenburg, C.E., I. Stolte, M.W. Langendam, F. Miedema, I.G. Williams, R. Colebunders, J.N. Weber, M. Fisher, and R.A. Coutinho. 2002. Long-term follow-up: no effect of therapeutic vaccination with HIV-1 p17/p24:Ty virus-like particles on HIV-1 disease progression. Vaccine. 20:2343–2347. [DOI] [PubMed] [Google Scholar]
- 8.Dikici, B., A.G. Kalayci, F. Ozgenc, M. Bosnak, M. Davutoglu, A. Ece, T. Ozkan, T. Ozeke, R.V. Yagci, and K. Haspolat. 2003. Therapeutic vaccination in the immunotolerant phase of children with chronic hepatitis B infection. Pediatr. Infect. Dis. J. 22:345–349. [DOI] [PubMed] [Google Scholar]
- 9.Nevens, F., T. Roskams, H. Van Vlierberghe, Y. Horsmans, D. Sprengers, A. Elewaut, V. Desmet, G. Leroux-Roels, E. Quinaux, E. Depla, et al. 2003. A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology. 38:1289–1296. [DOI] [PubMed] [Google Scholar]
- 10.Maini, M.K., C. Boni, G.S. Ogg, A.S. King, S. Reignat, C.K. Lee, J.R. Larrubia, G.J. Webster, A.J. McMichael, C. Ferrari, et al. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 117:1386–1396. [DOI] [PubMed] [Google Scholar]
- 11.Wherry, E.J., J.N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nisii, C., M. Tempestilli, C. Agrati, F. Poccia, G. Tocci, M.A. Longo, G. D'Offizi, R. Tersigni, O. Lo Iacono, G. Antonucci, and A. Oliva. 2006. Accumulation of dysfunctional effector CD8+ T cells in the liver of patients with chronic HCV infection. J. Hepatol. 44:475–483. [DOI] [PubMed] [Google Scholar]
- 13.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 14.Day, C.L., D.E. Kaufmann, P. Kiepiela, J.A. Brown, E.S. Moodley, S. Reddy, E.W. Mackey, J.D. Miller, A.J. Leslie, C. DePierres, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354. [DOI] [PubMed] [Google Scholar]
- 15.Petrovas, C., J.P. Casazza, J.M. Brenchley, D.A. Price, E. Gostick, W.C. Adams, M.L. Precopio, T. Schacker, M. Roederer, D.C. Douek, and R.A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trautmann, L., L. Janbazian, N. Chomont, E.A. Said, S. Gimmig, B. Bessette, M.R. Boulassel, E. Delwart, H. Sepulveda, R.S. Balderas, et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, J.Y., Z. Zhang, X. Wang, J.L. Fu, J. Yao, Y. Jiao, L. Chen, H. Zhang, J. Wei, L. Jin, et al. 2007. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 109:4671–4678. [DOI] [PubMed] [Google Scholar]
- 18.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 45:588–601. [DOI] [PubMed] [Google Scholar]
- 19.Radziewicz, H., C.C. Ibegbu, M.L. Fernandez, K.A. Workowski, K. Obideen, M. Wehbi, H.L. Hanson, J.P. Steinberg, D. Masopust, E.J. Wherry, et al. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, et al. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovas, C., D.A. Price, J. Mattapallil, D.R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, et al. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 110:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velu, V., S. Kannanganat, C. Ibegbu, L. Chennareddi, F. Villinger, G.J. Freeman, R. Ahmed, and R.R. Amara. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 81:5819–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutboul, F., D. Puthier, V. Appay, O. Pelle, H. Ait-Mohand, B. Combadiere, G. Carcelain, C. Katlama, S.L. Rowland-Jones, P. Debre, et al. 2005. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 19:1981–1986. [DOI] [PubMed] [Google Scholar]
- 26.Wherry, E.J., D.L. Barber, S.M. Kaech, J.N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 101:16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wherry, E.J., C.L. Day, R. Draenert, J.D. Miller, P. Kiepiela, T. Woodberry, C. Brander, M. Addo, P. Klenerman, R. Ahmed, and B.D. Walker. 2006. HIV-specific CD8 T cells express low levels of IL-7Ralpha: implications for HIV-specific T cell memory. Virology. 353:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migliaccio, M., P.M. Alves, P. Romero, and N. Rufer. 2006. Distinct mechanisms control human naive and antigen-experienced CD8+ T lymphocyte proliferation. J. Immunol. 176:2173–2182. [DOI] [PubMed] [Google Scholar]
- 29.Shin, H., S.D. Blackburn, J.N. Blattman, and E.J. Wherry. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hel, Z., D. Venzon, M. Poudyal, W.P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J.D. Altman, D. Watkins, et al. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6:1140–1146. [DOI] [PubMed] [Google Scholar]
- 31.Tryniszewska, E., J. Nacsa, M.G. Lewis, P. Silvera, D. Montefiori, D. Venzon, Z. Hel, R.W. Parks, M. Moniuszko, J. Tartaglia, et al. 2002. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J. Immunol. 169:5347–5357. [DOI] [PubMed] [Google Scholar]
- 32.Verrier, B. 2005. Therapeutic vaccination for chronic infectious diseases: lessons from HIV-1. J. Clin. Virol. 34(Suppl 1):S9–S12. [DOI] [PubMed] [Google Scholar]
- 33.Huang, Z., M.G. Murray, and J.A. Secrist III. 2006. Recent development of therapeutics for chronic HCV infection. Antiviral Res. 71:351–362. [DOI] [PubMed] [Google Scholar]
- 34.Michel, M.L., and M. Mancini-Bourgine. 2005. Therapeutic vaccination against chronic hepatitis B virus infection. J. Clin. Virol. 34(Suppl 1):S108–S114. [DOI] [PubMed] [Google Scholar]
- 35.Golden-Mason, L., J.R. Burton Jr., N. Castelblanco, J. Klarquist, S. Benlloch, C. Wang, and H.R. Rosen. 2006. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 44:1098–1109. [DOI] [PubMed] [Google Scholar]
- 36.Brooks, D.G., D.B. McGavern, and M.B. Oldstone. 2006. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brady, M.T., A.J. MacDonald, A.G. Rowan, and K.H. Mills. 2003. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 33:3448–3457. [DOI] [PubMed] [Google Scholar]
- 38.Cacciarelli, T.V., O.M. Martinez, R.G. Gish, J.C. Villanueva, and S.M. Krams. 1996. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 24:6–9. [DOI] [PubMed] [Google Scholar]
- 39.Clerici, M., C. Balotta, A. Salvaggio, C. Riva, D. Trabattoni, L. Papagno, A. Berlusconi, S. Rusconi, M.L. Villa, M. Moroni, and M. Galli. 1996. Human immunodeficiency virus (HIV) phenotype and interleukin-2/ interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood. 88:574–579. [PubMed] [Google Scholar]
- 40.Dolganiuc, A., K. Kodys, A. Kopasz, C. Marshall, T. Do, L. Romics Jr., P. Mandrekar, M. Zapp, and G. Szabo. 2003. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J. Immunol. 170:5615–5624. [DOI] [PubMed] [Google Scholar]
- 41.Rico, M.A., J.A. Quiroga, D. Subira, S. Castanon, J.M. Esteban, M. Pardo, and V. Carreno. 2001. Hepatitis B virus-specific T-cell proliferation and cytokine secretion in chronic hepatitis B e antibody-positive patients treated with ribavirin and interferon alpha. Hepatology. 33:295–300. [DOI] [PubMed] [Google Scholar]
- 42.Brooks, D.G., M.J. Trifilo, K.H. Edelmann, L. Teyton, D.B. McGavern, and M.B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ejrnaes, M., C.M. Filippi, M.M. Martinic, E.M. Ling, L.M. Togher, S. Crotty, and M.G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks, D.G., A.M. Lee, H. Elsaesser, D.B. McGavern, and M. Oldstone. 2008. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 205:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, L., Z. Zhang, W. Chen, Z. Zhang, Y. Li, M. Shi, J. Zhang, L. Chen, S. Wang, and F.S. Wang. 2007. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J. Immunol. 178:6634–6641. [DOI] [PubMed] [Google Scholar]
- 46.Curiel, T.J., S. Wei, H. Dong, X. Alvarez, P. Cheng, P. Mottram, R. Krzysiek, K.L. Knutson, B. Daniel, M.C. Zimmermann, et al. 2003. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9:562–567. [DOI] [PubMed] [Google Scholar]
- 47.Mueller, S.N., K.A. Hosiawa-Meagher, B.T. Konieczny, B.M. Sullivan, M.F. Bachmann, R.M. Locksley, R. Ahmed, and M. Matloubian. 2007. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 317:670–674. [DOI] [PubMed] [Google Scholar]
- 48.Grayson, J.M., L.E. Harrington, J.G. Lanier, E.J. Wherry, and R. Ahmed. 2002. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J. Immunol. 169:3760–3770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.