Abstract
The role of matricellular proteins in bacterial containment and in the induction of pathogen-specific adaptive immune responses is unknown. We studied the function of the matricellular protein secreted protein, acidic and rich in cysteine (SPARC/osteonectin) in the dissemination of locally injected Salmonella typhimurium and in the subsequent immune response. We show that SPARC was required for the development of organized acute inflammatory reactions with granuloma-like (GL) features and for the control of bacterial spreading to draining lymph nodes (DLNs). However, SPARC-related GL also inhibited dendritic cell (DC) migration to the DLNs and limited the development of adaptive immune response, thus conferring increased susceptibility to the pathogen. In SPARC-deficient mice, both DC migration and antigen-specific responses were restored against bacteria, leading to protective anti–S. typhimurium immunity. This highlights a new function of matricellular proteins in bacterial infection and suggests that initial containment of bacteria can have drawbacks.
Mycobacterium tuberculosis and Salmonella spp. have exploited their ability to grow intracellularly to avoid recognition and killing by circulating antibodies and the complement system. Thus, the main protective response toward intracellular bacteria is provided by cell-mediated immunity; soon after infection, a strong inflammatory reaction aimed at the containment and killing of the pathogens is initiated at the site of infection. The most complex examples of these structures are lung granulomas induced in response to M. tuberculosis (1). Differentiated macrophages, lymphocytes, and other immune cells are the main constituents of granulomas (2, 3). During chronic inflammatory reactions, activated macrophages undergo functional modifications, like the increase of cytoplasmic organelle numbers, and morphological transformation acquiring an epithelioid phenotype. Frequently, macrophages can fuse one with another to generate multinucleated giant cells with a high phagocytic potential. At early stages of infection, granulomas ensure mycobacterial containment, but at late stages, they can be exploited by the bacteria to hide and grow within the host. In this environment, the tubercle bacilli can survive for the lifetime of the host (4). Eventual breakdown of granulomas caused by tissue pathology can lead to the promotion of transmission of a high burden of bacilli to susceptible hosts. Thus, granuloma formation can be beneficial to the host, but also to the tubercle bacilli facilitating their spreading and pathology (4).
Granulomatous reactions have also been described in the spleens and liver in response to Salmonella spp., both in mice and humans (5–7). Whereas a functional adaptive immune response is required for the maintenance of the granuloma in response to M. tuberculosis (2, 3), the same is not true in response to S. typhimurium, where CD4 and CD8 T cells are not required (7). Soluble inflammatory mediators (TNF-α, IFN-γ, IL-18, and IL-12) are instead essential for S. typhimurium–induced granuloma formation, suggesting that the innate immune response plays a primary role (8–10). In our previous study, we showed that intradermal injection of S. typhimurium leads to the generation of an acute inflammatory reaction, which is consistent with a granuloma-like reaction (GLR) at the site of infection caused by the recruitment of inflammatory cells (11). This blocks the migration of DCs to the draining LN (DLN) and the subsequent generation of an adaptive immune response.
A characteristic of granulomatous disorders is the increased deposition of several extracellular matrix (ECM) proteins (12), but the role of the ECM in pathogen-induced granulomatous reactions still remains to be elucidated. Functional studies of unspecific inhibition of matrix metalloproteinase (MMP) have shown that MMP plays a role in facilitating dissemination of M. tuberculosis, likely via ECM degradation (13), suggesting a possible role for ECM in forming physical containment of the bacteria. The ECM is involved in tissue scaffolding and remodeling, as well as cell migration, proliferation, and differentiation. The ECM is composed of several structural proteins, growth factors, proteoglycans, and matricellular proteins. The latter is a growing family that comprises tenascin C, which accumulates in granulomas of the lung (14); thrombospondin 1 (TSP1) and TSP2; osteopontin; and secreted protein, acidic and rich in cysteine (SPARC/osteonectin). SPARC is evolutionarily highly conserved and participates in numerous physiological processes (15). It is involved in bone mineralization (16), cell proliferation and migration (17–20), tissue remodeling (21), and angiogenesis (22). SPARC-deficient mice have 50% less collagen deposition (23), which correlates with increased DC migration (24). After enhanced DC migration, the induction of a delayed type hypersensitivity and cutaneous contact hypersensitivity are faster because of a less structured ECM (24). Further, upon skin injury, SPARC is increased in the skin (23). Because massive collagen deposition is crucial at the onset of granulomatous inflammatory reaction (25), we sought to find out whether SPARC-deficient mice had defects in the development of granulomatous reactions and in the initiation of immune response to intracellular bacteria.
In this article, we have characterized the formation of a GL acute inflammatory reaction in response to intradermal injection of S. typhimurium in SPARC-deficient and -sufficient mice. In the absence of SPARC, mice failed to develop an organized GL reaction in response to S. typhimurium, leading to an accelerated spreading of the bacteria to the DLN and an increased immune response to S. typhimurium. However, this correlated with decreased systemic spreading of S. typhimurium and conferred higher resistance to the pathogens. These findings highlight a new function of matricellular proteins in bacterial infection and suggest that initial containment of bacteria could be detrimental to the host by limiting the induction of adaptive immune responses.
RESULTS
SPARC−/− mice do not develop organized GL reactions
I.v. injection of S. typhimurium in mice induces the generation of granulomas in the liver that are aimed at bacterial containment (8). We recently showed that intradermal (i.d.) S. typhimurium injection also leads to the induction of an acute inflammatory response in the skin that is reminiscent of a granulomatous reaction that blocks the migration of DCs, as well as of bacteria from the site of injection (11). This system would give us the unique opportunity to analyze factors or molecules involved in organized inflammatory structures and S. typhimurium containment after the dissemination of bacteria from the defined site of injection. An oral or an intravenous injection would lead to diffused spreading of the bacteria and would not allow us to follow their path in a precise way; an intradermal ear injection, on the contrary, allows us to follow bacterial dissemination to a single DLN. The first question that we asked was whether the matricellular protein SPARC played any role in the development of bacteria-induced GLRs. SPARC-deficient and control WT mice were injected in the ear pinna with 107 CFU of attenuated S. typhimurium SL3261 AT. This strain is deficient in the AroA gene and is dependent on p-aminobenzoic acid and 2,3-dihydroxybenzoate for synthesis of aromatic amino acids and growth. The availability of these compounds is limited in mammalian cells, thus restricting the growth of the bacteria (26). At different times, animals were killed, and ears were snap frozen in optimal cutting compound (OCT), sectioned, and stained with various markers to investigate the structure and the composition of the infiltrate.
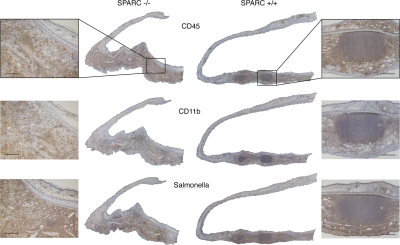
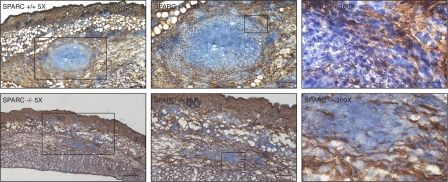
To be sure that the analyzed sections were effectively representative of the site of inflammation, a minimal dose of FITC-conjugated inert microbeads was coinjected with bacteria, and each slide was examined under the fluorescent microscope for the presence of emitting particles. Immunohistochemical decoration at day 5 after infection showed a clearly defined, compact organized structure in the infiltrate of SPARC+/+ ears; it was particularly rich in CD45+ leukocytes and surrounded by a ring of Cd11b+ cells, reminiscent of a granulomatous reaction, even though a fibrotic capsule was not observed, but this was expected for a primary reaction (Fig. 1). In contrast, the same markers in SPARC-deficient mice revealed a massive unorganized infiltrate (Figs. 1 and Figs.2). Further staining with anti–S. typhimurium antibody showed that bacteria were, for the most part (but not totally) contained within the GLR in wt mice, whereas they were spread in a wider area of inflammation in SPARC−/− ears (Fig. 1). The absence of GLR in KO mice corresponded to irregular deposition of collagen IV that, by failing to encapsulate the inflamed area, lead to a more dispersed leukocyte infiltrate, as assessed by immunohistochemical staining (Fig. 2) and by Masson trichromic stain of the inflamed area (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20071734/DC1). This suggests that SPARC is involved in the formation of an organized inflammatory structure with features of a granuloma, likely via the deposition of collagen fibers.
Figure 1.
SPARC−/− mice fail to develop an organized acute inflammatory reaction in response to S. typhimurium. SPARC+/+ (right) and SPARC−/− (left) mice were injected in the ear pinna with 107 CFU of attenuated S. typhimurium SL3261 AT. Immunohistochemical staining with anti-CD45 (top), anti-CD11b (middle), and anti–S. typhimurium (bottom) are shown on ear sections from mice killed 5 d after infection (brown stainings). Nuclei are stained in blue (hematoxylin). Images were merged by Photoshop to obtain whole ear sections. Original magnification of ear reconstructions was 5×, and of enlarged areas was 20×. Sections are representative of three independent experiments on four mice per group. Bar, 100 μm.
Figure 2.
Collagen IV staining reveals disorganized collagen deposition and more scattered infiltrate in SPARC−/− mice. SPARC+/+ (top row) and SPARC−/− (bottom row) mice were injected in the skin with 107 CFU of attenuated S. typhimurium SL3261 AT. Immunohistochemical staining with anti–collagen IV (brown) and counterstaining of nuclei with hematoxylin (blue) is shown on sections from mice killed 5 d after infection. Numbers show the original magnification of the sections. Boxes represent the magnified areas in the adjacent right images. Bars: (left) 50 μm; (middle) 25 μm; (right) 5 μm.
The composition of the leukocyte infiltrate is comparable in SPARC−/− and +/+ mice
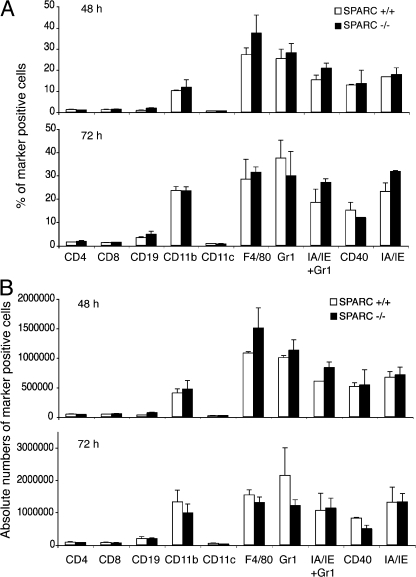
We considered the possibility that the observed structural differences of the inflamed site could be caused by an altered pattern of infiltrating cells in SPARC-deficient mice as compared with WT animals, which, for some reason, could be noncompatible with granuloma generation. Thus, we analyzed by flow cytometry the infiltrate composition over time after S. typhimurium injection. WT and SPARC-deficient mice were injected i.d. with bacteria, the inflamed area was excised, and constituent cells were analyzed at 48 and 72 h (Fig. 3). In these experiments, fluorescent beads were coinjected with bacteria to spot unequivocally the site of inflammation.
Figure 3.
Inflammatory cell infiltrate composition is very similar in SPARC+/+ and SPARC−/− mice. SPARC+/+ and SPARC−/− mice were injected intradermally in the back skin with 107 CFU of attenuated S. typhimurium SL3261 AT 48 and 72 h later, the sites characterized by an inflammatory reaction were excised, and cells were isolated by collagenase treatment. Cells were stained with fluorescent antibodies and analyzed by flow cytometry. (A) Y axes show percentage of marker-positive cells, x axes show the analyzed markers. (B) Y axes show total numbers of marker positive cells, x axes show the analyzed markers. Error bars represent the SD of three independent mice. One of three independent experiments is shown. SPARC −/−, black bars; SPARC+/+, white bars.
In both strains, F4/80 and Gr1+ cells represented the most abundant cell populations, both in frequency and absolute numbers. At these time points, Gr1+ cells are likely to corresponded to inflammatory monocytes, as they were also positive for MHC class II (IA/IE). Lymphocytes and DCs were present in very low percentages and numbers, the latter ranging from 1 to 6% without significant differences between strains and time.
No relevant discrepancies were outlined in the inflammation pattern, neither in the recruitment of cell populations nor in the kinetic of the recruitment itself, except for the macroscopic granulomatous formation that was detectable only in WT controls.
The absence of organized inflammatory reaction correlates with endogenous DC migration from infected site
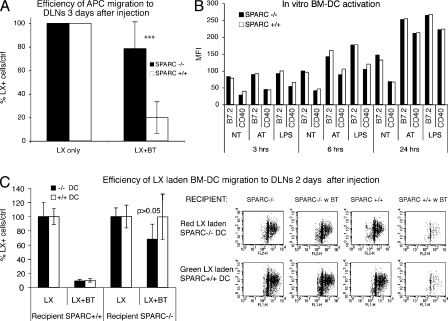
Generation of specific immune response consequent to inflammation normally requires DC migration from the periphery to DLNs. Nevertheless, we have previously demonstrated that subcutaneous injection of bacteria blocks DC migration from the site of inflammation to DLNs (11). We observed that impairment of granuloma formation in SPARC-deficient mice is associated with a restored capability of DCs to reach the DLN (Fig. 4 A). To monitor endogenous DC migration, FITC-conjugated latex beads were used as tracers (27). Mice were injected in four different sites of the dorsal skin, drained either by the inguinal or brachial LNs, with 107 particles. Injections were performed in the presence or absence of 107 bacterial CFU (SL 3261 AT), and migration of DCs carrying latex to DLNs was assessed. 3 d after injection, animals were killed, DLNs were collected and teased, and cells were released by treatment with collagenase. Cells were then stained for IA/IE or CD11c, and the entire population was acquired by flow cytometry, to determine the absolute numbers of DCs carrying latex beads in DLNs. To evaluate the effective role of bacteria on DC migration in the two strains, we evaluated the efficiency of DC migration expressed as the ratio of absolute numbers of DCs migrating in the absence versus the presence of bacteria. The efficiency of latex+ DC migration to DLNs after bacterial injection was dramatically and significantly higher in SPARC−/− than +/+ mice (Fig. 4 A). The presence of bacteria in KO mice decreased the efficiency of DC migration of only 20% versus 80% reduction observed in control WT animals. Also, when we analyzed the total numbers of migrating cells to the DLN, we found a striking difference when latex beads were injected together with bacteria in SPARC−/− versus +/+ mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20071734/DC1). As expected from our previous data, DCs in the SPARC−/− background migrated better than in the WT background (24). We recently ruled out the possibility that latex beads acquired the ability to reach the DLN in the absence of cellular transport because of the less organized collagen deposition in SPARC KO mice (24). Therefore, the latex+ cells that we find in DLN represent cells migrating from the injection site.
Figure 4.
DC migration from infected sites is inhibited by host-derived SPARC. (A) Endogenous DC migration is restored in SPARC−/− mice. 107 fluorescent latex (LX) microspheres/site were injected i.d. in mice in four different sites in the presence or absence of 107 CFU of S. typhimurium. Mice were killed at day 3 after treatment and the total number of IA/IE+ cells carrying FITC-latex particles per DLNs was assessed after acquisition of the whole sample. The efficiency of latex+ cell migration (the number of latex+ cells in DLN of mice injected only with latex particles is considered as 100% of migration) to the DLNs is shown. The difference between efficiency of migration of latex+ cells in the presence of bacteria (LX + BT) in SPARC+/+ (white bars) versus SPARC−/− (black bars) mice is highly significant (***, P < 0.0001). This is one experiment representative of three each with three mice per group. (B) BM-DCs from SPARC+/+ and SPARC−/− display the same kinetic of activation in vitro. BM-DCs were generated in GM-CSF–conditioned medium and activated with bacteria at a multiplicity of infection of 10:1 or with 1 μg of LPS. Up-regulation of costimulatory molecules was evaluated by flow cytometry at the indicated time points. One of two similar experiments is shown. (C) DC migration is inhibited by host-derived SPARC. BM-DCs from SPARC+/+ and SPARC−/− were loaded with green and red latex beads (LX), respectively. Mice were injected i.d. with BM-DCs from both strains in the presence or absence of bacteria (BT), and cell migration to the DLNs was assessed 2 d after injection. (left) The mean total efficiency of migration ± the SD of six different mice/group is shown. The difference in DC migration efficiency in the recipient SPARC+/+ mice in the presence of bacteria is not statistically significant (P > 0.05). (right) Representative dot plots showing total migrated cells from single DLNs in the different conditions. Recipient line shows the recipient background where BM-DCs from the two donor strains were injected in the presence or absence of bacteria (BT). (top) Dot plots show the recovery of Red LX laden SPARC−/− BM-DCs; (bottom) dot plots show the recovery of Green LX laden SPARC+/+ BM-DCs.
These data suggest that SPARC is essential for the process of blocking DC migration from the S. typhimurium–infected site into the DLN.
Tissue-derived, and not DC-derived, SPARC is responsible for block of DC migration
DCs express SPARC (24), which could play a role in cell differentiation and/or maturation; thus, to evaluate whether SPARC KO bacteria-treated DCs displayed a faster kinetic of maturation, and whether this was responsible for their capacity to migrate after bacterial injection, we tested their ability to mature in response to S. typhimurium or LPS.
BM-DCs were generated from WT and SPARC-deficient mice, and activated with S. typhimurium or LPS. At different time points, the expression of costimulatory molecules was evaluated by flow cytometry. As shown, no differences in terms of kinetics or intensity of activation were evidenced (Fig. 4 B). This, together with our previous observation that DCs generated from the two strains displayed similar migratory properties in vitro (24), suggested that the restored ability of DCs to migrate from the injection site to the DLN was likely not dependent on intrinsic differences of the DC population. To test this hypothesis, we evaluated whether tissue- or DC-derived SPARC was important to block DC migration from the infected site. We performed a double adoptive transfer experiment. WT and KO mice received contemporarily contiguous i.d. injections of red and green latex-laden BM-DCs from WT and KO strains, respectively. DC transfers were performed in the presence or absence of S. typhimurium SL 3261AT. Regardless of the type of DCs injected, their migration was abolished in the presence of bacteria only when inoculated into WT recipients (Fig. 4, C and D). This indicates that restored DC migration is caused by differences in the extracellular environment and not by different intrinsic properties of the cells.
Absence of GLR corresponds to an enhanced specific immune response in SPARC−/− mice
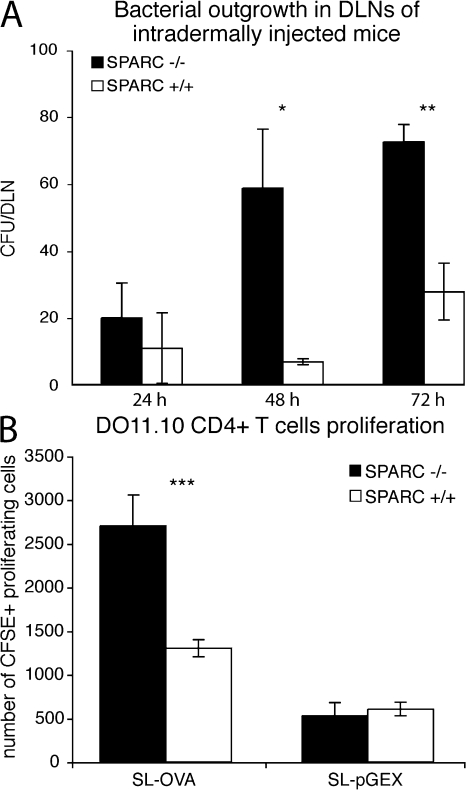
In our previous studies, we showed that both DC and bacteria spreading to DLNs was impaired in WT mice (11). Thus, we evaluated whether paralleling DC migration there was an increase of bacterial colonies recovered from DLNs. We show that a significant increase in bacterial CFU was detected in the DLNs of i.d. injected SPARC−/− in respect to WT counterparts (Fig. 5 A). Whether this is caused by bacteria reaching the DLN on their own or being carried by DCs is unknown; however, the low number of bacteria recovered in the whole DLN is more suggestive of a DC-mediated transport than a massive emigration of free bacteria from the injected site.
Figure 5.
Restored DC migration correlates with enhanced bacterial counts into DLN and increased T cell proliferation. (A) Bacterial load in SPARC−/− is higher than that in SPARC+/+ DLNs at days 2 and 3 after injection. DLNs of mice treated with bacteria were lysed with Na deoxycholate and plated on LB agar. Bacterial CFU were counted after overnight incubation at 37°C. The difference between bacterial counts at 48 and 72 h in DLN from SPARC+/+ versus SPARC−/− mice is highly significant (*, P < 0.01; **, P < 0.001). One of two similar experiments is shown. (B) OVA-expressing recombinant bacteria induce proliferation of OVA-specific CD4 T cells in SPARC−/−, but not in SPARC+/+ mice. SPARC WT and KO recipient mice were adoptively transferred with 3 × 106 CFSE labeled DO11.10 CD4+ T cells, and were injected i.d. 24 h later with recombinant S. typhimurium–expressing (SL-OVA) or not expressing (SL-pGEX) OVA. Proliferation of transferred T cells in DLNs was assessed 3 d after S. typhimurium injection. The number of CFSE+ proliferating cells is shown ± the SD of 8 mice per group. The difference in T cell proliferation in SPARC+/+ versus SPARC−/− mice is highly significant (***, P < 0.001).
As we showed that in the absence of DC migration or bacteria recovery in the DLN the adaptive immune response to bacteria-associated antigens was also impaired (11), we tested whether T cell activation to bacterial antigens was induced in SPARC−/− mice. Mice were transferred with CFSE-labeled OVA-specific transgenic T cells (from DO11.10 strain). 24 h after transfer, recipient animals were injected i.d. with recombinant bacteria expressing (SL-OVA) or not expressing (SL-pGEX) OVA (107 CFU in 4 different sites), and 3 d later proliferation of OVA-specific T cells in DLNs was evaluated by flow cytometry, as a reduction of CFSE fluorescence (Fig. 5 B).
The number of proliferating T cells was significantly higher in SPARC−/− mice treated with OVA-expressing recombinant bacteria compared with SPARC+/+ mice, indicating an efficient priming of antigen-specific T cells. OVA-specific T cells underwent five cycles of proliferation in SPARC−/− mice, and barely two cycles in WT mice (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20071734/DC1). As a control, SPARC+/+ and −/− animals injected with recombinant bacteria carrying the empty vector displayed no OVA-specific T cell proliferation. Together, these results indicate that in the absence of SPARC, bacteria can be recovered from the DLN, and this correlates with specific T cell activation.
Induction of adaptive immune response protects SPARC−/− mice from S. typhimurium infection
The presence of bacteria in DLNs could be interpreted both positively and negatively. On one hand, it could allow priming of S. typhimurium–specific T cells and, consequently, the generation of a specific immune response. On the other hand, it could represent the first step of infection spreading. Therefore, we evaluated whether the aforementioned specific response generated was sufficient to face spreading of infection by assessing the lethality of mice after injection of increasing doses of virulent S. typhimurium. We first tested whether DC migration was also impaired after subcutaneous injection of virulent S. typhimurium, i.e., bacteria able to replicate in vivo (SL1344). As shown in Fig. S4 (available at http://www.jem.org/cgi/content/full/jem.20071734/DC1), both 103 and 105 CFU of S. typhimurium inhibited DC migration to the DLN, a feature that was restored in SPARC−/− mice.
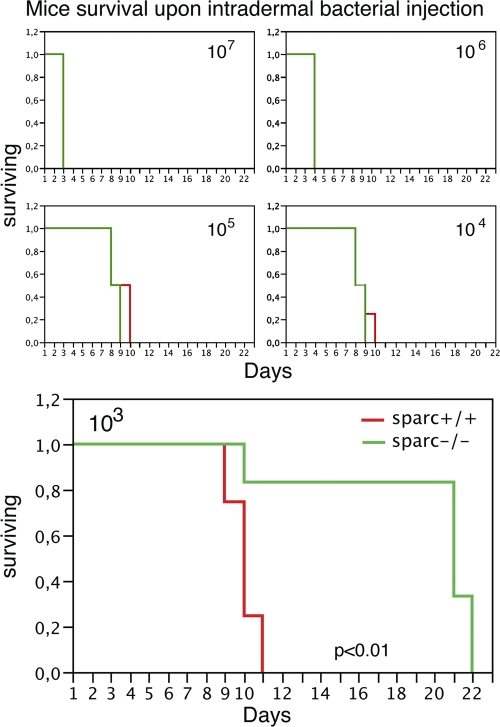
We then challenged SPARC−/− and +/+ mice with increasing doses of live, fully virulent S. typhimurium, ranging from 103 to 107 CFU (Fig. 6). At higher doses of bacteria, both strains displayed an overimposable mortality rate, suggesting that, at these doses, mice were similarly susceptible to S. typhimurium infection. In contrast, at low dose (103 CFU of bacteria), SPARC KO animals were dramatically more resistant when challenged with S. typhimurium, as the majority of mice survived till day 22 in the KO mice, whereas by day 11, all of the WT mice were dead. This indicates that even though SPARC−/− mice are impaired in their ability to form a GL structure, they do not display increased susceptibility to S. typhimurium infection; instead, at low doses they are more protected.
Figure 6.
SPARC−/− mice are more resistant to low dose intradermally injected pathogenic S. typhimurium. Different amounts of bacteria, ranging from 103 to 107 CFUs, were injected intradermally and mouse survival was analyzed. Kaplan-Meyer survival curves are shown. Whereas at 104 to 107 CFU there was no difference in survival curves, at 103 CFU, the difference between SPARC+/+ and SPARC−/− mice was statistically significant (P < 0.01, Log-Rank). This is one representative of three different experiments, each performed with five mice per group.
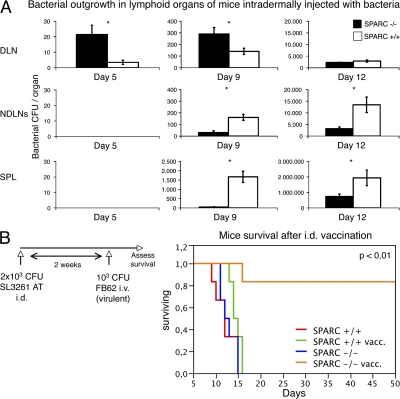
We hypothesized that the presence of a granuloma, by blocking the initiation of an adaptive immune response, was also impeding the control of the growth of S. typhimurium escaping the skin granulomatous reaction. To test this hypothesis, we evaluated whether the augmented survival of SPARC-deficient mice and the early presence of bacteria in DLNs, was associated with a retarded onset of systemic infection in these animals, measured as colonization of nondraining lymphoid organs. WT and KO mice received a single i.d. injection of 103 CFU of fully virulent and replicating S. typhimurium, and were killed at different time points. Bacterial outgrowth in DLN, nonDLNs, and the spleen was evaluated by plating serial dilution of lysed cells (Fig. 7 A). 5 d after i.d. injection, colonies were found only in DLNs, with a significant difference between SPARC+/+ and SPARC−/− animals, the latter showing a 10-fold higher number of recovered CFUs. This pattern of DLN colonization was conserved up to 9 d after injection. Conversely, at this time, bacteria had massively invaded the spleen and the nonDLNs of SPARC+/+ but not SPARC−/− mice, showing a 100-fold higher numbers in WT spleen and a 10-fold higher bacterial load in WT LNs versus the corresponding organs of SPARC-deficient animals. At day 12, DLNs of both strains were equally colonized by pathogens, but, again, a significantly higher number of bacteria was still found in the LNs and spleen of WT compared with SPARC−/− animals. The majority of WT mice did not survive day 12, making a comparison at later time points impossible.
Figure 7.
SPARC−/− mice display reduced systemic bacterial dissemination. (A) Bacterial dissemination was evaluated in SPARC+/+ and SPARC−/− animals injected intradermally with 103 CFU of pathogenic S. typhimurium. DLNs, nonDLNs, and spleens were lysed and plated at different time points. Total bacterial count/organ is shown ± the SD of 12 different mice per group. The difference in bacterial recovery in SPARC+/+ and SPARC−/− animals is statistically significant (*, P < 0.01). (B) Intradermal immunization with attenuated S. typhimurium can protect SPARC−/− but not SPARC+/+ mice from subsequent challenge with pathogenic S. typhimurium. SPARC+/+ and SPARC−/− mice were vaccinated subcutaneously with 2 × 103 CFU of attenuated S. typhimurium or with PBS as a control. 2 wk after vaccination, all mice were challenged i.v. with 103 CFU of pathogenic S. typhimurium. Only SPARC-deficient mice vaccinated with bacteria survive upon challenge with virulent S. typhimurium. Kaplan-Meyer survival curves are shown. This is one representative of four different experiments each performed on five mice per group. The difference between the SPARC−/−-vaccinated group versus all the others is statistically significant (P < 0.01, Log-Rank).
The sudden increase of bacteria in nondraining organs in WT animals at day 9 could suggest that within the granulomatous reaction, S. typhimurium grows undisturbed because of the inability to initiate a rapid anti–S. typhimurium response until the bacterial burden is such to disrupt the granuloma structure. The massive colonization of distal nondraining organs by S. typhimurium could be suggestive of a nonlymphaticly mediated dissemination.
Subcutaneous vaccination is protective only in SPARC−/− mice
Thus, if early dissemination of S. typhimurium is required to induce a protective anti–S. typhimurium response, we would then expect that only in SPARC−/− mice would a low dose of i.d. injected avirulent S. typhimurium vaccinate against the fully virulent strain. Both strains of animals were vaccinated with intradermal injection of 2 × 103 CFU of attenuated S. typhimurium SL3261 AT. 2 wk after immunization, mice were challenged i.v. with a lethal dose of virulent S. typhimurium (103 CFU; SL1344), and survival was assessed. All SPARC+/+ mice, irrespective of vaccination, and nonvaccinated SPARC−/− animals died between 6 and 15 d after injection (Fig. 7 B). On the contrary, 75% of vaccinated SPARC-deficient mice survived the challenge. These results suggest that the i.d. route of administration is highly inefficient for vaccination purposes, likely because of the inability of DCs to migrate to DLNs and to initiate pathogen-specific responses. In SPARC−/− mice where DCs can migrate to the DLN and initiate an antigen-specific response, the i.d. route of administration allows us to generate protection toward virulent S. typhimurium. This experiment also shows that the two strains of mice are equally susceptible to i.v. administration of S. typhimurium (see nonvaccinated mice), indicating that the differences observed in survival of mice challenged with 103 CFU of pathogens intradermally are not caused by strain-dependent intrinsic resistance to pathogens, but to skin-related differences that are presumably conferred by SPARC.
Altogether, these results suggest that although SPARC-proficient mice control infection spreading at early time points, SPARC−/− mice display a much better outcome because of the initiation of a systemic response to bacterial antigens.
DISCUSSION
The role of the microenvironment in the control of the immune function has recently been proposed (28–32), suggesting that the outcome of the immune response does not depend uniquely on the interaction between immune cells and antigens. Rather, environmental tissues provide the immune system with different signals that contribute to modulate its responses, according to specific organ requirements. We used an already established skin inflammatory model to monitor the dissemination and spreading of locally injected S. typhimurium and the subsequent immune response (11). Our experimental model suggests that the requirement of the skin to contain pathogen dissemination overcomes the default “program” of the immune system, which is to allow DC maturation and migration soon after pathogen encounter. This is achieved via tissue remodeling and the formation of an inflammatory reaction reminiscent of a granuloma that limits the spreading of the bacteria, similar to what has been described in the lung in response to M. tuberculosis (3, 4) or in the liver and spleen in response to oral S. typhimurium infection (33).
We show here that a major player for the observed effect is environmental SPARC. Matricellular protein SPARC participates in numerous physiological processes, and SPARC-deficient mice display an irregular and more lapsed deposition of collagen fibers (34). We found that in response to S. typhimurium, mice lacking SPARC have irregular collagen deposition and no signs of an organized inflammatory granulomatous reaction, which is consistent with the notion that massive collagen deposition is required for the onset of granulomatous inflammatory reaction (25).
It has been described that the absence of granuloma leads to an increased susceptibility of animals to pathogens (35). Most of these studies were performed in TNF-deficient mice that fail to develop granulomas in response to Bacillus Calmette–Guerin (36) and are highly susceptible to M. tuberculosis infection (37). However, TNF plays a major role in inflammatory responses and intracellular bacterial clearance by inducing macrophages to produce nitric oxide and chemokines (CCL2, CCL5, CCL9, and CXCL10) involved in the subsequent recruitment of inflammatory cells (38). TNF-deficient mice also show a defective homing of T cells that localize in the pulmonary perivascular and peribronchial region (39). Thus, it is very difficult to ascertain whether the observed increased susceptibility to bacterial infection in these mice is caused by the absence of a granuloma or to the lack of TNF-mediated antibacterial effect.
In our model, flow cytometric analysis of inflammatory cells from mice intradermally injected with S. typhimurium reveals no differences in terms of cell populations or cell numbers between SPARC WT and KO animals. However, the histochemical analysis of the injection site reveals that the inflammatory reaction is not organized in SPARC−/− mice. This observation suggests that the absence of SPARC mainly affects the structure and the organization of the infiltrate, rather than its composition, making this a very valuable model to study the role of acute inflammatory reactions in bacterial infection. We observed, by means of immunohistochemical staining that most, but not all of the injected bacteria were confined inside the GLRs in WT animals, whereas they were largely spread in KO counterparts. This correlated with an increased spreading of S. typhimurium to DLN and to the ability of bacteria-laden DCs to reach the DLN. At later time points, we found that WT but not SPARC−/− mice had uncontrolled proliferation of bacteria in nonDLNs and were more susceptible to S. typhimurium infection, suggesting that initial spreading to DLNs is beneficial to control subsequent bacterial spreading. This correlated with the development of an anti–S. typhimurium response: injection of recombinant S. typhimurium expressing OVA in mice adoptively transferred with DO11.10 CD4 T cells revealed that in the absence of SPARC, OVA-specific T cells proliferated in DLNs, suggesting that a specific response is mounted. Thus, whereas granulomatous reaction is actually effective in an initial containment of bacteria, it is disadvantageous at later time points, as it also impedes the development of a protective immune response to the bacteria and their control after escape from the site of infection. Indeed, at low doses of bacteria, the ability of SPARC-deficient animals to mount a rapid and specific immune response was beneficial, and resulted in an increased survival to pathogenic bacteria. Interestingly, at high doses of injected bacteria, both SPARC−/− and +/+ mice underwent rapid bacterial colonization and died. Two nonmutually exclusive possibilities could be envisaged: one in which the induced immune response can better control bacteria dissemination, and thus the absence of an organized granuloma in SPARC−/− mice does not impact on mouse survival; and another, in which the formation of a granuloma could also represent a favorable microenvironment for bacteria replication, protected from a possible secondary specific immune response (4). The sudden dissemination of S. typhimurium in WT animals between day 5 and 9 to nondraining organs would favor this hypothesis, suggesting that bacteria grow undisturbed in the GLR until the granuloma falls apart. The role of protective immune response initiated by DC migration to the DLN is supported by the finding that i.d. injection with low doses of nonvirulent S. typhimurium lead to protective immunity to virulent S. typhimurium injected i.v. only in SPARC−/− and not +/+ mice. As nonimmunized strains were both similarly susceptible to S. typhimurium injected i.v., we can rule out intrinsic differences in the ability of SPARC−/− mice to resist intradermal S. typhimurium injection.
After our previous study on the role of environmental SPARC in DC migration, we show here that ECM also plays a role in DC migration to DLNs during bacterial infection. This is likely caused by the formation of a physical block generated by SPARC-mediated collagen deposition. This finding suggests that ECM can be an obstacle for DC exodus from the tissue, and is in agreement with the observation that ECM degradation by matrix metalloproteases is required for DC migration (40–44). In the absence of SPARC, the more lapsed collagen deposition could create holes in the ECM, rendering it traversable by the DCs. Thus, the inability of DCs to migrate to DLNs for T cell activation, as well as the generation of an environment that is conducive to bacterial replication, could account for the observed increased susceptibility of WT mice to S. typhimurium infection. We cannot exclude that SPARC could also play a role in blocking the differentiation of monocytes into DCs in the skin, as we showed that intradermal bacterial injection impedes DC differentiation from monocytes (11). However, our adoptive transfer experiments using DCs from SPARC WT or KO mice revealed a direct effect of environmental SPARC on fully differentiated DCs, suggesting that this effect is already exerted on mature DCs. This is a very rapid event, as it occurs 1 d after infection, which is consistent with active tissue remodeling (11). Notably, soluble antigens released by S. typhimurium, such as flagellin, that do not require cellular transport to reach the DLN, rapidly induce an immune response (45), thereby confirming our previous data showing that the presence of S. typhimurium does not inhibit the presentation of soluble proteins (11). It remains to be established whether highly expressed cell-associated antigens can also find a preferential route of presentation, possibly via nearby recruited phagocytes that are not involved within the granulomatous reaction, but with a delayed kinetic (45). Either way, it is striking that when bacteria cannot gain access to DLNs during infection in WT animals (11, 45), establishment of a protective anti–S. typhimurium response is also lacking.
Thus, our results suggest that during skin bacterial infection, two events occur: on one side, DC differentiation from monocytes is impaired; and on the other side, already differentiated DCs, as well as bacteria, are retained at the site of infection by SPARC-dependent mechanisms. This feature does not seem to be unique to bacteria, as in response to Leishmania monocytogenes infection there is also a block of DC migration into the DLN during the first week of infection, which then resolves at later time points (46). It would be interesting to evaluate whether in this case there is also SPARC involvement in parasite containment and whether persistence of L. monocytogenes disrupts the ECM mesh in its own interest.
In conclusion, these results tell us that when the skin is accidentally injured, e.g., by a cut, and is exposed to environmental microorganisms, the immune system responds with the induction of a vigorous innate response culminating with a granulomatous reaction that is aimed at microbial containment. This response, however, retards the establishment of antibacterial-specific responses and is inefficient in controlling S. typhimurium's growth, thus favoring its dissemination. A possible therapeutic implication of these results is that during intradermal bacterial vaccination, one might consider administering a molecule for ECM degradation to favor DC migration into the DLNs and the subsequent immune response. Notably, an old strategy to heighten vaccination to tuberculosis was to coadminister hyaluronidase with Bacillus Calmette-Guerin (47).
MATERIALS AND METHODS
Mice.
5–6-wk-old BALB/c mice were purchased from Harlan. DO11.10 OVA-TCR transgenic T cells were provided by D. Lo (Scripps Research Institute, La Jolla, CA). SPARC KO mice, originally on a mixed 129SV/C57BL/6 background, were backcrossed for 12 generations with BALB/cAnNCrl (Charles River Laboratories) to obtain congenic SPARC KO mice. All experiments were performed in accordance with the guidelines established in the Principles of Laboratory Animal Care (directive 86/609/EEC) and approved by the Italian Ministry of Health.
Antibodies and flow cytometry.
The following monoclonal antibodies and matched isotypes controls were purchased from BD Biosciences: CD4 (L3T4), CD8a (Ly2), CD19, IA/IE, CD11b (Mac-1), CD11c (N418), CD45R (B220), Gr1 (Ly-6G). Rat anti–mouse F4/80 was obtained from Caltag Laboratories. Pure rabbit S. typhimurium antiserum was purchased from ViroStat.
Flow cytometry analysis was performed with a FACSCalibur (CellQuest software; BD Biosciences).
Bacterial strains and preparation for injections.
The S. typhimurium derivative SL3261 AT is a metabolically defective aroA strain generated from the SL1344 WT according to the methodology described by Datsenko and Wanner (48). A recombinant strain of SL3261 AT was generated expressing either the gene coding for GST-OVA fusion (SL-OVA) or with the GST alone (SL pGEX). 107 CFUs of SL-OVA express nearly 1 μg protein. Single colonies were grown overnight at 37°C in Luria broth (LB; Difco) and restarted the next day at 1:10 of the original volume to reach an OD600 = 0.35, which corresponds to 7 × 108 CFU/ml.
Mice treatments, skin inflammation induction, and latex migration.
Mice were anesthetized i.p. with Avertin 2.5% and shaved at four sites of the dorsal skin, which were drained either by the inguinal or brachial LNs. 107 CFUs of bacteria were diluted to a final volume of 10 μl PBS and injected i.d. using a Hamilton syringe (Thermo Fisher Scientific). 107 FITC-conjugated latex particles of 1 μm in diameter (Polysciences) were injected as tracers in the presence or absence of bacteria. Migration of IA/IE+ or CD11c+ cells carrying latex to DLNs was assessed 3 d after treatment. At indicated times, mice were killed, DLNs were collected and teased, and cells were released by treatment with 0.25% collagenase at 37°C for 25 min (Collagenase D; Roche). Cells were stained for IA/IE or CD11c expression. For quantification, the entire population of DLN cells was acquired by cytofluorometry and absolute numbers of CD11c+ or IA/IE+ cells carrying latex beads were determined per LN. The region of the skin corresponding to the site of injection, as identified by faint visual appearance of green latex beads, was separated and cells were released after collagenase treatment and stained for FACS analysis as described in Antibodies and flow cytometry. To visualize the inflammatory granulomatous reaction, SPARC+/+ and −/− mice were injected in the ear pinna or i.d. with 107 CFU of attenuated S. typhimurium SL3261 AT, and mice were killed 5 d later. The specimens were immersed in OCT and snap-frozen in liquid nitrogen.
In vitro loading of DCs with latex microspheres and mouse treatment.
Murine DCs were generated from BM of SPARC+/+ and −/− mice. In brief, BM was harvested by flushing femurs with PBS. Cells were resuspended at 2 × 106 cells/ml in IMDM (Invitrogen) supplemented with 30% supernatant of GM-CSF expressing fibroblasts. On day 6–8, the differentiation of DCs was assessed by flow cytometry using antibodies to CD11c, B7.2, CD40, H-2Kd, I-A/I-E. DCs were incubated with green-LX- or red-LX-microspheres (1:100, DCs/microspheres) for 30 min at 37°C, and uptake of the LX-microspheres was determined by FACS analysis of CD11c-gated cells. 3 × 105 red-LX–loaded DCs from SPARC WT and 3 × 105 green-LX–loaded DCs from SPARC KO mice (or vice versa) were injected intradermally in the dorsal skin of anesthetized SPARC WT and SPARC KO mice. Mice were killed 48 h after injection, DLNs were collected, and cells were analyzed by FACS.
TCR transgenic T cell adoptive transfer.
DO11.10 TCR-OVA T cells specific for the OVA327–339 peptide in association with I-Ad, were collected from spleen, brachial, and inguinal LNs. CD4+ T cells were purified by positive selection using anti-CD4 (L3T4) magnetic microbeads (Mini-MACS; Miltenyi Biotec), and cells were labeled with 5 μM carboxyl-fluorescein-succinimidyl ester (CFSE) for 15 min at 37°C (Invitrogen).
SPARC+/+ or −/− recipient mice were injected i.v. with 3 × 106 DO11.10 CFSE-labeled CD4 T cells and immunized with 107 CFUs of SLpOVA or SLpGEX (as a control) after 24 h. Mice injected with 10 μl of 0.25 μg OVA emulsified in CFA were always included in each experiment as a control. 3 d later, DLNs were collected and DO11.10 T cell proliferation was assessed as a reduction in CFSE labeling.
Bacterial outgrowth.
Cells from spleen, popliteal LNs, and DLNs (brachial or inguinal) of mice injected i.d. in the dorsal skin with 103 CFU of WT S. typhimurium (FB62, SL1344) were lysed with 0.5% sodium deoxycholate. CFUs were counted after serial dilution of cellular lysates on LB agar to quantify the number of migrated live intracellular bacteria.
Immunohistochemistry.
For immunohistology, tissues were embedded in OCT, snap frozen in liquid nitrogen, and sectioned at 7 μm. Sections were subsequently fixed in acetone for 10 min, placed in 3% H2O2/MeOH solution for 5 min to block endogenous peroxidase activity, rinsed in PBS, and blocked in 10% FCS for 20 min.
Specimens were then incubated for 1 h with biotinylated primary antibodies or with rabbit anti–S. typhimurium antibody (ViroStat) or rabbit polyclonal antibody against mouse collagen type IV (AB756; Chemicon). After washing, sections were overlaid with avidin–peroxidase complex for 30 min (Sigma-Aldrich). Peroxidase-conjugated mouse anti–rabbit antibody (DakoCytomation) was used as secondary antibody for sections incubated with anti–S. typhimurium and anti–collagen IV primary antibodies. Antigen was revealed with 3,3′-diaminobenzidine (Sigma-Aldrich) according to the manufacturer's instructions. Sections were counterstained with Mayer's hematoxylin, dehydrated in graded alcohol (70, 90, and 100% ethanol), and mounted in Eukitt mounting medium (Merck Eurolabs). All images were captured on a microscope (Leica) equipped with a digital camera (DXM1200; Nikon), analyzed using ACT1 software, and merged by Photoshop (Adobe).
Mouse vaccinations and challenges.
Mice were injected intradermally in the dorsal skin with 2 × 103 CFU of avirulent SL3261 AT. 2 wk later mice were challenged i.v. with a lethal dose of virulent S. typhimurium (103 CFU, SL1344), and survival was assessed.
Statistical analysis.
Statistical significance was evaluated by nonparametric Mann-Whitney U test (Wilcoxon-Kruskal Wallis) to analyze variables that were not normally distributed or by the Student's t test for continuous variables. Significance was defined at P < 0.05 (two-tailed test). Statistic calculations and Kaplan Meyer's curves were performed by JMP 5.1 software (SAS Cary).
Online supplemental materials.
Fig. S1 shows that different collagen organization characterizes the inflammatory reaction in the infected mouse skin of SPARC−/− mice. Fig. S2 shows that DC migration from infected sites is inhibited by host-derived SPARC. Fig. S3 shows that restored DC migration correlates with increased T cell proliferation. Fig. S4 shows that endogenous DC migration is also restored in SPARC−/− mice after injection of fully virulent S. typhimurium. A Supplemental materials and methods is also provided. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071734/DC1.
Supplementary Material
Acknowledgments
M. Rescigno is supported by grants from the Crohn's and Colitis Foundation of America, the Italian Association for Cancer Research, and the Italian Ministry of Health (Ricerca Finalizzata). G. Matteoli is a recipient of a Fondazione Italiana Ricerca sul Cancro fellowship.
The authors have no conflicting financial interests.
Abbreviations used: DLN, draining LN; ECM, extracellular matrix; GL, granuloma-like; GLR, GL reaction; i.d., intradermal; LB, Luria broth; MMP, matrix metalloproteinase; OCT, optimal cutting compound; SPARC, secreted protein, acidic and rich in cysteine; TSP, thrombospondin.
G. Rotta and G. Matteoli contributed equally to this work.
References
- 1.Ulrichs, T., and S.H. Kaufmann. 2006. New insights into the function of granulomas in human tuberculosis. J. Pathol. 208:261–269. [DOI] [PubMed] [Google Scholar]
- 2.Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann, S.H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 1:20–30. [DOI] [PubMed] [Google Scholar]
- 4.Flynn, J.L., and J. Chan. 2005. What's good for the host is good for the bug. Trends Microbiol. 13:98–102. [DOI] [PubMed] [Google Scholar]
- 5.Mert, A., F. Tabak, R. Ozaras, R. Ozturk, H. Aki, and Y. Aktuglu. 2004. Typhoid fever as a rare cause of hepatic, splenic, and bone marrow granulomas. Intern. Med. 43:436–439. [DOI] [PubMed] [Google Scholar]
- 6.Nakoneczna, I., and H.S. Hsu. 1983. Histopathological study of protective immunity against murine salmonellosis induced by killed vaccine. Infect. Immun. 39:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hormaeche, C.E., P. Mastroeni, A. Arena, J. Uddin, and H.S. Joysey. 1990. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 70:247–250. [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroeni, P., J.N. Skepper, and C.E. Hormaeche. 1995. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 63:3674–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everest, P., M. Roberts, and G. Dougan. 1998. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect. Immun. 66:3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastroeni, P., B. Villarreal-Ramos, and C.E. Hormaeche. 1992. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro-Salmonella vaccines. Microb. Pathog. 13:477–491. [DOI] [PubMed] [Google Scholar]
- 11.Rotta, G., E.W. Edwards, S. Sangaletti, C. Bennett, S. Ronzoni, M.P. Colombo, R.M. Steinman, G.J. Randolph, and M. Rescigno. 2003. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J. Exp. Med. 198:1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman, J., Y.J. Jeon, A. Gal, and R.L. Perez. 1995. Distribution of extracellular matrices, matrix receptors, and transforming growth factor-beta 1 in human and experimental lung granulomatous inflammation. Am. J. Med. Sci. 309:124–133. [DOI] [PubMed] [Google Scholar]
- 13.Taylor, J.L., J.M. Hattle, S.A. Dreitz, J.M. Troudt, L.S. Izzo, R.J. Basaraba, I.M. Orme, L.M. Matrisian, and A.A. Izzo. 2006. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 74:6135–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaarteenaho-Wiik, R., O. Sademies, P. Paakko, J. Risteli, and Y. Soini. 2007. Extracellular matrix proteins and myofibroblasts in granulomas of sarcoidosis, atypical mycobacteriosis, and tuberculosis of the lung. Hum. Pathol. 38:147–153. [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw, A.D., and E.H. Sage. 2001. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Invest. 107:1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Termine, J.D., H.K. Kleinman, S.W. Whitson, K.M. Conn, M.L. McGarvey, and G.R. Martin. 1981. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 26:99–105. [DOI] [PubMed] [Google Scholar]
- 17.Sage, H., R.B. Vernon, S.E. Funk, E.A. Everitt, and J. Angello. 1989. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 109:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasselaar, P., and E.H. Sage. 1992. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J. Cell. Biochem. 49:272–283. [DOI] [PubMed] [Google Scholar]
- 19.Francki, A., K. Motamed, T.D. McClure, M. Kaya, C. Murri, D.J. Blake, J.G. Carbon, and E.H. Sage. 2003. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J. Cell. Biochem. 88:802–811. [DOI] [PubMed] [Google Scholar]
- 20.Schiemann, B.J., J.R. Neil, and W.P. Schiemann. 2003. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol. Biol. Cell. 14:3977–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremble, P.M., T.F. Lane, E.H. Sage, and Z. Werb. 1993. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J. Cell Biol. 121:1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupprion, C., K. Motamed, and E.H. Sage. 1998. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J. Biol. Chem. 273:29635–29640. [DOI] [PubMed] [Google Scholar]
- 23.Bradshaw, A.D., M.J. Reed, and E.H. Sage. 2002. SPARC-null mice exhibit accelerated cutaneous wound closure. J. Histochem. Cytochem. 50:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Sangaletti, S., L. Gioiosa, C. Guiducci, G. Rotta, M. Rescigno, A. Stoppacciaro, C. Chiodoni, and M.P. Colombo. 2005. Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J. Cell Sci. 118:3685–3694. [DOI] [PubMed] [Google Scholar]
- 25.Singh, K.P., H.C. Gerard, A.P. Hudson, and D.L. Boros. 2004. Dynamics of collagen, MMP and TIMP gene expression during the granulomatous, fibrotic process induced by Schistosoma mansoni eggs. Ann. Trop. Med. Parasitol. 98:581–593. [DOI] [PubMed] [Google Scholar]
- 26.Hoiseth, S.K., and B.A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 291:238–239. [DOI] [PubMed] [Google Scholar]
- 27.Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, M., H. Tang, Z. Guo, H. An, X. Zhu, W. Song, J. Guo, X. Huang, T. Chen, J. Wang, and X. Cao. 2004. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5:1124–1133. [DOI] [PubMed] [Google Scholar]
- 29.Svensson, M., A. Maroof, M. Ato, and P.M. Kaye. 2004. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 21:805–816. [DOI] [PubMed] [Google Scholar]
- 30.Tang, H., Z. Guo, M. Zhang, J. Wang, G. Chen, and X. Cao. 2006. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 108:1189–1197. [DOI] [PubMed] [Google Scholar]
- 31.Alpan, O., E. Bachelder, E. Isil, H. Arnheiter, and P. Matzinger. 2004. ‘Educated’ dendritic cells act as messengers from memory to naive T helper cells. Nat. Immunol. 5:615–622. [DOI] [PubMed] [Google Scholar]
- 32.Rimoldi, M., M. Chieppa, P. Larghi, M. Vulcano, P. Allavena, and M. Rescigno. 2005. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 106:2818–2826. [DOI] [PubMed] [Google Scholar]
- 33.Mastroeni, P., and N. Menager. 2003. Development of acquired immunity to Salmonella. J. Med. Microbiol. 52:453–459. [DOI] [PubMed] [Google Scholar]
- 34.Bradshaw, A.D., P. Puolakkainen, J. Dasgupta, J.M. Davidson, T.N. Wight, and E. Helene Sage. 2003. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Invest. Dermatol. 120:949–955. [DOI] [PubMed] [Google Scholar]
- 35.Dannenberg, A.M. Jr. 1982. Pathogenesis of pulmonary tuberculosis. Am. Rev. Respir. Dis. 125:25–29. [DOI] [PubMed] [Google Scholar]
- 36.Kindler, V., A.P. Sappino, G.E. Grau, P.F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 56:731–740. [DOI] [PubMed] [Google Scholar]
- 37.Flynn, J.L., M.M. Goldstein, J. Chan, K.J. Triebold, K. Pfeffer, C.J. Lowenstein, R. Schreiber, T.W. Mak, and B.R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 2:561–572. [DOI] [PubMed] [Google Scholar]
- 38.Algood, H.M., P.L. Lin, and J.L. Flynn. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41(Suppl 3):S189–S193. [DOI] [PubMed] [Google Scholar]
- 39.Bean, A.G., D.R. Roach, H. Briscoe, M.P. France, H. Korner, J.D. Sedgwick, and W.J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504–3511. [PubMed] [Google Scholar]
- 40.Zozulya, A.L., E. Reinke, D.C. Baiu, J. Karman, M. Sandor, and Z. Fabry. 2007. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J. Immunol. 178:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, M.X., X. Qu, B.H. Kong, Q.L. Lam, Q.Q. Shao, B.P. Deng, K.H. Ko, and L. Lu. 2006. Membrane type 1-matrix metalloproteinase is involved in the migration of human monocyte-derived dendritic cells. Immunol. Cell Biol. 84:557–562. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, W., S. Darmanin, Q. Fu, J. Chen, H. Cui, J. Wang, F. Okada, J. Hamada, Y. Hattori, T. Kondo, et al. 2005. Hypoxia suppresses the production of matrix metalloproteinases and the migration of human monocyte-derived dendritic cells. Eur. J. Immunol. 35:3468–3477. [DOI] [PubMed] [Google Scholar]
- 43.Baratelli, F.E., N. Heuze-Vourc'h, K. Krysan, M. Dohadwala, K. Riedl, S. Sharma, and S.M. Dubinett. 2004. Prostaglandin E2-dependent enhancement of tissue inhibitors of metalloproteinases-1 production limits dendritic cell migration through extracellular matrix. J. Immunol. 173:5458–5466. [DOI] [PubMed] [Google Scholar]
- 44.Osman, M., M. Tortorella, M. Londei, and S. Quaratino. 2002. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases define the migratory characteristics of human monocyte-derived dendritic cells. Immunology. 105:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravindran, R., L. Rusch, A. Itano, M.K. Jenkins, and S.J. McSorley. 2007. CCR6-dependent recruitment of blood phagocytes is necessary for rapid CD4 T cell responses to local bacterial infection. Proc. Natl. Acad. Sci. USA. 104:12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leon, B., M. Lopez-Bravo, and C. Ardavin. 2007. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 26:519–531. [DOI] [PubMed] [Google Scholar]
- 47.Bergqvist, S. 1950. Nord. Med. Increased reaction to BCG vaccination caused by hyaluronidase. 43:955–956. [PubMed]
- 48.Datsenko, K.A., and B.L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 97:6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.