Abstract
Increases in neuronal activity of hypocretin (HCRT), a peptide involved in arousal, and in HCRT-1 receptor mRNA expression have recently been identified in association with lactation. HCRT is released within brain regions regulating maternal behaviour and it is possible that increased HCRT neurotransmission during lactation supports maternal care. This study examined for the first time the behavioural effects of HCRT on lactating mice. At intermediate doses, intracerebroventricular (i.c.v.) injections of HCRT-1 (0.06 and 0.1 μg) elevated levels of licking and grooming of pups (but not self-grooming) and number of nursing bouts without affecting other behaviours. At the highest dose, i.c.v. HCRT-1 (0.3 μg) delayed latency to nurse, decreased nursing, increased time off nest, and decreased maternal aggression. Intraperitoneal injections of the HCRT-1 receptor antagonist, SB-334867, exhibited a general trend towards increasing time spent low-arched back nursing (p=0.053) and decreasing licking and grooming of pups while high-arched back nursing (p=0.052). This suggests that the endogenous release of HCRT, working independently or dependently with other neuromodulators, may be necessary for full maternal behaviour expression. Possible sites of HCRT action in enhancing and impairing maternal care were identified via examinations of c-Fos immunoreactivity in association with i.c.v. HCRT injections. Together, these finding support the idea of HCRT modulating maternal behaviour, with intermediate levels (0.06 and 0.1 μg) supporting, even augmenting some behaviours, but with levels too high (0.3 μg i.c.v. HCRT) maternal behaviour and aggression are suppressed.
Keywords: bed nucleus of the stria terminalis, lateral septum, maternal care, maternal defense, grooming
Lactation involves changes in arousal and wakefulness. Lactating rats exhibit increased sleep disruptions to tend to offspring (1-4). Also, lactating women experience elevated nighttime sleep disruptions and increased time awake associated with caring for offspring (5). These and other studies suggest a close connection between production of maternal behaviour and altered arousal during lactation.
Extensive evidence suggests that the neuropeptide, hypocretin (HCRT), modulates arousal (6). HCRT exists in two peptide forms, HCRT-1 and HCRT-2, also called orexin A and orexin B, respectively. Centrally administered HCRT increases wakefulness and arousal in rats (7) and also reduces REM sleep (8). A correlation has also been suggested between low HCRT neurotransmission and narcolepsy in mice (9). These links between HCRT and arousal suggest that HCRT might play a role in modulating arousal and sleep during lactation.
A recent study found increased diurnal c-Fos activity of HCRT neurones in lactating versus virgin female mice (10). Another study found that on postpartum day 1, lactating rats had increased mRNA levels of the HCRT-1 receptor (preferential binding site of HCRT-1) relative to pregnant females (11). While HCRT neurones are found exclusively in lateral hypothalamus (LH) and adjacent brain regions, HCRT receptors are found in a number of regions, including, locus coeruleus (LC), paraventricular hypothalamic nucleus (PVN), arcuate nucleus, septal regions, nucleus accumbens (NuAC), medial preoptic area (mPOA), and bed nucleus of the stria terminalis (BNST) (12,13). Many of these regions are implicated in maternal behaviour (14) and/or arousal (13). Intracerebroventricular (i.c.v.) injections of HCRT trigger increases in c-Fos activity in most of these regions (13,15). Hence, it is possible that HCRT could directly or indirectly influence maternal behaviour.
Despite indirect evidence linking HCRT to behaviour during lactation, no study has directly examined HCRT’s effect on maternal behaviour. By conducting i.c.v. injections with a range of HCRT doses into lactating mice, this study addresses whether or how HCRT supports reproductive behaviour.
We hypothesised that at intermediate levels, HCRT may enhance maternal behaviour, including maternal aggression, by elevating general arousal. At high levels of release, HCRT triggers the release of corticotropin-releasing factor (CRF) (12,16), a peptide shown to impair maternal aggression in mice (17) and maternal behaviour in maternally primed virgin rats when injected centrally (18). Therefore, we hypothesised that at high doses HCRT might inhibit maternal aggression and other maternal behaviours. We also predicted that the HCRT-1 receptor antagonist, SB-334867, would impair maternal behaviour and aggression by preventing normal HCRT activity. To gain insights into where HCRT-1 is acting within the CNS, we also examined where and how HCRT-1 altered c-Fos expression in association with triggering changes in behaviour.
Materials and Methods
Mice
Outbred (hsd:ICR) female mice (Mus musculus; :~ 42-56 days postpartum) (Harlan, Madison, WI) were housed with a single breeder male of the same strain. Following impregnation (~2 weeks), each female was housed individually for the remainder of the study. Female mice were given ad lib access to Breeder Chow (Harlan) and tap water. Just prior to parturition, female mice were given precut nesting material. Polypropylene cages were cleaned weekly prior to parturition, but afterwards cages were unchanged until the end of the experiment. Sexually naïve male mice of the same strain (~ 42-56 days postpartum) were used as intruders during aggression tests. Intruder males were group housed (4 mice/cage) and never used more than once per day (days between tests ranged between 3-7 days) and not for more than 3 total tests each. Over the course of testing, each female was exposed to three different intruder males and due to the counterbalanced nature of the study (see below), males with varying experience were equally distributed over the various peptide treatments. Males in this study were highly quiescent. As standard procedure intruder males are removed if they show signs of aggression and only in ~ 4% of cases (6 out of 147 tests) were intruder males removed. All mice were housed on a 14:10 light/dark cycle with lights on at 0600 CST. All testing was performed between 0930 and 1500 h. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the University of Wisconsin.
Cannulae surgeries
On postpartum Day 1 (parturition is postpartum Day 0), litters were culled to 11 pups and on this same day each lactating female was fitted with cannulae to the lateral ventricle. Females with less than 9 pups were omitted from the study. Before surgery, the hair above the skull was shaved and the skin cleaned and treated with alcohol and Betadine (Purdue Frederick, Stamford, CT). Under isoflurane anaesthesia, using a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), a midline incision across the top of the skull was made and the periosteum removed. A 1 mm hole was drilled −0.6 mm (posterior) to and 1.6 mm (lateral) to Bregma. In the hole, a 26 gauge stainless-steel indwelling cannula (Plastics One, Roanoke, VA) was implanted to −2.5 mm below the skull surface into the lateral ventricle. Two small screws were drilled into the skull lateral and anterior to the cannula to help stabilise the cannula. Each cannula was secured to the skull using dental cement (Plastics One). A dummy cannula was inserted to maintain patency until injections were made. Injections were made manually using a 33 gauge stainless-steel injector, which extended 1 mm beyond the guide, attached to PE-50 tubing (Becton Dickenson, Sparks, MD) fitted to a 10 μl Hamilton syringe.
Intracerebroventricular injections (i.c.v.) of HCRT-1
Beginning 2 days after surgery (postpartum Day 4), single injections were delivered each day for up to 3 consecutive days (postpartum Days 4-6) to mice under light isoflurane anaesthesia to minimise an acute stress response. Previous studies show that mice continue to display high levels of maternal behaviour when isoflurane is used for brief anesthetization, indicating that this approach does not adversely affect the behaviour of interest (17,19). All injections were made using a 1 μl volume over a 60 second time span. Infusions were verified by following movement of an air bubble in the tubing and the injector remained in place for 60 sec following each injection. Thirty minutes after injection, each female was tested for maternal aggression for 5 min and maternal behaviour for 55 min as described below. After the completion of the testing series and just prior to fixation (described below), a 1 μl volume of 0.01 % Chicago sky blue (Sigma, St. Louis, MO) in saline was injected into the brain to verify cannulae placement. Only results from cannulae correctly directed into the lateral ventricle were used. The following doses were injected into two groups: HCRT-1/Orexin-A (Phoenix Pharmaceuticals, Belmont, CA); 0.03 μg (0.008 nmol), 0.06 μg (0.016 nmol), 0.1 μg (0.028 nmol) and 0.3 μg (0.084 nmol) dissolved in saline. Saline was used as the vehicle solution and control for each mouse. A range of 4 doses of HCRT-1 were tested across two groups of mice (1 vehicle and 2 doses per group) as each mouse underwent a maximum of three tests. Limiting each mouse to three tests was done to ensure that all behavioural testing occurred during the window in which high levels of maternal behaviour (Days 2-8) (20), including aggression, are expressed and is consistent with previous studies (17,19). To test the behavioural effects of Group A (0.03 μg and 0.3 μg HCRT-1 versus vehicle) 19 mice were used. For testing the behavioural effects of Group B (0.06 μg and 0.1 μg HCRT-1 versus vehicle) 18 mice were used. Each mouse was tested as part of a group (either A or B) and received each treatment within that group over 3 consecutive days (e.g. 0.3 μg, 0.03 μg HCRT-1 and vehicle in Group A) employing a within animal repeated measures experimental design. I.c.v. HCRT-1 has previously been shown to have a behavioural timecourse of about 90 minutes (7,15) and site-directed injections have been shown to have waking effects up to 4 hours (21), therefore repeated treatment would have limited impact on the effects of HCRT-1 over time. The orders of all injections of HCRT-1 were counterbalanced such that an equal number of mice received each of the three doses (including vehicle) on the three different test days. Doses were chosen based on previous i.c.v. studies of the behavioural effects of HCRT in rats (7).
Intraperitoneal (i.p.) injections of HCRT-1 receptor antagonist SB-334867
A separate set of lactating mice from those used to test the effects of the i.c.v. injections were used to investigate the effects of the HCRT-1 receptor antagonist, SB-334867 (Tocris Bioscience, Ellisville, MO). On postpartum Day 4, 30 minutes following injection, dams underwent a maternal aggression and behaviour test (without treatment) to determine individual baseline levels. On postpartum Day 6 a single i.p. injection was delivered to mice under light isoflurane anaesthesia to minimise an acute stress response of the mice. The following two doses along with vehicle were injected i.p. into three discrete groups: SB-334867; 10 mg/kg and 30 mg/kg dissolved in a 60:40 dimethyl sulfoxide (DMSO)/saline solution, and vehicle; 60:40 DMSO/saline solution was used as the control. To test the behavioural effects of SB-334867, 6 mice were used in each group for a total of 18 mice. Doses were chosen based on previous i.p. studies of the behavioural effects of SB-334867 in rats (22,23). The range of timing of the antagonist injections mirrored those used for the agonist, HCRT-1.
Maternal aggression and behaviour testing
Prior to each injection (whether i.c.v. or i.p.), pups were removed from the home cage and placed under a warming lamp until they were reunited with dam following the maternal aggression test. After each injection, females were promptly returned to their home cages and after 30 min elapsed, females were moved into the testing room, where they were tested in their home cages, an environment kept constant during the experiment. Previously, females had not entered the testing room, but had resided in their home cages since pairing. The maternal aggression test began by introducing a male intruder for 5 min. Removal of pups from the home cage of a dam before an aggression test does not diminish the expression of maternal aggression in mice (24). After the intruder male was removed, the pups were scattered evenly away from the nest allowing the female to retrieve and/or interact with her pups and perform maternal behaviour for 55 minutes. The total testing lasted an hour. Each test session was recorded on videotape and subsequently analyzed off-line to quantify maternal behaviour by individuals blind to testing conditions. For quantification of maternal aggression the following features were measured: latency to first attack, number of attacks, and total duration of attacks (25,26). Pup retrieval was quantified by measuring the time elapsed to retrieval of first, fourth and all pups (17,19). Other maternal behaviour measures quantified included latency to nurse, number of nursing bouts (nursing bout could include shifts in mother’s nursing as long as pups remained in nipple contact and end of nursing bout was defined as all pups being off nipples), duration of each nursing bout, total time nursing, time spent in the high-arched back nursing (HAN) position (determined by females adopting a crouched position that arched over pups with legs splayed and fully extended), time spent in the low-arched back (LAN) or supine nursing position (determined by female adopting a “blanket” posture in which the dam lays almost flat over the pups with minimal leg extension or if the dam is laying on her side while the pups are nursing), total duration of nest building activity, total duration of licking and grooming of pups by the female, latency for a dam to be on nest, total time a dam was on her nest (often while engaging in other maternal behaviour), total time spent off the nest, time spent off the nest subsequent to first nest bout, and total time the dam spent self-grooming. Also analysed was time the dam spent licking and grooming her pups while HAN and LAN. Nursing postures were determined from previous studies (27,28).
Immunohistochemistry for c-Fos
One day following the last behavioural test (postpartum Days 8) mice were injected with either HCRT-1 or saline (described below), returned to their home cage with their pups and brains were collected 105 minutes later (±5 min). No behavioural tests were performed. To analyze how HCRT-1 affected c-Fos activity in Group A, 19 mice were randomly assigned to 2 groups: saline-injected (n=11) and 0.3 μg HCRT-1-injected (n=8). The dose of 0.3 μg HCRT-1 was chosen because it was the lowest effective dose of HCRT-1 to reduce measures of maternal aggression in Group A. To analyze how HCRT-1 affected c-Fos activity in Group B, 15 mice were randomly assigned to 2 groups: 0.06 μg HCRT-1 (n=9) and 0.1 μg HCRT-1-injected (n=9). Both doses of 0.06 and 0.1 μg HCRT-1 were chosen and a saline-injected control group not collected because at the time of the collection, the behavioral analysis was not complete and therefore it was not clear which dose, if any, would have an effect on maternal behaviour. However, both doses of HCRT-1 were effective at elevating levels of licking and grooming of pups in Group B lactating females and only the brains injected with 0.1 μg HCRT-1 were further examined. A small number of brains were collected on Day 9 or 10 (rather than Day 8), therefore, a correlation analysis was performed only using those brains fixed on Day 8 (2 brains were fixed on Day 9). It was found that results from Day 8 only animals were highly consistent with results from the correlation of all animals regardless of day of fixation (data not shown). Therefore, all animals in Group B were included in the analysis.
Following isoflurane anaesthesia, mice were sacrificed and the brains removed. Brains were post-fixed overnight in 6% acrolein in phosphate buffered saline (PBS) and cryoprotected in 30% sucrose in PBS for two days. Brains were frozen on a platform and cut into 40 micron thick sections using a sliding microtome (Leica, Microsystems, Heidelberg, Germany) and stored in a cryoprotectant solution at −20 degrees C until processing for immunohistochemistry with antibodies that are specific to recognizing c-Fos proteins. Immunohistochemistry was run separately for Group A and Group B. Group A consisted of one of two sets of alternate sections of 0.3 μg and vehicle and Group B consisted of one of two sets of alternate sections of 0.1 μg HCRT-1. For both groups, sections underwent previously described immunohistochemical processes, which included incubating the sections for two days at 4 degrees C with rabbit anti-Fos antibodies (1:15,000; Calbiochem, San Diego, CA, catalog # PC38) (17,19). The sections were then mounted, dehydrated in a series of ethyl alcohols and xylenes, and coverslipped.
Analysis of c-Fos immunoreactivity (Fos-IR)
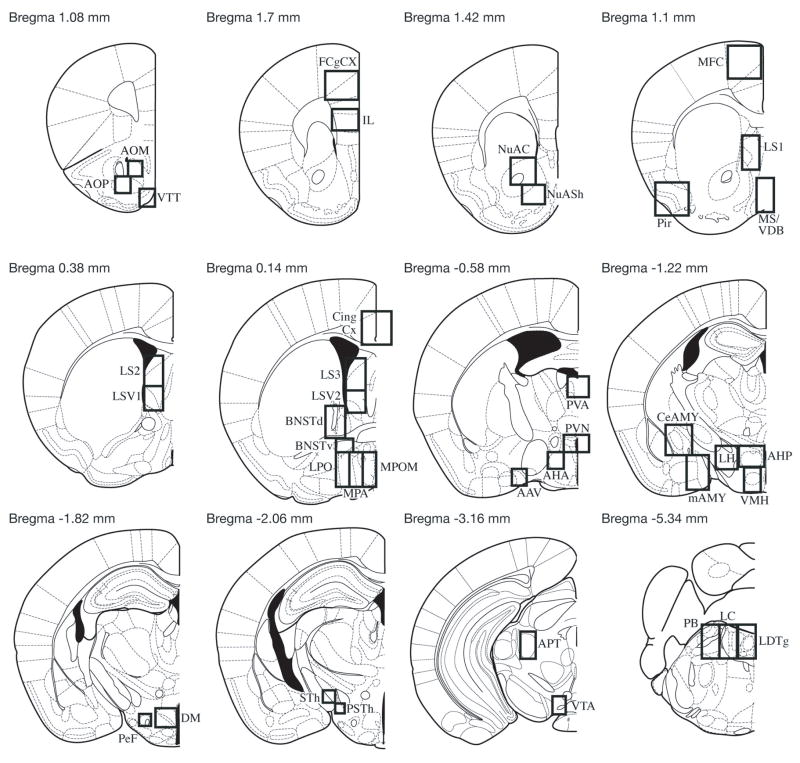
For both groups, previously described bright field microscopy was used for counting c-Fos-positive cells (17,19). Dimensions and locations of boxes used to count cells in specific regions are shown in Fig 1. All group sections were run in one batch.
Fig 1.
Schematic representation of the brain regions analyzed. Not shown is Bregma 0.86 mm (which includes SHi), Bregma 0.02 mm (which includes Tu and VLPO), Bregma −0.46 mm (which includes SCN), Bregma −0.82 mm (which includes SON), Bregma −1.34 mm (which includes CA1, CA2, CA3, DG and SI), Bregma −2.3 mm (which includes PH) and Bregma −4.84 mm (which includes cPAG and DRD). Reprinted from The Mouse Brain in Stereotaxic Coordinates, 2nd ed., G. Paxinos and K.B.J. Franklin, Figures 14, 17,19, 22, 28, 30, 36, 41, 46, 48, 57, 75, Copyright 2001, with permission from Elsevier. AAV, anterior amygdaloid area; AOM, anterior olfactory nucleus medial; AOP, anterior olfactory nucleus posterior; AHA, anterior hypothalamic area; AHP, AHA posterior; APT, anterior pretectal nucleus, BNSTd, bed nucleus of the stria terminalis dorsal; BNSTv, BNST ventral; CeAmy, central amygdala; CA1, field CA1 of hippocampus; CA2, field CA2 of hippocampus; CA3, field CA3 of hippocampus; Cg Cx, cingulate cortex; cPAG, caudal periaqueductal grey; DG, dentate gyrus; DM, dorsomedial nucleus; DRD, dorsal raphe nucleus; F Cg Cx, frontal cingulate cortex; IL, infralimbic cortex; LC, locus coeruleus; LDTg, laterodorsal tegmental area; LH, lateral hypothalamus; LPO, lateral preoptic area; LS, lateral septum; LSV, LS ventral; mAmy, medial amygdala; MFC, medial frontal cortex; MS/VDB, medial septal nucleus/vertical limb of the diagonal band; MPA, medial preoptic area; MPOM, medial preoptic nucleus; NuAC, nucleus accumbens core; NuASh, nucleus accumbens shell; Tu, olfactory tubercle; PB, parabrachial nucleus; PeF, perifornical nucleus; PH; posterior hypothalamic area; Pir Cx, piriform cortex; PSth, parasubthalamic nucleus; PVA, paraventricular thalamic nucleus; PVN, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus; SHi, septohippocampal nucleus; SI, subtantia innominata; SON, supraoptic nucleus; STh, subthalamic nucleus; VMH, ventromedial hypothalamus; VLPO, ventrolateral preoptic area; VTA, ventral tegmental area; VTT, ventral tenia tecta.
Data analysis
Data was analysed with the assistance of the statistical software, SigmaStat 3.0 (SPSS Inc, Chicago), in which the normality of the data was determined and the correct parametric or non-parametric test utilised. For i.c.v. injections, maternal aggression and other maternal behaviour testing variables were analyzed using a repeated measures (RM) ANOVA. In the cases where the data were not normally distributed, (i.e. latency to first attack) a non-parametric Friedman RM ANOVA on Ranks test was performed. In the case of latency to first attack, if an animal was non-aggressive, a time of 300 s (the maximum time of the aggression test) was assigned. Likewise, if an animal did not retrieve at least 4 pups or any pups at all, a time of 120 seconds was assigned (the maximum assigned in previous studies) (17,19). In addition, the effect of day on behaviour or treatment did not significantly affect of the results, therefore, this was not included in the analysis. No mortality of pups occurred following any injections therefore this measure was not analysed. The standard p-value cutoff of 0.05 was used to evaluate the significance of the behavioural data.
It was found that Group A mice, when given the highest injections of HCRT-1 (0.3 μg), displayed a longer latency to nurse (see Results). As a result these mice spent a shorter time participating in maternal behaviour relative to when they were injected to vehicle (because they took longer to become maternal). Therefore maternal behaviour in Group A was also analysed to evaluate the percentage of time the dams engaged in maternal behaviour once becoming maternal. Becoming maternal was defined as the dam spending 10 or more continuous seconds on-nest before engaging in off-nest behaviour. Percentage of time dams engaged in maternal behaviour while on-nest was also evaluated for Group A.
All HCRT-1 receptor antagonist, SB-334867, behavioural data was analysed by first establishing differences from baseline for each mouse with each treatment to incorporate individual variability into the statistical model. A parametric one-way analysis of variance (ANOVA) on difference scores was then performed between the three groups (e.g. vehicle, n=6; 10 mg/kg SB-334867, n=6; 30 mg/kg SB-334867, n=6) for each behavioural measure. This approach is modeled after standard statistical method used in other behavioral paradigms (Toufexis et al., 2004; Walker et al., 2003). In cases where data were not normally distributed (i.e. latency to nurse), a non-parametric test Kruskal-Wallis One Way Analysis of Variance on Ranks was used.
For c-Fos analysis, separate one-way ANOVAs were used to test the effect of Group A (vehicle and 0.3 μg HCRT-1) on number of c-Fos-positive cells in 52 separate brain regions. In cases where the data were not normally distributed, then a non-parametric Kruskal-Wallis ANOVA on Ranks test was used. For all other measures parametric tests were employed. In Group A, the number of mice per group were, saline, n=11 and 0.3 μg HCRT-1, n=8. Areas evaluated included brain regions containing HCRT-1 receptors (due to the high binding affinities of HCRT-1 to this receptor) and brain regions previously implicated in maternal aggression, maternal behaviour, and/or HCRT-1 signalling. In order to correct for multiple comparisons (52 one-way ANOVAs) the False Discovery Rate method of Storey (2002) was applied using the open source software Qvalue (29). This method can be used to estimate the p-value cutoff to use for each test that will yield a global, experiment-wide, false discovery rate of 5%. This method has been used previously to correct for multiple comparisons (17,19,30). Our data set consisted of a combination of large and small p-values (13 out of 52 total tests yielded a p-value of less than 0.05) (Table 2). Applying the p-value standard cutoff of .05 would only yield a false discovery rate of 0.7% (e.g. 0.7 out of 100 positive results will be false positives). Therefore, despite the performance of multiple tests, it remains appropriate to apply the standard p value of 0.05 to the data set.
Table 2.
Mean (±SEM) time (in sec) of differences in maternal behaviour (including aggression) performed in lactating mice with i.p. injections of HCRT-1 receptor antagonist SB-334867 relative to baseline. No values differed significantly from vehicle (determined by One way ANOVA (see Methods). Measures showing general trends (p<0.06), shown in italics.
| Maternal Behaviour | ||||
|---|---|---|---|---|
| Vehicle (n=6) | SB-334867 10 mg/kg (n=6) | SB-334867 30 mg/kg (n=6) | One Way ANOVA | |
| Latency to first attack | 90.0±59.7 | 213.5±55.1 | 176.7±55.9 | p = 0.317 |
| # of attacks | −8.2±5.4 | −12.8±4.3 | −11.2±3.5 | p = 0.760 |
| Total time aggressive | −10.7±7.4 | −16.8±5.4 | −15.0±4.7 | p = 0.757 |
| Time retrieve pup 1 | −5.8±21.5 | 47.8±15.3 | 51.2±22.8 | p = 0.113 |
| Time retrieve pup 4 | 9.0±9.0 | 27.7±17.6 | 15.3±10.7 # | p = 0.668 # |
| Latency to nurse | −5.8±659.6 | −130.0±814.9 | −241.3±158.4 # | p =0.994 # |
| # of nursing bouts | 1.0±0.9 | −1.8±2.4 | 0.3±0.3 # | p =0.851 # |
| Total nursing | −94.8±429.6 | −5.3±710.9 | 128.8±204.9 | p =0.950 |
| Total HAN | 253.8±343.0 | −912.7±641.6 | −750.8±395.2 | p = 0.208 |
| Total LAN | −348.7±213.8 | 907.3±480.0 | 998.8±442.4 | p = 0.053 |
| Total nest building | −48.0±22.3 | −69.2±29.2 | −113.5±48.4 | p = 0.425 |
| Latency to LG | 332.3±743.3 | −835.0±811.7 | −132.2±293.4 | p = 0.486 |
| Total LG | 52.0±41.0 | −39.5±30.3 | −64.5±53.0 | p = 0.158 |
| LG while HAN | 40.8±27.4 | −46.8±33.1 | −89.3±42.5 | p = 0.052 |
| Latency to on-nest | 508.3±436.4 | −580.7±572.8 | 6.8±45.5 | p = 0.214 |
| Total on-nest | −151.8±290.8 | 371.2±171.3 | −200.2±135.5 | p = 0.137 |
| Total self-groom | −14.8±169.9 | −10.7±61.6 | −47.0±80.2 # | p = 0.755 # |
| Total off-nest | −202.5±223.1 | −195.8±262.0 | 63.5±209.0 | p = 0.661 |
- data non-normal, analyzed with the Kruskal-Wallis One Way Analysis of Variance on Ranks
For the brain regions in Group B (for which no control group existed); 0.1 μg HCRT-1, n=9, Pearson correlation coefficients were calculated between each brain region among the individuals to assess the pattern of c-Fos co-activation triggered by the peptide, as previously performed (31). After running a Pearson correlation on all data in Group B, clusters of positively correlated regions were identified. Pearson correlations were also run on vehicle and 0.3 μg injected brains from Group A.
Results
Effects of i.c.v. HCRT-1 on maternal aggression
Group A
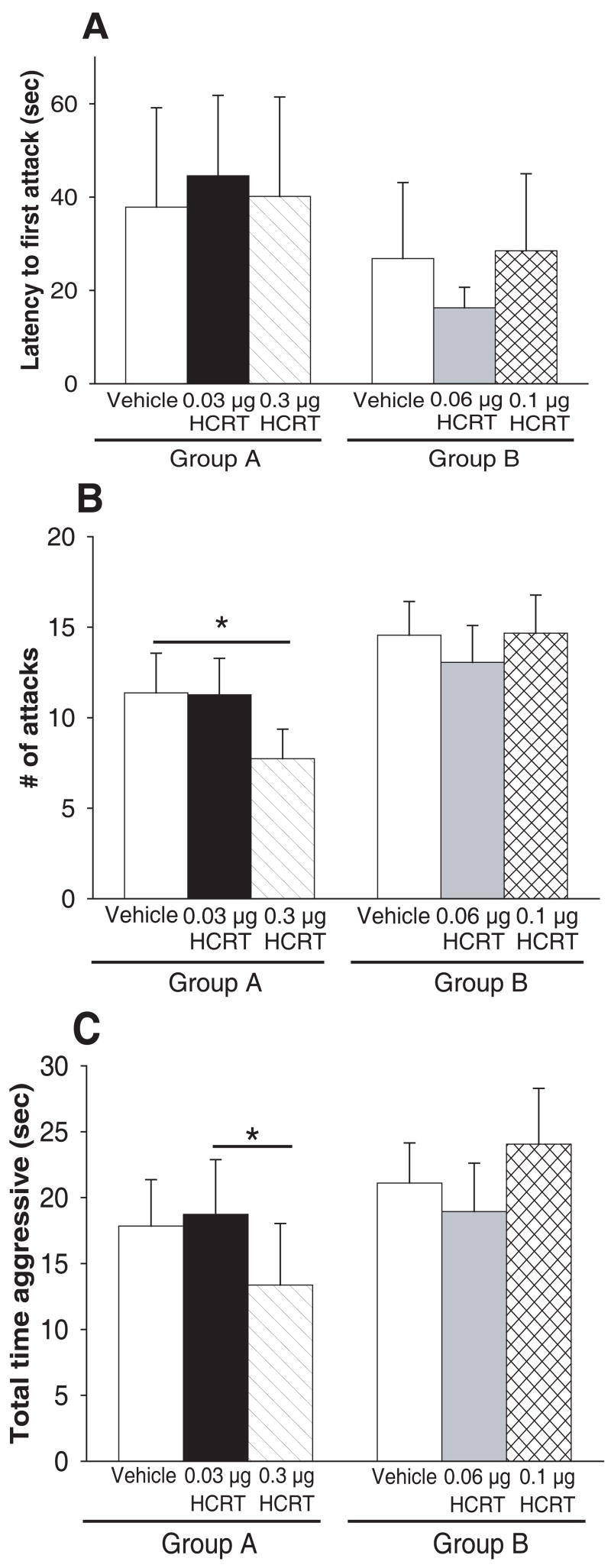
There was a significant overall effect of treatment on Group A on maternal aggression expression in 2 different behavioural measures (described below) (Fig. 2B-C), but not on latency to first attack (X² (2) = 2.657, p = 0.265; Friedman RM ANOVA on Ranks) (Fig. 2A).
Fig 2.
Effects of i.c.v. HCRT-1 on measures of maternal aggression in outbred mice. A) mean latency to first bite is not significantly delayed by any dose of HCRT-1 in either Group A or B. B) Group A: mean number of attacks is significantly reduced by 0.3 μg HCRT-1 relative to vehicle (One way RM ANOVA). Group B: mean number of attacks is not significantly affected by either 0.06 or 0.1 μg HCRT-1 relative. C) Group A: mean total time aggressive is significantly decreased by 0.3 μg HCRT-1 relative to 0.03 μg HCRT-1 (One-way RM ANOVA). Group B: mean total time aggressive is not significantly altered by either 0.06 or 0.1 μg HCRT-1. Bars represent means ± SE. For all tests, order of injections was counterbalanced. * = p<0.05.
Group A injections significantly altered the mean number of attacks (X² (2) = 6.29, p = 0.043; Friedman RM ANOVA on Ranks) (vehicle, median= 9, 25%=3.25, 75%=18.5; HCRT-1, 0.03 μg median= 8, 25%=3.5, 75%=17.75; HCRT-1, 0.3 μg median= 9, 25%=1.25, 75%=13) (Fig. 2B). Dunn’s Method post-hoc tests revealed that the lowest dose of HCRT-1 in Group A (0.03 μg) had no effect on the mean number of attacks relative to vehicle injections. In contrast, 0.3 μg HCRT-1 significantly decreased the mean number of attacks relative to vehicle (Q = 2.028, p<0.05; Dunn’s Method) (Fig. 2B).
Group A injections also significantly affected the total mean time aggressive (X² (2) = 9.233, p= 0.010; Friedman RM ANOVA on Ranks) (vehicle, median= 13, 25%=4.75, 75%=29.0; HCRT-1, 0.03 μg median= 17, 25%=5, 75%=31.75; HCRT-1, 0.3 μg median= 9, 25%=1.25, 75%=17) (Fig. 2C). Dunn’s Method post-hoc tests revealed that neither dose of HCRT-1 in Group A (0.03 or 0.3 μg) had an effect on the mean time aggressive relative to vehicle injections. However, 0.3 μg HCRT-1 decreased the mean time aggressive relative to 0.03 μg HCRT-1 (Q = 2.433, p<0.05; Dunn’s Method) (Fig 2C).
Group B
There was not a significant overall effect of treatment on Group B in any of the 3 behavioural measures of maternal aggression (Fig 2A-C). Neither injection (0.06 or 0.1 μg HCRT-1) affected latency to first attack (X² (2) = 0.116, p = 0.944; Friedman RM ANOVA on Ranks), number of attacks (F (2,17) = 0.406, p = 0.669; One Way RM ANOVA), or total time aggressive (X² (2) = 3.855, p = 0.146; Friedman RM ANOVA on Ranks).
Effects of i.c.v. HCRT-1 on maternal behaviour
Group A
There was no overall effect of treatment for Group A injections (0.03 and 0.3 μg HCRT-1) on pup retrieval in relation to vehicle injections (Table 1), including time to retrieve all pups (data not shown). There was an overall significant treatment effect of Group A injections on 4 different measures of maternal behaviour (see below).
Table 1.
Mean (±SEM) time (in sec) of maternal behaviour performed in lactating mice in Group A (i.c.v. injections of vehicle and 0.03 μg and 0.3 μg of HCRT-1) and Group B (i.c.v. injections of vehicle and 0.06 μg and 0.1 μg HCRT-1). Values that differ significantly from vehicle are shown in bold; p < 0.05.
| Maternal Behaviour | Group A | (n=19) | Group B | (n=18) | ||
|---|---|---|---|---|---|---|
| Vehicle | HCRT 0.03 μg | HCRT 0.3 μg | Vehicle | HCRT 0.06 μg | HCRT 0.1 μg | |
| Time retrieve pup 1 | 63.7±11.7 | 85.7±10.8 | 69.7±12.1 # | 65.4±12.0 | 62.9±10.3 | 78.8±11.9# |
| Time retrieve pup 4 | 110.6±6.4 | 104.9±6.3 | 103.1±7.2 | 101.1±7.8 | 105.2±7.1 | 95.8±8.9 # |
| Latency to nurse | 393.1±45.1 | 755.4±168.0 | 1094.1±214.8* | 653.6±182.2 | 437.6±80.1 | 603.9±122.1 # |
| # of nursing bouts | 5.7±0.7 | 4.6±0.6 | 4.9±0.8 | 3.3±0.7 | 4.3±0.6 | 4.7±0.6 * # |
| Total nursing | 2243.4±106.9 | 2000.3±160.4§ | 1326.5±205.7† | 2079.8±219.6 | 2331.2±117.2 | 2139.2±165.9 # |
| Total HAN | 1911.8±543.9 | 1180.1±208.1 | 1018.4±210.1 | 1283.6±241.7 | 1561.7±172.8 | 1428.4±199.1 |
| Total LAN | 819.7±163.2 | 801.9±200.9§ | 302.4±111.9† | 856.1±203.7 | 1010.3±271.0 | 688.0±146.2 # |
| Total nest building | 38.45±13.4 | 31.8±9.7 | 34.1±8.8 | 32.6±10.7 | 37.7±10.2 | 35.7±10.4 # |
| Latency to LG | 710.9±163.1 | 637.1±106.4 | 1165.1±201.9 # | 1141.3±287.6 | 813.9±190.7 | 674.6±150.0 # |
| LG while HAN | 59.7±17.6 | 42.0±11.5 | 58.6±21.6 | 28.1±11.7 | 62.2±18.0* | 61.0±17.1* # |
| LG while LAN | 32.9±13.2 | 13.1±6.1 | 23.1±11.5 | 16.4±7.6 | 14.6±7.3 | 26.6±10.6 # |
| Latency to on-nest | 337.3±44.8 | 512.6±104.8 | 781.6±207.5 | 375.7±176.0 | 311.9±67.7 | 348.9±74.2 # |
| Total on-nest | 349.7±78.1 | 404.2±128.7 | 316.3±73.1 | 375.0±62.92 | 273.8±46.5 | 375.0±62.9 # |
| Total self-groom | 322.6±53.1 | 307.7±35.3 | 467.4±60.4 | 198.3±38.3 | 198.9±28.3 | 286.1±45.3 |
| Total off-nest | 729.5±107.7 | 945.6±157.6 ‡ | 1524.9±199.3 * | 767.9±222.2 | 602.2±102.8 | 814.2±177.8 # |
- data non-normal, analyzed with the Friedman Repeated Measures Analysis of Variance on Ranks
- increase relative to vehicle, p<0.05
- decrease relative to vehicle, p<0.05
-increase relative to 0.3 μg, p<0.05
-decrease relative to 0.3 μg, p<0.05
There was a significant overall effect of treatment Group A on latency for the dam begin nursing (F (2,17) = 5.376, p = 0.009; One Way RM ANOVA). Holm-Sidak post-hoc tests revealed that 0.3 μg HCRT-1 significantly increased latency to nurse relative to vehicle injections (t= 3.268, p= 0.002; Holm-Sidak), but there was no effect of the lowest dose of HCRT-1 (0.03 μg) on latency to nurse (Table 1).
In addition, there was a significant overall effect of treatment on Group A on the total time spent nursing by the lactating dam (F (2,19) = 6.985, p = 0.003; One Way RM ANOVA). Holm-Sidak post-hoc tests revealed that 0.3 μg HCRT-1 decreased total time nursing relative to vehicle injections (t=3.644, p<0.001; Holm-Sidak) and 0.3 μg HCRT-1 decreased total time nursing relative to 0.03 μg HCRT-1 (t = 2.555, p =0.015; Holm-Sidak) (see Table 1).
Also, Group A treatment had an overall significant effect on total time the dam spent in the LAN or supine nursing position (F (2,19) = 3.956, p = 0.028; One Way RM ANOVA). Holm-Sidak post-hoc tests revealed that 0.3 μg HCRT-1 decreased total time LAN relative to vehicle injections (t= 2.610, p= 0.013; Holm-Sidak) and 0.3 μg HCRT-1 decreased total time LAN relative to 0.03 μg HCRT-1 ( t= 2.219, p= 0.033; Holm-Sidak) (Table 1), but no other effects were observed.
Group A had an overall effect of treatment on the total amount of time a dam spent off her nest (F (2,17) = 5.723, p = 0.007; One Way RM ANOVA). Holm-Sidak post-hoc tests revealed that 0.3 μg HCRT-1 increased total time spent off-nest relative to vehicle injections (t= 3.290, p= 0.002; Holm-Sidak) and 0.3 HCRT-1 increased total time spent off-nest relative to 0.03 μg HCRT-1 injections (t= 2.339, p= 0.025; Holm-Sidak). No other measures of maternal behaviour were significantly altered by either Group A dose of HCRT-1 (0.03 μg and 0.3 μg), however, there was a general trend of treatment to increase the latency of a dam to lick and groom her pups (F (2,19) = 3.151, p = 0.055; One Way RM ANOVA) (see Table 1).
Because mice, when injected with the highest level of HCRT-1 (0.3 μg), displayed an increased latency to nurse, as an additional analysis, the percentage of time dams engaged in maternal behaviour once they became maternal was evaluated. Of the 3 other previously affected maternal behaviours (total time nursing, total time LAN, and total time off-nest), 2 of the same measures were still found to be significantly altered by treatment. These included decreased the percentage of time dams spent in the LAN or supine nursing position (F (2,17) = 4.622, p = 0.017; One Way RM ANOVA) and increased percentage of time the dam spent off her nest (F (2,19) = 7.009, p = 0.003; One Way RM ANOVA). In addition, 0.3 μg HCRT-1 also increased the percentage of time a dam spent self-grooming while nursing (F (2,19) = 4.388, p = 0.020; One Way RM ANOVA).
Group A mice were also evaluated for the percentage of time dams engaged in maternal behaviour while on-nest. None of the Group A injections (0.03 μg and 0.3 μg HCRT-1) had any effect on maternal behaviour performed on-nest (data not shown).
Group B
None of the Group B injections (0.06 and 0.1 μg HCRT-1) had any effect on pup retrieval in relation to vehicle injections (Table 1), including time to retrieve all pups (data not shown). There was an overall significant effect of Group B HCRT-1 injections on 3 different measures of maternal behaviour (see below). There was no effect of day of treatment on any measure of maternal behaviour (data not shown).
Group B had an overall effect of treatment on the number of nursing bouts ( X² (2) = 6.303, p=0.043; Friedman RM Analysis of Variance on Ranks) (vehicle, median=3, 25%=1.00, 75%=4.00; HCRT-1, 0.06 μg median= 4.000, 25%=2.00, 75%=5.00; HCRT-1, 0.1 μg median=4, 25%=3.00, 75%=7.00). Dunn’s Method post-hoc tests revealed that 0.1 μg HCRT-1 significantly increased the number of nursing bouts relative to vehicle injections (Q= 2.333, p<0.05; Dunn’s Method) (Table 1), but no other effects were detected.
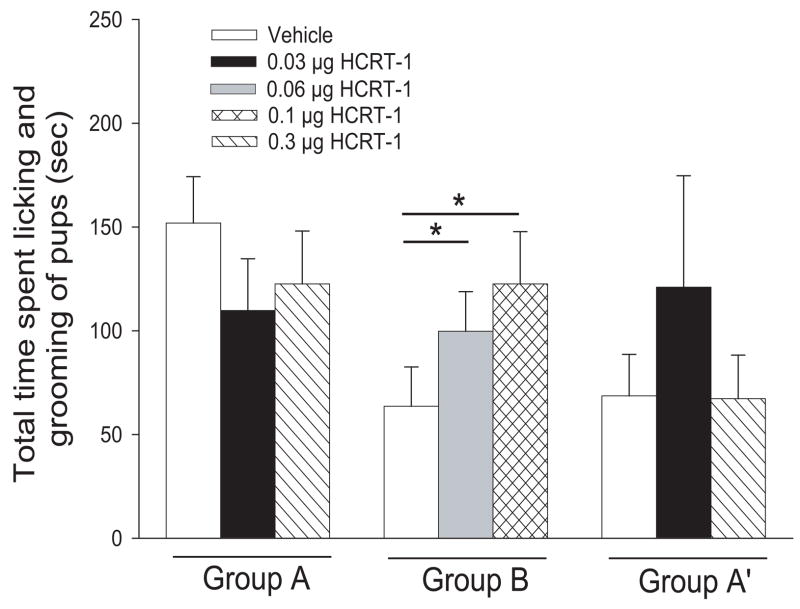
In addition, there was a significant Group B overall effect of treatment on the level of licking and grooming of pups by the dam ( X² (2) = 12.444, p=0.002; Friedman RM Analysis of Variance on Ranks) (vehicle, median=21.5, 25%=107.00, 75%=4.00; HCRT-1, 0.06 μg median= 78.50, 25%=40.00, 75%=153.00; HCRT-1, 0.1 μg median=118.500, 25%=34.00, 75%=154.00). Dunn’s Method post-hoc tests revealed that 0.06 μg significantly increased levels of licking and grooming of pups relative to vehicle injections (Q= 2.667, p<0.050; Dunn’s Method) and 0.1 μg HCRT-1 also significantly increased levels of licking and grooming of pups relative to vehicle injections (Q= 3.333, p<0.05; Dunn’s Method) (Fig. 3). Due to the difference in vehicle levels of licking and grooming behaviour seen between the two groups (higher in Group A), data was also analysed just including mice with comparable vehicle levels. When vehicle levels for Group A (68.6±20.0 sec; n=9) were made equivalent to those of Group B (63.8±19.0 sec, n=18), there still was not a significant overall effect of treatment of Group A on licking and grooming behavior (F (2,11) = 0.465, p = 0.635; One Way RM ANOVA) (Fig. 3A’). Therefore, regardless of baseline levels, Group B injections still significantly elevated levels of pup licking and grooming, while Group A injections did not.
Fig 3.
Effects of i.c.v. HCRT-1 on licking and grooming behaviour of pups by females in outbred mice. Group A: mean total time spent licking and grooming was not affected by either 0.03 or 0.3 μg HCRT-1 relative to vehicle. Group B: mean total time spent licking and grooming behaviour was significantly increased by both 0.06 and 0.1 μg HCRT-1 relative to vehicle (One-way RM ANOVA). Group A’: vehicle levels of licking and grooming were adjusted to match the vehicle levels seen in Group B (see Results and Methods for details). Mean total time spent licking and grooming behaviour of pups from baseline (vehicle) was not affected by either 0.03 or 0.3 μg HCRT-1. Bars represent means ± SE. For all tests, order of injections was counterbalanced. * = p<0.05.
Also, there was a significant Group B overall effect of treatment on the level of licking and grooming of pups by the dam while in the HAN position (X² (2) = 6.533, p=0.038; Friedman RM Analysis of Variance on Ranks) (vehicle, median=10.00, 25%=0.00, 75%=41.00; HCRT-1, 0.06 μg median= 22.50, 25%=40.00, 75%=119.00; HCRT-1, 0.1 μg median= 39.50, 25%=0.00, 75%=99.00). Dunn’s Method post-hoc tests revealed that only 0.1 μg HCRT-1 significantly increased levels of licking and grooming of pups relative while HAN to vehicle injections (Q= 2.333, p<0.05; Dunn’s Method) (Table 1).
Behavioural effects of HCRT-1 receptor antagonist SB-334867
There was not a significant overall effect of treatment in any of the 3 behavioural measures of maternal aggression (Table 2). Neither injection (10 mg/kg or 30 mg/kg SB-334867) affected latency to first attack (F (2,17) = 1.241, p = 0.317; One Way ANOVA), number of attacks (F (2,17) = 0.279, p = 0.760; One Way ANOVA), or total time aggressive (F (2,17) = 0.284, p = 0.757; One Way ANOVA).
Neither dose of SB-334867 significantly affected any measure of maternal behaviour (Table 2). However, while not significant, overall SB-334867 tended to increase total time LAN (F (2,17) = 3.605, p = 0.053; One Way ANOVA), and decrease licking and grooming while HAN (F (2,17) = 3.619, p = 0.052; One Way ANOVA) (see Table 2).
Effects of i.c.v. injections of HCRT-1 on Fos-IR
0.1 μg HCRT-1
I.c.v. injections of 0.1 μg HCRT-1 (one of the doses of peptide shown to increase licking and grooming of pups) was injected into Group B mice in the absence of behavioural testing to identify sites of action within the CNS using Fos-IR.
Because no mice from this group were injected with vehicle (see Methods above) vehicle-treatment comparisons could not be made. Using an alternative approach of examining correlations of activity among the different brain regions (31), 11 brain regions (out of the 55 examined) were found to be significantly correlated with one another (Table 3), suggesting that 0.1 μg HCRT-1 injections either directly or indirectly affected activity in these regions. As indicated below, each of these regions is enriched with HCRT-1 receptors. A correlation analysis was also performed only using those brains fixed on Day 8 (2 brains were fixed on Day 9) and similar results from this correlation were found as when all animals from the group (regardless of day of fixation) were used (data not shown). Correlation analysis of Fos-IR in the 0.3 μg HCRT-1 and vehicle groups (described above) failed to show either a similar pattern of activation to brains injected with 0.1 μg HCRT-1 or a unique significant pattern of activation (data not shown). Table 3 contains Pearson correlation coefficients of subset of brain regions examined that show highly intercorrelated (r>0.70, p<0.05) c-Fos activity with treatment.
Table 3.
Pearson correlation coefficients of brain regions with highly intercorrelated (r>0.70) c-Fos activity with i.c.v. 0.1 μg HCRT-1, the effective dose that increased licking and grooming of pups by lactating dams.
| Brain Regions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AOM | IL | BNSTv | AHA | AHP | CeAmy | mAmy | LH | DM | VTA | LC | |
| AOM | 1 | ||||||||||
| IL | 0.762 | 1 | |||||||||
| BNSTv | 0.968 | 0.906 | 1 | ||||||||
| AHA | 0.946 | 0.892 | 0.970 | 1 | |||||||
| AHP | 0.927 | 0.762 | 0.787 | 0.920 | 1 | ||||||
| CeAmy | 0.802 | 0.846 | 0.819 | 0.836 | 0.808 | 1 | |||||
| mAmy | 0.885 | 0.935 | 0.913 | 0.956 | 0.926 | 0.951 | 1 | ||||
| LH | 0.874 | 0.926 | 0.927 | 0.978 | 0.959 | 0.826 | 0.951 | 1 | |||
| DM | 0.935 | 0.929 | 0.965 | 0.956 | 0.878 | 0.740 | 0.878 | 0.933 | 1 | ||
| VTA | 0.880 | 0.883 | 0.910 | 0.899 | 0.776 | 0.911 | 0.945 | 0.927 | 0.826 | 1 | |
| LC | 0.960 | 0.978 | 0.856 | 0.863 | 0.762 | 0.931 | 0.941 | 0.823 | 0.860 | 0.869 | 1 |
All correlations, p<0.05, except between LH and LC
0.3 μg HCRT-1
I.c.v. injections of 0.3 μg HCRT-1 (the dose of peptide that impaired maternal aggression, decreased time on two measures of nursing, and elevated time off nest) or vehicle were injected into Group A mice in the absence of behavioural testing to determine sites of action within the CNS using Fos-IR. Twelve out of the 52 brain regions examined showed a significant increase in Fos-IR relative to vehicle injections and these include, AOM, AOP, F Cg Cx, LS 1 (Fig. 5A and B), LS2, LSV 1, LSV2, PVA, AHA, SCN, mAmy, VMH, and LC (Table 4). One out of 52 brain regions examined, the subthalamic nucleus (STh), showed a significant decrease in Fos-IR relative to vehicle injections (Table 4). Table 4 contains means and standard errors for a subset of brain regions examined that showed either significant or close to significant changes with treatment (for additional regions examined see Fig 1). For those significant brain regions in which non-parametric tests were performed due to non-normal data distribution, the following features were found: AOM (vehicle, median= 4, 25%=1.5, 75%=5.25; 0.3 μg HCRT-1, mean= 20, 25%= 7, 75%= 27.25), AOP (vehicle, median= 1, 25%=0.75, 75%=4.25; 0.3 μg HCRT-1, mean= 11, 25%= 2.5, 75%= 33.0), F Cg Cx (vehicle, median= 32, 25%=11.75, 75%=49.5; 0.3 μg HCRT-1, mean= 63.5, 25%= 51.0, 75%= 82.0), LS2 (vehicle, median= 6, 25%=5.25, 75%=12.75; 0.3 μg HCRT-1, mean= 35.5, 25%= 13, 75%= 39.5), LSV2 (vehicle, median= 50, 25%=33.5, 75%=66.0; 0.3 μg HCRT-1, mean= 134.0, 25%= 62.5, 75%= 190.0), STh (vehicle, median= 10, 25%=9, 75%=12; 0.3 μg HCRT-1, mean= 3, 25%= 1.5, 75%= 8.5).
Table 4.
Mean (±SEM) number of c-Fos-positive cells in brain regions of mice injected with HCRT 0.3 μg or vehicle. All regions included with p<0.15. As determined by Q-value software (see Methods), regions with p<0.05 reach significance and are shown in bold.
| Brain Region | Vehicle (n) | HCRT 0.3 (n) | One-way ANOVA |
|---|---|---|---|
| AOM | 3.8±1.0 (9) | 18.0±4.5 (7) | p= 0.011# |
| AOP | 4.4±2.9 (9) | 15.9±6.6 (7) | p= 0.041# |
| FCgCx | 46.1 ±16.5 (11) | 81.4±22.8 (8) | p = 0.047# |
| LS1 | 48.1±13.0 (11) | 98.4±19.4 (8) | p = 0.039 |
| LSV1 | 4.9±15.8 (11) | 114.9±14.2 (8) | p = 0.015 |
| LSV2 | 56.8±11.6 (11) | 122.4±25.8 (8) | p = 0.039 # |
| SCN | 104.1±20.5 (10) | 157.5±11.1 (8) | p = 0.049 |
| AHA | 23.3±4.6 (10) | 37.8±3.8 (8) | p = 0.032 |
| PVA | 318.8±34.2 (9) | 429.4±33.5 (8) | p = 0.036 |
| AHP | 54.9±8.6 (11) | 116.6±18.0 (8) | p = 0.004 |
| VMH | 13.1±2.8 (10) | 32.4±7.4 (8) | p = 0.018 |
| STh | 10.9±1.6 (10) | 5.6±2.4 (8) | p = 0.040# |
| LC | 48.1±13.0 (11) | 98.4±19.4 (8) | p = 0.039 |
|
| |||
| NuAC | 34.6±6.8 (11) | 76.4±18.9 (7) | p = 0.062 # |
| NuASh | 18.2±5.2 (10) | 37.6±8.1 (7) | p = 0.051 |
| BNSTd | 27.4±4.2 (11) | 41.4±5.4 (8) | p = 0.053 |
| IL | 67.7±15.4 (11) | 114.6±21.4 (8) | p = 0.098# |
| MS/VDB | 22.2±8.5 (9) | 49.1±13.3 (7) | p = 0.097 |
| SHi | 20.2±6.6 (11) | 39.5±9.1 (8) | p = 0.095 |
| Cg Cx | 46.8±11.1 (11) | 91.3±21.9 (8) | p = 0.066 |
| LS2 | 14.3±5.9 (11) | 33.4±9.5 (8) | p = 0.052 # |
| MPA | 43.5±9.2 (11) | 70.4±8.4 (7) | p = 0.061 |
| MPOM | 63.5±12.3 (11) | 92.6±12.1 (7) | p = 0.131 |
| CA1 | 6.6±2.5 (11) | 14±4.5 (8) | p = 0.144 |
| DG | 18.455±3.4 (11) | 28.5±6.0 (8) | p = 0.136 |
| PH | 129.7±24.3 (7) | 207.7±31.6 (6) | p = 0.072 |
| APT | 15.6±6.6 (11) | 27.5±5.3 (8) | p = 0.063 # |
- data non-normal, analyzed with the Kruskal-Wallis One Way ANOVA on Ranks
Discussion
In the current study, we present evidence for the first time suggesting HCRT plays an important role in the production of maternal care. Our findings suggest that HCRT modulates maternal behaviour with intermediate levels supporting care and even specifically enhancing some behaviour, such as licking and grooming of pups. However, with HCRT levels that are too high (high dose i.c.v. HCRT) maternal care and aggression are impaired.
HCRT-1 modulation of maternal behaviour
Licking and grooming of pups by dams is considered a critical aspect of care-giving in rodents (14). For example, levels of licking and grooming of pups alters the HPA axis and behavioural responses to stress of pups when examined as adults (20,32). Interestingly, levels of licking and grooming of pups can also be transmitted epigenetically (33). This is the first study to show that HCRT-1 (0.06 and 0.1 μg both) can elevate levels of licking and grooming of pups. The increased licking and grooming found at these doses occurred mostly within high arched-backed nursing which itself was modestly increased (Table 1). Interestingly, when vehicle levels from Group A were adjusted to mirror those of Group B, the low dose of HCRT (0.03 μg) also displayed a trend toward increased time spent licking and grooming, though hat did not reach significance likely due to the large error (see Fig 3). Although, removal of pups is a standard procedure to induce the onset maternal behaviour, it is possible that the preceding maternal aggression test may have altered the behavioural response of the dam to the peptide. However, this is unlikely. Current results show that at the doses of HCRT-1 (0.06 μg and 0.1 μg) that elevated maternal care, no effect was found on maternal aggression. This demonstrates the ability of maternal behaviour to be altered independently of aggressive responding. However, to ensure no effect of the preceding aggression test, maternal aggression and maternal behaviour could be evaluated in separate groups of mice or an additional group of mice could be tested for maternal behaviour only. It may also be advantageous to allow for more recovery time between i.c.v. surgery and the onset of behavioural testing assuring limited surgical effects. Future studies examining remote HCRT injections while dams are behaving maternally (including finer distinctions of nursing behavior, such as the differences between still upright nursing and upright nursing while engaged in other behaviour) would be another approach to examine how HCRT alters maternal behaviour.
Importantly, self-grooming by dams was not significantly altered with treatment. Previously, HCRT-1 has been shown to elevate self-grooming activity (6,34) and HCRT cells exhibit a high discharge rate during self-grooming behaviour (35). Hence, alterations in the lactating brain could have switched the response to HCRT from self-grooming to grooming (and licking) of offspring. Centrally administered HCRT-1 and intra-VTA HCRT-1 infusions trigger increases in dopamine levels in prefrontal cortex that positively correlate with self-grooming (36). VTA sends projections to NuASh, which has a high density of HCRT fibres (37), and in which dopamine is released prior to the onset of and during a pup licking and grooming bout (38). Also, haloperidol (dopamine-2 receptor antagonist) inhibited self-grooming behaviour induced by i.c.v. HCRT (39). Together, these results suggest HCRT and dopamine may interact to regulate pup licking and grooming. A role for serotonin (40) and oxytocin in HCRT’s action is also possible. Recent reports have indicated that pup-licking levels are significantly lower in oxytocin knock-out mice (41) and decreased by i.c.v. injections of oxytocin antagonist in lactating rats (42). Other insights into how HCRT positively modulates licking and grooming of pups were provided by Fos-IR described below.
At the highest tested dose of HCRT-1 (0.3 μg, the only dose that impaired aggression), a subset of maternal behaviours were impaired, including latency to nurse, total time nursing, and total low arched-back nursing (Table 1). Time spent off the nest also increased significantly. These behaviours could be due to the state of hyperarousal linked with HCRT-1 (15) that may disrupt maternal care. Originally HCRT was cited as having a principal role in feeding behavior (13) via such experiments as central administration of both HCRT-1 and 2 which elevated food consumption levels in rats (43). However, recent studies have shown evidence that HCRT has a prominent role in the modulation of arousal and energy homeostasis, which may indirectly affect other behaviours (44-46).
Another explanation for HCRT’s behavioural effects at this high level is by triggering the central release of corticotropin-releasing factor (CRF) (12,16), a peptide previously shown to impair maternal behaviour in maternally primed virgin rats (18). HCRT neurones project to and have receptors in the PVN and amygdala (47,48), both of which are enriched with CRF-positive neurones. Because CRF neurones in PVN project to and contact HCRT neurones in the lateral hypothalamus (49,50), a regulatory loop between HCRT and CRF has been suggested. Hence, some of HCRT’s actions could be CRF-dependent, but whether or how the high dose of HCRT (0.3 μg) interacts with CRF to impair maternal care needs to be addressed. In addition, CRF release stimulates the hypothalamic-pituitary axis which in turn facilities corticosterone release. Previously corticosterone has been shown to modulate maternal behaviour in postpartum (51) and maternally sensitised virgin rats (52). Thus, HCRT could also be modulating maternal responding and defense indirectly via corticosterone release. Future studies monitoring and/or manipulating corticosterone in association with HCRT injections would be useful for possible mechanisms of HCRT’s actions.
HCRT-1 modulation of maternal aggression
At doses where HCRT positively modulates certain maternal behaviours, maternal aggression is unaffected (Table 1; Fig. 2). Only the highest HCRT-1 dose (0.3 μg) negatively affects maternal aggression. One possible mechanism for HCRT-induced decreases of aggression is via the triggering of CRF release, as indicated above. CRF dose-dependently impairs maternal aggression (17) as do two related peptides, urocortin (Ucn) 1 and Ucn 3 (19). Fos-IR with i.c.v. HCRT-1 described below provides additional indirect evidence for a link between HCRT and CRF. HCRT could also affect aggression via interactions with other peptides, such as oxytocin. Recent work indicates oxytocin decreases maternal aggression in rats when infused into the BNST and CeAmy (53), while an infusion of an oxytocin antagonist into the CeAmy increases maternal aggression (54). In contrast, another study showed that oxytocin injections into the PVN increase maternal aggression while oxytocin release in the CeAmy and PVN corresponds with displays of aggression during maternal defense tests (55). Oxytocin, then, can regulate maternal aggression in an interesting and complex manner that appears to depend on levels and sites of action. Understanding whether or how HCRT interacts with oxytocinergic pathways to negatively regulate maternal aggression would provide important insights into the neural basis of this behavior.
Behavioural Effects of HCRT-1 Antagonist, SB-334867
SB-334867 did not significantly affect maternal aggression or maternal behaviour (Table 2). However, general trends were seen in increases in latency to retrieve first pup (p=0.113), decreases in pup licking and grooming (p=0.158), decreases in time spent on-nest (p=0.137), increases in LAN (p=0.053) and decreases in pup licking and grooming while HAN (p=0.052). These findings are consistent with the idea that a certain tone of HCRT facilitates maternal care. The previous finding of elevated activity in HCRT neurones in association with lactation (10) suggested the possibility that HCRT is elevated above normal (non-lactating) levels to facilitate maternal care. It is believed that these trends were specific to maternal behaviour because all behaviour during a one-hour period was monitored and analysed. No effects were found on the non-maternal behaviour of self-grooming or off-nest activity which was used to survey general locomotor activity. One possible reason full behavioural effects were not seen is that the full effects of an antagonist are often not observed in the absence of peptide treatment. For example, when administered peripherally, SB-334867 decreases i.c.v. HCRT-induced locomotor activity and grooming in rats (23,40), but alone only decreases grooming frequency and not duration (22,56). Further studies utilising another HCRT-1 antagonist would be valuable in evaluating the effects of HCRT receptor blockage on maternal behaviour and aggression.
HCRT injections and Fos-IR
To examine possible sites where HCRT facilitated licking and grooming of pups, Fos-IR was evaluated in mice injected with 0.1 μg HCRT-1. Because vehicle-injected control sections were not available (see Methods above), we used a correlational analysis (31) to gain insights into HCRT altered brain activity. Significant correlations of activity among 11 brain regions were identified including: AOM, VTA, LH, LC, BNSTv, CeA, MeA, and AHA (Table 3). Most of these regions contains HCRT-1 receptors (48) or HCRT-IR fibres (37). An increase in c-Fos is also seen with injections of 0.3 μg HCRT-1 in 4 of the regions (AOM, AHA, AHP and LC), suggesting these regions are being activated via HCRT (see Table 4). Because most of these regions have been implicated in maternal behaviour (14,57-60), it is possible that HCRT is acting directly on any or all regions to elevate licking and grooming of pups and site-specific injections of HCRT would be required to evaluate these possibilities. HCRT has recently been linked to reward responding (60) and an array of studies indicates that maternal care is a rewarding or addictive behaviour (14,59,61-63). One possibility is that HCRT action in the lactating dam (possibly via VTA) is to reinforce this reward although this would need to be explored in subsequent studies.
It is also possible that these c-Fos results reflect behavioral output due to treatment. Therefore, it is important to perform future studies that can clarify these results. For example, studies using site-directed injections of HCRT into some of the regions highlighted above will help to further provide insights into where and how HCRT endogenously alters maternal care behaviour. A detailed examination of altered expression of HCRT receptors during lactation could also provide insights into normal sites of HCRT modulation of maternal care.
How HCRT-1 (0.3ag) may have impaired aggression and nursing behaviour was evaluated by comparing Fos-IR in vehicle and HCRT injected mice. HCRT-1 (0.3ag) triggered Fos-IR increases in 13 regions relative to vehicle (see Table 4). Most of the regions activated by HCRT-1 are HCRT-1 receptor enriched, except for the HCRT-2 receptor enriched lateral septal regions (47,48), however, HCRT-1 terminals fields have been detected there (64). Interestingly, some sites just above significance with altered Fos-IR activity are central to maternal care, such as MPA (p = 0.06), NuAC (p = 0.06), and NuASh (p = 0.05).
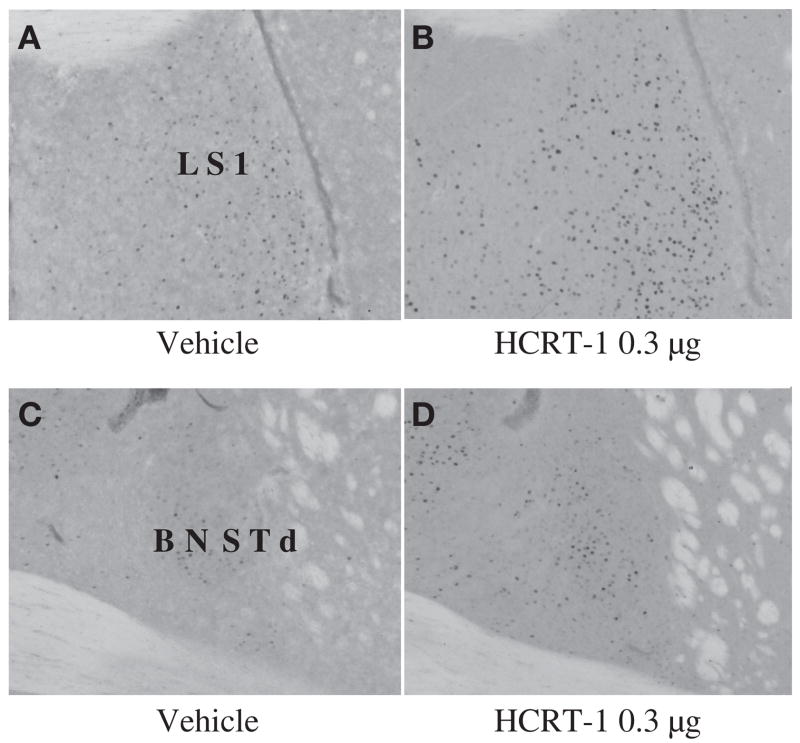
While any or all of the other regions with altered activity due to 0.3ag HCRT may contribute to decreased nursing and maternal aggression, LS is of particular interest for aggression (Fig 4). Previously, increased Fos-IR in LS occurred with the injection of three other peptides at doses that inhibit maternal aggression, CRF (17), Ucn 1, and Ucn 3 (19). BNSTd was also activated by these three related peptides and approached significance with HCRT in this study (p=0.053) (see Fig 4). It should be noted that these related peptides induced Fos-IR increases in slightly more posterior portion of the lateral septum than that seen here (19). This suggests that the peptides may negatively regulate maternal aggression through similar pathways and that LS, and possibly BNSTd, are critical regions involved in the negative regulation of maternal aggression. Whether Fos-IR increases are due directly to HCRT or indirectly via CRF (or Ucn 1 or 3) and whether LS plays a role in negatively regulating maternal aggression and altering maternal behaviour needs to be addressed in future studies. Coupling HCRT injections with the performance of behavior, such as pup reintroduction or introduction of a male intruder, and/or double-labeling of Fos and either HCRT or CRF receptors in future studies would provide insights into how HCRT is negatively affecting these behaviours.
Fig 4.
Examples of c-Fos immunoreactivity (Fos-IR) 2 h following i.c.v. infusion of either vehicle or HCRT-1 (0.3 μg). Injections were given 105 min (±5 min) before brain fixation. (A and C), vehicle, (B and D), HCRT-1 0.3 μg. Sites of significant increases in Fos-IR for HCRT-1 0.3 μg include LS1 (p= 0.039) and BNSTd (p=0.053) shows a general increasing trend (see Results).
Summary
Taking previous findings with those of the present study, we suggest that HCRT-1 is elevated in association with lactation and that HCRT modulates maternal care and aggression. With optimal levels, HCRT supports maternal behaviour (as seen with enhancement of some maternal behaviours), but if HCRT levels are too high (i.c.v. 0.3 μg HCRT) maternal behaviour and aggression are suppressed, as may also be the case if HCRT receptors are blocked (HCRT antagonist). To our knowledge, this is the first study to link directly HCRT to the regulation of maternal behaviour and to show HCRT can positively affect licking and grooming. How high HCRT levels negatively regulate some maternal behaviours is not known, but we propose that HCRT could inhibit both maternal defense and care by activating CRF, although this would need to be established experimentally. Together, these results add important new insights into how HCRT modulates maternal care and aggression.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH066086 to S.C.G. The authors wish to Dr. Justin Rhodes for statistical advice, Bill Feeny for assistance with figures and Emily Bethea, Kelly Clinkenbeard, Jose Evans, Mike Foley, Allen Irgens, Matthew Sutterer, Michelle Sulzer, Sharon Stevenson, Grace Lee, and Nina Hasen for technical assistance and Kate Skogen and Jeff Alexander for animal care.
References
- 1.Levin R, Stern JM. Maternal influences on ontogeny of suckling and feeding rhythms in the rat. J Comp Physiol Psychol. 1975;89:711–721. doi: 10.1037/h0077038. [DOI] [PubMed] [Google Scholar]
- 2.Leon M, Woodside B. Energetic limits on reproduction: maternal food intake. Physiol Behav. 1983;30:945–57. doi: 10.1016/0031-9384(83)90260-3. [DOI] [PubMed] [Google Scholar]
- 3.Stern JM, Levin R. Food availability as a determinant of the rats' circadian rhythm in maternal behavior. Dev Psych. 1976;9:137–148. doi: 10.1002/dev.420090206. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C, Johnson R, Nielsen M. Maternal behavior in mice selected for large litter size. Applied Animal Behaviour Science. 2002;79:63–73. [Google Scholar]
- 5.Nishihara K, Horiuchi S. Changes in sleep patterns of young women from late pregnancy to postpartum: relationships to their infants' movements. Percept Mot Skills. 1998;87:1043–1056. doi: 10.2466/pms.1998.87.3.1043. [DOI] [PubMed] [Google Scholar]
- 6.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:669–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 8.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 9.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 10.España R, Berridge C, Gammie S. Diurnal levels of Fos immunoreactivity are elevated within hypocretin neurons in lactating mice. Peptides. 2004;25:1927–1934. doi: 10.1016/j.peptides.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Murata T, Narita K, Kazumasa H, Higuchi T. Variation in the expression of orexin and orexin receptors in the rat hypothalamus during the estrous cycle. Endocrine. 2003;22:127–134. doi: 10.1385/ENDO:22:2:127. [DOI] [PubMed] [Google Scholar]
- 12.Samson WK, Taylor MT. Hypocretin/orexin suppresses corticotroph responsiveness in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1140–R1145. doi: 10.1152/ajpregu.2001.281.4.R1140. [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Bloom SR. Orexins/hypocretins: waking up the scientific world. Clin Endocrinol. 2001;54:421–429. doi: 10.1046/j.1365-2265.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 14.Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- 15.España RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 16.Jasberenyi M, Bujdoso E, Pataki I, Telegdy G. Effects of orexin on the hypothalamic-pituitary-adrenal system. J Endocrinol. 2000;12:1174–1178. doi: 10.1046/j.1365-2826.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CA, Caldwell JD, McGuire M, Evans DL. Corticotropin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sci. 1991;48:1537–46. doi: 10.1016/0024-3205(91)90278-j. [DOI] [PubMed] [Google Scholar]
- 19.D'Anna K, Stevenson SA, Gammie S. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:1061–71. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- 20.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe J, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–65. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii Y, Blundell J, Halford J, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ. Anorexia and weight loss in male rates 24h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–341. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers RJ, Halford J, Nunes de Souza R, Canto de Souza A, Piper DC, Arch J, Upton N, Porter R, Johns A, Blundell J. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioral satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- 24.Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiol Behav. 1981;26:253–8. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- 25.Gammie SC, Huang PL, Nelson RJ. Maternal aggression in endothelial nitric oxide synthase-deficient mice. Hormones & Behavior. 2000;38:13–20. doi: 10.1006/hbeh.2000.1595. [DOI] [PubMed] [Google Scholar]
- 26.Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–35. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- 28.Champagne F, Francis D, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 29.Dabney A, Storey JD. QVALUE [Computer software] Retrieved from http://facultywashingtonedu/~jstorey/qvalue/2002.
- 30.Rhodes JS, Ryabinin A, Crabbe J. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- 31.Day H, Masini C, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-Trimethylthiazoline (TMT), in rats, suggests both systematic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. [see comments] Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 33.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 34.Ida T, Nakahara K, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- 35.Mileykovskiy B, Kiyashchenko L, Siegel J. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vittoz N, Berridge CW. Hypocretin/Orexin selectively increases dopamine efflux within the prefrontal cortex: Involvement of the ventral tegmental area. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300807. in press. [DOI] [PubMed] [Google Scholar]
- 37.Peyron C, Tighe D, van de Poll NA, De Lecea L, Heller H, Sutcliffe J, Kilduff T. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Champagne F, Chretien P, Stevenson C, Zhang T, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuzaki I, Sakurai T, Kunii K, Nakamura T, Yanagisawa M, Goto K. Involvement of serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 2002;104:119–123. doi: 10.1016/s0167-0115(01)00355-x. [DOI] [PubMed] [Google Scholar]
- 40.Duxon M, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter R, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes, Brain and Behavior. 2003 doi: 10.1111/j.1601-183X.2005.00162.x. online publication. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams' oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–41. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski G, Wilson S, Arch J, Buckingham R, Haynes A, Carr S, Annan R, McNulty D, Liu W-S, Terrett J, Elshourbagy N, Bergsma D, Yanagisawa M. Orexin and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 44.Siegel J. Hypocretin (Orexin): Role in Normal Behavior and Neuropathology. Annu Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Medicine Reviews. 2005;9:231–41. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Samson WK, Resch Z. The hypocretin/orexin story. Trends in Endocrinology and Metabolism. 2000;11:257–262. doi: 10.1016/s1043-2760(00)00273-3. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi P, Yu H, MacNeil D, Van der Ploeg L, Guan X. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 48.Marcus J, Aschkenasi C, Lee C, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 49.Stricker-Krongrad A, Beck B. Modulation of hypothalamic hypocretin/orexin mRNA expression by glucocorticoids. Biochem Biophys Res Commun. 2002;296:129–133. doi: 10.1016/s0006-291x(02)00848-3. [DOI] [PubMed] [Google Scholar]
- 50.Paneda C, Winsky-Sommerer R, Boutrel B, De Lecea L. The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drug News & Perspectives. 2005;18:250–255. doi: 10.1358/dnp.2005.18.4.908659. [DOI] [PubMed] [Google Scholar]
- 51.Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on maternal behavior in the postpartum rat. Hormones and Behavior. 2004;46:411–419. doi: 10.1016/j.yhbeh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Rees SL, Panesar S, Steiner M, Fleming AS. The effects of adrenalectomy and corticosterone replacement on induction of maternal behavior in the virgin female rat. Hormones and Behavior. 2005 doi: 10.1016/j.yhbeh.2005.08.012. in press. [DOI] [PubMed] [Google Scholar]
- 53.Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85:354–62. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J Neurosci. 2005;25:6807–15. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii Y, Blundell J, Halford J, Upton N, Porter R, Johns A, Rodgers RJ. Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan T, Cirrito J, Numan M, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–52. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- 58.Calamandrei G, Keverne EB. Differential expression of Fos protein in the brain of female mice dependent on pup sensory cues and maternal experience. Behav Neurosci. 1994;108:113–120. doi: 10.1037//0735-7044.108.1.113. [DOI] [PubMed] [Google Scholar]
- 59.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup reinforcement. Behav Brain Res. 2000;108:215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 60.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 61.Thompson A, Kristal MB. Opiod stimulation in the ventral tegmental area facilitates the onset of maternal behavior in rats. Brain Res. 1996;743:184–201. doi: 10.1016/s0006-8993(96)01041-4. [DOI] [PubMed] [Google Scholar]
- 62.Mattson B, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 63.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:184–186. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 64.De Lecea L, Kilduff T, Peyron C, Gao X-B, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett F, II, Frankel W, van den Pol A, Bloom FE, Gautvik K, Sutcliffe J. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]