Abstract
Background and purpose:
An oral, single dose of 7.5 mg kg−1 of unfractionated heparin (UFH) reduces thrombosis by 50% in a rat model of venous thrombosis. As long-term use is required clinically, our objectives were to study the antithrombotic effects following repeated oral UFH administration.
Experimental approach:
Bovine lung UFH was administered by oral gavage to rats in 3 doses of 7.5 mg kg−1 each 12, 24, 48, and 72 h apart; and in 3 or 15 doses of 1 mg kg−1 every 48 h. The last dose was given immediately after thrombus initiation where 10% formalin in methanol was applied to the jugular vein. The vessel was examined for thrombosis 4 h later. Amounts of heparin in tissue and endothelium, and plasma anticoagulant activity were measured.
Key results:
When 3 × 7.5 mg kg−1 heparin was given, thrombotic incidence was most reduced at 48 h dose-intervals and was significantly less than single dose treatment. There was a negative correlation between endothelial heparin content and thrombotic incidence, but not anticoagulant activity. When 3 doses of 1 mg kg−1 every 48 h were given, thrombotic incidence was similar to single dose treatment. When 15 doses were given, total thrombotic incidence was less than for 3 doses and was similar to that after s.c. administration.
Conclusions and implications:
Antithrombotic activity increased with repeated doses of oral UFH, with antithrombotic effects similar to s.c. administration. Antithrombotic activity was related to heparin on endothelium.
Keywords: heparin, oral heparin, venous thrombosis, rats, endothelium, anticoagulant activity
Introduction
Heparins are important drugs used to prevent venous thromboembolism (Dentali et al., 2007). Unfractionated heparins (UFH) are administered by both intravenous and subcutaneous (s.c.) routes, while lower molecular weight derivatives are injected s.c. The high negative charge and size of the heparin molecule and the low pH as well as digestive enzymes in the gut have led to the assumption that heparin is unsuitable for absorption (Arbit et al., 2006). Little change in anticoagulant activity following oral administration also supports this assumption (Dryjski et al., 1989). However, our studies show that oral heparins prevent thrombosis in rat venous and arterial thrombosis models (Hiebert et al., 1996, 2000a, 2001; Pinel et al., 2004). In the venous model, incidence of stable thrombi was reduced by 50% after 7.5 mg−1 kg−1 of UFH was given by gavage. Thus, the oral route may be a way to administer heparin.
Oral heparin would be particularly beneficial when long-term use is required. It would prevent the hospitalization needed for intravenous heparin, making treatment more convenient for the patient and would reduce costs. It would also prevent bruising and haematomas that occur with s.c. administration. Long-term oral heparin may also allow for additional heparin uses. Reported beneficial effects of heparin where oral administration may be effective include the following: prevention of atherosclerosis (Engelberg, 1996); adjuncts to chemotherapeutic agents (Levine et al., 2003); as anti-inflammatory (Cornet et al., 2007) and anti-viral agents (Bagasra and Lischner, 1988); for control of angiogenesis (Folkman et al., 1989); treatment of hypertension (Mandal et al., 1995); fibromyalgia (Berg et al., 1999); and Crohn's disease (Prajapati et al., 2002).
There are few studies describing the effects of repeated doses of oral heparin without the use of delivery agents. Thus, effects of repetitive administration and selection of the appropriate dose for long-term therapy are essential. The objective of this study was to determine the antithrombotic effect of UFH when given in repetitive doses in a rat venous model and whether the drug accumulated in the body. We also wished to select an effective repetitive dosing regimen for oral heparin in a rat thrombosis model. Plasma anticoagulant activity and recovered heparin in endothelium and tissues were also monitored.
Methods
Heparin
Unfractionated bovine lung heparin (156. 2 U mg−1) was obtained from Scientific Protein Labs, Division of Viobin Corporation, WI, USA and was dissolved in water at 3, 20 and 25 mg ml−1.
Animals
All animal experiments, handling and housing were conducted according to the Principles of Animal Care of the Canadian Federation of Biological Societies. A total of 283 male Wistar rats, weighing 305±4 g (mean±standard error of the mean (s.e.m.)), were obtained from Charles River Canada, St Constant, Quebec, Canada. Animals were fasted overnight prior to treatment and were anaesthetized with barbital and methoxyflurane for experimental procedures.
Animal model
As indicated in Table 1, bovine lung UFH was given as a single dose of 7.5 mg kg−1; in three repeated doses of 7.5 mg kg−1 each 12, 24, 48 and 72 h apart; three repeated doses of 1 mg kg−1 each 48 h apart; and 15 repeated doses of 1 mg kg−1 each 48 h apart. Heparin was given by oral gavage using a steel needle with a blunted end, 7.5 cm in length, to which a 1-ml syringe could be attached. Volumes given were 0.04–0.16 ml of heparin solution depending on rat weight, followed by 0.2 ml saline. Control animals were given 0.4 ml saline by oral gavage. In addition, 7.5 mg kg−1 bovine lung UFH was injected s.c., in volumes ranging from 0.08 to 0.13 ml depending on body weight, in 15 doses, 48 h apart. Saline and the last heparin dose were given 4 h prior to killing the animals. Rats were placed in metabolic cages to collect urine and faecal samples on a daily basis when three repetitive doses were given; and for a 24 h period, prior to and on days 29–30 when 15 repetitive doses were given. Animals were weighed prior to heparin and on days 15 and 30.
Table 1.
Experimental groups used in study
| Dose mg kg−1 | Route | Number of rats | Number of doses | Frequency (h) |
|---|---|---|---|---|
| 0 | Oral | 51 | 1 | Single |
| 7.5 | Oral | 37 | 1 | Single |
| 7.5 | Oral | 20 | 3 | 12 |
| 7.5 | Oral | 26 | 3 | 24 |
| 7.5 | Oral | 20 | 3 | 48 |
| 7.5 | Oral | 26 | 3 | 72 |
| 1 | Oral | 20 | 3 | 48 |
| 1 | s.c | 20 | 15 | 48 |
| 1 | Oral | 20 | 15 | 48 |
| 0 | Oral | 43 | 15 | 48 |
Thrombosis test
A thrombus was initiated by a modified method of Blake et al. (1959) immediately prior to the last heparin dose. Several drops of 10% formalin in 65% methanol were applied to the exposed jugular vein and the incision closed. After 4 h, the rat was anaesthetized and examined for signs of external bleeding. The incision was opened and the jugular vein directly inspected for a thrombus by pressing gently with a cotton pledget. The thrombus was scored as positive (stable thrombus) if a thrombus was visible in the jugular vein and blood flow was restricted. The thrombus was scored as unstable if the thrombus could be seen in the jugular vein, but on examination with a cotton pledget, the thrombus moved and blood flow was maintained. The thrombus was scored as negative if a thrombus was never seen and blood flow was not disrupted. The scoring was performed by the same observer who was unaware of the treatments. Per cent incidence of total thrombotic events, and stable and unstable thrombi were calculated by dividing the number of events by the total number of animals observed for the treatment.
Tissue collection
Tissue samples were collected immediately after examination of the vessel. Blood samples were collected in 3.8% sodium citrate (1 part sodium citrate to 9 parts blood) from the abdominal aorta. As a source of endothelium, the thoracic aorta and inferior vena cava were removed and placed in saline. A blood sample was sent for complete blood counts to Prairie Diagnostic Services, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Canada. Immediately following, the activated partial thromboplastin time (APTT) was determined from plasma derived from blood samples obtained with a minimum of tissue trauma. Additional plasma aliquots were frozen for later determination of anti-Factor Xa or anti-Factor IIa activity. Liver, kidney and lung, were collected. Stomach, duodenum, jejunum, ileum and colon were removed and washed with distilled water. Tissues and washes were frozen for later heparin extraction.
Heparin extraction and measurement from endothelium and tissues
Endothelium was obtained using a modified published procedure (Hiebert and Jaques, 1976). Vessels were slit open, pinned to dental wax lumen side up and rinsed in saline. Cellulose acetate membrane (Schleicher & Schuel BioScience, GmbH, Dassel, Germany), was applied to the lumenal surface and when lifted, endothelium was removed. Mean areas for aortic and vena caval endothelium, measured to the nearest mm from the monolayer on cellulose acetate paper, were 2.60±0.04 and 0.45±0.01 cm2 (mean±s.e.m.), respectively. Cellulose acetate membrane was removed from endothelium by two repeats of dissolving in cold acetone followed by centrifuging and discarding the supernatant. The precipitates were air-dried and then digested by 10 μl pronase (from Streptomyces griseus, Sigma-Aldrich Canada Ltd., Oakville, ON, Canada, 40 mg kg−1 in 1 M Tris buffer) for 48 h at 37 °C. Digests were centrifuged at 8000 × g for 10 min, supernatant was collected and the precipitate washed twice with 100 μl of 26.8% NaCl with washes added to the supernatant. Glycosaminoglycans were precipitated from the supernatant with five volumes of methanol and the precipitate was dried.
Heparin was extracted from tissue, gut washes and faeces by modified methods as described previously (Jaques, 1977). When three repeated doses were used, gut tissues were washed with saline and washes were collected. Average wet weights of tissue were 1.9, 1.8, 1.9, 1.8, 1.4, 9.1, 2.0 and 1.2 g for stomach, duodenum, jejunum, ileum, colon, liver, lung and kidney, respectively. Average weights of gut washes, obtained by subtracting the difference in gut weight before and after washing, were 1.1, 0.4, 0.7, 0.8 and 2.7 g for stomach, duodenum, jejunum, ileum and colon washes, respectively. The weights of faeces accumulated over 24 h averaged 10.4 g. Minced tissues, gut washes and faeces were defatted with acetone and isoproterenol:petroleum ether (1:1), and digested at 37 °C by pronase, 40 mg kg−1 in 1 M Tris buffer in 0.1 M CaCl2 at pH 8. Digests were purified by precipitating with 1% NaCl in acetone and then methanol. Precipitates were dried, dissolved in water and analysed. Heparin was isolated from urine by exhaustive dialysis against water using 1000 molecular weight cut-off dialysis tubing (Spectrum Laboratories Inc., RanchoDominguez, CA, USA).
Agarose gel electrophoresis was used to identify and measure heparin in all tissue, washes, urine and endothelial extracts (Jaques et al., 1990). Slides were stained with 0.04% Toluidine Blue in 80% acetone and background colour was removed with 1% acetic acid. Heparin was identified by electrophoretic migration as compared to reference material and amounts determined by densitometry. For endothelial samples, 5 μl of distilled water was added to each endothelial extract and 2 μl of the dissolved extract was added to two lanes on the agarose gel electrophoresis slide. After determining the density of the band, as compared to reference heparin, the total amount of heparin in each endothelial sample was calculated and was expressed as heparin recovered in μg cm−1. The percentage of endothelial samples positive for heparin was calculated by dividing the number of samples in which heparin was recovered by the total number of samples examined in each experimental group followed by multiplying by 100.
Measurement of anticoagulant activity in plasma
Chromogenic assays were used to measure anti-Factor Xa activity (Accucolor Heparin (Sigma)) and anti-Factor IIa activity (DiaPharma Group Inc., West Chester, OH, USA). The absorbance of the sample was read at 405 nm. Heparin concentrations in samples based on anti-Factor Xa and anti-Factor IIa were obtained by comparing absorbance obtained in the test samples to a standard curve constructed using known amounts of heparin added to control plasma. The APTT (Biopool, Ventura, CA, USA) was also measured.
Data analyses
All results are expressed as mean±s.e.m. Thrombosis data are expressed as a percentage. The χ2-test for difference between proportions was used to compare thrombotic incidences and the percentage of endothelial samples positive for heparin. Heparin concentrations in tissues and gut washes were subjected to a logarithmic transformation prior to analyses to ensure similar variances between groups. The mean and s.e.m. are expressed as the antilog; thus positive and negative s.e.m. are different and are included in the text and tables. Differences in endothelial and plasma heparin concentrations obtained by anticoagulant tests were analysed using a one-way analysis of variance followed by Tukey's multiple comparison test. Single dose and 30-day controls were combined when endothelial and plasma heparin concentrations were considered. Differences in tissue heparin concentrations were determined using a one-tailed t-test. A Pearsons correlation was used to determine the correlation between mean endothelial concentrations and the total thrombotic incidence. Probability P<0.05 was considered significant.
Results
Initial studies were performed to determine whether orally administered heparin had an accumulative antithrombotic effect when administered in three repetitive doses compared with a single dose. The duration between dosages was varied to see whether this affected antithrombotic activity. Furthermore, to determine whether a cumulative effect could be seen following repetitive dosing with oral heparin, 15 doses of 1 mg per kg per 48 h given by stomach tube was compared with the effects of 3 doses of 1 mg per kg per 48 h by oral gavage and with the effects of 15 doses of 1 mg per kg per 48 h given by s.c. injection.
Antithrombotic activity
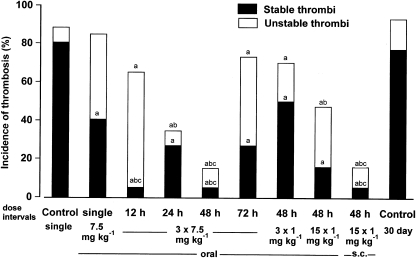
A single oral dose of 7.5 mg kg−1 resulted in a significant reduction in incidence of stable thrombi, when compared to that in control rats (Figure 1). Total thrombotic incidence (stable plus unstable thrombi) was not significantly different from control rats. When 7.5 mg kg−1 was given in three consecutive oral doses 12, 24, 48 and 72 h apart, total thrombotic incidence was decreased with time between doses up to 48 h. Total thrombotic incidence was significantly less than after saline administration, with dose intervals of 12, 24 and 48 h, but not 72 h. Total thrombotic incidence was significantly less than for a single dose with intervals of 24 and 48 h but not 12 and 72 h. Incidences of stable thrombi for 12, 24, 48 and 72 h dose intervals were significantly reduced compared to saline treatment. Incidences of stable thrombi after repeated doses were significantly less than single dose administration with 12 and 48 h, but not 24 and 72 h dose intervals.
Figure 1.
Antithrombotic effects in a rat jugular vein model when unfractionated heparin (UFH) was given by the oral route in repeated doses. UFH was given orally at 7.5 mg kg−1 as a single dose, three repeated doses 12, 24, 48 and 72 h apart; or at 1 mg kg−1 in three repeated doses 48 h apart. In addition UFH was given orally or s.c. in 15 repeated doses at 1 mg kg−1, 48 h apart. Control rats were given oral saline as a single dose or for 30 days. The final dose was given immediately following thrombus initiation and vessels were examined 4 h later. Significantly different from a, controls; b, single-dose UFH 7.5 mg kg−1; c, 3 × 1 mg kg−1 48 h apart (χ2-test for differences between proportions).
As repetitive dosing at 7.5 mg kg−1 resulted in a reduced incidence of stable thrombi, a lower dose (1 mg kg−1), was administered as three doses at the optimum interval (48 h), determined from the results already shown in Figure 1. Incidence of stable thrombi, but not total thrombotic events, was significantly less than saline administration (Figure 1).
Incidence of stable thrombi was decreased when oral heparin was administered in 15 doses as compared with 3 doses of 1 mg per kg per 48 h or with 30-day control rats (Figure 1). There was no difference in incidence of stable thrombi between oral and s.c. administration. Total thrombotic incidence was significantly less than that of 30-day controls after 15, but not 3 doses, of 1 mg per kg per 48 h. Total thrombotic incidence did not differ significantly between oral and s.c. administration. Total thrombotic incidence following s.c., but not oral, administration of 15 doses of 1 mg per kg per 48 h was significantly less than that after 3 doses of 1 mg per kg per 48 h of UFH.
Endothelial concentrations
Heparin was found with both aortic and vena caval endothelium following oral administration (Table 1). The percentage of aortic endothelial samples positive for heparin was greatest when 3 doses of 7.5 mg kg−1 were given at 48 and 24 h dose intervals, which was significantly greater than controls or for 12 and 72 h intervals. The amount recovered from aortic endothelium was greatest at 48 h intervals and was significantly greater than controls or for 12 or 72 h dose intervals. The percentage of vena caval endothelial samples positive for heparin was greatest at 48 h intervals. The percentage of positive samples was significantly greater than controls for 12, 24 and 48 h, but not 72 h intervals. The amounts found on vena caval endothelium were greatest for 48 h followed by 24 intervals. Amounts found at 48 h intervals were significantly greater than controls or when heparin was given at 12 or 72 h intervals.
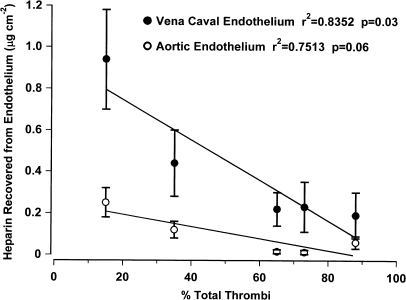
There was a significant negative correlation (P=0.03) between the mean amount of heparin found with vena caval endothelium and the total thrombotic incidence when rats were treated with three doses of 7.5 mg kg−1 heparin (Figure 2). The negative correlation between mean amount of heparin found with aortic endothelium and the total thrombotic incidence approached significance (P=0.057).
Figure 2.
Correlation between antithrombotic activity and heparin recovered from vena caval and aortic endothelium following repeated dose administration. Heparin (7.5 mg kg−1) was administered in three repeated doses 12, 24, 48 or 72 h apart immediately following thrombus initiation. Control rats were given saline. Thrombosis was scored and aorta and superior vena cava were harvested 4 h after thrombus initiation. Mean±s.e.m. are shown for concentrations on aortic and vena caval endothelium. Pearson correlation calculation r2=0.8352, P=0.030 for vena cava; r2=0.7513, P=0.057 for aorta.
When heparin was given in three doses of 1 mg per kg per 48 h, incidence and amounts found on aortic or vena caval endothelium did not differ from controls. Heparin was also found with endothelium after 15 doses of 1 mg per kg per 48 h (Table 2). Per cent incidence of samples positive for heparin, for both oral and s.c. administration of 15 doses, was significantly greater than that of controls or after 3 doses of 1 mg per kg per 48 h. The amount found on vena caval endothelium after oral heparin was significantly greater than s.c. administration and in controls (Table 2).
Table 2.
Heparin with endothelium following administration of unfractionated heparin in repetitive doses
| Route | Dose (mg kg−1) | Dose intervals (h) | No. of doses |
Aortic endothelium |
Vena caval endothelium |
||
|---|---|---|---|---|---|---|---|
| % Positive | μg cm−2 | % Positive | μg cm−2 | ||||
| Oral | 7.5 | Single | 1 | 27 | 0.01±0.01 | 19 | 0.05±0.04 |
| Oral | 7.5 | 12 | 3 | 15 | 0.02±0.01 | 45a,d | 0.22±0.08 |
| Oral | 7.5 | 24 | 3 | 58a,b,c,d | 0.12±0.04 | 54a,d | 0.43±0.16 |
| Oral | 7.5 | 48 | 3 | 60a,b,c,d | 0.25±0.06a,b,c,d | 65a,c,d | 0.94±0.24a,b,c,d,e |
| Oral | 7.5 | 72 | 3 | 23 | 0.01±0.01 | 27 | 0.23±0.12 |
| Oral | 1 | 48 | 3 | 5 | 0.02±0.02 | 10 | 0.23±0.17 |
| Oral | 1 | 48 | 15 | 52a,b,d | 0.07±0.02 | 68a,c,d | 0.81±0.21a |
| s.c. | 1 | 48 | 15 | 68a,b,c,d | 0.04±0.01 | 79b,c,d | 0.21±0.04 |
| Oral | Saline | 16 | 0.06±0.02 | 9 | 0.19±0.11 | ||
Abbreviation: ANOVA, analysis of variance; s.c., subcutaneous.
Significantly different than acontrol, b12 h, c72 h, d1 mg kg−1 48 h−1 3 doses, esubcutaneous.
χ2 for differences between proportions for % positive; one-way ANOVA for μg cm−2.
Plasma anticoagulant activity
Heparin administered in three doses of 7.5 mg kg−1 in 12 and 24 h intervals showed a small but significant increase in APTT versus controls, single-dose treatment and repetitive dosing at 48 and 72 h (Table 3). There was no correlation between APTT and antithrombotic activity. There was no difference in heparin concentrations as determined by anti-Factor Xa activity between groups. Plasma heparin concentrations determined by anti-Factor Xa and IIa activity following 15 × 1 mg per kg per 48 h oral or s.c. heparin administration did not differ significantly from controls. A small but significant decrease in APTT was observed in rats receiving 15 doses of 1 mg per kg per 48 h when compared to control values or those given 3 doses of 1 mg per kg per 48 h (Table 3).
Table 3.
Plasma anticoagulant activity following repetitive oral unfractionated heparin administration
| Route | Dose (mg kg−1) | Dose intervals (h) | No. of dosages | APTT (s) | Anti-Xa activity (μg ml−1) | Anti-Iia activity (μg ml−1) |
|---|---|---|---|---|---|---|
| Oral | 7.5 | Single | 1 | 18.67±0.8 | — | — |
| Oral | 7.5 | 12 | 3 | 25.07±0.9a,b,c,d | 1.96±0.0 | — |
| Oral | 7.5 | 24 | 3 | 22.91±0.8a,b,c,d | 0.84±0.2 | — |
| Oral | 7.5 | 48 | 3 | 17.78±0.6 | 0.95±0.0 | — |
| Oral | 7.5 | 72 | 3 | 17.18±0.5a | 2.81±0.2 | — |
| Oral | 1 | 48 | 3 | 21.43±1.0 | — | — |
| Oral | 1 | 48 | 15 | 16.88±0.6a,e | 0.44±0.2 | 0.05±0.05 |
| s.c. | 1 | 48 | 15 | 17.26±0.5a,e | 4.35±0.6 | 0.00±0.00 |
| Oral | Saline | 20.04±0.7 | 3.81±0.7 | 0.00±0.00 |
Abbreviations: ANOVA, analysis of variance; APTT, activated partial thromboplastin time; s.c., subcutaneous.
Significantly different than acontrol, bsingle, c48 h, d72 h, e3 × 1 mg kg−1 48 h−1. One-way ANOVA, Tukey's multiple comparison test. —, not determined
Distribution in tissues
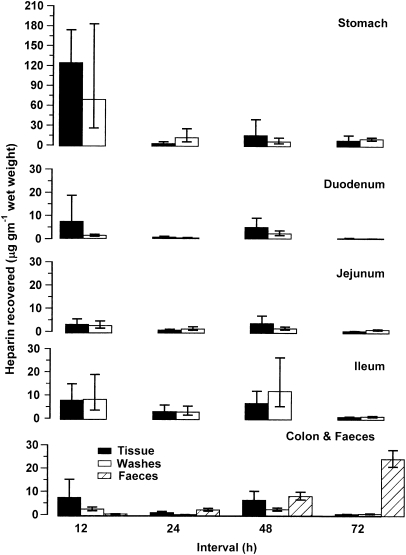
Heparin was found in all gut tissues and washes at all time intervals. When heparin was given at 3 × 7.5 mg kg−1, most heparin was found in the stomach and stomach washes at 12 h intervals (Figure 3). The concentration of heparin in faeces increased as the intervals between administrations increased. Total amounts found in faeces were (mean±s.e.m. after log transformation; see Methods) 1.8 (+0.7, −0.5), 12.2 (+4.8, −3.5), 57.2 (+12.5, −10.2), 327 (+46.8, −54.6) micrograms after oral heparin given at 12, 24, 48 and 72 h dose intervals, respectively. In the same animals, heparin was recovered from all non-gut tissue analysed. Heparin recovered from lung were 56.1 (+59.7, −28.9), 3.4 (+2.9, −1.4), 27.9 (+55.1, +18.5) and 23.4 (+23.2, −11.6) micrograms per gram of wet weight; from liver were 29.2 (+39.2, −16.7), 2.2 (+3.7, −1.4), 4.6 (+3.5, −2.0) and 0.6 (+1.3, −0.7) micrograms per gram of wet weight; and from kidney were 7.6 (+3.9, −2.6), 0.6 (+0.4, −0.2), 30.7 (+4.7, −4.0) and 1.4 (+1.2, −0.7) micrograms per gram of wet weight for 12, 24, 48 and 72 h, respectively. Most heparin was recovered from lung and liver samples when given at 12 h dose intervals. Total amounts recovered from urine were 0.86 (+0.4, −0.3), 1.3 (+0.7, −0.5), 3.1 (+0.1, −0.7) and 0.6 (+0.2, −0.7) microgram for 12, 24, 48 and 72 h, respectively. There was no increase in amounts found in urine as intervals between dosing increased.
Figure 3.
Heparin found in gut tissue, gut washes and faeces following oral administration of three repeated doses of 7.5 mg kg−1 at different time intervals. Time intervals were 12, 24, 48 and 72 h apart. Tissue and washes were harvested 4 h after the last dose from six rats per group selected at random. Mean±s.e.m. are shown. Faeces were collected from 20 rats per group throughout the time of heparin exposure.
When heparin was given as 15 doses of 1 mg per kg per 48 h over a 30-day period, only trace amounts were found in gut tissue following oral or s.c. administration (Table 4). Nevertheless, significantly more heparin was found in stomach, jejunum and ileum tissue following oral versus s.c. heparin. Heparin was also found in lung and liver, which differed significantly from control but not s.c. administration. Amounts found in lung were significantly greater for s.c. versus oral administration. Amounts found in urine and faecal samples did not differ significantly between groups (Table 4).
Table 4.
Heparin found in gut and non-gut tissues following administration of 15 dosages of 1 mg per kg per 48 h by the oral or subcutaneous route
| Stomach (μg gm−1) | Duodenum (μg gm−1) | Jejunum (μg gm−1) | Ileum (μg gm−1) | Colon (μg gm−1) | Lung (μg gm−1) | Liver (μg gm−1) | Urine (μg)a | Faeces (μg)a |
|---|---|---|---|---|---|---|---|---|
| Oral | ||||||||
| 9.0b | 0.4 | 1.1b | 5.9b,c | 0.3 | 53.6c | 3.4c | 1.3 | 6.8 |
| +21.9, −6.4 | +0.2, −0.2 | +0.6, −0.4 | +5.6,−2.9 | +0.1, −0.1 | +75.1, −15.3 | +16.4, −2.7 | +0.4,−0.3 | +1.0, −0.9 |
| s.c. | ||||||||
| 1 | 0.2 | 0.2 | 0.4 | 0.4 | 97.4c,d | 0.6 | 0.7 | 7 |
| +3.0, −0.8 | +0.1, −0.0 | +0.1, −0.0 | +0.2, −0.1 | +0.1, −0.1 | +73.9, −42.0 | +1.1, −0.4 | +0.2, −0.2 | +8.4, −1.1 |
| Control | ||||||||
| 1.4 | 0.5 | 0.6 | 1.4 | 0.5 | 1.9 | 0.1 | 0.5 | 3.5 |
| +4.2, −1.0 | +1.3, −0.4 | +0.8, −0.4 | +3.6, −1.0 | +1.1, −0.3 | +13.4, −1.6 | +0.0, −0.0 | +0.0, −0.0 | +2.3, −1.4 |
Number in bold face are averages of 6–20 determinations. Light-face numbers are positive and negative standard errors after log transformation.
Total micrograms for 24-h period
Greater than subcutaneous.
Greater than control.
Greater than oral administration.
Animal health
When heparin was given in 15 doses of 1 mg per kg per 48 h, weight gain over the 30-day period was 128.8±33.3, 144.5±28.9 g and 145.0±17.8 g for oral, subcutaneous and control treated, respectively, and did not differ significantly between groups. There was no evidence of bleeding and all haematological values including RBC count, mean corpuscular volume, mean corpuscular haemoglobin concentration and leukocyte counts were within normal ranges. There were no significant differences in haematological indices except mean corpuscular volume. In rats given oral heparin, this was 54.8 fl and was significantly different from controls (57.33 fl) but similar to those given s.c. heparin (55.0 fl) (P=0.047, one-way analysis of variance). Platelet count was normal although there was evidence of a few enlarged platelets in 40% of the oral heparin-treated group.
Discussion
Although heparin is considered to be ineffective when given by the oral route, our laboratory and others have collected considerable data supporting the hypothesis that heparin is absorbed, without delivery agents (Gorski and Lagodzinski, 1991; Engelberg, 1995; Hiebert, 2002). Oral heparin is considered ineffective due to certain facts: it is believed that heparin is degraded by stomach acids (Money and York, 2001); the high negative charge and molecular weight suggest that absorption is unlikely (Money and York, 2001); there is little change in anticoagulant activity following oral administration (Dryjski et al., 1989). However, it is unlikely that heparin is broken down by stomach acidity since heparin in 0.1 N HCl at 30 °C for 100 h showed no degradation, while in 0.1N HCl at 60 °C for 10 h showed only 2% breakdown (Jandik et al., 1996). We and others observed small but significant changes in plasma anticoagulant activity following oral administration to both rats and humans (Hiebert et al., 2000b, 2005). Engelberg showed a significant but small increase in APTT after giving oral heparin to 45 individuals (Engelberg, 1995). Although changes in anticoagulant activity are minimal, considerable amounts of heparin are recovered from endothelium such that the endothelium can be considered as the prime distribution site (Hiebert, 2002). Heparin can also be recovered from urine in human studies (Hiebert et al., 2005). We have also demonstrated that single doses of orally administered UFH and low molecular weight heparins have antithrombotic activity in rat venous (Hiebert, 2002) and arterial models (Pinel et al., 2004), despite low plasma levels.
Although, clinically, heparin is given in repeated doses, only a few studies have considered the effects of repeated dosing with orally administered heparin, without delivery agents. All report significant changes with evidence of absorption. A study in humans showed that oral heparin at a dose of 20 000 U twice weekly for 2–8 months showed slight prolongation of the APTT (Engelberg, 1995). Unfractionated or low molecular heparin at 0.5 mg ml−1 in drinking water, given for 9–11 weeks to spontaneously hypertensive rats, returned systolic blood pressure to normal (Vasdev et al., 1992, 1994). Oral heparin was also found to be effective in the survival of skin allografts and the treatment of rheumatoid arthritis (Gorski and Lagodzinski, 1991; Gorski et al., 1991, 1993; Imiela et al., 1995).
Our previous single-dose studies in a rat jugular vein model of venous thrombosis indicated that the dose required to reduce thrombosis by 50% was 7.5 mg kg−1, while 3.25 mg kg−1 had no antithrombotic effect (Hiebert et al., 1996). In the present study, three oral doses of 7.5 mg kg−1 of UFH, 24 or 48 h apart, significantly reduced total thrombotic incidence, while doses given 12 and 48 h apart, reduced the incidence of stable clots, compared to single-dose administration. This suggested that repetitive dosing was more effective than single-dose administration. This conclusion was supported by the observation that three doses of 1 mg kg−1 of UFH, 48 h apart, reduced thrombotic incidence by 50%, an effect similar to that observed after a single dose of 7.5 mg kg−1, and was more effective than a single dose of 3.25 mg kg−1 which was ineffective in previous studies (Hiebert et al., 1996). Furthermore, 15 doses of 1 mg per kg per 48 h exerted an increased antithrombotic effect, compared to those after 3 doses. These observations clearly indicate accumulation of an antithrombotic effect following oral heparin administration.
Although we have previously observed heparin on endothelium after oral administration, this is the first time we have shown a clear negative correlation between thrombotic incidence and endothelial heparin. No such correlation was seen with anticoagulant activity, agreeing with previous observations indicating a poor correlation between APTT or anti-Factor Xa activity and antithrombotic activity (Bara et al., 1999; Morris, 2003). This clearly indicates that heparin's antithrombotic activity is more dependent on heparin on endothelium than plasma anticoagulant activity.
The mechanism of accumulation of an antithrombotic effect is not entirely clear but may be related to increased heparin levels on endothelium. This is shown in the present study where more endothelial heparin was found when oral heparin was given for 15 doses of 1 mg per kg per 48 h versus 3 doses (Table 2). Furthermore, heparin can be found in all tissues investigated, indicating that it is widely distributed and thus may reflect attachment to, or accumulation within, endothelium. Considerable amounts of heparin were recovered from the lung, which may be due to the large vascular surface area in lung. In support, our previous studies found radioactivity in all tissues examined 24 h following administration of 14C-labelled and -unlabelled porcine mucosal heparin, including gut tissue and washes and liver, lung, kidney, spleen, thymus, bone marrow, muscle, skin and so on (Hiebert et al., 2000b). In the present study, only small amounts were recovered from faeces and urine after repeated doses, which agrees with previous observations where averages of 1.5 and 0.5% were found with urine and faeces, respectively, suggesting a slow excretion rate (Hiebert et al., 2000b). The present study also suggests that redistribution takes place. This was particularly evident in stomach tissue where more heparin was found after three doses of 7.5 mg per kg per 12 h than when the doses were given at longer intervals (Figure 3). The accumulation of effect was not related to anticoagulant activity in plasma.
There was little difference in antithrombotic activity when heparin was administered by the s.c. versus the oral route. This further supports the fact that orally administered UFH is absorbed. The effective dose of 1 mg per kg per 48 h is well within the dose recommended for s.c. administration to humans, initial dose 333 U kg−1 followed by a fixed dose of 250 U per kg per 12 h (Kearon et al., 2006).
The 15-dose regimen of orally administered heparin (by oral gavage) had no deleterious effect on the health of the animals. No bleeding was recorded and blood parameters were similar to controls. As thrombocytopenia is a side effect of heparin administration, platelets were monitored. Although enlarged platelets were observed, there was no change in platelet number. Failure to observe platelet changes could be due to species differences.
Thus studies on repetitive dosing further support the idea that orally administered UFH is absorbed and is an effective antithrombotic agent. Repeated dosing at 48 h intervals reduces the dose required compared with single dosing, indicating that accumulation occurs. Measurements of the amount recovered from tissue following repeated dosing suggests that heparin is only slowly excreted and that redistribution occurs. Antithrombotic activity was correlated with endothelial heparin but not anticoagulant activity. Repeated oral and s.c. heparin at the same dose had similar antithrombotic effects. The used effective oral dose of 1 mg (150 U) per kg per 48 h is well within the range of effective clinical s.c. doses suggested for humans to prevent thrombosis and suggests an oral dosing regimen that should be tested further. Bleeding is not a side effect of oral UFH, but further studies are needed on the effect on platelets. Although orally administered UFH has an accumulative antithrombotic effect, further studies are needed to determine whether accumulation occurs for other effects of oral UFH or for oral low molecular weight heparins.
Acknowledgments
This project was funded by the Heart & Stroke Foundation of Saskatchewan.
Abbreviations
- APTT
activated partial thromboplastin time
- UFH
unfractionated heparin
Conflict of interest
The authors state no conflict of interest.
References
- Arbit E, Goldberg M, Gomez-Orellana I, Majuru S. Oral heparin: status review. Thromb J. 2006;4:6. doi: 10.1186/1477-9560-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lischner HW. Activity of dextran sulfate and other polyanionic polysaccharides against human immunodeficiency virus. J Infect Dis. 1988;158:1084–1087. doi: 10.1093/infdis/158.5.1084. [DOI] [PubMed] [Google Scholar]
- Bara L, Planes A, Samama MM. Occurence of thrombosis and haemorrhage, relationship with anti-Xa, anti-IIa activities, and D-dimer plasma levels in patients receiving low molecular weight heparin, enoxaparin or tinzaparin, to prevent deep vein thrombosis after hip surgery. Br J Haematol. 1999;104:230–240. doi: 10.1046/j.1365-2141.1999.01153.x. [DOI] [PubMed] [Google Scholar]
- Berg D, Berg LH, Couvaras J, Harrison H. Chronic fatigue syndrome and/or fibromyalgia as a variation of antiphospholipid antibody syndrome: an explanatory model and approach to laboratory diagnosis. Blood Coagul Fibrinolysis. 1999;10:435–438. doi: 10.1097/00001721-199910000-00006. [DOI] [PubMed] [Google Scholar]
- Blake OR, Ashwin JG, Jaques LB. An assay for the antithrombotic activity of anticoagulants. J Clin Path. 1959;12:118–122. doi: 10.1136/jcp.12.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98:579–586. [PubMed] [Google Scholar]
- Dentali F, Douketis JD, Gianni M, Lim W, Crowther MA. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- Dryjski M, Schneider DE, Mojaverian P, Kuo B, Bjornsson TD. Investigations on plasma activity of low molecular weight heparin after intravenous and oral administrations. Br J Clin Pharm. 1989;28:188–192. doi: 10.1111/j.1365-2125.1989.tb05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg H. Orally ingested heparin is absorbed in humans. Clin Appl Thromb Hemost. 1995;1:283–285. [Google Scholar]
- Engelberg H. Actions of heparin in the atherosclerotic process. Pharmacol Rev. 1996;48:327–352. [PubMed] [Google Scholar]
- Folkman J, Weisz PB, Joullie MM, Li WW, Ewing WR. Control of angiogenesis with synthetic heparin substitutes. Science. 1989;243:1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- Gorski A, Lagodzinski Z. Oral heparin prolongs survival of skin allografts. Arch Immunol Ther Exp. 1991;39:557–562. [PubMed] [Google Scholar]
- Gorski A, Wasik M, Nowaczyk M, Korczak-kowalska G. Immunomodulating activity of heparin. FASEB J. 1991;5:2287–2291. doi: 10.1096/fasebj.5.9.1860620. [DOI] [PubMed] [Google Scholar]
- Gorski A, Imiela J, Nosarzewski J.Oral heparin in the treatment of rheumatoid-arthritis J Immun 1993V150,N8PA239(Abstract) [PubMed] [Google Scholar]
- Hiebert LM. Oral heparins. Clin Lab. 2002;48:111–116. [PubMed] [Google Scholar]
- Hiebert LM, Jaques LB. The observation of heparin on endothelium after injection. Thromb Res. 1976;8:195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- Hiebert LM, Ping T, Wice SM. Antithrombotic activity of orally administered low molecular weight heparin (logiparin) in a rat model. Haemostasis. 2000a;30:196–203. doi: 10.1159/000054135. [DOI] [PubMed] [Google Scholar]
- Hiebert LM, Wice SM, Jaques LB. Antithrombotic activity of oral unfractionated heparin. J Cardiovasc Pharmacol. 1996;28:26–29. doi: 10.1097/00005344-199607000-00005. [DOI] [PubMed] [Google Scholar]
- Hiebert LM, Wice SM, Ping T. Increased plasma anti-Xa activity and recovery of heparin from urine suggest absorption of orally administered unfractionated heparin in human subjects. J Lab Clin Med. 2005;145:151–155. doi: 10.1016/j.lab.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hiebert LM, Wice SM, Ping T, Herr D, Laux V. Antithrombotic efficacy in a rat model of the low molecular weight heparin, reviparin sodium, administered by the oral route. Thromb Haemost. 2001;85:114–118. [PubMed] [Google Scholar]
- Hiebert LM, Wice SM, Ping T, Hileman RE, Capila I, Linhardt RJ. Tissue distribution and antithrombotic activity of unlabeled or 14C-labeled porcine intestinal mucosal heparin following administration to rats by the oral route. Can J Physiol Pharm. 2000b;78:307–320. [PubMed] [Google Scholar]
- Imiela J, Nosarzewski J, Gorski A. Oral heparins in the treatment of rheumatoid arthritis. Arch Immun Ther Exp. 1995;43:313–315. [PubMed] [Google Scholar]
- Jandik KA, Kruep D, Cartier M, Linhardt RJ. Accelerated stability studies of heparin. J Pharm Sc. 1996;85,1:45–51. doi: 10.1021/js9502736. [DOI] [PubMed] [Google Scholar]
- Jaques LB. Determination of heparin and related sulfated mucopolysaccharides. Methods Biochem Anal. 1977;24:203–312. doi: 10.1002/9780470110447.ch4. [DOI] [PubMed] [Google Scholar]
- Jaques LB, Wice SM, Hiebert LM. Determination of absolute amounts of heparin and of dextran sulfate in plasma in microgram quantities. J Lab Clin Med. 1990;115,4:422–432. [PubMed] [Google Scholar]
- Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, Ockelford P, et al. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006;296:935–942. doi: 10.1001/jama.296.8.935. [DOI] [PubMed] [Google Scholar]
- Levine MN, Lee AY, Kakkar AK. From Trossseau to targeted therapy:new insights and innovations in thrombosis and cancer. J Thromb Haemost. 2003;1:1456–1463. doi: 10.1046/j.1538-7836.2003.00275.x. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Lyden T, Saklayen MG. Heparin lowers blood pressure:Biological and clinical perspectives. Kidney Int. 1995;47:1017–1022. doi: 10.1038/ki.1995.147. [DOI] [PubMed] [Google Scholar]
- Money SR, York JW. Development of oral heparin therapy for prophylaxis and treatment of deep venous thrombosis. Cardiovasc Surg. 2001;9:211–218. doi: 10.1016/s0967-2109(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Morris T. Heparin and low molecular weight heparin: background and pharmacology. Clin Chest Med. 2003;24:39–47. doi: 10.1016/s0272-5231(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Pinel C, Wice SM, Hiebert LM. Orally administered heparins prevent arterial thrombosis in a rat model. Thromb Haemost. 2004;91:919–926. doi: 10.1160/TH03-08-0527. [DOI] [PubMed] [Google Scholar]
- Prajapati DN, Newcomer JR, Emmons J, Abu-Hajir M, Binion DG. Successful treatment of an acute flare of steroid-resistant Crohn's colitis during pregnancy with unfractionated heparin. Inflamm Bowel Dis. 2002;8:192–195. doi: 10.1097/00054725-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Ford C, Longerich L, Barrett B, Parai S, Campbell N. Oral treatment with low molecular weight heparin normalizes blood pressure in hypertensive rats. Artery. 1994;21,1:1–28. [PubMed] [Google Scholar]
- Vasdev S, Sampson CA, Longerich L, Prabhakaran VM, Parai S. Oral heparin normalizes blood pressure and elevated cytosolic calcium in hypertensive rats. Artery. 1992;19,3:124–146. [PubMed] [Google Scholar]