Abstract
Background and purpose:
Curcumin has been used to treat cancer, diabetes and other pathologies. However, little is known regarding its role in altering post-translational modifications of histone H3. A recent report suggests that acute hyperglycaemia induces a global down-regulation of gene expression in human tissues and epigenetic regulation of gene expression could be a novel mechanism underlying the pathological processes of hyperglycaemia. The present study was undertaken to examine changes in histone modification by curcumin treatment which prevents development of type I diabetic nephropathy.
Experimental approach:
Male Sprague-Dawley rats were rendered diabetic using a single dose of streptozotocin (55 mg kg−1, i.p.). Diabetic nephropathy was assessed by measurements of blood urea nitrogen, albumin and creatinine levels. Post-translational modifications of histone H3, heat shock protein-27 (HSP-27) and mitogen-activated protein (MAP) kinase p38 expression were examined by western blotting.
Key results:
Treatment of diabetic rats with curcumin significantly decreased blood urea nitrogen and creatinine and increased albumin; variables associated with the development of diabetic nephropathy. There were also increased levels of HSP-27 and MAP kinase (p38) in diabetic kidney. However, curcumin treatment prevented this increase in HSP-27 and p38 expression. Moreover, at nuclear level curcumin prevented the decrease in dephosphorylation and increases acetylation of histone H3.
Conclusions and implications:
Our results suggested that protection against development of diabetic nephropathy by curcumin treatment involved changes in post-translational modifications of histone H3, expression of HSP-27 and MAP kinase p38 in diabetic kidney.
Keywords: curcumin, histone acetylation, histone phosphorylation, diabetic nephropathy, oxidative stress, HSP-27, p38
Introduction
Diabetic nephropathy is one of the most serious complications of diabetes and the most common cause of end-stage renal failure. At present, diabetic kidney disease affects about 15–25% of type I diabetes patients (Hovind et al., 2003) and 30–40% of patients with type II diabetes (Yokoyama et al., 2000). Diabetic nephropathy is characterized by specific renal morphological and functional alterations. Features of early diabetic renal changes are glomerular hyperfiltration, glomerular and renal hypertrophy, increased urinary albumin excretion, increased basement membrane thickness and mesangial expansion with the accumulation of extracellular matrix proteins such as collagen, fibronectin and laminin. Diabetic nephropathy is characterized by proteinuria, a decline in renal function, glomerulosclerosis and interstitial fibrosis. Diabetic nephropathy is diagnosed on the basis of the extent of albumin protein excretion in urine (proteinuria). The usual daily limits of proteinuria are as follows: normal <30 mg, microalbuminuria 30–300 mg and macroalbuminuria >300 mg.
Persistent hyperglycaemia generates intracellular reactive oxygen species (ROS) in mesangial and tubular epithelial cells via protein kinase C, nicotinamide adenine dinucleotide phosphate oxidase and mitochondrial metabolism. Moreover, it also upregulates transforming growth factor-β1 (Oh et al., 1998) and extracellular matrix expression (Ayo et al., 1990; Ziyadeh et al., 1994; Oh et al., 1998) in the glomerular mesangial cells. Transforming growth factor-β1, advanced glycation end products and angiotensin II can also induce ROS and these further activate stress-activated signalling pathways in diabetic kidney. Stress-activated signalling pathways such as those of nuclear factor-κB, p38 mitogen-activated protein (MAP) kinase and Jun kinases underlie the development of diabetic complications (Evans et al., 2002). It has also been reported that high glucose levels can activate the p38 MAP kinase pathway in many cell types, including renal cells (Adhikary et al., 2004; Susztak et al., 2006). There is significantly increased apoptosis in the tubular and interstitial cells during the course of progression of diabetic nephropathy (Zhang et al., 1997; Kumar et al., 2004).
Antioxidants effectively inhibit high-glucose- and H2O2-induced transforming growth factor-β1 and fibronectin upregulation, thus providing evidence that ROS play an important role in high glucose -induced renal injury (Ha and Lee, 2000; Iglesias-De La Cruz et al., 2001). Among the spices, turmeric is used as flavouring and colouring agent in the Indian diet every day and is known to possess antioxidant properties (Sharma et al., 2006b). Dietary supplementation with curcumin has been reported to have beneficial effects on diabetic nephropathy, and these have been attributed to its ability to lower blood cholesterol levels (Suresh Babu and Srinivasan, 1998). In addition, curcumin is also reported to prevent the acute renal failure and related oxidative stress caused by chronic administration of cyclosporine in rats (Tirkey et al., 2005). Moreover, curcumin also has beneficial effects in retarding glomerulosclerosis caused by adriamycin (Venkatesan et al., 2000).
On a molecular level, curcumin has been reported to cause histone hypoacetylation in human hepatoma cells, but its in vivo target molecules remain to be clarified. Recently, Meugnier et al. (2007) have shown that glucose toxicity induces a global downregulation of gene expression in humans. Moreover, a recent report suggests a role for histone deacetylase inhibitors in diabetic nephropathy (Lee et al., 2007). In addition, several reports suggest the role of post-translational modification of histones in general and particularly histone deacetylases in regulating gene expression. However, little is known regarding the role of curcumin in modulating post-translational modifications of histone H3 in kidneys during type I diabetes. Hence, the present study was designed to elucidate the role of curcumin on histones and related molecules in streptozotocin (STZ)-induced type I diabetic nephropathy.
Materials and methods
Animal treatment
All the experiments were approved by the Institutional Animal Ethics Committee and complied with the NIH guidelines on handling of experimental animals. Experiments were performed on male Sprague–Dawley rats in the weight range of 240 to 260 g, which were procured from the central animal facility of the institute, kept under controlled environmental conditions at room temperature 22±2 °C and 12 h light/dark cycles. After 1 week of acclimatization, animals were randomly divided into two groups at the start of the experiment. In the first group, type I diabetes was induced as described previously (Tikoo et al., 2007b). Briefly, diabetes was induced by injecting a single dose of STZ (55 mg kg−1, intraperitoneally, dissolved in ice-cold sodium citrate buffer (0.01 M, pH 4.4). Age-matched control rats received only sodium citrate buffer. Animals included in the study as diabetic animals had plasma glucose levels >16.7 mmol l−1 after 48 h post induction of diabetes. After 2 weeks, diabetic animals were divided into two groups, namely diabetic/control (n=6) and diabetic/treated with curcumin (50 mg kg−1 day−1, postoperatively, for 6 weeks, n=6). Along with these groups, there was one age-matched normal/control group (n=6) and one normal/curcumin-treated group (n=6). Treatment of curcumin was started from the third week and continued till the end of the eighth week (6-week treatment). Each animal in the control group received vehicle (1% sodium carboxymethyl cellulose) (2 ml kg−1 day−1, per oral).
Estimation of plasma glucose, albumin, blood urea nitrogen and creatinine
Blood samples were collected from the retro-orbital plexus of rats under mild ether anaesthesia, in heparinized centrifuge tubes and immediately centrifuged at 2300 g for separation of plasma. Plasma was stored at −80 °C until assays were performed. The plasma was used for the estimation of glucose, albumin, blood urea nitrogen (BUN) and creatinine as described previously (Tikoo et al., 2007a, 2007b).
Assessment of renal oxidative stress markers
Oxidative stress markers were measured as described previously (Tikoo et al., 2007b). The lipid peroxide level in animal tissues was measured according to the method described by Ohkawa et al. (1979). Superoxide dismutase (SOD) activity was estimated according to the method described by Paoletti and Mocali (1990).
Protein isolation and western blotting
Nuclei, histone isolation and western blotting were performed as described previously (Tikoo et al., 1997, 2007a, 2007b). Immunoblot analysis was performed using anti-acetylated histone H3 (rabbit, 1:5000; Upstate, Lake Placid, NY, USA), anti-phosphorylated histone H3 Ser10 (rabbit, 1:2000), anti-histone H3 (rabbit, 1:5000, Upstate), anti-p38 (rabbit, 1:500), anti-heat-shock protein-27 (HSP-27) (rabbit, 1:500), anti-actin (rabbit, 1:2500; Sigma, St Louis, MO, USA) and horseradish peroxidase-conjugated secondary antibodies (anti-rabbit) from Santa Cruz Biotechnology, Inc., Delaware Avenue, CA, USA. Proteins were detected with the enhanced chemiluminescence system and ECL Hyperfilm (Amersham Pharmacia Biotech, UK Ltd, Little Chalfont, Buckinghamshire, England).
Statistical analysis
Experimental values are expressed as mean±s.e.mean. Comparison of mean values between various groups was performed by one-way analysis of variance followed by multiple comparisons by Tukey's test. P-value<0.05 was considered significant.
Chemicals
All the chemicals were purchased from Sigma, unless otherwise mentioned.
Results
In this study, the STZ-treated diabetic rats developed uncontrolled type 1 insulin-dependent diabetes mellitus. All the rats had well-developed signs of diabetes after 2 weeks of STZ administration, that is, hyperglycaemia, glycosuria, polyuria, increased water consumption and weight loss.
Change in body weight and kidney weight/body weight ratio by curcumin treatment
Streptozotocin-induced diabetic animals showed significant decrease in body weight and increase in kidney weight. However, kidney weight/body weight ratio was doubled as compared with normal rats. Treatment of curcumin prevented body weight loss in diabetic rats as compared with untreated diabetic rats. In addition, it also reduced the increase in kidney weights in diabetic rats. Diabetic rats had increased kidney weight/body weight ratio, a marker for the development of diabetic nephropathy, and this ratio was significantly decreased by treatment with curcumin (Table 1).
Table 1.
Effect of curcumin on body weight (g), kidney weight (g) and kidney weight/body weight ratio in diabetic animals
| Group | Body weight (BW) (g) | Kidney weight (KW) (g) | KW/BW ratio*1000 |
|---|---|---|---|
| Normal/control | 411±13 | 0.67±0.02 | 1.7±0.03 |
| Normal/curcumin treated | 394±15 | 0.69±0.06 | 1.7±0.04 |
| Diabetic/control | 186±8*** | 1.13±0.11* | 6.0±0.05*** |
| Diabetic/curcumin treated | 215±8 | 0.89±0.04† | 3.9±0.15††† |
Body weight and kidney weight were measured after 8 weeks.
All values represent means±s.e.mean (n=6). ***P<0.001; *P<0.05, significantly different from normal/control. †††P<0.001; †P<0.05, significantly different from diabetic/control.
Effect of curcumin treatment on plasma glucose, BUN, plasma creatinine and plasma albumin
After 8 weeks of STZ treatment, plasma glucose level of diabetic rats was significantly higher than in the normal control group. Treatment with curcumin did not show any significant effect on plasma glucose level in diabetic rats (Table 2). The diabetes-induced increase in plasma creatinine/body weight ratio of diabetic animals was reduced by curcumin treatment. In addition, curcumin significantly reduced the elevated BUN of diabetic rats (Table 2). Increased plasma creatinine level and BUN are indications of the development of diabetic nephropathy in rats (Makino et al., 2002; Breyer et al., 2005). Plasma albumin level was significantly decreased in diabetic animals as compared with age-matched control rats, and this decrease was prevented by curcumin treatment. Maintenance of these biochemical variables closer to those in control rats by curcumin treatment suggests that curcumin plays a role, either directly or indirectly, in providing protection against diabetic nephropathy or delay in its development.
Table 2.
Effect of curcumin on plasma glucose (PGL), BUN, plasma creatinine (PCR)/body weight (BW) ratio and plasma albumin (PAL) in diabetic rats
| Group | PGL (mmol l−1) | BUN (mmol l−1) | PCR/BW ratio*1000 | PAL (g l−1) |
|---|---|---|---|---|
| Normal/control | 7±0.3 | 3±0.08 | 2.4±0.2 | 27±0.4 |
| Normal/curcumin treated | 7±0.2 | 3±0.09 | 2.6±0.1 | 28±0.7 |
| Diabetic/control | 31±0.8*** | 7±0.79*** | 9.7±1.1*** | 20±1.2*** |
| Diabetic/curcumin treated | 29±0.7 | 4±0.21†† | 5.9±0.5†† | 23±0.4†† |
Abbreviation: BUN, blood urea nitrogen.
Biochemical variables were estimated after 8 weeks.
All the values represent mean±s.e.mean (n=6). ***P<0.001, significantly different from normal/control. ††P<0.01, significantly different from diabetic/control.
Curcumin prevents changes in oxidative stress markers in diabetic rats
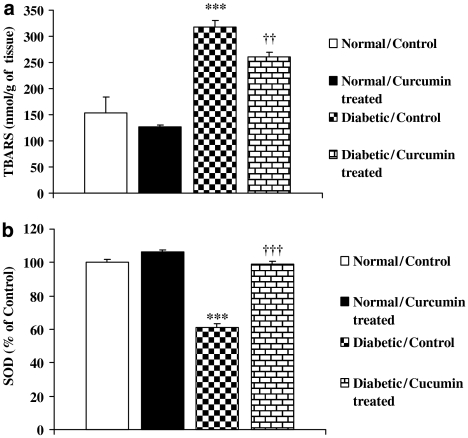
Curcumin treatment showed significant change in the levels of thiobarbituric acid-reacting substances and SOD in diabetic rat kidney. Diabetic rats show higher levels of thiobarbituric acid-reacting substances as compared with control (Figure 1a), and curcumin significantly reduced the levels of thiobarbituric acid-reacting substances in diabetic rat kidney. SOD activity in the kidneys was also significantly lower in the diabetic animals as compared with the control animals. However, treatment with curcumin prevented this decrease in SOD activity in diabetic animals (Figure 1b).
Figure 1.
Effect of curcumin on the level of thiobarbituric acid-reacting substances (a) and SOD (b) in diabetic rats. All the values represent mean±s.e.mean (n=6). ***P<0.001, significantly different from normal/control; †††P<0.001; ††P<0.01, significantly different from diabetic/control. SOD, superoxide dismutase; TBARS, thiobarbituric acid-reacting substances.
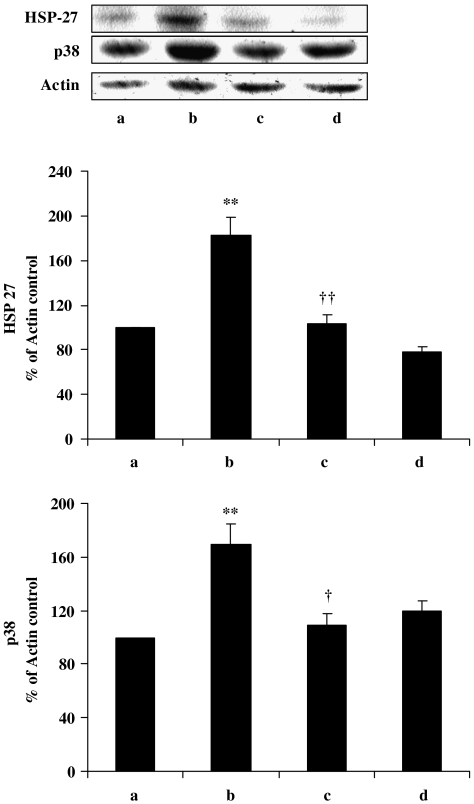
Curcumin decreases the expression of HSP-27 and p38 in STZ-induced diabetic rat kidney
Persistent hyperglycaemia has been reported to upregulate the endogenous stress protein HSP-27 (Chen et al., 2005), and this protein plays a cytoprotective role through its antioxidant, antiapoptotic and actin-stabilizing properties during cell stress (Djamali et al., 2005). Moreover, it has also been reported that hyperglycaemic conditions stimulate the activation of MAP kinase p38 (Igarashi et al., 1999). This kinase is a proapoptotic protein and plays a pathological role in diabetic condition (Susztak et al., 2006). Our data also show increased expression of HSP-27 and MAP kinase p38 in diabetic kidney. However, treatment with curcumin prevented this increase in the expression of HSP-27 and p38 (Figure 2, lanes b and c).
Figure 2.
Western blot of HSP-27 and MAP kinase p38 in kidney after treatment with curcumin in diabetic rats. Lane a, normal/control; lane b, diabetic/control; lane c, diabetic/curcumin treated and lane d, control/curcumin treated. Results were normalized with respect to actin in respective controls. Similar results were obtained in three independent sets of experiments. All the values represent mean±s.e.mean (n=3). **P<0.01, significantly different from normal/control; ††P<0.01; †P<0.05, significantly different from diabetic/control. HSP-27, heat-shock protein-27; MAP, mitogen-activated protein.
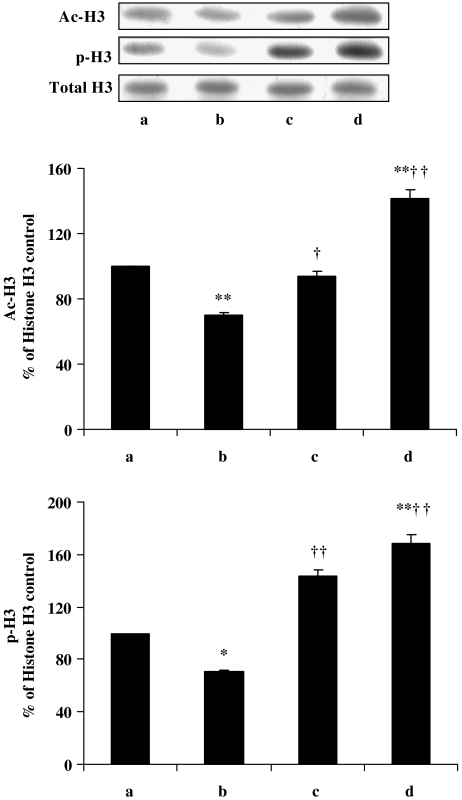
Change in histone H3 acetylation by curcumin treatment in diabetic rats
Histone acetylation plays an important role in eukaryotic gene transcription. Histone acetyltransferase and histone deacetylase are the enzymes controlling the state of histone acetylation in vivo. In vitro, curcumin at high concentrations (100 μM) causes loss of cell viability, which involves histone hypoacetylation (Kang et al., 2005). However, our in vivo results show that treatment with curcumin leads to hyperacetylation of histone H3 in diabetic rats (Figure 3, upper graph, bars b and c). Similarly, curcumin alone in control rats induces hyperacetylation of histone H3 as compared with control untreated rats (Figure 3, upper graph, bars a and d).
Figure 3.
Western blot of acetylation and phosphorylation of histone H3 in kidney after treatment with curcumin in diabetic rats. Lane a, normal/control; lane b, diabetic/control; lane c, diabetic/curcumin treated and lane d, control/curcumin treated. Results were normalized with respect to total histone H3. Similar results were obtained in three independent set of experiments. All the values represent mean±s.e.mean (n=3). **P<0.01; *P<0.05, significantly different from normal/control; ††P<0.01; †P<0.05, significantly different from diabetic/control. Ac-H3, acetylated histone-H3; p-H3, phosphorylated histone-H3.
Treatment with curcumin changes the phosphorylation of histone H3 in diabetic rats
Activation of p38 is known to be involved in the phosphorylation of histone H3, which leads to modified chromatin structure (Dong et al., 2004). Phosphorylation of histone H3 at Ser10 occurs usually when cells enter into mitosis. Several toxicants have also been shown to induce histone H3 phosphorylation, which results in premature chromatin condensation and cell death, suggesting that cells are undergoing growth arrest (Li et al., 2002). The lower graph in Figure 3 shows a significant reduction of phosphorylated histone H3, that is, increased dephosphorylation of this protein, in kidneys from diabetic rats. However, treatment with curcumin of diabetic animals prevented this dephosphorylation of histone H3. In addition, curcumin on its own increases the phosphorylation of histone H3 in control rat kidney. This change in histone H3 phosphorylation can only be explained if we assume that curcumin either directly or indirectly prevented cells undergoing mitotic arrest under diabetic conditions.
Discussion
We here provide evidence that protection against the development of diabetic nephropathy by curcumin treatment involves changes in the expression of HSP-27 and MAP kinase p38. Our data show that curcumin treatment prevented the development of diabetic nephropathy by significantly lowering BUN and plasma creatinine/body weight ratio in diabetic animals. This could be explained if there was increased clearance of blood urea and creatinine by the kidney, or if there was decreased protein degradation. Moreover, curcumin also prevented the decrease in plasma albumin concentration in diabetic rats.
Various biological actions of curcumin are mediated by inhibiting cell proliferation (Sikora et al., 1997), oxidative stress (Sharma et al., 2006b) and inflammation (Sharma et al., 2006a). Curcumin has been reported to show its cytoprotection against oxidative stress by increasing heme oxygenase activity in vascular endothelial cells (Motterlini et al., 2000). In addition, it is also reported to inhibit lipid peroxidation, resulting in protection against the cytotoxic action of hydrogen peroxide in renal epithelial cells (Cohly et al., 1998). Moreover, curcumin has been reported to mediate its effects by modulating several important molecular targets, including transcription factors (nuclear factor-κB and activating protein-1) (Brennan and O'neill, 1998; Bengmark, 2006), enzymes (cyclooxygenase-2, 5-lipooxygenase and inducible nitric oxide synthase inhibitor) (Plummer et al., 1999) and cytokines (tumour necrosis factor-α, interleukin-1, interleukin-6 and chemokines) (Bengmark, 2006).
Small heat-stress proteins, such as HSP-27, are molecular chaperones that modulate the ability of cells to respond to several types of injuries (Arrigo, 2001). Protection generated by the expression of HSP-27 against cell death induced by oxidative stress has already been described (Arrigo et al., 2005). Accumulation of abnormal proteins in the kidney and other tissues takes place due to constitutive alterations of intracellular protein recognition, assembly, and turnover in type 1 diabetes mellitus with diabetic nephropathy. In this regard, increased levels of HSP-27 in skin fibroblasts of patients with type I diabetes and diabetic nephropathy have recently been reported (Tessari et al., 2007), suggesting these changes in HSP-27 present in fibroblasts might reflect those in the kidney and be pathophysiologically related to the development of nephropathy in type 1 diabetes mellitus. Our results also indicate that there was an increase in expression of HSP-27 in diabetic kidney. However, treatment of curcumin prevented this increase in HSP-27 expression, either directly or indirectly decreasing the oxidative stress.
High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells (Allen et al., 2003, 2005) and in mesangial cells (Mishra and Simonson, 2005), which is mediated by multiple caspases that are activated by ROS, such as peroxynitrite (ONOO−). Moreover, glucose-induced ROS production initiates podocyte apoptosis and podocyte depletion in vitro and in vivo, and suggest that podocyte apoptosis/depletion represents a novel early pathomechanism(s) leading to diabetic nephropathy in murine type 1 and type 2 diabetic models (Susztak et al., 2006). Increased phosphorylation of MAP kinase p38 plays an important role in human and experimental diabetic nephropathy (Adhikary et al., 2004). In the present study, we also found an increase in MAP kinase p38 expression in diabetic kidney. These results are consistent with our previous report (Tikoo et al., 2007b). Moreover, treatment with curcumin significantly reduced the expression of this kinase in diabetic kidney, indicating its antiapoptotic effect. Changes in the expression of HSP-27 and MAP kinase p38 clearly suggested that curcumin was preventing the cells from undergoing apoptosis, which results in the development of diabetic nephropathy.
Recently, global gene expression analysis in healthy men during a hyperglycaemic–euglycaemic clamp, suggested that modifications of gene expression could be an additional effect of glucose toxicity in vivo (Meugnier et al., 2007). Acetylation of histones and non-histone proteins is an important post-translational modification involved in the regulation of gene expression in mammalian cells. Dysfunction of histone acetyltransferase is often associated with the manifestation of several diseases (Kang et al., 2006). Recent studies showed that oxidative stress could also regulate gene transcription by differentially affecting changes in histone modifications. Oxidative stress and proinflammatory mediators have been suggested to influence histone acetylation and phosphorylation, via a mechanism dependent on the activation of the MAP kinase pathway (Rahman et al., 2004). Recently, we have reported the dephosphorylation of histone H3 and the activation of MAP kinase p38 in diabetic kidney (Tikoo et al., 2007b). Similarly, in the present study, we also observed a decrease in histone H3 phosphorylation (Ser10) in the diabetic rat kidney. Interestingly, this decrease in phosphorylation of histone H3 is prevented by curcumin treatment, suggesting that cells were not undergoing mitotic arrest.
Phosphorylation of histone H3 at Ser10 facilitates the transcription of immediate-early genes (Mahadevan et al., 1991; Thomson et al., 1999; Labrador and Corces, 2003; Soloaga et al., 2003), whereas during mitosis such phosphorylation facilitates chromosome remodelling and condensation (Wei et al., 1998, 1999; Squires et al., 2003). However, phosphorylation of H3 and apoptotic chromosome condensation are unrelated events and chromosome condensation can occur without phosphorylation of Ser10 (Hendzel et al., 1998). Thus, decreased phosphorylation of histone H3 under diabetic conditions does not necessarily imply that cells are protected against apoptotic cell death. However, these results do demonstrate the regulatory role of histone phosphatases and histone kinases in diabetic conditions. The increase in phosphorylation of histone H3 with curcumin treatment might be due to specific inhibition of histone phosphatases or activation of histone kinases. Our data provide indirect evidence that curcumin increases the phosphorylation of histone H3, which is not histone kinase-dependent, but is dependent on histone phosphatase.
Until now, there have been no reports regarding the effect of curcumin on histone H3 acetylation in diabetic kidney. Several reports indicate that oxidative stress induces hypoacetylation of histones (Kang et al., 2005), which may lead to a decrease in eukaryotic gene transcription and cell-cycle arrest. In contrast to the report that curcumin induces hypoacetylation of histone H3 and H4 in neural progenitor cells (Kang et al., 2006), our in vivo data show that treatment of curcumin leads to hyperacetylation of histone H3 in diabetic kidney, as well as in control rat kidney. This can be explained because of the differences in the two cell systems. Increase in histone acetylation suggests an increase the transcription of various genes. Results of the present study suggest that curcumin treatment increased transcription of certain genes, which are repressed under diabetic conditions and may be involved in the pathophysiology of the disease. However, further studies are required to warrant any conclusion.
In conclusion, our results show for the first time that protection observed in the development of diabetic nephropathy by curcumin treatment in STZ-induced diabetic rats involves changes in post-translational modifications of histone H3 and also changes in the expression of HSP-27 and p38.
Acknowledgments
This work was supported by grant from Department of Biotechnology, Government of India (Grant BT/PR 4005/BRB/10/331/2003) and National Institute of Pharmaceutical Education and Research (NIPER).
Abbreviations
- BUN
blood urea nitrogen
- HSP
heat-shock protein
- MAP
mitogen-activated protein
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STZ
streptozotocin
Conflict of interest
The authors state no conflict of interest.
References
- Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–1222. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17:908–910. doi: 10.1096/fj.02-0130fje. [DOI] [PubMed] [Google Scholar]
- Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16:705–713. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Firdaus WJ, Mellier G, Moulin M, Paul C, Diaz-Latoud C, et al. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods. 2005;35:126–138. doi: 10.1016/j.ymeth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ayo SH, Radnik RA, Garoni JA, Glass WF, Kreisberg JI. High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol. 1990;136:1339–1348. [PMC free article] [PubMed] [Google Scholar]
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- Brennan P, O'neill LA. Inhibition of nuclear factor kappaB by direct modification in whole cells—mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers. Biochem Pharmacol. 1998;55:965–973. doi: 10.1016/s0006-2952(97)00535-2. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Bottinger E, Brosius FC, III, Coffman TM, Harris RC, Heilig CW, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- Chen H, Wu XJ, Lu XY, Zhu L, Wang LP, Yang HT, et al. Phosphorylated heat shock protein 27 is involved in enhanced heart tolerance to ischemia in short-term type 1 diabetic rats. Acta Pharmacol Sin. 2005;26:806–812. doi: 10.1111/j.1745-7254.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Cohly HH, Taylor A, Angel MF, Salahudeen AK. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24:49–54. doi: 10.1016/s0891-5849(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Djamali A, Reese S, Oberley T, Hullett D, Becker B. Heat shock protein 27 in chronic allograft nephropathy: a local stress response. Transplantation. 2005;79:1645–1657. doi: 10.1097/01.tp.0000164319.83159.a7. [DOI] [PubMed] [Google Scholar]
- Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol. 2004;287:F1049–F1058. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl. 2000;77:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Nishioka WK, Raymond Y, Allis CD, Bazett-Jones DP, Th'ng JP. Chromatin condensation is not associated with apoptosis. J Biol Chem. 1998;273:24470–24478. doi: 10.1074/jbc.273.38.24470. [DOI] [PubMed] [Google Scholar]
- Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, et al. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem Pharmacol. 2005;69:1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–174. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem. 2004;259:67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- Labrador M, Corces VG. Phosphorylation of histone H3 during transcriptional activation depends on promoter structure. Genes Dev. 2003;17:43–48. doi: 10.1101/gad.1021403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Noh H, Seo JY, Yu MR, Ha H. Histone deacetylase inhibitors: a novel class of therapeutic agents in diabetic nephropathy. Kidney Int Suppl. 2007;106:S61–S66. doi: 10.1038/sj.ki.5002388. [DOI] [PubMed] [Google Scholar]
- Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991;65:775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- Makino H, Tanaka I, Mukoyama M, Sugawara A, Mori K, Muro S, et al. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–1765. doi: 10.1097/01.asn.0000019782.37851.bf. [DOI] [PubMed] [Google Scholar]
- Meugnier E, Faraj M, Rome S, Beauregard G, Michaut A, Pelloux V, et al. Acute hyperglycemia induces a global downregulation of gene expression in adipose tissue and skeletal muscle of healthy subjects. Diabetes. 2007;56:992–999. doi: 10.2337/db06-1242. [DOI] [PubMed] [Google Scholar]
- Mishra R, Simonson MS. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol. 2005;4:2. doi: 10.1186/1475-2840-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998;54:1872–1878. doi: 10.1046/j.1523-1755.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006a;536:256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006b;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- Sikora E, Bielak-Zmijewska A, Piwocka K, Skierski J, Radziszewska E. Inhibition of proliferation and apoptosis of human and rat T lymphocytes by curcumin, a curry pigment. Biochem Pharmacol. 1997;54:899–907. doi: 10.1016/s0006-2952(97)00251-7. [DOI] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, et al. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol. 2003;65:361–376. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- Suresh Babu P, Srinivasan K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol Cell Biochem. 1998;181:87–96. doi: 10.1023/a:1006821828706. [DOI] [PubMed] [Google Scholar]
- Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- Tessari P, Puricelli L, Iori E, Arrigoni G, Vedovato M, James P, et al. Altered chaperone and protein turnover regulators expression in cultured skin fibroblasts from type 1 diabetes mellitus with nephropathy. J Proteome Res. 2007;6:976–986. doi: 10.1021/pr060443n. [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative map kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo K, Bhatt DK, Gaikwad AB, Sharma V, Kabra DG. Differential effects of tannic acid on cisplatin induced nephrotoxicity in rats. FEBS Lett. 2007a;581:2027–2035. doi: 10.1016/j.febslet.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Tikoo K, Gupta S, Hamid QA, Shah V, Chatterjee B, Ali Z. Structure of active chromatin: isolation and characterization of transcriptionally active chromatin from rat liver. Biochem J. 1997;322 Part 1:273–279. doi: 10.1042/bj3220273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett. 2007b;581:1071–1078. doi: 10.1016/j.febslet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tirkey N, Kaur G, Vij G, Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005;5:15. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N, Punithavathi D, Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br J Pharmacol. 2000;129:231–234. doi: 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Khanna P, Chan LL, Campbell G, Ansari NH. Diabetes-induced apoptosis in rat kidney. Biochem Mol Med. 1997;61:58–62. doi: 10.1006/bmme.1997.2592. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]